Abstract
Transient global ischemia induces selective delayed cell death, primarily of principal neurons in the hippocampal CA1. However, the molecular mechanisms underlying ischemia-induced cell death are as yet unclear. The present study shows that global ischemia triggers a pronounced and cell-specific reduction in GluR2 [the subunit that limits Ca2+ permeability of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors] in vulnerable CA1 neurons, as evidenced by immunofluorescence of brain sections and Western blot analysis of microdissected hippocampal subfields. At 72 h after ischemia (a time before cell death), virtually all CA1 pyramidal neurons exhibited greatly reduced GluR2 immunolabeling throughout their somata and dendritic processes. GluR2 immunolabeling was unchanged in pyramidal cells of the CA3 and granule cells of the dentate gyrus, regions resistant to ischemia-induced damage. Immunolabeling of the AMPA receptor subunit GluR1 was unchanged in CA1, CA3, and dentate gyrus. Western analysis indicated that GluR2 subunit abundance was markedly reduced in CA1 at 60 and 72 h after the ischemic insult; GluR1 abundance was unchanged in all subfields at all times examined. These findings, together with the previous observation of enhanced AMPA-elicited Ca2+ influx in postischemic CA1 neurons, show that functional GluR2-lacking, Ca2+-permeable AMPA receptors are expressed in vulnerable neurons before cell death. Thus, the present study provides an important link in the postulated causal chain between global ischemia and delayed death of CA1 pyramidal neurons.
Keywords: excitotoxicity, neuronal death, excitatory amino acid, glutamate, calcium
Transient severe global ischemia, occurring in patients during cardiorespiratory arrest or cardiac surgery or experimentally in animals, induces selective, delayed neuronal cell death. Pyramidal neurons in the hippocampal CA1 and some hilar neurons are particularly vulnerable (1). In rats and gerbils, cell loss is not prominent until 2–5 days after ischemia (2). Although the molecular mechanisms underlying ischemia-induced neuronal death are as yet unclear, the substantial delay between insult and onset of death suggests that transcriptional changes play a critical role.
α-Amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) mediate fast excitatory transmission and are assembled from subunits encoded by four genes, GluR1–4 (or GluR-A–D) subunits (3). AMPARs assembled from combinations of GluR1, GluR3, and/or GluR4 subunits are permeable to Ca2+ and have doubly rectifying current-voltage relations caused by voltage-dependent block by intracellular polyamines (4). The presence of edited GluR2 subunits greatly reduces Ca2+ permeability (5, 6), voltage-dependent block by polyamines (7, 8), and single-channel conductance (9) of recombinant AMPARs expressed in Xenopus oocytes and mammalian cell lines. Moreover, patch-clamp recording combined with reverse transcription-PCR demonstrate that AMPAR permeability to Ca2+ varies inversely with abundance of GluR2 mRNA in a wide range of cell types. Excitatory principal neurons such as hippocampal and neocortical pyramidal cells and dentate gyrus (DG) granule cells express abundant GluR2 mRNA and exhibit low AMPAR Ca2+ permeability (5, 10, 11). Thus, a reduction in the level of GluR2 expression would be expected to have significant physiological consequences.
The relative expression of GluR2 subunit mRNA and protein in neurons is not static but is regulated in a cell-specific manner during development (12) and may be remodeled after seizures (13–16) and ischemic insult (17, 18) and by administration of antipsychotics (19), drugs of abuse (20, 21), or corticosteroids (22). Considerable evidence implicates GluR2-lacking, Ca2+-permeable AMPARs in the delayed neuronal death associated with global ischemia. AMPAR antagonists administered as late as 16 h after the induction of global ischemia afford neuroprotection against ischemia-induced damage (23, 24). Global ischemia triggers an acute suppression of GluR2 mRNA abundance in CA1 pyramidal neurons (17, 18) and delayed increase in AMPAR-mediated Ca2+ influx in vulnerable CA1 neurons before cell death (17). However, little is known about GluR2 protein abundance in postischemic pyramidal neurons. Because under physiological conditions, GluR2 is under translational control (25) and translational regulation may be altered after ischemia, ischemia-induced changes in GluR2 mRNA abundance may not be predictive of changes in GluR2 protein abundance.
The present study was undertaken to examine global ischemia-induced changes in expression of AMPAR subunits. Immunolabeling revealed intense expression of GluR1 and greatly reduced expression of GluR2 within the cell somata and dendrites of virtually all CA1 pyramidal neurons at 72 h after ischemia, a time that clearly precedes detectable cell death. Western blot analysis revealed significant reduction of GluR2 subunit abundance at 60 h and 72 h after ischemia. GluR1 abundance was unchanged in all subfields at all times examined. These results show that ischemia triggers a cell-specific suppression of GluR2 protein, which would lead to remodeling of AMPAR subunit composition in neurons destined to die. These findings together with the findings of ischemia-induced increase in AMPAR Ca2+ permeability (17) and GluR2 knockdown-induced neuronal death (26) implicate Ca2+-permeable AMPARs as critical mediators in ischemia-induced neuronal death.
