Abstract
Age-related macular degeneration (AMD) causes severe vision loss in the elderly; early identification of AMD risk could help slow or prevent disease progression. Toward the discovery of AMD biomarkers, we quantified plasma protein Nε-carboxymethyllysine (CML) and pentosidine from 58 AMD and 32 control donors. CML and pentosidine are advanced glycation end products that are abundant in Bruch membrane, the extracellular matrix separating the retinal pigment epithelium from the blood-bearing choriocapillaris. We measured CML and pentosidine by LC-MS/MS and LC-fluorometry, respectively, and found higher mean levels of CML (∼54%) and pentosidine (∼64%) in AMD (p < 0.0001) relative to normal controls. Plasma protein fructosyl-lysine, a marker of early glycation, was found by amino acid analysis to be in equal amounts in control and non-diabetic AMD donors, supporting an association between AMD and increased levels of CML and pentosidine independent of other diseases like diabetes. Carboxyethylpyrrole (CEP), an oxidative modification from docosahexaenoate-containing lipids and also abundant in AMD Bruch membrane, was elevated ∼86% in the AMD cohort, but autoantibody titers to CEP, CML, and pentosidine were not significantly increased. Compellingly higher mean levels of CML and pentosidine were present in AMD plasma protein over a broad age range. Receiver operating curves indicate that CML, CEP adducts, and pentosidine alone discriminated between AMD and control subjects with 78, 79, and 88% accuracy, respectively, whereas CML in combination with pentosidine provided ∼89% accuracy, and CEP plus pentosidine provided ∼92% accuracy. Pentosidine levels appeared slightly altered in AMD patients with hypertension and cardiovascular disease, indicating further studies are warranted. Overall this study supports the potential utility of plasma protein CML and pentosidine as biomarkers for assessing AMD risk and susceptibility, particularly in combination with CEP adducts and with concurrent analyses of fructosyl-lysine to detect confounding factors.
Age-related macular degeneration (AMD)1 is a progressive, multifactorial disease and a major cause of severe vision loss in the elderly (1). Deposition of debris (drusen) in the macular region of Bruch membrane, the extracellular matrix separating the choriocapillaris from the retinal pigment epithelium (RPE), is an early, hallmark risk factor of AMD. The disease can progress to advanced dry AMD (geographic atrophy), which is characterized by regional degeneration of photoreceptor and RPE cells, or to advanced wet AMD (choroidal neovascularization (CNV)), which is characterized by abnormal blood vessels growing from the choriocapillaris through Bruch membrane beneath the retina. CNV accounts for over 80% of debilitating vision loss in AMD; however, only 10–15% of AMD cases progress to CNV.
There is growing consensus that AMD is an age-related inflammatory disease involving dysregulation of the complement system; however, triggers of the inflammatory response have yet to be well defined. Oxidative stress appears to be involved as smoking significantly increases the risk of AMD (2), antioxidant vitamins can selectively slow AMD progression (3), and a host of oxidative protein and DNA modifications have been detected at elevated levels in AMD Bruch membrane, drusen, retina, RPE, and plasma (4–11). Oxidative protein modifications like carboxyethylpyrrole (CEP) and Nε-carboxymethyllysine (CML), both elevated in AMD Bruch membrane, stimulate neovascularization in vivo (12, 13), suggesting possible roles in CNV. Other studies have shown that mice immunized with CEP protein modifications develop an AMD-like phenotype (14). Accordingly oxidative modifications may be catalysts or triggers of AMD pathology (6).
AMD has long been hypothesized to be a systemic disease (15) based in part on the presence of retinal drusen in patients with membranoproliferative glomerulonephritis type II (16) and systemic complement activation in AMD (17). Support for this hypothesis also comes from mounting evidence that advanced glycation end products (AGEs) may play a role in AMD (4, 5, 7, 18, 19). AGEs are a heterogeneous group of mostly oxidative modifications resulting from the Maillard nonenzymatic glycation reaction that have been associated with age-related diseases and diabetic complications (20, 21). In 1998, CML was the first AGE to be found in AMD Bruch membrane and drusen (4). Other AGEs have since been detected in AMD ocular tissues (5, 7, 18) and in Bruch membrane, drusen, RPE, and choroidal extracellular matrix from healthy eyes (6, 22). CML, a nonfluorescent AGE, and pentosidine, a fluorescent cross-linking AGE, increase with age in Bruch membrane (18, 23). Receptors for AGEs (RAGE and AGE-R1) appear elevated on RPE and photoreceptor cells in early and advanced dry AMD (7) especially in RPE overlying drusen-like deposits on Bruch membrane (19). AGE-R3, also known as galectin-3, is elevated in AMD Bruch membrane (24).
Although AMD susceptibility genes now account for over 50% of AMD cases (25), many individuals with AMD risk genotypes may never develop advanced disease with severe vision loss. Nevertheless the prevalence of advanced AMD is increasing (26). Toward the discovery of better methods to detect those at risk for advanced AMD, we quantified CML and pentosidine in plasma proteins from AMD and control patients and compared their discriminatory accuracy with plasma CEP biomarkers. CEP biomarkers have been shown to enhance the AMD predictive accuracy of genomic AMD biomarkers (11). This report shows CML and pentosidine to be elevated in AMD plasma proteins and demonstrates their potential biomarker utility in assessing AMD risk and susceptibility especially in combination with CEP biomarkers.
EXPERIMENTAL PROCEDURES
Case-Control Study Design
Clinically documented AMD and control blood donors were recruited prospectively between 2005 and 2008 from the Cole Eye Institute, Cleveland Clinic Foundation with Institutional Review Board approval and according to Declaration of Helsinki principles. All patients received a comprehensive eye examination by a clinician in the Clinical Study Group and provided written informed consent. Blood samples were collected after clinical examination and diagnosis and without the possibility of systematic bias. Human identifiers were removed, and the specimens were encoded by the Clinical Study Group to protect donor confidentiality. The study design a priori was to compare CML and pentosidine plasma protein levels in a statistically significant number of control, early/mid-stage AMD, and advanced AMD plasma from age- and gender-matched donors. AMD disease progression was categorized based on fundus examination, and patients were included in the study from Age-Related Eye Disease Study AMD categories 2, 3, and 4 (3). Briefly early/mid-stage AMD patients (Age-Related Eye Disease Study categories 2 and 3) exhibited multiple small to intermediate drusen in the macula or one or more large drusen, RPE pigmentary abnormalities, or any combination of these in one or both eyes or geographic atrophy that did not involve the macula and at least one eye having visual acuity 20/30 or better. AMD category 4 patients exhibited advanced AMD with substantial CNV or geographic atrophy involving the macula in one or both eyes; this cohort included 28 CNV and three geographic atrophy patients. Control donors lacked macular drusen and exhibited no clinical evidence of any retinal disorder. Study population characteristics are summarized in Table I, including age, gender, race, smoking status, and health history.
Table I. Characteristics of the study population.
| Property and category | Control (n = 32) | Early/mid-stage AMD (n = 27) | Advanced AMD (n = 31) |
|---|---|---|---|
| Age (year) | |||
| Mean ± S.D. | 72 ± 6 | 73 ± 9 | 74 ± 6 |
| Range | 56–84 | 57–91 | 64–89 |
| Gender | |||
| Male | 15 (46.9%) | 14 (51.9%) | 16 (51.6%) |
| Female | 17 (53.1%) | 13 (48.1%) | 15 (48.4%) |
| Race | |||
| Caucasian | 32 (100%) | 27 (100%) | 31 (100%) |
| Smoking status | |||
| Non-smoker | 30 (93.8%) | 21 (77.8%) | 30 (96.8%) |
| Smoker | 2 (6.2%) | 6 (22.2%) | 1 (3.2%) |
| Health history | |||
| Hypertension | 21 (65.6%) | 14 (51.9%) | 20 (64.5%) |
| Hyperlipidemia | 15 (46.9%) | 14 (51.9%) | 10 (32.3%) |
| Diabetes | 0 (0.0%) | 3 (11.1%) | 2 (6.5%) |
| Cardiovascular disease | 6 (18.8%) | 7 (25.9%) | 5 (16.1%) |
Human Plasma Preparation
Nonfasting blood specimens were collected in BD Vacutainer® K2EDTA tubes, and plasma was prepared within 6 h and aliquoted to vials containing the antioxidant butylated hydroxytoluene (22 μg/ml plasma) and a protease inhibitor mixture (Sigma product number P 8340; 10 μl/ml plasma) (11). The plasma was flushed with argon, quench frozen in liquid nitrogen immediately, and stored at −80 °C until analysis. For the plasma used in this study storage time at −80 °C ranged from 4 to 34 months and averaged 13 months. All samples were frozen and thawed only once.
