Abstract
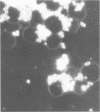
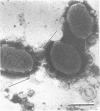
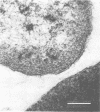
A total of 53 isolates of Bordetella bronchiseptica from dogs and pigs were tested for their ability to agglutinate chicken, horse, sheep, dog, pig, and guinea pig erythrocytes. No differences in hemagglutinating activity were attributed to the animal origin of the bordetella isolates. Horse and dog erythrocytes consistently resulted in the strongest hemagglutination reactions, whereas only 4% of the B. bronchiseptica isolates produced weak agglutination of chicken erythrocytes. A total of 85% of the isolates agglutinated horse, sheep, dog, pig, and guinea pig erythrocytes. One canine isolate with hemagglutinating activity, strain 110H, was examined to determine the nature of the hemagglutinin(s) involved. Hemagglutination was always accompanied by hemadsorption, as determined by dark-field or phase-contrast microscopy. Treatment of cells and cell extracts with heat or protease K inhibited the hemagglutination reaction. Sonicated bacterial cells had a greater hemagglutinating ability than did unsonicated live bacteria. The hemagglutination reaction was not inhibited by any of 17 sugars nor by N- acetylglucosamine or ethylene glycol-bis-(β-aminoethyl ether)-N, N-tetraacetic acid. Hemagglutinins were not detected in sonic extracts nor in several bacterial subunit fractions, including isolated pili. Antigens in some of these preparations were, however, detectable by indirect hemagglutination with anti-B. bronchiseptica serum. Isolated pili could not be detected on the erythrocyte surface by electron microscopy; however, serial sections of erythrocytes agglutinated by the live Bordetella organisms showed that the bacterial outer membrane and the erythrocyte surface were separated by a space of approximately 20 nm. This study provided additional circumstantial evidence that B. bronchiseptica pili or at least heat-labile surface proteins which extend some distance from the bacterial surface are involved in hemagglutination. Multiple hemagglutinins are likely to exist within this species since one isolate lacking pili also agglutinated canine erthyrocytes. The hemagglutinins of B. bronchiseptica need to be isolated and characterized before the hemagglutination reaction can be applied to studies of attachment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Munoz J. J. Purification and crystallization of fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1979 Jul;25(1):460–462. doi: 10.1128/iai.25.1.460-462.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis D. A., Greisen H. A., Appel M. J. Bacteriological variation among Bordetella bronchiseptica isolates from dogs and other species. J Clin Microbiol. 1977 Apr;5(4):471–480. doi: 10.1128/jcm.5.4.471-480.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis D. A., Greisen H. A., Appel M. J. Pathogenesis of canine bordetellosis. J Infect Dis. 1977 May;135(5):753–762. doi: 10.1093/infdis/135.5.753. [DOI] [PubMed] [Google Scholar]
- Bemis D. A., Kennedy J. R. An improved system for studying the effect of Bordetella bronchiseptica on the ciliary activity of canine tracheal epithelial cells. J Infect Dis. 1981 Oct;144(4):349–357. doi: 10.1093/infdis/144.4.349. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M. R., Sellwood R., Gibbons R. A. Haemagglutinating and adhesive properties associated with the K99 antigen of bovine strains of Escherichia coli. J Gen Microbiol. 1976 Oct;96(2):269–275. doi: 10.1099/00221287-96-2-269. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Höhne C., Noble M. A., Haldane E. V., Lior H., Young L. S. Hemolysin and K antigens in relation to serotype and hemagglutination type of Escherichia coli isolated from extraintestinal infections. J Clin Microbiol. 1981 Jan;13(1):171–178. doi: 10.1128/jcm.13.1.171-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLAGHER G. L. ISOLATION OF BORDETELLA BRONCHISEPTICA FROM HORSES. Vet Rec. 1965 May 29;77:632–633. [PubMed] [Google Scholar]
- Heckels J. E., Blackett B., Everson J. S., Ward M. E. The influence of surface charge on the attachment of Neisseria gonorrhoeae to human cells. J Gen Microbiol. 1976 Oct;96(2):359–364. doi: 10.1099/00221287-96-2-359. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. K., Koshimizu K., Ogata M. Studies on the etiology of infectious atrophic rhinitis of swine. II. Agglutination test on Bordetella bronchiseptica infection. Nihon Juigaku Zasshi. 1970 Dec;32(6):295–306. doi: 10.1292/jvms1939.32.295. [DOI] [PubMed] [Google Scholar]
- Koransky J. R., Scales R. W., Kraus S. J. Bacterial hemagglutination by Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):495–498. doi: 10.1128/iai.12.3.495-498.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASRY F. L. G. Production, extraction and purification of haemagglutinin of Haemophilus pertussis. J Gen Microbiol. 1952 Nov;7(3-4):201–210. doi: 10.1099/00221287-7-3-4-201. [DOI] [PubMed] [Google Scholar]
- Old D. C., Scott S. S. Hemagglutinins and fimbriae of Providencia spp. J Bacteriol. 1981 Apr;146(1):404–408. doi: 10.1128/jb.146.1.404-408.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry S. H., Porter P. Immunological aspects of cell membrane adhesion demonstrated by porcine enteropathogenic Escherichia coli. Immunology. 1978 Jan;34(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E. Hemagglutination by Neisseria meningitidis. Can J Microbiol. 1981 Jun;27(6):586–593. doi: 10.1139/m81-089. [DOI] [PubMed] [Google Scholar]
- Sedlock D. M., Bartus H. F., Zajac I., Actor P. Analysis of parameters affecting the hemagglutination activity of Escherichia coli possessing colonization factor antigens: improved medium for observing erythrocyte agglutination. J Clin Microbiol. 1981 Feb;13(2):301–308. doi: 10.1128/jcm.13.2.301-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIBAULT P., SZTURM-RUBINSTEN S., PIECHAUD-BOURBON D. Haemophilus bronchisepticus et Alcaligenes faecalis. I. De quelques caractères différentiels. Ann Inst Pasteur (Paris) 1955 Feb;88(2):246–250. [PubMed] [Google Scholar]
- Yokomizo Y., Shimizu T. Adherence of Bordetella bronchiseptica to swine nasal epithelial cells and its possible role in virulence. Res Vet Sci. 1979 Jul;27(1):15–21. [PubMed] [Google Scholar]