Materials and Methods
Global Ischemia.
Animals were treated in accordance with the principles and procedures of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Adult male Mongolian gerbils (Tumblebrook Farms, Wilmington, MA) weighing 50–80 g were maintained in a temperature- and light-controlled environment with a 14-h light/10-h dark cycle. Animals were fasted overnight and anesthetized with i.p. ketamine (80 mg/kg) and xylazine (6 mg/kg). Global ischemia was induced by temporary (5 min) bilateral occlusion of the carotid arteries, followed by reperfusion. Body temperature was maintained at 37°C with a rectal thermistor and heat lamp during administration of anesthesia. Control gerbils were sham-operated.
Histological Analysis.
Neuronal damage was assessed by histological examination of brain sections at the level of the dorsal hippocampus from animals at 0 h, 24 h, 48 h, 72 h, and 1 week after ischemia (n = 7 per time point) or at 1 week after sham operation (n = 7). Animals were placed under deep anesthesia, and their brains were removed. Hippocampi were quickly dissected out and cut into 1-mm thick transverse sections by using a McIlwain tissue chopper. After fixation in 2.5% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 at 4°C overnight, slices were osmicated (2% OsO4 in 0.1 M sodium cacodylate buffer, pH 7.4, 2 h), dehydrated, and embedded in Eponate 12 resin (Ted Pella, Redding, CA). Two-micrometer sections were cut and stained with toluidine blue.
GluR1 and GluR2 Immunolabeling.
GluR1 and GluR2 protein expression was assessed by immunolabeling of brain sections at the level of the dorsal hippocampus (3.3 to 4.0 from bregma) as described (14). In brief, animals were deeply anesthetized with pentobarbital (50 mg/kg, i.p.) and perfused transcardially with ice-cold 4% paraformaldehyde in PBS at 72 h after ischemia (n = 4) or sham operation (n = 3). Brains were removed, cut into 5-mm blocks, postfixed (6 h), and cut into sections (50 μm) by vibratome. Tissue sections were processed for immunolabeling with two subunit-specific antibodies: (i) GluR1C Ab, an antibody directed to a C-terminal epitope of GluR1 (Pharmigen); and (ii) GluR2C Ab, an antibody directed to residues 827–842 in the C terminus of GluR2 (gift of R. J. Wenthold, National Institutes of Health, Bethesda, MD). These antibodies have been shown to have a high degree of subunit specificity (27). Sections were incubated with GluR1 and GluR2 primary Abs at concentrations of 2.5 μg/ml and 2 μg/ml, respectively (overnight, 4°C) and then with biotinylated goat anti-rabbit IgG. Sections then were incubated with fluorescein avidin DCS (GluR2) or Texas red avidin D (GluR1; Vector Laboratories), dry-mounted, and cover-slipped with Vectashield to reduce quenching. Images were collected with a Bio-Rad MRC 600 Kr/Ar laser scanning confocal microscope. Settings were held constant for imaging of sections from control and experimental animals. To assess specificity of immunofluorescent probes, separate control sections were processed with nonimmune rabbit IgG in place of primary Ab. These control sections showed no labeling.
Western Blotting.
Subunit abundance in hippocampal subfields was assessed by Western blot analysis as described (14). In each group of four animals, one was killed at 48, 60, or 72 h after ischemia or 72 h after sham operation, hippocampi were dissected out, and thick (1 mm) transverse slices were cut. The first and fifth slices of each hippocampus were fixed and used for histology as above. For Westerns, the CA1 was rapidly separated from the CA3-DG region from remaining slices by microdissection, placed in ice-cold PBS supplemented with protease inhibitors [1 mM EDTA, 300 μM 4-(2-aminoethyl) benzenesulfonyl fluoride, 15 μg/ml leupeptin; Sigma], and stored at −70°C until use. CA1 and CA3 samples (10 μg) from a given group of four animals were loaded on the same polyacrylamide (4–20%) minigel and separated by gel electrophoresis. Four replicate gels were run for each of six groups. Protein bands were transferred to nitrocellulose membranes (Amersham Pharmacia) in blotting buffer containing 0.192 M glycine and 20% methanol. For all groups two membranes were probed with GluR2C Ab. For two groups two membranes were probed with GluR1C Ab. For the remaining four groups, one membrane was probed with GluR1C Ab and one with GluR1N Ab, an Ab to an N-terminal epitope of GluR1 (gift of R. J. Wenthold). Membranes were blocked (30 min) with 25 mM Tris⋅HCl buffer, pH 8.0, 125 mM NaCl, 0.1% Tween 20 and 4% skim milk, incubated with primary Ab (2 h, room temperature) and then with donkey anti-rabbit IgG-HRP (1:1,000; 1 h, room temperature; Amersham Pharmacia) followed by enhanced chemiluminescence reagents (Amersham Pharmacia). Moist membranes were apposed to XAR-5 x-ray film (Eastman Kodak). Band ODs were determined with a Scan Jet 4-C computing densitometer by using NIH image 1.61. OD values for postischemic animals were normalized to the OD for the control animal from the same group and averaged. Statistical significance was assessed by means of the Kruskal-Wallis test, followed by Dunn's Multiple Comparison Test.