Sample Preparation and Amino Acid Analysis
Plasma (∼200 μl; ∼10-mg protein) was transferred to 6 × 50-mm glass hydrolysis tubes, and protein was precipitated with 2 volumes of cold acetone. After incubation at 4 °C for 10–20 min, the preparation was centrifuged briefly in a Microfuge, the supernatant was discarded, and the pellet was washed once with 67% acetone (400 μl) and vacuum-dried (27). Plasma protein was prepared for hydrolysis by adding 60 μl of 6 n HCl to each dried pellet, and then the hydrolysis tubes were placed in a 40-ml screw cap vial containing ∼300 μl of 6 n HCl with a few small crystals of phenol. The 40-ml vial was capped with a Mininert slide valve, the valve was connected to a vacuum pump and argon source via a three-way stopcock, and the vial was alternately evacuated and flushed with argon three times and then sealed under vacuum (27). Protein was hydrolyzed at 110 °C for 16 h, then vacuum-dried, flushed with argon, and stored at −20 °C until analysis. Protein was quantified by PTC amino acid analysis (∼80 μg was derivatized, and ∼3 μg was analyzed) using an Agilent 1100 HPLC system, a Haisil PTC C18 column (220 × 2.1 mm; Applied Biosystems), and a Gilson model 116 UV detector (27). Protein was also quantified by AccQ·TagTM amino acid analysis (∼3.5 μg was derivatized, and ∼35 ng was analyzed) using an Acquity Ultra Performance LC system (Waters) and AccQ·Tag Ultra column (100 × 2.1 mm) according to the vendor (28). Bovine serum albumin from the National Bureau of Standards was used as a protein standard and hydrolysis control. Amino acid calibration standards were obtained from Pierce and Thermo Scientific.
Fructosyl-lysine Quantification
Fructosyl-lysine was quantified as furosine derivative (ε-N-(2-furoylmethyl)lysine) by duplicate AccQ·Tag amino acid analyses (∼15 μg was derivatized, and ∼2.7 μg was analyzed) using the Acquity LC system (Waters) described above. Furosine contains a primary and a secondary amino group, and both are derivatized by the AccQ·Tag reagent, yielding two different monoderivatized forms and a diderivatized species. The apparent diderivatized species was well separated from other amino acids, exhibited a constant response factor up to ∼55 pmol derivatized, and was used for quantification of furosine in protein hydrolysates. Furosine standard was purchased from NeoMPS, Inc.
CML and Pentosidine Quantification
Plasma protein HCl hydrolysates (∼8 mg in 40 μl of H2O) were spiked with a S-β(4-pyridylethyl)-l-cysteine (PEC) internal standard (50 pmol) and fractionated on LC system 1 composed of an Agilent 1100 HPLC system, a HypercarbTM porous graphite carbon column (5-μm particles, 50 × 10 mm; Thermo Scientific) maintained at 30 °C with an Applied Biosystems 112A column oven, and aqueous trifluoroacetic acid/acetonitrile solvents using gradient elution (0–100% acetonitrile over 13 min) and a flow rate of 1 ml/min. The eluant was monitored for fluorescence (335-nm excitation, 385-nm emission) with a WatersTM 474 scanning fluorometer and initially split with 20% directed to an API 3000 triple quadrupole electrospray mass spectrometer (Applied Biosystems) and 80% directed to a fraction collector. After determining reproducible elution times using control plasma spiked with standard AGEs and PEC, 100% of the eluant was directed to the fraction collector and three fractions were collected, one each for CML, PEC, and the co-eluting pentosidine plus argpyrimidine. The fractions were vacuum dried and re-fractionated on LC system 2 composed of the same HPLC equipment but with an aqueous normal phase Cogent Diamond Hydride™ Column (4.2 μm particles, 150 × 2.1 mm) used at room temperature. Fractions containing CML were re-chromatographed using aqueous acetic acid/acetonitrile solvents and gradient elution (95–0% acetonitrile in 13.6 min) at a flow rate of 400 μl/min. Fractions containing PEC, argpyrimidine and pentosidine were re-chromatographed using 10 mm ammonium acetate, pH 6.0/acetonitrile solvents, gradient elution (100–0% acetonitrile in 15 min) at a flow rate of 400 μl/min. Aqueous normal phase chromatography was monitored by fluorescence detection followed by 100% of the eluant directed to the mass spectrometer. CML and PEC were quantified by multiple reaction monitoring (MRM), and pentosidine and argpyrimidine were measured by fluorescence; final CML and pentosidine amounts were adjusted based on the recovery of the PEC internal standard. Plasma protein argpyrimidine concentrations were below reliable detection limits in this analytical system and are not reported. Calibration curves were developed in triplicate each day of analysis using LC system 2 and external standards. CML standard was purchased from NeoMPS, Inc., pentosidine was obtained from the International Maillard Reaction Society (Case Western Reserve University, Cleveland, OH), argpyrimidine was prepared in our laboratories by R. H. N., and PEC was purchased from Sigma.
Mass Spectrometry
The mass spectrometer was operated with Analyst 1.4.1 software (Applied Biosystems); MS/MS spectra were generated on singly charged precursor ions for CML, pentosidine, argpyrimidine, and PEC; and specific transition ions for each modified amino acid were analyzed by MRM. The declustering potential, focusing potential, collision energy, and exit potential were optimized for each ion to ±0.1 Da and ±1 V. Ion spray voltage was set at 5300 V, and source temperature was set at 425 °C in LC system 1 and at 490 °C in LC system 2. The m/z of the precursor ions and each of the transitions ions and their optimized voltages were transcribed, respectively, into an LCsync method in the Analyst 1.4.1 software. CML was quantified by MRM using precursor ion 205.1 and product ion 84.1, PEC was quantified using precursor ion 227.3 and product ion 106.1, pentosidine was monitored by MRM using precursor ion 379.2 and product ion 187.2, and argpyrimidine was monitored using precursor ion 255.2 and product ion 237.3. Peak areas and external standard calibration curves were used for quantification of CML and PEC by MRM and of pentosidine by fluorescence.
Pentosidine and CML Autoantibody Assays
Pentosidine autoantibody titers were measured by direct ELISA using pentosidine conjugated to bovine serum albumin (pentosidine-BSA) or unmodified BSA as the coating antigen (∼8 μg/well). CML autoantibody titers were measured with the same methodology using CML-BSA or BSA as the coating agent (∼15 μg/well). Plasma (100 μl of a 1:10 dilution) was applied to coating agents, and the ELISA was developed as described previously for the CEP autoantibody assay (9, 11). Anti-CML (R&D Systems product MAB3247) or anti-pentosidine monoclonal antibodies (Trans Genic Inc. product KH012) were used for positive ELISA controls. Titer was defined as the ratio of plasma binding to antigen (A) versus binding to BSA (A0) (9).
Preparation of AGE-BSA
Pentosidine-BSA was prepared by mixing pentosidine (1 mg; NeoMPS, Inc. product SC1535) in dimethylformamide (100 μl) with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl (5 mg; Pierce) and N-hydroxysuccinimide (6 mg; Pierce) at room temperature for 2 h followed by the addition of BSA (2.5 mg in 0.5 ml PBS; Sigma product A6003) and continued incubation at 37 °C for 48 h. The reaction was stopped by dialysis against 25 mm Tris-HCl (pH 8.0) for 2 h at 4 °C and then dialysis against PBS for another 24 h with two changes. CML-BSA was prepared by mixing BSA in PBS with 25 mm glyoxylic acid and 50 mm NaCNBH3 for 48 h at room temperature followed by dialysis against PBS for 24 h with two changes (29). Protein was quantified by the BCA assay (Pierce), and pentosidine and CML modifications were confirmed by Western analysis before and after the modification reactions using anti-CML monoclonal antibody (R&D Systems product MAB3247) or anti-pentosidine monoclonal antibody (Trans Genic Inc. product KH012).
