Abstract
Acetate, a glial-specific substrate, is an attractive alternative to glucose for the study of neuronal-glial interactions. The present study investigates the kinetics of acetate uptake and utilization in the rat brain in vivo during infusion of [2-13C]acetate using NMR spectroscopy. When plasma acetate concentration was increased, the rate of brain acetate utilization (CMRace) increased progressively and reached close to saturation for plasma acetate concentration > 2-3 mM, whereas brain acetate concentration continued to increase. The Michaelis-Menten constant for brain acetate utilization ( ) was much smaller than for acetate transport through the blood-brain barrier ( ). The maximum transport capacity of acetate through the blood-brain barrier ( ) was nearly two-fold higher than the maximum rate of brain acetate utilization ( ). We conclude that, under our experimental conditions, brain acetate utilization is saturated when plasma acetate concentrations increase above 2-3 mM. At such high plasma acetate concentration, the rate-limiting step for glial acetate metabolism is not the blood-brain barrier, but occurs after entry of acetate into the brain.
Keywords: Acetate, 13C, transport, brain, LCModel, NMR spectroscopy
Introduction
The use of magnetic resonance spectroscopy combined with the administration of 13C-enriched substrates is a powerful tool to study brain metabolism (Behar et al. 1986; Ross et al. 1988; Beckmann et al. 1991; Gruetter et al. 1992; de Graaf et al. 2003; Gruetter et al. 2003; Henry et al. 2006b). In particular, analysis of 13C turnover curves with two-compartment neuronal-glial models has been used to measure multiple metabolic rates, such as the rate of neuronal (VTCA(n)) and glial (VTCA(g)) TCA cycles, the rate of glutamate-glutamine cycle (Vnt) and the rate of pyruvate carboxylase (See (Gruetter et al. 2003) for review). However most metabolic modeling studies with two-compartment models have been performed using [1-13C]glucose or [1,6-13C2]glucose as the infused substrate. We recently reported that using [1-13C]glucose or [1,6-13C2]glucose alone may not allow precise determination of all metabolic fluxes in the model (Shestov et al. 2007).
An attractive alternative to glucose for the study of neuronal-glial interactions is 13C-acetate. Acetate is metabolized almost exclusively in glia and not in neurons (Nicklas and Clarke 1969; Badar-Goffer et al. 1990; Cerdan et al. 1990; Waniewski and Martin 1998; Cruz et al. 2005) and therefore may provide more precise metabolic information about glial-specific processes than glucose. A few in vivo NMR studies have been reported recently in the human brain (Bluml et al. 2002; Lebon et al. 2002; Mason et al. 2006) and in the animal brain (Chassain et al. 2005; Yang et al. 2007). Furthermore a number of NMR studies have been reported using 13C-labeled acetate either in brain extracts or in brain slices (Cerdan et al. 1990; Hassel et al. 1995; Patel et al. 2005). However, in previous dynamic studies with 13C-acetate, the determination of metabolic fluxes (such as VTCA(g) or Vnt) from 13C-acetate data was performed using data at isotopic steady-state (Bluml et al. 2002; Lebon et al. 2002; Patel et al. 2005; Mason et al. 2006). Such analysis at isotopic steady-state does not take full advantage of the dynamic information available from in vivo 13C NMR spectroscopy data and in particular does not allow determination of absolute metabolic rates but only relative metabolic rates (e.g. the ratio Vnt/VTCA(n) or the ratio VTCA(g)/(VTCA(total)). To our knowledge, there has been no attempt to perform dynamic metabolic modeling using metabolic rates as free parameters in the fitting procedure, as has been done previously with 13C-glucose. This may be explained in part by the fact that such dynamic metabolic modeling would require prior knowledge of the transport and uptake kinetics of the infused substrate. However there is little data available about acetate transport and utilization in vivo during prolonged acetate infusion.
In this context, the aim of this study was to determine kinetic parameters for acetate transport and utilization in the brain and to identify rate-limiting steps. The rationale was to acquire sufficient prior knowledge for future metabolic modeling of 13C turnover curves obtained during [2-13C]acetate infusion. The experimental approach was to measure incorporation of [2-13C]acetate into brain acetate and brain amino acids (glutamate and glutamine) using 1H{13C} NMR spectroscopy during infusion of [2-13C]acetate at different infusion rates. Analysis of these data using relevant models of acetate transport and utilization allows determination of the desired transport parameters.