Results
Global Ischemia Induces Selective, Delayed Neurodegeneration in CA1.
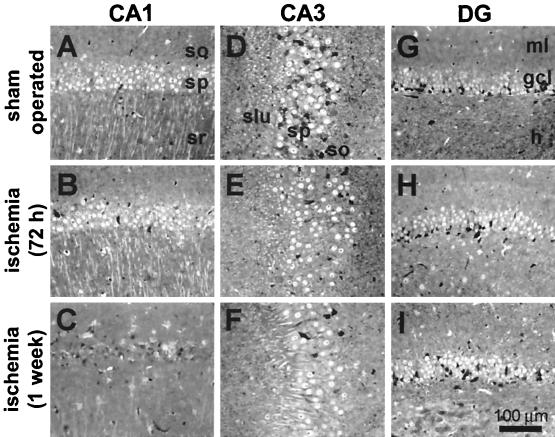
Experiments were performed on adult male gerbils subjected to sham operation or global ischemia by bilateral occlusion of the carotid arteries. Gerbils offer an advantage compared with rats in that forebrain ischemia can be produced by the relatively simple two-vessel occlusion model. To assess neuronal loss, brain sections of experimental and control animals were subjected to histological analysis. Brain sections at the level of the hippocampus revealed no detectable cell loss in CA1 at 24 h, 48 h (data not illustrated), or 72 h after induction of global ischemia (Fig. 1B). At 1 week after ischemia there was virtually complete loss of neurons in the CA1 pyramidal cell layer (Fig. 1C). The few surviving neurons exhibited pycnotic nuclei, indicative of early neurodegeneration. The CA3 and DG exhibited no detectable cell loss at all times examined (Fig. 1 D–I). These data are in confirmation of others (17, 28).
Figure 1.
Global ischemia induces selective, delayed neurodegeneration in hippocampal CA1. Toluidine blue staining of plastic-embedded sections through the dorsal hippocampus. (A, D, and G) Control brain from an animal killed at 7 days after sham operation. (B, E, and H) At 72 h after transient (5 min) global ischemia there was no histologically detectable neuronal death in any hippocampal subfield. (C, F, and I) By 7 days after ischemia, the pyramidal cell layer of CA1 exhibited essentially complete loss of neurons, whereas CA3 and DG showed no damage.
Global Ischemia Specifically Reduces GluR2 Immunolabeling in CA1 Pyramidal Neurons Before Cell Death.
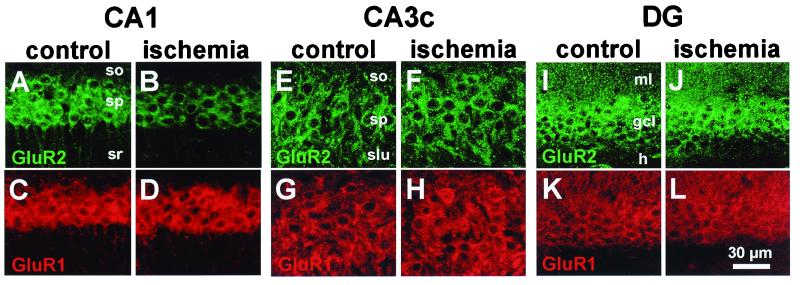
To assess the effect of global ischemia on AMPAR subunit expression in individual neurons, we performed immunolabeling on brain sections of control and experimental animals at 72 h after ischemia with two subunit-specific antibodies, GluR1C and GluR2C. Immunolabeling was performed at 72 h because: (i) there is no histologically detectable cell loss at this time; (ii) GluR2 mRNA is markedly reduced by 48 h after ischemia (17); and (iii) the half-life for GluR2/3 subunits measured in primary cultures of cerebellar granule cells is on the order of 18 ± 5 h (ref. 29, but see also ref. 30). In CA1 of control animals, immunolabeling of GluR2 (Fig. 2A) and GluR1 (Fig. 2C) was intense in the somata of pyramidal neurons (stratum pyramidale) and extended into the apical dendrites (stratum radiatum). In CA3, immunolabeling of GluR2 (Fig. 2E) and GluR1 (Fig. 2G) exhibited patterns similar to those in CA1. In DG, immunolabeling of GluR2 (Fig. 2I) and GluR1 (Fig. 2K) was intense in granule cell somata and proximal dendrites. These patterns of expression in gerbil hippocampus are in accord with studies in rats and monkeys (31–33).
Figure 2.