CEP Adduct and CEP Autoantibody Assays
The CEP adduct and autoantibody concentrations of plasma used in this study were reported previously among 1404 plasma samples (11). Briefly CEP adducts were quantified with a competitive ELISA using rabbit anti-CEP polyclonal antibody, CEP conjugated to BSA as coating agent, and known amounts of CEP-modified human serum albumin as reference protein. CEP autoantibody titers were measured by direct ELISA using CEP-BSA as coating antigen. These methods are well documented (9, 11).
Statistics
Continuous measures were summarized using means, standard deviations, medians, and interquartile ranges, whereas categorical factors were described using frequencies and percentages. Differences between control and AMD patients in plasma concentrations of CML, pentosidine, and CEP adducts as well as in CML, pentosidine, and CEP autoantibodies were evaluated using two-sample t tests in Excel 2003 (Microsoft® Office). To evaluate a relationship between CML and pentosidine with AMD susceptibility, a logistic regression model was fit with both variables as predictors using SAS 9.1 software (SAS Institute). C-statistics measured the ability of the model to discriminate between AMD and controls, and odds ratios (ORs) showed the change in risk of AMD based on the predictors. ORs, c-statistics, and p values were determined based on log-transformed marker concentrations. Validation of c-statistics was performed using 2000 bootstrap (random) resamplings to calculate empirical 95% confidence intervals (CIs) and by performing 10-fold cross-validation. Sensitivity and specificity were calculated to maximize the sum of the two values using receiver operating characteristic (ROC) curves constructed with SAS 9.1 from the output of logistic regression analysis fit with either CML, pentosidine, or CEP adducts alone or in combination. C-statistics and p values comparing ROC curves were determined with SAS 9.1. For association analyses of combined effects of CML and pentosidine with CEP adducts, ORs with 95% CI and Fisher exact p values were also calculated with SAS 9.1 software. Pearson's correlation analysis in Minitab Release 15 (Minitab Inc.) was used to compare concentrations of AGE markers with plasma donor age.
RESULTS
Elevated CML and Pentosidine in AMD Plasma
Fluorometry and MRM tandem mass spectrometry coupled with two LC separations were used to quantify plasma protein-bound AGEs. MS/MS spectra used to identify high intensity product ions for MRM monitoring of CML, pentosidine, argpyrimidine, and the PEC internal standard are shown in supplemental Fig. 1. Representative extracted ion and fluorescence LC profiles are shown for LC system 1 in supplemental Fig. 2 and for LC system 2 in supplemental Fig. 3. MRM standard calibration curves for CML and PEC and fluorescence calibration curves for pentosidine exhibited excellent linearity (supplemental Fig. 4).
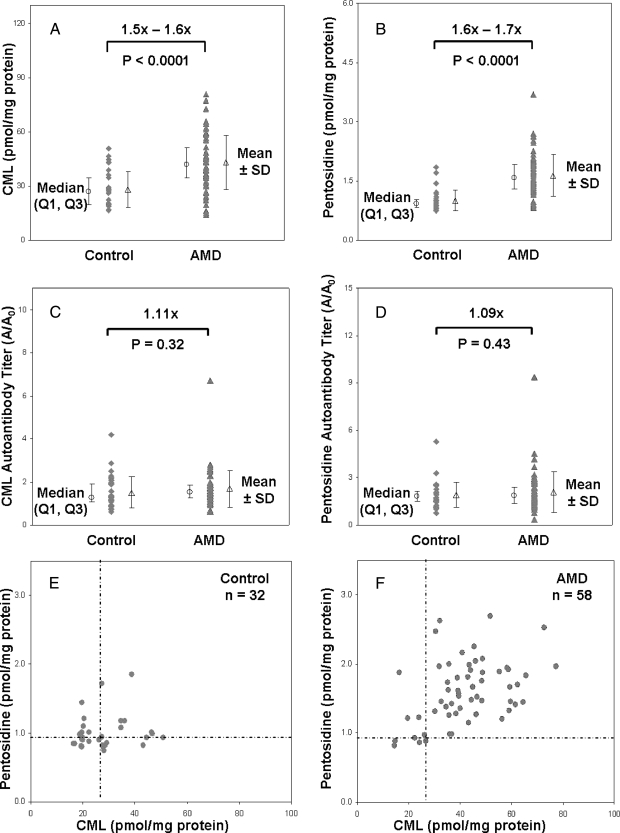
Plasma from a total of 32 control subjects and 58 AMD patients was analyzed, including 27 early/mid-stage dry AMD and 31 advanced AMD patients. Protein was quantified by both PTC and AccQ·Tag amino acid analysis, yielding well defined protein concentrations with excellent agreement between the amino acid analysis methods (<4% average difference). Amounts of CML and pentosidine in each HCl hydrolysate were corrected based on the percent recovery of the PEC internal standard that averaged 70.1 ± 12.4 (mean ± S.D., n = 90). Overall AMD patients exhibited ∼54% higher CML and ∼64% higher pentosidine concentrations relative to control plasma (Fig. 1, A and B, and Table II). Comparison of log-transformed values confirmed the results with p < 0.0001 for both AGEs. Mean variability averaged ∼36% relative standard deviation (R.S.D.) for CML and ∼24% R.S.D. for pentosidine (n = 90). Relative to early/mid-stage AMD, advanced AMD patients exhibited ∼9% higher CML (p = 0.35) and ∼17% higher pentosidine (p = 0.06) (Table II). CML and pentosidine autoantibody titers measured by ELISA were ∼9–11% higher in AMD patients; however, these differences were not statistically significant (Fig. 1, C and D).
Fig. 1.
CML and pentosidine are elevated in AMD plasma. CML (A) and pentosidine concentrations (B) quantified by LC-MS/MS and LC-fluorometry from control (n = 32) and AMD (n = 58) plasma donors and autoantibody titers for CML (C) and pentosidine (D) quantified by ELISA (32 control and 57 AMD plasma samples) are shown with median (○) results ±first and third quartiles (Q1, Q3) and mean (△) results ±S.D. p values (two-sided t test) were determined from log-transformed concentrations. Correlation between CML and pentosidine concentrations is shown for the control (E) and AMD (F) cohorts with horizontal and vertical dashed lines indicating median control values. Significantly more donors with both CML and pentosidine elevated are apparent in AMD patients than in the controls (upper right quadrants in E and F).
Table II. CML and pentosidine markers in control and AMD plasma.
CML and pentosidine concentrations were determined by LC-MS and LC-fluorometry; protein was quantified by amino acid analysis. Determined mean concentrations of CML expressed in fmol of CML/nmol of Lys were: control, 33.8 ± 11.9; early/mid-stage AMD, 49.8 ± 19.8; advanced AMD, 54.6 ± 17.4; and all AMD, 52.3 ± 18.5. Determined mean concentrations of pentosidine expressed in fmol of pentosidine/nmol of Lys were: control, 1.2 ± 0.3; early/mid-stage AMD, 1.8 ± 0.5; advanced AMD, 2.2 ± 0.8; and all AMD, 2.0 ± 0.7. The OR reflects the AMD risk for donors exhibiting elevated levels of either CML or pentosidine markers relative to median control levels. The p values were determined using the Fisher exact test. Odds ratios, 95% CI, and p values are based on log-transformed CML and pentosidine concentrations.