Materials and Methods
Animal Preparation
All studies were approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Overnight fasted male adult Sprague-Dawley rats (n = 10, weight 275 ± 8 g; mean ± SD) were anesthetized with a mixture of 30% oxygen, 70% nitrous oxide and 5% isoflurane. They were then intubated and maintained anaesthetized with 1.8% isoflurane during surgery. Two femoral arteries were cannulated with polyethylene tubing for the measurement of blood gases, glucose and acetate levels, and for monitoring arterial blood pressure and heart rate. One femoral vein was cannulated for intravenous infusion of 13C-labeled acetate. In addition, an intraperitoneal line was inserted for administration of morphine sulfate and pancuronium bromide. After surgery, isoflurane was replaced by a bolus injection of morphine sulfate (50 mg/kg) and pancuronium (1.0 mg/kg) followed by continuous infusion (33.3 mg/kg/hr) of a mixture of morphine sulfate and pancuronium in a molar ratio of 3:1.
Animals were placed in a cradle and the head secured with bite-bar and ear rods. The body temperature was monitored throughout the study with a rectal thermo sensor and maintained at 37°C using a hot water circulation tube. Blood gases were kept within physiological limits throughout the duration of the study (pH = 7.3 ± 0.1, PCO2 = 41.3 ± 9.0 mm Hg and PO2 = 180.0 ± 28.7 mm Hg).
Acetate Infusion Protocol
A bolus of 1.0 g/kg of 99%-enriched labeled [2-13C]acetate (1.6 M and pH = 4.0) was administered with an infusion rate decreasing exponentially over 8 minutes, followed by a constant infusion rate for 2 hours in each animal. Four different groups (groups 1, 2, 3, 4; n = 2 in each group, except n = 4 in group 3) were studied with different rates of continuous infusion (0.5, 1.0, 1.5 and 1.75 g/kg/hr respectively). Blood samples were collected every 15 or 30 minutes to measure the plasma acetate concentration and enrichment using high-resolution NMR spectroscopy as described below.
It is known that injecting sodium acetate at a pH of 7.0 causes metabolic alkalosis in the blood of both animals and humans (Ward et al. 1985; Burnier et al. 1992). To avoid metabolic alkalosis in this study, the acetate solution was infused at an acidic pH (of 4.0) as previously used in rats (Sugimoto et al. 1997).
In Vivo NMR Spectroscopy
All measurements were performed on a 9.4 Tesla horizontal 31 cm bore magnet, interfaced to a Varian INOVA spectrometer (Varian, Palo Alto, CA). Localized in vivo 1H{13C} NMR spectra from the rat brain were obtained using the ACED-STEAM sequence as described previously (Pfeuffer et al. 1999). The RF coil consisted of a 13C rectangular surface coil (16×30 mm2) and two quadrature 1H coils (15 mm diameter for each loop). A volume-of-interest of 405 μl (9×5×9 mm3) was positioned in the rat brain with the aid of transverse and sagittal RARE images. Shimming was performed using FAST(EST)MAP resulting in a 16 to 22 Hz water linewidth.
Spectra were acquired in an interleaved fashion with the 13C inversion pulse applied every other scan with a repetition time (TR) of 2.5 s and 5 K data points. In addition a macromolecule spectrum (256 averages) was collected before the start of the 13C-acetate infusion using inversion-recovery with an inversion time chosen to null the signal from metabolites (“metabolite-nulled spectrum”).
Processing and Quantitation of In Vivo Spectra
Frequency variations due to the magnetic field frequency drift were corrected scan-by-scan using the N-acetyl-aspartate resonance. The difference spectra were summed in batches of 64 averages, giving a temporal resolution of 5.43 min. The 1H spectra measured without 13C inversion were also added with the same temporal resolution in order to determine the concentration of cerebral metabolites.
In vivo spectra were analyzed using LCModel version 6.1-4A (Stephen Provencher Inc., Oakville, ON, Canada). No baseline correction, zero-filling or apodization functions were applied to the in vivo data prior to the analysis. Two basis sets for LCModel analysis were generated: one for the non-edited STEAM spectrum (without 13C inversion) and one for the POCE difference spectra. The spectrum for each metabolite was simulated using in-house written programs (Henry et al. 2006a) based on density matrix formalism in Matlab (The MathWorks Inc., Natick, MA, USA) and using measured and published values of the proton-proton coupling constants (JHH) and chemical shifts (Govindaraju et al. 2000). In addition one bond and long-range JCH coupling constants (Deelchand et al. 2006) were used as required for generating the POCE spectra. The simulation readily took into account strong coupling effects (Henry et al. 2006a).