Global ischemia induces cell-specific suppression of GluR2, but not GluR1, immunolabeling in CA1 pyramidal neurons. GluR2 (Upper) and GluR1 (Lower) immunolabeling in the CA1 pyramidal cell layer (A–D), the CA3a pyramidal cell layer (E–H) and DG granule cell layer (I–L) in sections from a control animal (A, C, E, G, I, and K) and an experimental animal 72 h after ischemia (B, D, F, H, J, and L). Data are typical of two control and four ischemic animals. Ischemia induced down-regulation of GluR2, but not GluR1, in CA1 pyramidal neurons. Ischemia did not alter GluR1 or GluR2 immunolabeling in CA3a pyramidal neurons or DG granule cells. so: stratum oriens; sp: stratum pyramidale; sr: stratum radiatum; slu: stratum lucidum; ml: molecular layer; gcl: granule cell layer; h: hilus.
At 72 h after global ischemia, changes were observed only for GluR2 in CA1 (Fig. 2B). GluR2 immunolabeling was markedly reduced in the somata and basilar and apical dendrites of virtually all pyramidal neurons in CA1, relative to that of control animals. In contrast, GluR1 immunolabeling, examined in adjacent sections from the same animals, was robust and not detectably altered at 72 h postischemia, relative to that in sections from control animals (Fig. 2D). No changes were detectable in either GluR1 or GluR2 immunolabeling in the CA3 (Fig. 2 F and H) or DG (Fig. 2 J and L) at 72 h after global ischemia, relative to that in control animals. Together with the histology data illustrated in Fig. 1D, these data show a pronounced reduction in GluR2 subunit expression in CA1 pyramidal neurons before cell death.
Global Ischemia Specifically Reduces GluR2 Abundance in CA1, as Assessed by Western Analysis.
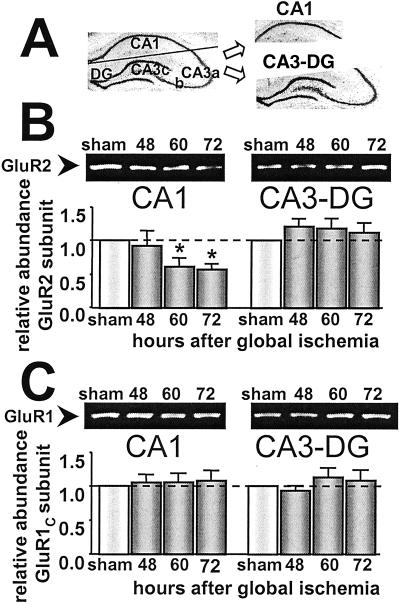
To assess quantitatively the effects of global ischemia on AMPAR subunit expression, we performed Western analysis. Individual hippocampal subfields were microdissected from brains of control animals at 72 h and experimental animals at 48, 60, and 72 h after ischemia. Because in situ hybridization studies revealed no changes in either GluR1 or GluR2 mRNA expression in CA3 or DG after ischemia (17), these subfields were combined (CA3-DG; Fig. 3A), a simplification validated by the immunolabeling data above. Samples of total cell protein were subjected to electrophoresis and blots were probed with GluR2C (Fig. 3B) and GluR1C (Fig. 3C), subunit-specific antibodies directed to the GluR1 or GluR2 subunit, respectively. Global ischemia led to a marked reduction in the level of GluR2 protein in CA1 at 60 and 72 h (to 61.5 ± 11.9% and 57.6 ± 7.9% of control, respectively; n = 6 per time point; P < 0.05; Fig. 3B). GluR2 protein expression was not significantly altered in CA3-DG at any time examined (121.2 ± 11.4%, 119 ± 14.5%, and 112.4 ± 9.2% of control at 48, 60, and 72 h after ischemia, respectively). GluR1 subunit abundance was unaltered in CA1 and CA3-DG at 48, 60, or 72 h after ischemia (Fig. 3C). These findings indicate that ischemia-induced changes in GluR2 subunit expression at 60 and 72 h are subunit- and subfield-specific. Moreover, the reduction in GluR2 in CA1 was caused by a reduction in subunit abundance per pyramidal neuron and not by cell loss (see Fig. 2).
Figure 3.
Global ischemia induces subunit-specific down-regulation of GluR2 abundance in CA1. (A) Microdissection of the hippocampus for Western blot analysis. (B) Representative Western blots probed with GluR2C Ab (Upper) and relative GluR2 subunit abundance (defined as the ratio of band densities for experimental vs. control samples; Lower) for CA1 (Left) and CA3-DG (Right) in control and postischemic animals. Protein samples of hippocampal CA1 and CA3-DG were from control animals at 72 h and experimental animals at 48, 60, and 72 h after brief (5 min) global ischemia. Relative GluR2 subunit abundance was markedly decreased in CA1 at 60 h (to 61.5 ± 11.9% of control; n = 6 per time point; P < 0.05) and 72 h after ischemia (to 57.6 ± 7.9% of control, P < 0.05; n = 6 per time point). GluR2 subunit abundance was unchanged in CA3-DG. (C) Western blots probed with GluR1C Ab (Upper) and relative GluR1 subunit abundance displayed as in B. Aliquots of the same protein samples (hippocampal CA1 and CA3-DG) used in B were run on different gels. GluR1 subunit abundance was not altered in the CA1 or CA3-DG at any times examined after ischemia (n = 6 per time point). Band densities were corrected for protein concentration. Bars are means ± SEMs. Asterisks indicate significant difference from control values (P < 0.05).