| n | CML (pmol/mg protein) |
CML elevated above control median |
||||
|---|---|---|---|---|---|---|
| Mean ± S.D. | Median (Q1, Q3) | Odds Ratio | 95% CI | p value | ||
| Control | 32 | 27.9 ± 10.0 | 26.7 (19.7, 34.7) | 1 | Reference | |
| Early/mid-stage AMD | 27 | 41.0 ± 16.1 | 39.6 (28.4, 57.3) | 2.9 | 1.0, 8.6 | 0.068 |
| Advanced AMD | 31 | 44.8 ± 14.0 | 43.3 (35.5, 48.6) | 30.0 | 3.6, 247.3 | <0.0001 |
| All AMD | 58 | 43.0 ± 15.0 | 42.0 (34.7, 51.0) | 6.3 | 2.3, 17.3 | <0.0001 |
| n | Pentosidine (pmol/mg protein) |
Pentosidine elevated above control median |
||||
|---|---|---|---|---|---|---|
| Mean ± S.D. | Median (Q1, Q3) | Odds Ratio | 95% CI | p value | ||
| Control | 32 | 1.00 ± 0.25 | 0.93 (0.84, 1.02) | 1 | Reference | |
| Early/mid-stage AMD | 27 | 1.51 ± 0.41 | 1.48 (1.22, 1.82) | 8.0 | 2.0, 32.0 | 0.002 |
| Advanced AMD | 31 | 1.76 ± 0.59 | 1.71 (1.34, 1.99) | 14.5 | 3.0, 71.2 | <0.0001 |
| All AMD | 58 | 1.64 ± 0.53 | 1.59 (1.29, 1.92) | 10.6 | 3.4, 33.5 | <0.0001 |
Correlation of CML and pentosidine concentrations (Fig. 1, E and F) showed both markers to be elevated above median control levels in 84% of AMD patients (49 of 58) compared with only 28% of controls (nine of 32). Logistic regression modeling of the CML and pentosidine data for all AMD donors yielded c-statistics (Table III) of 0.79 and 0.88, respectively. This indicates that for a randomly selected AMD case and healthy control, the case will have elevated CML 79% of the time and elevated pentosidine 88% of the time. Small differences in c-statistics were observed between early/mid-stage AMD and advanced AMD with slightly higher values obtained for advanced stage disease. Bootstrap resampling and 10-fold cross-validation verified all c-statistics and 95% CIs (Table III). Risk of AMD was estimated by OR for donors with elevated CML and pentosidine (Table II). For all AMD, the data generated OR ∼ 6.3 for CML and OR ∼ 10.6 for pentosidine; ORs for advanced AMD were greater than for early/mid-stage disease. Each OR exhibited a large CI (Table II), indicating that these are preliminary estimates that lack precision.
Table III. C-statistics for CML and pentosidine.
The c-statistic and 95% CI were determined by a logistic regression model fit with log-transformed CML and pentosidine concentrations as independent variables and was validated by bootstrap resampling and 10-fold cross-validation.
| CML |
|||||||
|---|---|---|---|---|---|---|---|
| n | Total |
Bootstrap validation |
10-Fold cross-validation |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Control | 32 | ||||||
| Early/mid-stage AMD | 27 | 0.74 | 0.60, 0.87 | 0.74 | 0.59, 0.87 | 0.71 | 0.57, 0.84 |
| Advanced AMD | 31 | 0.84 | 0.74, 0.94 | 0.85 | 0.74, 0.94 | 0.82 | 0.71, 0.93 |
| All AMD | 58 | 0.79 | 0.70, 0.89 | 0.80 | 0.69, 0.89 | 0.78 | 0.68, 0.88 |
| Pentosidine |
|||||||
|---|---|---|---|---|---|---|---|
| n | Total |
Bootstrap validation |
10-Fold cross-validation |
||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Control | 32 | ||||||
| Early/mid-stage AMD | 27 | 0.87 | 0.80, 0.98 | 0.88 | 0.79, 0.97 | 0.86 | 0.75, 0.96 |
| Advanced AMD | 31 | 0.94 | 0.89, 0.99 | 0.95 | 0.88, 0.99 | 0.93 | 0.87, 0.99 |
| All AMD | 58 | 0.88 | 0.81, 0.95 | 0.89 | 0.80, 0.95 | 0.87 | 0.79, 0.94 |
Plasma CEP Adducts and Autoantibodies in the Study Population
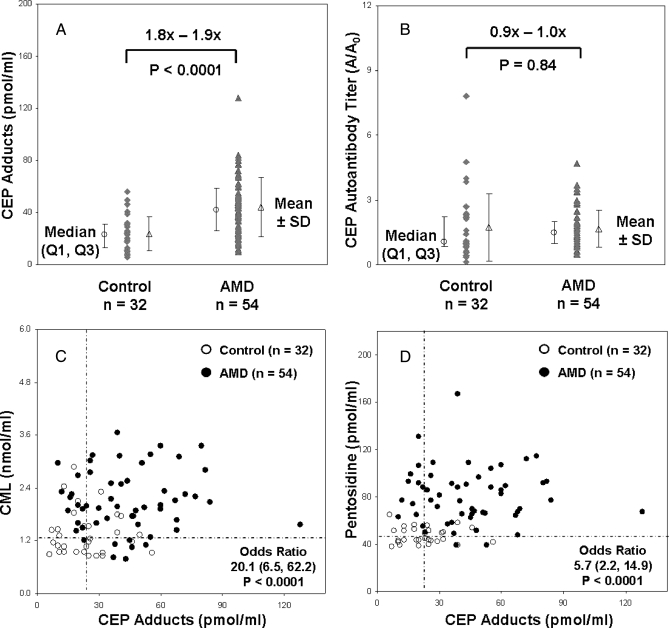
CEP plasma biomarkers offer potential utility in assessing AMD susceptibility (11), therefore it was of interest to compare CEP biomarkers with CML and pentosidine. In this study population, mean CEP adduct levels were ∼86% higher (p < 0.0001), but mean CEP autoantibodies were not significantly higher in AMD plasma (p = 0.84) (Fig. 2, A and B). CEP autoantibody titers were higher than CML and pentosidine autoantibody titers in the AMD cohort, but none were significantly elevated relative to the control cohort. Correlation of CEP adducts with CML and pentosidine (Fig. 2, C and D) shows that all three oxidative protein modifications were elevated 69–74% above median control levels. Predicted risk of AMD yielded OR = 13.9 (95% CI, 4.9, 39.7) for pentosidine plus CML, OR = 20.1 (95% CI, 6.5, 62.2) for CML plus CEP adducts, and OR = 5.7 (95% CI, 2.2, 14.9) for pentosidine plus CEP adducts. Again the large confidence intervals indicate that the ORs are elevated but lack precision.
Fig. 2.
Correlation between CML, pentosidine, and CEP adducts in AMD plasma. Plasma CEP adduct concentrations (A) and CEP autoantibody titers (B) quantified by ELISA from control (n = 32) and AMD donors (n = 54) are shown with median (○) results ±first and third quartiles (Q1, Q3) and mean (△) results ± S.D. p values (two-sided t test) were determined from log-transformed concentrations. Correlation between CML and CEP adduct concentrations (C) and between pentosidine and CEP adduct concentrations (D) are shown with horizontal and vertical dashed lines indicating median control values. Odds ratios for AMD risk and 95% confidence intervals were determined based on two markers elevated relative to the median control values; p values were determined using the Fisher exact test.
AMD Discriminatory Capability of Plasma CML, Pentosidine, and CEP Adducts
Sensitivity and specificity measures were determined with ROC curves from all AMD cases and controls for CML, pentosidine, and CEP adducts alone and for the combined markers (Table IV). Calculated to maximize the sum of the two values, the sensitivity of CML or pentosidine alone were the same (84%) and greater than for CEP (67%) alone. The specificities of pentosidine (88%) and CEP (81%) alone were higher than that for CML alone (72%). The area under the ROC curve (c-statistic) is a measure of the overall discriminating accuracy of the markers, and comparison of c-statistics suggested no significant difference in discriminatory accuracy between CEP (∼78%) and CML (∼79%) alone (p ∼ 0.90) and between pentosidine and CEP alone (p ∼ 0.10); however, pentosidine exhibited significantly higher discrimination accuracy (∼88%) than CML (p ∼ 0.05). c-statistics for the joint effect of combined markers were verified by bootstrap resampling and by 10-fold cross-validation. Comparison of c-statistics for combined markers suggested no significant difference in discriminatory accuracy between CEP plus CML (∼87%) versus either marker alone (p ∼ 0.1) or between CEP plus pentosidine (∼92%) versus pentosidine alone (p ∼ 0.2). However, CEP plus pentosidine appeared to be a better discriminator than CEP alone (p ∼ 0.02), and CML plus pentosidine (∼89%) may be a better discriminator than CEP alone (p ∼ 0.06). These results suggest that the use of all three biomarkers may provide the best approach to a prognostic test for AMD.