Metabolite concentrations were obtained relative to the concentration of creatine + phosphocreatine (assumed to be 8.0 μmol/g). The 13C isotopic enrichments of acetate C2, glutamate and glutamine were determined by the relative concentration ratio of the 13C concentration obtained from the edited 1H-13C spectra divided by the total concentration (12C + 13C) obtained from the unedited 1H spectrum for each resonance of interest.
Measurement of Plasma Concentration and Isotopic Enrichment of Glucose and Acetate
The total glucose concentration in plasma was measured with a glucose analyzer (Analox, London, UK). High-resolution 1H NMR spectra were used to determine plasma acetate concentration (using glucose as a concentration reference) and isotopic enrichment in plasma samples. Prior to high-resolution NMR measurements, 450 μL D2O was added to each plasma sample. Measurements were performed at 37°C on a 14.1 Tesla UNITY INOVA spectrometer (Varian, Palo Alto, CA, USA) equipped with a 5 mm triple resonance probe with single Z-axis gradient. A STEAM diffusion sequence (TE = 9 ms, TM = 64 ms, TR = 10 s, 32 averages) was used (de Graaf and Behar 2003) in order to simplify the 1H NMR spectrum from macromolecules and lipoproteins present in blood plasma. Briefly two 1H spectra were acquired; one with a low diffusion factor (4.3 s/mm2) and one with a high diffusion factor (10500 s/mm2). The difference between these spectra resulted in a spectrum consisting of metabolites resonances only. A Lorentzian line broadening of 0.5 Hz was applied prior to Fourier transformation of the difference spectrum and line fitting was performed using the Varian built-in software for acetate C2 and glucose C1α proton resonances.
The acetate C2 resonance (1.90 ppm) consisted of three peaks (a singlet S and a doublet D) whose signal integrals are noted as AceS and AceD respectively. Based on the total peak integral of glucose C1α proton resonance at 5.23 ppm (denoted as Glc1α), the concentration of acetate was calculated as: [Ace] = [Glc] × fp × (AceS + AceD) / (Glc1α), where fp (= 0.13) is a correction factor for differences in the number of protons between acetate and glucose C1α. The isotopic enrichment for acetate C2 was also computed as IEAce = AceD / (AceS + AceD).
Acetate Transport Kinetics
Since transport through the blood-brain barrier (BBB) is a bidirectional process, a reversible non-steady-state Michaelis-Menten model was used to obtain kinetics parameters for acetate transport (using the measured time courses of acetate level in plasma and brain from group 3):
| (1) |
where Splasma and Sbrain denote acetate concentration in plasma and brain respectively, ES is the acetate-transporter complex (the transporter being the monocarboxylase transporter), and CMRace is the cerebral metabolic rate of acetate. This model is described mathematically by the following equation (Voet and Voet 2004):
| (2) |
where is the Michaelis-Menten constant for transport of acetate through the BBB, is the maximal transport rate of acetate and Vd is the physical distribution volume (0.77 ml/g). CMRace is modeled by a standard non-reversible Michaelis-Menten model, i.e.
| (3) |
Where and represent the apparent Michaelis-Menten constant and the maximal rate respectively, for acetate transport through the mitochondrial inner membrane and acetyl-CoA synthetase from acetate.
Unknown parameters in these equations are , , and while the measured data are [Splasma] and [Sbrain]. The optimal value of the four unknown parameters was determined by simultaneously fitting the following two curves: 1) the time course of [Sbrain] measured in animals in group 3 (n = 4) using [Splasma] as input function and 2) the relationship [Sbrain] = f([Splasma]) obtained by plotting the values of [Splasma] and [Sbrain] at steady-state for each animal. Least-square fitting of the two experimental curves was performed with the Levenberg-Marquardt algorithm in Matlab. Monte-Carlo simulations confirmed that this procedure allows precise estimation of , , and without requiring any assumption on any of these four parameters.