The ratio of GluR2/GluR1 subunit abundance, which is inversely related to AMPAR Ca2+ permeability, was calculated from the data in Fig. 3. In CA1, the GluR2/GluR1 ratio decreased to 0.55 of control at 60 h (P < 0.05) and to 0.54 of control at 72 h (P < 0.05; data not illustrated). Because GluR1 subunit abundance was essentially constant, this ratio closely tracked the changes in GluR2. The finding of a marked reduction in GluR2 subunit and in the GluR2/GluR1 ratio in CA1 neurons is consistent with expression of GluR2-lacking, Ca2+-permeable AMPARs.
Global Ischemia Does Not Promote Proteolysis of the GluR1 Subunit.
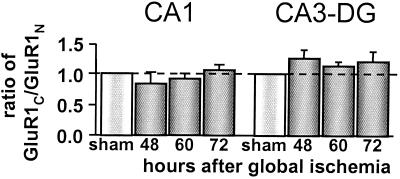
Calpain is activated in postischemic brain (34) and is known to cleave at a site within the C-terminal tail of the GluR1 subunit (35). To examine the structural stability of the GluR1 subunit in postischemic CA1, we subjected the same protein samples used for experiments in Fig. 3 (see Materials and Methods) to Western analysis using GluR1N Ab, an antibody that recognizes a sequence in the amino-terminal domain of the GluR1 subunit, as probe. We reasoned that if the GluR1 C-terminal tail were cleaved, the GluR1C/GluR1N ratio would decrease after ischemia. In CA1 and CA3-DG, the GluR1C/GluR1N ratio was unchanged as late as 72 h after ischemia (Fig. 4). Moreover, the mobility of the band corresponding to GluR1 was not significantly altered (data not illustrated). These data together with those of Fig. 3C argue against significant proteolysis of the GluR1 subunit in postischemic CA1.
Figure 4.
The GluR1 C terminus is not cleaved in CA1 after global ischemia. The ratio of GluR1 subunit abundance in CA1 (Left) and CA3-DG (Right) calculated from Westerns probed with GluR1C Ab or GluR1N Ab. Protein samples were from control animals and experimental animals at 48, 60, and 72 h after ischemia. In CA1 and CA3-DG, the GluR1c/GluR1N ratio was not significantly changed at any times examined after ischemia, indicating stability of the GluR1 C terminus after ischemia. Band densities were corrected for protein concentration and normalized to corresponding control values. Bars are means ± SEMs from four experiments.
Discussion
The present study demonstrates that AMPAR subunit expression is remodeled in the hippocampal CA1 after global ischemia, but before the onset of histologically detectable neuronal death. The conclusions of the study are (i) global ischemia induces down-regulation of GluR2 subunit expression specifically in vulnerable CA1 neurons, as assessed by Western analysis and immunolabeling; neurons in CA3 and DG, regions resistant to ischemia-induced damage, exhibit no change in GluR2 protein; (ii) GluR1 subunit expression is stable in all subfields at all times examined; hence, the GluR2:GluR1 ratio is reduced; (iii) GluR1 abundance detected with an N-terminally directed antibody was not significantly different from that measured with a C-terminally directed antibody, arguing against GluR1 proteolysis in postischemic neurons; and (iv) virtually all CA1 pyramidal neurons expressing high GluR1 show essentially complete loss of GluR2, consistent with expression of GluR2-lacking AMPARs in the pyramidal cell layer. Our finding of a cell-specific suppression of GluR2 in CA1 differs with the findings of Kjoller and Diemer (30) that global ischemia induced in rats by two vessel occlusion with hypotension causes a reduction in GluR2 protein in CA1 and DG/CA3.
Our previous study involving electrophysiology and Ca2+ imaging indicates that AMPAR-mediated Ca2+ influx increases in CA1 neurons after global ischemia, but before the onset of neuronal death (17). Together, the two studies suggest that ischemia triggers down-regulation of GluR2 and subsequent surface expression of functional GluR2-lacking, Ca2+-permeable AMPARs in CA1 neurons. Our previous study involving GluR2 antisense oligonucleotides indicates that GluR2 knockdown, even in the absence of a neurological insult, is sufficient to induce selective, delayed death of hippocampal pyramidal neurons (26). These studies suggest that Ca2+-permeable AMPARs are critical mediators of ischemia-induced neuronal death.
The impact of GluR2 down-regulation on AMPAR Ca2+ permeability depends on a number of factors including: (i) the number of GluR2 subunits per receptor assembly in hippocampal principal neurons; (ii) the number of GluR2 subunits per receptor required to disrupt Ca2+ permeability; and (iii) the degree of variability in AMPAR subunit stoichiometry within a neuronal population. Studies involving functional properties of recombinant AMPARs indicate that the number of GluR2 subunits per receptor is graded, rather than fixed (36). Moreover, studies involving reverse transcription–PCR of single neurons show that GluR2 mRNA abundance is inversely correlated with Ca2+ permeability (5, 11). In contrast, neuronal nicotinic, γ-aminobutyric acid type A, and glycine receptors tend to form assemblies with fixed stoichiometries (5, 37–39). The presence of a single GluR2 subunit in AMPARs appears sufficient to disrupt Ca2+ permeability (5, 36). Thus, neurons with high GluR2 would require a greater loss of GluR2 for a change in permeability.