Table IV. Sensitivity and specificity of CML, pentosidine, and CEP adducts.
Sensitivity and specificity were determined from ROC curves to maximize the sum of the two values and constructed from the output of logistic regression analysis fit with either CEP adduct, CML, or pentosidine concentrations alone or in combination. The concentration of CML was expressed in nmol/ml, and pentosidine and CEP adducts were expressed in pmol/ml. C-statistics, 95% CI, and p values derived from single and joint markers were determined with SAS 9.1 based on log-transformed marker concentrations. Verification of c-statistics and 95% CI was performed by bootstrap resampling and 10-fold cross-validation. The c-statistic is a measure of the area under the ROC curve and of the accuracy of the markers to discriminate between AMD cases and controls with 1.0 equivalent to 100% accuracy and 0.5 equal to no discrimination.
| Markers alone |
|||
|---|---|---|---|
| CEP adducts | CML | Pentosidine | |
| Sensitivity (%) | 67 | 84 | 84 |
| Specificity (%) | 81 | 72 | 88 |
| c-statistic | 0.78 | 0.79 | 0.88 |
| 95% CI | 0.68, 0.88 | 0.70, 0.89 | 0.81, 0.95 |
| c-statistic (bootstrap validation) | 0.79 | 0.80 | 0.89 |
| 95% CI (bootstrap validation) | 0.67, 0.87 | 0.69, 0.89 | 0.80, 0.95 |
| c-statistic (10-fold cross-validation) | 0.76 | 0.78 | 0.87 |
| 95% CI (10-fold cross-validation) | 0.66, 0.86 | 0.68, 0.88 | 0.79, 0.94 |
| p value | 0.90 (vs. CML) | 0.05 (vs. pentosidine) | 0.10 (vs. CEP) |
| Joint effect of markers |
|||
|---|---|---|---|
| CML + pentosidine | CEP + CML | CEP + pentosidine | |
| Sensitivity (%) | 83 | 69 | 89 |
| Specificity (%) | 97 | 91 | 91 |
| c-statistic | 0.89 | 0.87 | 0.92 |
| 95% CI | 0.81, 0.96 | 0.80, 0.94 | 0.86, 0.99 |
| c-statistic (bootstrap validation) | 0.90 | 0.88 | 0.93 |
| 95% CI (bootstrap validation) | 0.82, 0.96 | 0.80, 0.94 | 0.86, 0.98 |
| c-statistic (10-fold cross-validation) | 0.87 | 0.86 | 0.90 |
| 95% CI (10-fold cross-validation) | 0.80, 0.95 | 0.78, 0.93 | 0.83, 0.98 |
| p value | 0.06 (vs. CEP) | 0.10 (vs. CML) | 0.20 (vs. pentosidine) |
| 0.13 (vs. CEP) | 0.02 (vs. CEP) | ||
The Influence of Demographic and Health Factors on Plasma CML and Pentosidine
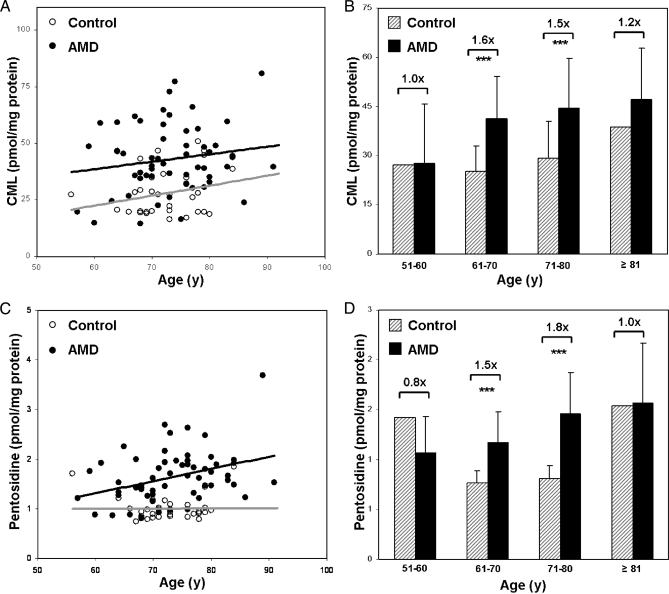
Comparisons by donor age revealed that CML concentrations increased gradually with age in both control and AMD donors (Fig. 3A) and that AMD patients exhibit significantly higher mean levels of CML than controls at all ages (Fig. 3B). Mean pentosidine increased gradually with age in AMD donors, remained stable with age in controls (Fig. 3C), and was higher in AMD than in control plasma at all ages analyzed (Fig. 3D).
Fig. 3.
Plasma CML and pentosidine by donor age. Plasma protein CML (A) and pentosidine (C) levels in the control (n = 32) and AMD (n = 58) cohorts are shown plotted by donor age. Pearson's correlation analysis revealed gradual increases with age for CML in both control and AMD donors and for pentosidine in AMD donors. Control donors exhibited little change in pentosidine levels with age. Plasma protein CML (B) and pentosidine (D) levels in control and AMD donors are plotted by age group, including 51–60 years (y) (control, n = 1; AMD, n = 3), 61–70 years (control, n = 12; AMD, n = 18), 71–81 years (control, n = 18; AMD, n = 28), and ≥81 years (control, n = 1; AMD n = 9). -Fold differences in CML and pentosidine concentrations are indicated between control and AMD groups. Asterisks reflect p values from a two-sample t test (***, p < 0.001). Error bars reflect standard deviation.
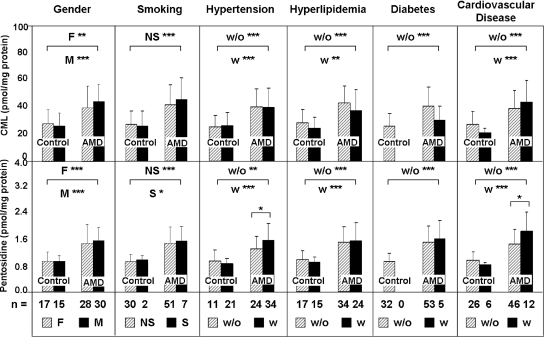
A comparison of plasma protein CML and pentosidine concentrations by gender and health history is shown in Fig. 4, including smoking, hypertension, hyperlipidemia, diabetes, and cardiovascular disease. For each comparison, significant differences in CML and pentosidine concentrations were observed between AMD and control donors. The detection of differences within the AMD and control cohorts was limited by sample size particularly for the control cohorts, and no significant differences in CML concentrations were detected within any of the cohorts. Small but significant differences were detected in pentosidine levels within two of the AMD cohorts. Specifically mean pentosidine levels were higher in AMD patients with hypertension and cardiovascular disease.
Fig. 4.
Plasma CML and pentosidine concentrations stratified by demographic and health factors. Plasma protein CML and pentosidine levels in the AMD and control cohorts are plotted based on donor status with regard to gender, status of smoking, hypertension, hyperlipidemia, diabetes, and cardiovascular diseases. Sample size per group is indicated, and asterisks reflect p values from a two-sample t test of log-transformed marker concentrations (***, p < 0.001; **, p < 0.01; *, p < 0.05). F, female, M, male; S, smokers; NS, non-smokers; w, with; w/o, without. Error bars reflect standard deviation.
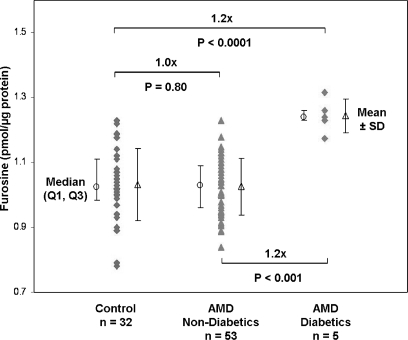
Because diabetes is such a prevalent disease and because both CML (30, 31) and pentosidine (32, 33) have been reported to be elevated in diabetic plasma, we considered the possibility that undiagnosed diabetes in our study group contributed to the observed CML and pentosidine concentrations. For this purpose we quantified plasma protein fructosyl-lysine as furosine, a marker of early glycation, in all donor samples (Fig. 5) as shown by the representative furosine amino acid analysis chromatograms in supplemental Fig. 5. Protein quantification from the furosine analyses was in good agreement with previous amino acid analyses of these samples (average quantitative difference, ∼1.7%; n = 90). The average concentration of plasma protein furosine was 1.03 ± 0.11 pmol/μg in normal controls (n = 32), 1.03 ± 0.09 pmol/μg in non-diabetic AMD donors (n = 53), and 1.24 ± 0.05 pmol/μg in previously diagnosed diabetic AMD patients (n = 5). No difference was found in mean furosine concentrations between controls and non-diabetic AMD patients; however, the five diabetic AMD donors exhibited ∼20% higher furosine levels than the controls.