Results
Plasma Acetate Levels
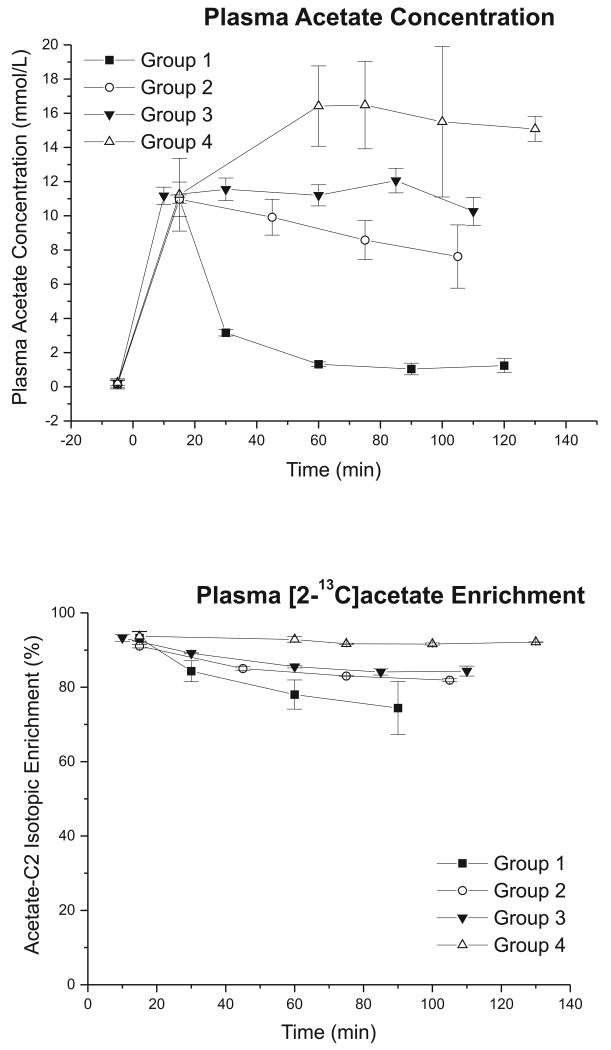
Plasma acetate concentration and isotopic enrichment increased rapidly after starting the 13C infusion (Figure 1). The total plasma acetate concentration rose quickly from baseline (0.19 ± 0.05 mmol/L) to 11.09 ± 0.13 mmol/L at 15 min, and quickly reached 93 ± 1% enrichment in [2-13C]acetate (Figure 1). Thereafter, the blood concentration and isotopic enrichment of acetate were dependent on the rate of continuous infusion. At a low infusion rate (0.5 g/kg/hr, group 1), the concentration progressively decreased to 1.3 ± 0.1 mmol/L (at 60 min) and enrichment dropped from 93% to 78%. Doubling the rate of infusion (group 2), the reduction was slower (8.6 ± 2.1 mmol/L at 75 min). Further increasing the infusion rate to 1.5 g/kg/hr (group 3), the concentration of plasma acetate was constant throughout the experiment, although its enrichment decreased slowly (from 93% to 86 %). At the highest infusion rate (1.75 g/kg/hr, group 4) the plasma acetate concentration was much higher (16.4 ± 2.4 mmol/L at 60 min) with a nearly constant isotopic enrichment throughout the experiment (Figure 1).
Figure 1.
(A) Total plasma acetate concentration (mmol/L) and (B) 13C isotopic enrichment (%) measured from high-resolution 1H NMR spectra following [2-13C]acetate infusion (TR = 10 s, 32 averages per time point). The time courses were found to be dependent on the constant continuous infusion rate of the labeled substrate. Error bars represent standard deviation. The rate of acetate infusion was 0.5, 1.0, 1.5 and 1.75 g/kg/hr for groups 1, 2, 3 and 4 respectively.
13C Metabolites Concentration in the Brain
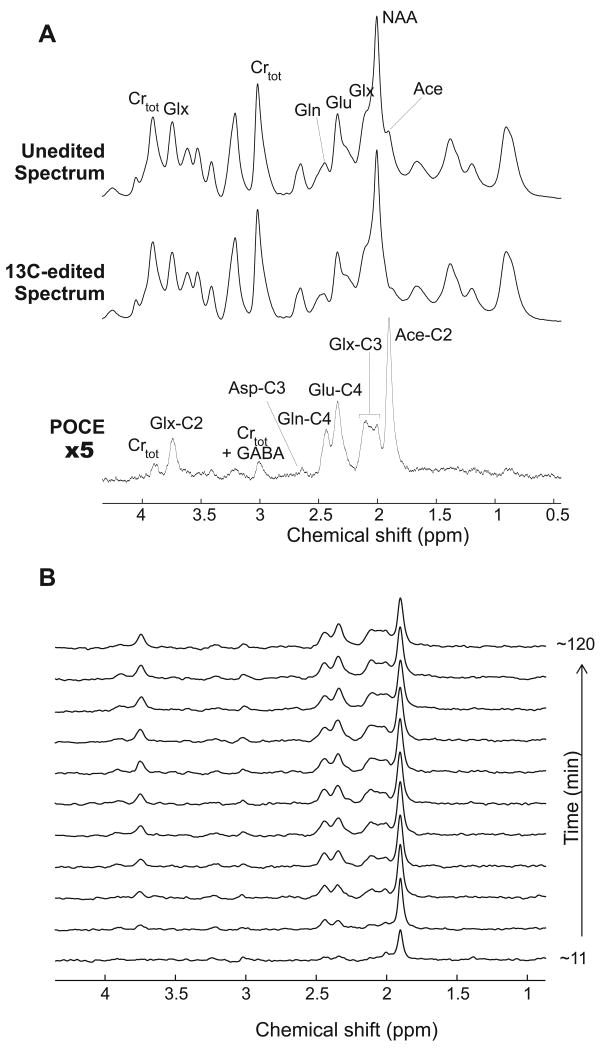
In vivo carbon-edited 1H NMR spectra (Figure 2) showed 13C label incorporation into multiple resonances, such as the acetate C2 proton resonance at 1.90 ppm, glutamate-C4 at 2.34 ppm, and glutamine-C4 at 2.44 ppm. After the bolus of 13C-labeled acetate, [2-13C]acetate was quickly detected in the brain with an isotopic enrichment of 99 ± 5% (at 11 min) as determined from analysis of in vivo spectra with LCModel. This enrichment was consistent, within the margin of error, with the 93% enrichment measured in the blood. Similar brain acetate levels were measured in all groups in the first 10 min of 13C infusion, as expected, since the same bolus was given in all groups.