Neurons that express low GluR2 (e.g., hippocampal interneurons) and hippocampal pyramidal neurons of the GluR2(−/−) knockout mouse (40) survive. A possible explanation is that these cells cope with AMPAR-mediated Ca2+ influx by expressing higher levels of Ca2+ buffering and/or extrusion proteins and/or by differing in number, localization, and interaction of AMPARs with signaling and anchoring proteins. The viability of these neurons suggest that acute reduction of GluR2 in neurons that normally express high GluR2 may be required for neuronal death. The GluR2(−/−) knockout was made on a 129/SvEMS × C57BL/6 hybrid, a strain with high resistance to glutamate-induced excitotoxicity (5, 41), and susceptibility of wild-type littermates to excitotoxic cell death is unknown.
Reliability of Subunit Composition Estimates by Western Analysis.
Because an objective of the present study was to assess ischemia-induced changes in AMPAR subunit abundance quantitatively, the following steps were taken: (i) control and experimental samples were processed under identical conditions; and (ii) subunit abundance in experimental samples was normalized to that of control samples.
Although both immunolabeling and Western analysis revealed a reduction in GluR2 subunit after ischemia, the reduction observed by immunofluorescence was greater. Several factors inherent to Western analysis might cause underestimation of GluR2 down-regulation at CA1 synapses. First, Westerns do not distinguish between principal and nonprincipal neurons. Because >95% of neurons in CA1 are pyramidal cells (42, 43), this factor is unlikely to be significant. Second, Westerns do not distinguish between neuronal and nonneuronal cell protein. Cultured hippocampal astrocytes exhibit intense GluR1 and weak GluR2 immunolabeling (44). Although GluR2 immunolabeling is strongest in cell somata and proximal dendrites of neurons, labeling of distal dendrites and astrocytes in a larger volume might contribute significantly. Finally, immunolabeling may not accurately reflect subunit abundance per cell.
Targeting and Trafficking of AMPARs.
The number of functional AMPARs at synapses depends not only on transcriptional and translational efficiency, but on targeting and trafficking of receptor subunits to spines and on internalization and degradation of receptors expressed at the cell surface. Targeting and trafficking of AMPARs at CA1 synapses are regulated by synaptic activity (45, 46). Moreover, individual spines along a single dendrite may express AMPARs that differ in subunit composition and functional properties including conductance, kinetics, and Ca2+ permeability (47, 48). Recent studies reveal a very rapid recycling of GluR2-containing AMPARs at CA1 synapses under physiological conditions via an N-methyl-maleimide-sensitive fusion (NSF) protein-dependent mechanism (49–51). Loading of postsynaptic hippocampal neurons with peptides corresponding to the NSF binding site on GluR2 or with a mAb against NSF causes rapid (but incomplete) rundown (<15 min) in the amplitude of AMPAR excitatory postsynaptic currents (49, 50). Support for rapid recycling of AMPARs is further strengthened by the demonstration of pools of AMPARs within spines (52), the high concentration of NSF in the hippocampus (53, 54), its high localization within hippocampal postsynaptic densities (PSDs) (55), and its accumulation in PSDs after transient cerebral ischemia (56).
Unlike recycling of AMPARs, which occurs very rapidly, degradation of AMPAR subunits occurs over many tens of hours. In vitro studies involving pulse–chase labeling of newly synthesized proteins indicate half-lives of 18 ± 5 h for GluR2/3 in cerebellar granule neurons (29), 31 h for GluR1 in spinal cord neurons (57), and 23 h for GluR4 in cerebellar granule cells (29). In vivo studies involving pulse–chase labeling of CA1 indicate a metabolic half-life for GluR2 of 108 h (for i.v. infusion of radiolabel) to 144 h (for intrahippocampal injection of radiolabel; ref. 30). However, the half-life for GluR2 degradation in postischemic brain is unknown. Using the metabolic half-life of ≈18 h reported in ref. 29 and assuming that GluR2 mRNA in CA1 is reduced by about 50% at 48 h after ischemia and remains constant thereafter, we predict GluR2 subunit abundance to approach 50% of control at 72 h. The observed value is 57.6 ± 7.9% of control at 72 h, as assessed by Westerns.
Stability of the GluR1 Subunit in Postischemic Brain.
Because calpain is activated in postischemic brain and is known to cleave a fragment within the C-terminal tail of the GluR1 subunit (35), we examined structural stability of the GluR1 subunit in ischemic brain. The findings of nearly identical values for GluR1 abundance in CA1 after ischemia, detected by either the GluR1C or GluR1N Ab, and of unchanged mobility of the GluR1 band indicate stability of the GluR1 subunit after an ischemic insult.