Fig. 5.
Furosine in AMD and control plasma. Fructosyl-lysine concentrations quantified as furosine by amino acid analysis are shown with median (○) ±first and third quartiles (Q1, Q3) and mean (△) ± S.D. Non-diabetic AMD and control plasma donors exhibited ∼20% lower mean furosine levels than diabetic AMD donors. p values (two-sided t test) were determined from log-transformed concentrations. Error bars reflect standard deviation.
DISCUSSION
CML is a lysine modification, and pentosidine is a fluorescent lysine-arginine cross-link, and both are formed through the ubiquitous Maillard reaction, which combines sugar carbonyls with primary amino groups to form glycated residues called Amadori products. Amadori products undergo subsequent reactions including intramolecular rearrangements and oxidative fragmentations to produce heterogeneous modifications that are collectively known as AGEs. Extracellular matrix proteins like collagen are particularly susceptible to AGE modification because of slow turnover rates; tissue and circulating AGE levels are higher in smokers and in those on a high AGE diet (21). AGE formation can lead to a myriad of effects, including altered protein function and activation of intracellular signaling pathways. Previous studies have implicated AGEs in the pathogenesis of AMD (4, 5, 7, 18, 19) as well as other age-associated diseases, including atherosclerosis, arthritis, Alzheimer disease, and diabetic complications (21, 30–36). Notably AGE formation and diabetic retinopathy in rodents have been reduced or prevented by treatment with pyridoxamine (37), a derivative of vitamin B6, and the risk of AMD in women (38) has been reduced by treatment with vitamin B6 (in combination with folic acid and vitamin B12).
The purpose of this study was to discover biomarkers for assessing AMD risk and susceptibility. We hypothesized that CML and pentosidine could be elevated in plasma proteins based on similar histological localization of CML, pentosidine, and CEP in Bruch membrane and drusen and the fact that CEP adducts are elevated in AMD plasma (11). Other direct evidence of oxidative damage in AMD tissues in addition to CML (4) and CEP (6, 9, 11) includes elevated levels of glycoaldehyde-derived AGEs in the RPE and photoreceptor cells in early and advanced dry AMD (7); 4-hydroxynonenal, acrolein, nitrotyrosine, and the DNA modification 8-hydroxy-2′-deoxyguanosine in advanced dry AMD retina (8); and nitrotyrosine in AMD plasma (10). Recently fluorescent AGEs were elegantly demonstrated in human RPE, Bruch membrane, and choroidal extracellular matrix, and RPE grown in vitro on AGEs was shown to contain increased lipofuscin (39), which accumulates in AMD with potential adverse consequences (40). Notably RAGE belongs to the family of multiligand, pattern recognition receptors associated with AMD pathology (41–47) and appears to mediate AGE-induced damage of RPE cells in vitro (7) and of neurons in vivo (48).
In the present study of 58 AMD and 32 control subjects, we found mean CML and pentosidine to be significantly elevated in AMD plasma proteins over a broad age range and in those with early/mid-stage dry AMD. Comparison of early/mid-stage AMD with advanced AMD donors revealed no significant difference in CML concentrations; however, the average pentosidine plasma protein level was ∼17% higher in advanced AMD (p ∼ 0.06), suggesting that analysis of larger cohorts could reveal a significant difference with disease progression. Mean CEP adducts were elevated 86% in the AMD cohort (p < 0.0001), but autoantibody titers for CEP, CML, and pentosidine were not elevated significantly. The CEP autoantibody results differ from our previous report (11) where mean levels were elevated ∼30% (p < 0.0001). This discrepancy is due in part to sample size, which was smaller in the present study (n = 86) compared with the previous study (n = 1404), and in part to the variability associated with the autoantibody ELISA procedure. The present analyses detected no confounding influences regarding CML plasma protein concentrations; however, pentosidine levels were significantly higher in AMD donors with hypertension or cardiovascular disease as found previously for CEP adducts (11). Epidemiological studies have inconsistently associated hypertension and cardiovascular disease with AMD (49). This preliminary analysis involved relatively small AMD and control cohorts, and further studies are warranted of demographic and health factor influences and CML and pentosidine autoantibody levels.
We quantified furosine, an acid hydrolysis product of fructosyl-lysine, because of its association with diabetic complications and other diseases (34–36). Control and non-diabetic AMD plasma protein samples exhibited mean furosine concentrations of ∼1.0 pmol/μg of protein in excellent agreement with reported values for non-diabetic plasma, i.e. 0.9–1.0 pmol of furosine/μg of protein (34–36). Except for five diagnosed diabetic AMD donors with mean furosine of 1.24 pmol/μg, no other donor in the study population exhibited furosine >2 S.D. above the mean control level (i.e. >1.25 pmol/μg). Arguably furosine concentrations within 1 S.D. of the mean diabetic level (i.e. ≥1.19 pmol/μg) might be suspect diabetics, and five donor plasma samples in our study group fit this criteria. For these three control and two AMD patients, CML and pentosidine concentrations were <1 S.D. from the mean control or AMD level, respectively, and medical records provided no indication that they were diabetics. These five plasma samples also did not exhibit significantly altered CEP adduct levels, consistent with our previous finding (11) that CEP adducts are not elevated in AMD diabetic compared with AMD non-diabetic plasma (from the analysis of 796 diabetic and 130 non-diabetic AMD donors). Overall the furosine analyses showed no unknown diabetics were present in the study population and support an association between AMD pathology and increased levels of plasma protein CML and pentosidine.
The present results compellingly demonstrated higher CML and pentosidine levels in AMD plasma proteins; therefore their potential AMD biomarker efficacy was compared with each other and with CEP adducts. Odds ratios for AMD risk based on elevated CML (OR, 6.3; 95% CI, 2.3, 17.3) and pentosidine (OR; 10.6; 95% CI, 3.4, 33.5) for all AMD cases suggest that CML and pentosidine offer prognostic potential. However, the large confidence intervals indicate that more analyses are required to refine precision. Nevertheless these OR estimates were greater than the OR based on elevated CEP adducts (OR, 3.4; 95% CI, 1.3, 9.0). For comparison, elevated CEP adducts and CEP autoantibodies combined yielded an OR of 3.2 (95% CI, 2.5, 4.0) for AMD risk in a larger study population (n = 1404) (11). The area under the ROC curves (c-statistics) suggest that CML and CEP adducts alone can discriminate between AMD and control plasma donors with approximately equal accuracy (∼78–79%) and that pentosidine alone can discriminate with higher accuracy (88%). C-statistics for joint effect were increased relative to the single markers with significant differences associated with pentosidine plus CEP, a combination with better discrimination accuracy than CEP alone (p ∼ 0.02), and possibly with CML plus pentosidine (∼89%), a combination that also may be a better discriminator than CEP alone (p ∼ 0.06). Nevertheless all three markers may offer the most accurate assessment of AMD risk.
For clinical usefulness, standardization of AGE measurements is needed. Although pentosidine and CML are well studied, comparison of AGE results across multiple studies remains complicated by variations in sample preparation, specimen storage, AGE assay methods, protein quantification methods, and the array of formats used to report quantitative AGE data (e.g. pmol/ml, pmol/mg of protein, fmol/nmol of Lys, and mmol/mol of hydroxyproline, among others). In this study, the variability of the LC-MS and LC-fluorometry measurements for CML and pentosidine (∼36 and ∼24% R.S.D., respectively) was significantly lower than the variability of ELISA measurements for CEP adducts (∼50–52% R.S.D.) (11). The variability of ELISA-measured autoantibody titers for CML, pentosidine, and CEP (11) was even greater (51–67% R.S.D.) perhaps due in part to plasma storage time (i.e. 4–34 months) and possible antibody inactivation. In this study, storage at −80 °C under argon in the presence of butylated hydroxytoluene antioxidant probably had little impact on CML and pentosidine because immediately upon thawing the plasma protein was precipitated, washed, and hydrolyzed. Protein was quantified by amino acid analysis using two methods, yielding <4% average difference in amount per method and <5% average R.S.D. per sample. Toward improved methodological standardization, we suggest the use of chromatographic rather than ELISA methods to measure AGEs and the expanded use of amino acid analysis to quantify protein and furosine.