Transcriptional Regulation of the GluR2 Gene.
Although the molecular mechanisms whereby global ischemia alters GluR2 expression have not been determined, regulatory elements and transcriptional factors implicated in the regulation of GluR2 gene expression have been identified (58). Functional promoter studies indicate that the GluR2 gene is under transcriptional control by the silencer element REST (RE1 element silencing transcription factor, also known as NRSF/XBR), which renders it highly neuron-specific (58, 59). Repression of GluR2 expression by REST is observed in non-neuronal cells under physiological conditions and hippocampal pyramidal neurons after severe limbic seizures (60). These observations raise the intriguing possibility that brief ischemic insults also may induce REST expression in vulnerable pyramidal neurons leading to selective suppression of GluR2 relative to other AMPAR subunits.
Physiological Significance AMPAR Remodeling after Ischemia.
AMPAR Ca2+ permeability varies inversely with abundance of GluR2 mRNA in a wide range of cell types (5, 10, 11). Principal neurons of adult hippocampus express high levels of edited GluR2 and exhibit low AMPAR-mediated Ca2+ permeability (11). An acute reduction in GluR2 expression in CA1 pyramidal neurons would be expected to increase the number of Ca2+-permeable AMPARs and, thereby, their vulnerability to excitotoxicity of endogenous glutamate. Remodeling of AMPAR subunit composition and increased AMPAR-mediated Ca2+ influx may contribute more generally to delayed neuronal death after a neuronal insult.
Acknowledgments
This work was supported by National Institutes of Health Grants NS 20752 and NS 31282 (to R.S.Z.) and NS 07512 (to M.V.L.B.). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience.
Abbreviations
- AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor
DG, dentate gyrus
References
- 1.Choi D W. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 2.Pulsinelli W A, Brierley J B, Plum F. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 3.Seeburg P H. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 4.Verdoorn T A, Burnashev N, Monyer H, Seeburg P H, Sakmann B. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- 5.Geiger J R, Melcher T, Koh D S, Sakmann B, Seeburg P H, Jonas P, Monyer H. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann M, Hartley M, Heinemann S. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 7.Bowie D, Mayer M L. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 8.Donevan S D, Rogawski M A. Proc Natl Acad Sci USA. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson G T, Kamboj S K, Cull-Candy S G. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Neuron. 1994;12:383–388. doi: 10.1016/0896-6273(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 11.Jonas P, Racca C, Sakmann B, Seeburg P H, Monyer H. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini-Giampietro D E, Bennett M V, Zukin R S. Proc Natl Acad Sci USA. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman L K, Pellegrini-Giampietro D E, Sperber E F, Bennett M V, Moshe S L, Zukin R S. J Neurosci. 1994;14:2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grooms S Y, Opitz T, Bennett M V, Zukin R S. Proc Natl Acad Sci USA. 2000;97:3631–3636. doi: 10.1073/pnas.050586497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollard H, Heron A, Moreau J, Ben-Ari Y, Khrestchatisky M. Neuroscience. 1993;57:545–554. doi: 10.1016/0306-4522(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 16.Prince H K, Conn P J, Blackstone C D, Huganir R L, Levey A I. J Neurochem. 1995;64:462–465. doi: 10.1046/j.1471-4159.1995.64010462.x. [DOI] [PubMed] [Google Scholar]
- 17.Gorter J A, Petrozzino J J, Aronica E M, Rosenbaum D M, Opitz T, Bennett M V, Connor J A, Zukin R S. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellegrini-Giampietro D E, Zukin R S, Bennett M V, Cho S, Pulsinelli W A. Proc Natl Acad Sci USA. 1992;89:10499–10503. doi: 10.1073/pnas.89.21.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald L W, Deutch A Y, Gasic G, Heinemann S F, Nestler E J. J Neurosci. 1995;15:2453–2461. doi: 10.1523/JNEUROSCI.15-03-02453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald L W, Ortiz J, Hamedani A G, Nestler E J. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz J, Fitzgerald L W, Lane S, Terwilliger R, Nestler E J. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 22.Nair S M, Werkman T R, Craig J, Finnell R, Joels M, Eberwine J H. J Neurosci. 1998;18:2685–2696. doi: 10.1523/JNEUROSCI.18-07-02685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulsinelli W, Sarokin A, Buchan A. Prog Brain Res. 1993;96:125–135. doi: 10.1016/s0079-6123(08)63262-8. [DOI] [PubMed] [Google Scholar]
- 24.Sheardown M J. Stroke. 1993;24:I146–I147. [PubMed] [Google Scholar]
- 25.Myers S J, Dingledine R, Borges K. Annu Rev Pharmacol Toxicol. 1999;39:221–41. doi: 10.1146/annurev.pharmtox.39.1.221. [DOI] [PubMed] [Google Scholar]
- 26.Oguro K, Oguro N, Kojima T, Grooms S Y, Calderone A, Zheng X, Bennett M V, Zukin R S. J Neurosci. 1999;19:9218–9227. doi: 10.1523/JNEUROSCI.19-21-09218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenthold R J, Yokotani N, Doi K, Wada K. J Biol Chem. 1992;267:501–507. [PubMed] [Google Scholar]
- 28.Kirino T. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 29.Huh K H, Wenthold R J. J Biol Chem. 1999;274:151–157. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- 30.Kjoller C, Diemer N H. Neurochem Int. 2000;37:7–15. doi: 10.1016/s0197-0186(00)00008-5. [DOI] [PubMed] [Google Scholar]
- 31.Blackstone C D, Moss S J, Martin L J, Levey A I, Price D L, Huganir R L. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- 32.Rogers S W, Hughes T E, Hollmann M, Gasic G P, Deneris E S, Heinemann S. J Neurosci. 1991;11:2713–2724. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vissavajjhala P, Janssen W G, Hu Y, Gazzaley A H, Moran T, Hof P R, Morrison J H. Exp Neurol. 1996;142:296–312. doi: 10.1006/exnr.1996.0199. [DOI] [PubMed] [Google Scholar]
- 34.Lipton P. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 35.Bi X, Chen J, Dang S, Wenthold R J, Tocco G, Baudry M. J Neurochem. 1997;68:1484–1494. doi: 10.1046/j.1471-4159.1997.68041484.x. [DOI] [PubMed] [Google Scholar]
- 36.Washburn M S, Numberger M, Zhang S, Dingledine R. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper E, Couturier S, Ballivet M. Nature (London) 1991;350:235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- 38.Kuhse J, Laube B, Magalei D, Betz H. Neuron. 1993;11:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- 39.Tretter V, Ehya N, Fuchs K, Sieghart W. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna F A, Velumian A, MacDonald J, Carlen P, et al. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 41.Schauwecker P E, Steward O. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenthold R J, Petralia R S, Blahos J, II, Niedzielski A S. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard C, Wheal H V. Hippocampus. 1994;4:497–529. doi: 10.1002/hipo.450040502. [DOI] [PubMed] [Google Scholar]
- 44.Fan D, Grooms S Y, Araneda R C, Johnson A B, Dobrenis K, Kessler J A, Zukin R S. J Neurosci Res. 1999;57:557–571. [PubMed] [Google Scholar]
- 45.Hayashi Y, Shi S H, Esteban J A, Piccini A, Poncer J C, Malinow R. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 46.Shi S H, Hayashi Y, Petralia R S, Zaman S H, Wenthold R J, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 47.Landsend A S, Amiry-Moghaddam M, Matsubara A, Bergersen L, Usami S, Wenthold R J, Ottersen O P. J Neurosci. 1997;17:834–842. doi: 10.1523/JNEUROSCI.17-02-00834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubio M E, Wenthold R J. J Neurosci. 1999;19:5549–5562. doi: 10.1523/JNEUROSCI.19-13-05549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishimune A, Isaac J T, Molnar E, Noel J, Nash S R, Tagaya M, Collingridge G L, Nakanishi S, Henley J M. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 50.Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir R L. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 51.Osten P, Srivastava S, Inman G J, Vilim F S, Khatri L, Lee L M, States B A, Einheber S, Milner T A, Hanson P I, Ziff E B. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- 52.Richmond S A, Irving A J, Molnar E, McIlhinney R A, Michelangeli F, Henley J M, Collingridge G L. Neuroscience. 1996;75:69–82. doi: 10.1016/0306-4522(96)00217-5. [DOI] [PubMed] [Google Scholar]
- 53.Hong R M, Mori H, Fukui T, Moriyama Y, Futai M, Yamamoto A, Tashiro Y, Tagaya M. FEBS Lett. 1994;350:253–257. doi: 10.1016/0014-5793(94)00778-0. [DOI] [PubMed] [Google Scholar]
- 54.Puschel A W, O'Connor V, Betz H. FEBS Lett. 1994;347:55–58. doi: 10.1016/0014-5793(94)00505-2. [DOI] [PubMed] [Google Scholar]
- 55.Walsh M J, Kuruc N. J Neurochem. 1992;59:667–678. doi: 10.1111/j.1471-4159.1992.tb09421.x. [DOI] [PubMed] [Google Scholar]
- 56.Hu B R, Park M, Martone M E, Fischer W H, Ellisman M H, Zivin J A. J Neurosci. 1998;18:625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mammen A L, Kameyama K, Roche K W, Huganir R L. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 58.Myers S J, Peters J, Huang Y, Comer M B, Barthel F, Dingledine R. J Neurosci. 1998;18:6723–6739. doi: 10.1523/JNEUROSCI.18-17-06723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Y, Myers S J, Dingledine R. Nat Neurosci. 1999;2:867–872. doi: 10.1038/13165. [DOI] [PubMed] [Google Scholar]
- 60.Palm K, Belluardo N, Metsis M, Timmusk T. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]