Overall this study supports the hypothesis that AMD is a systemic disease (15, 17) and demonstrates the potential utility of plasma protein CML and pentosidine as biomarkers for AMD risk or susceptibility, particularly in combination with CEP. The statistical analyses suggest that plasma levels of CML together with pentosidine discriminate between AMD and control patients with 89% accuracy and that pentosidine in combination with CEP adducts can discriminate with 92% accuracy. However, factors potentially confounding plasma protein CML and pentosidine as AMD biomarkers should not be ignored. Concurrent monitoring of plasma protein furosine provides an effective method to narrow the cause of increased AGEs. When furosine, CML, and pentosidine are all elevated, an accurate clinical assessment will require more information. In such cases, CEP adduct levels could help rule out diabetic complications because plasma CEP adducts are elevated in AMD but not in diabetes (11). The present results warrant a much larger investigation, both prospective and longitudinal, of clinical applications of plasma protein CML, pentosidine, and CEP as AMD biomarkers.
Acknowledgments
The Clinical Genomic and Proteomic AMD Study Group for this study was composed of the following clinicians who performed ophthalmic patient examinations: Dr. Peter K. Kaiser, Dr. Hilel Lewis, Dr. Gregory S. Kosmorsky, Dr. Roger H. S. Langston, Dr. Andrew P. Schachat, Dr. Jonathan E. Sears, Dr. Rishi Singh, Dr. Mindy Toabe, Dr. Elias I. Traboulsi, and Dr. Nadia Waheed. The Study Group also included the following individuals who obtained written informed consent and blood specimens from patients and performed patient chart reviews: Dr. Sonya Bamba, Dr. Emma Lessieur, Patrice Nerone, Tiffany Ruez, and Ellen Simpson. We thank Dr. Vincent Monnier for select reagents, methodology for preparing pentosidine-BSA, and valuable discussions. We are grateful to James Bena for assistance with the statistical analyses.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grants EY015638, EY014239, and EY09912. This work was also supported by State of Ohio Grant BRTT 05-29, a Foundation Fighting Blindness center grant (to the Cole Eye Institute), a Research to Prevent Blindness (RPB) challenge grant (to the Cole Eye Institute), a RPB senior investigator award (to J. W. C.), a Steinbach Award (to J. W. C.), and the Cleveland Clinic Foundation.
 The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
1 The abbreviations used are:
- AMD
- age-related macular degeneration
- AGE
- advanced glycation end product
- AccQ
- 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate
- CEP
- carboxyethylpyrrole
- CI
- confidence interval
- CML
- Nε-carboxymethyllysine
- CNV
- choroidal neovascularization
- OR
- odds ratio
- PEC
- S-β(4-pyridylethyl)-l-cysteine
- PTC
- phenylthiocarbamyl
- ROC
- receiver operating characteristic
- RPE
- retinal pigment epithelium
- R.S.D.
- relative S.D.
- RAGE and AGE-R
- receptors for AGEs
- MRM
- multiple reaction monitoring.
REFERENCES
- 1.Jager R. D., Mieler W. F., Miller J. W. ( 2008) Age-related macular degeneration. N. Engl. J. Med. 358, 2606– 2617 [DOI] [PubMed] [Google Scholar]
- 2.Seddon J. M., Willett W. C., Speizer F. E., Hankinson S. E. ( 1996) A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 276, 1141– 1146 [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Group ( 2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch. Ophthalmol. 119, 1417– 1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishibashi T., Murata T., Hangai M., Nagai R., Horiuchi S., Lopez P. F., Hinton D. R., Ryan S. J. ( 1998) Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 116, 1629– 1632 [DOI] [PubMed] [Google Scholar]
- 5.Hammes H. P., Hoerauf H., Alt A., Schleicher E., Clausen J. T., Bretzel R. G., Laqua H. ( 1999) N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 40, 1855– 1859 [PubMed] [Google Scholar]
- 6.Crabb J. W., Miyagi M., Gu X., Shadrach K., West K. A., Sakaguchi H., Kamei M., Hasan A., Yan L., Rayborn M. E., Salomon R. G., Hollyfield J. G. ( 2002) Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 99, 14682– 14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howes K. A., Liu Y., Dunaief J. L., Milam A., Frederick J. M., Marks A., Baehr W. ( 2004) Receptor for advanced glycation end products and age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 45, 3713– 3720 [DOI] [PubMed] [Google Scholar]
- 8.Shen J. K., Dong A., Hackett S. F., Bell W. R., Green W. R., Campochiaro P. A. ( 2007) Oxidative damage in age-related macular degeneration. Histol. Histopathol. 22, 1301– 1308 [DOI] [PubMed] [Google Scholar]
- 9.Gu X., Meer S. G., Miyagi M., Rayborn M. E., Hollyfield J. G., Crabb J. W., Salomon R. G. ( 2003) Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 278, 42027– 42035 [DOI] [PubMed] [Google Scholar]
- 10.Gu J., Zhan X., Crabb J. S., Bala E., Renganathan K., Hagstrom S. A., Lewis H., Salomon R. G., Crabb J. W. ( 2007) Oxidative modifications as biomarkers for AMD. Invest. Ophthalmol. Vis. Sci. 48, E-abstract 34 [Google Scholar]
- 11.Gu J., Paeur G. J., Yue X., Narendra U., Sturgill G. M., Bena J., Gu X., Peachey N. S., Salomon R. G., Hagstrom S. A., Crabb J. W.Clinical Genomic And Proteomic Study Group ( 2009) Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Mol. Cell. Proteomics 10.1074/mcp.M800453-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamoto T., Tanaka S., Stan A. C., Koike T., Kase M., Makita Z., Sawa H., Nagashima K. ( 2002) Advanced glycation end products induce angiogenesis in vivo. Microvasc. Res. 63, 186– 195 [DOI] [PubMed] [Google Scholar]
- 13.Ebrahem Q., Renganathan K., Sears J., Vasanji A., Gu X., Lu L., Salomon R. G., Crabb J. W., Anand-Apte B. ( 2006) Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: implications for age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 103, 13480– 13484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollyfield J. G., Bonilha V. L., Rayborn M. E., Yang X., Shadrach K. G., Lu L., Ufret R. L., Salomon R. G., Perez V. L. ( 2008) Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 14, 194– 198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hageman G. S., Luthert P. J., Victor Chong N. H., Johnson L. V., Anderson D. H., Mullins R. F. ( 2001) An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 20, 705– 732 [DOI] [PubMed] [Google Scholar]
- 16.Huang S. J., Costa D. L., Gross N. E., Yannuzzi L. A. ( 2003) Peripheral drusen in membranoproliferative glomerulonephritis type II. Retina 23, 429– 431 [DOI] [PubMed] [Google Scholar]
- 17.Scholl H. P., Charbel Issa P., Walier M., Janzer S., Pollok-Kopp B., Börncke F., Fritsche L. G., Chong N. V., Fimmers R., Wienker T., Holz F. G., Weber B. H., Oppermann M. ( 2008) Systemic complement activation in age-related macular degeneration. PLoS ONE 3, e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handa J. T., Verzijl N., Matsunaga H., Aotaki-Keen A., Lutty G. A., te Koppele J. M., Miyata T., Hjelmeland L. M. ( 1999) Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest. Ophthalmol. Vis. Sci. 40, 775– 779 [PubMed] [Google Scholar]
- 19.Yamada Y., Ishibashi K., Ishibashi K., Bhutto I. A., Tian J., Lutty G. A., Handa J. T. ( 2006) The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp. Eye Res. 82, 840– 848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baynes J. W. ( 2001) The role of AGEs in aging: causation or correlation. Exp. Gerontol. 36, 1527– 1537 [DOI] [PubMed] [Google Scholar]
- 21.Goh S. Y., Cooper M. E. ( 2008) Clinical review: the role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 93, 1143– 1152 [DOI] [PubMed] [Google Scholar]
- 22.Farboud B., Aotaki-Keen A., Miyata T., Hjelmeland L. M., Handa J. T. ( 1999) Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch's membrane of the aging eye. Mol. Vis. 5, 11. [PubMed] [Google Scholar]
- 23.Glenn J. V., Beattie J. R., Barrett L., Frizzell N., Thorpe S. R., Boulton M. E., McGarvey J. J., Stitt A. W. ( 2007) Confocal Raman microscopy can quantify advanced glycation end product (AGE) modifications in Bruch's membrane leading to accurate, nondestructive prediction of ocular aging. FASEB J. 21, 3542– 3552 [DOI] [PubMed] [Google Scholar]
- 24.Gu X., Yuan X., Crabb J. S., Shadrach K., Hollyfield J. G., Crabb J. W. ( 2009) Comparison of proteins in dry and wet AMD Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 50, E-abstract 2343 [Google Scholar]
- 25.Fritsche L. G., Loenhardt T., Janssen A., Fisher S. A., Rivera A., Keilhauer C. N., Weber B. H. ( 2008) Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet. 40, 892– 896 [DOI] [PubMed] [Google Scholar]
- 26.Friedman D. S., O'Colmain B. J., Muñoz B., Tomany S. C., McCarty C., de Jong P. T., Nemesure B., Mitchell P., Kempen J. ( 2004) Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 122, 564– 572 [DOI] [PubMed] [Google Scholar]
- 27.Crabb J. W., West K. A., Dodson W. S., Hulmes J. D. ( 1997) Amino acid analysis, in Current Protocols in Protein Science ( Coligan J. E., Ploegh H. L., Smith J. A., Speicher D. W. eds) Unit 11.9, Suppl. 7, pp. 11.09.01– 11.09.42, John Wiley & Sons, Inc., New York [Google Scholar]
- 28.Cohen S. A., Michaud D. P. ( 1993) Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 211, 279– 287 [DOI] [PubMed] [Google Scholar]
- 29.Reddy S., Bichler J., Wells-Knecht K. J., Thorpe S. R., Baynes J. W. ( 1995) N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 34, 10872– 10878 [DOI] [PubMed] [Google Scholar]
- 30.Lieuw-A-Fa M. L., van Hinsbergh V. W., Teerlink T., Barto R., Twisk J., Stehouwer C. D., Schalkwijk C. G. ( 2004) Increased levels of N(epsilon)-(carboxymethyl)lysine and N(epsilon)-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: correlation with markers of endothelial dysfunction. Nephrol. Dial. Transplant. 19, 631– 636 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed A. A., Muniandy S., Ismail I. S. ( 2007) Role of N-(carboxymethyl)lysine in the development of ischemic heart disease in type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 41, 97– 105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odetti P., Fogarty J., Sell D. R., Monnier V. M. ( 1992) Chromatographic quantitation of plasma and erythrocyte pentosidine in diabetic and uremic subjects. Diabetes 41, 153– 159 [DOI] [PubMed] [Google Scholar]
- 33.Yoshida N., Okumura K., Aso Y. ( 2005) High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism 54, 345– 350 [DOI] [PubMed] [Google Scholar]
- 34.Friedlander M. A., Wu Y. C., Elgawish A., Monnier V. M. ( 1996) Early and advanced glycosylation end products. Kinetics of formation and clearance in peritoneal dialysis. J. Clin. Investig. 97, 728– 735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riviere S., Birlouez-Aragon I., Vellas B. ( 1998) Plasma protein glycation in Alzheimer's disease. Glycoconj. J. 15, 1039– 1042 [DOI] [PubMed] [Google Scholar]
- 36.Floridi A., Trizza V., Paolotti P., Lucarelli C. ( 1999) Analytical strategy for the assessment of the protein glycation status in uremic patients by high-performance liquid chromatography. J. Chromatogr. A 846, 65– 71 [DOI] [PubMed] [Google Scholar]
- 37.Stitt A., Gardiner T. A., Anderson N. L., Canning P., Frizzell N., Duffy N., Boyle C., Janusewski A. S., Chachich M., Baynes J. W., Thorpe S. R. ( 2002) The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes 51, 2826– 2832 [DOI] [PubMed] [Google Scholar]
- 38.Christen W. G., Glynn R. J., Chew E. Y., Albert C. M., Manson J. E. ( 2009) Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women's Antioxidant and Folic Acid Cardiovascular Study. Arch. Intern. Med. 169, 335– 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glenn J. V., Mahaffy H., Wu K., Smith G., Nagai R., Simpson D. A., Boulton M. E., Stitt A. W. ( 2009) Advanced glycation end product (AGE) accumulation on Bruch's membrane: links to age-related RPE dysfunction. Invest. Ophthalmol. Vis. Sci. 50, 441– 451 [DOI] [PubMed] [Google Scholar]
- 40.Ng K. P., Gugiu B., Renganathan K., Davies M. W., Gu X., Crabb J. S., Kim S. R., Rózanowska M. B., Bonilha V. L., Rayborn M. E., Salomon R. G., Sparrow J. R., Boulton M. E., Hollyfield J. G., Crabb J. W. ( 2008) Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteomics 7, 1397– 1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards A. O., Ritter R., 3rd, Abel K. J., Manning A., Panhuysen C., Farrer L. A. ( 2005) Complement factor H polymorphism and age-related macular degeneration. Science 308, 421– 424 [DOI] [PubMed] [Google Scholar]
- 42.Hageman G. S., Anderson D. H., Johnson L. V., Hancox L. S., Taiber A. J., Hardisty L. I., Hageman J. L., Stockman H. A., Borchardt J. D., Gehrs K. M., Smith R. J., Silvestri G., Russell S. R., Klaver C. C., Barbazetto I., Chang S., Yannuzzi L. A., Barile G. R., Merriam J. C., Smith R. T., Olsh A. K., Bergeron J., Zernant J., Merriam J. E., Gold B., Dean M., Allikmets R. ( 2005) A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 102, 7227– 7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haines J. L., Hauser M. A., Schmidt S., Scott W. K., Olson L. M., Gallins P., Spencer K. L., Kwan S. Y., Noureddine M., Gilbert J. R., Schnetz-Boutaud N., Agarwal A., Postel E. A., Pericak-Vance M. A. ( 2005) Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419– 421 [DOI] [PubMed] [Google Scholar]
- 44.Klein R. J., Zeiss C., Chew E. Y., Tsai J. Y., Sackler R. S., Haynes C., Henning A. K., SanGiovanni J. P., Mane S. M., Mayne S. T., Bracken M. B., Ferris F. L., Ott J., Barnstable C., Hoh J. ( 2005) Complement factor H polymorphism in age-related macular degeneration. Science 308, 385– 389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gold B., Merriam J. E., Zernant J., Hancox L. S., Taiber A. J., Gehrs K., Cramer K., Neel J., Bergeron J., Barile G. R., Smith R. T., Hageman G. S., Dean M., Allikmets R. ( 2006) Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 38, 458– 462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yates J. R., Sepp T., Matharu B. K., Khan J. C., Thurlby D. A., Shahid H., Clayton D. G., Hayward C., Morgan J., Wright A. F., Armbrecht A. M., Dhillon B., Deary I. J., Redmond E., Bird A. C., Moore A. T. ( 2007) Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 357, 553– 561 [DOI] [PubMed] [Google Scholar]
- 47.Maller J. B., Fagerness J. A., Reynolds R. C., Neale B. M., Daly M. J., Seddon J. M. ( 2007) Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat. Genet. 39, 1200– 1201 [DOI] [PubMed] [Google Scholar]
- 48.Hassid B. G., Nair M. N., Ducruet A. F., Otten M. L., Komotar R. J., Pinsky D. J., Schmidt A. M., Yan S. F., Connolly E. S. ( 2009) Neuronal RAGE expression modulates severity of injury following transient focal cerebral ischemia. J. Clin. Neurosci. 16, 302– 306 [DOI] [PubMed] [Google Scholar]
- 49.Klein R. ( 2007) Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 14, 184– 187 [DOI] [PubMed] [Google Scholar]