Figure 2.
(A) Summed 13C-edited 1H NMR spectrum (TR = 2.5 s, TE = 7.7 ms, TM = 20 ms) acquired for 2 hours from the brain of one rat in group 3 during infusion of [2-13C]acetate. The POCE difference spectrum (scaled up by 5-fold) was obtained by subtracting the spectra acquired with the 13C inversion pulse on (edited spectrum) and off (unedited spectrum). (B) The corresponding time course of 13C-edited spectra shows incorporation of 13C labels into the cerebral pools of acetate C2, glutamate and glutamine C2, C3 and C4 with a temporal resolution of ∼11 min. Asp: aspartate; Crtot: Cr + PCr; GABA: gamma-aminobutyric acid; Glu: glutamate; Gln: glutamine; Glx: (glutamate + glutamine); and NAA: N-acetyl aspartate.
After the first 10 min, the time course of brain [2-13C]acetate concentration depended on the rate of continuous acetate infusion (Figure 3A). With the low infusion rate of 0.5 g/kg/hr (group 1), brain acetate decreased to levels undetectable from in vivo spectra within 50 min. With the rate doubled (group 2), brain acetate concentration also decreased, but more slowly. With 1.5 g/kg/hr (group 3), the brain 13C-acetate concentration was stable at about 1.5 mM. Finally, at a high infusion rate (1.75 g/kg/hr, group 4), brain acetate concentration increased steadily, reaching 3.5 mM after 120 min of infusion.
Figure 3.
Effect of four different continuous infusion rates on the in vivo 13C-metabolite concentration time courses of (A) acetate-C2, (B) glutamine-C4 and (C) glutamate-C4 following [2-13C]acetate infusion in the rat brain. The temporal resolution was 5.3 min and each time curve represents the average of two animals. Error bars represent mean Cramér-Rao Lower Bound. Constant infusion rate of acetate was 0.5, 1.0, 1.5 and 1.75 g/kg/hr for group 1, 2, 3 and 4 respectively.
13C-labeling of Brain Amino Acids: Glutamate and Glutamine
13C label from [2-13C]acetate entered the glial TCA cycle and was subsequently incorporated into the glutamate C4 (Figure 3C) and glutamine C4 carbon positions (Figure 3B). Interestingly 13C glutamate and glutamine labeling curves were nearly identical for groups 2, 3 and 4, whereas brain acetate concentration increased together with plasma acetate concentration. This indicates saturation of acetate entry from the brain into the glial TCA cycle rather than saturation of acetate transport through the blood-brain barrier. In addition in group 1 13C glutamine levels began to decrease at approximately 40 min, when the corresponding plasma acetate concentration dropped below 2-3 mM (Figure 1A) and brain acetate concentration approached zero (Figure 3A). Taken together, these observations show that, under our experimental conditions, brain acetate utilization becomes saturated when plasma acetate concentration increases above 2-3 mM.
In groups 2, 3 and 4, glutamine reached steady-state more quickly (at 40 min) compared to glutamate which was still increasing even after 2 hours of infusion. The observation that glutamate lags behind glutamine is consistent with the fact that acetate is primarily taken up by astrocytes in the brain resulting in astrocytic glutamine being labeled before neuronal glutamate (Berl et al. 1961; van den Berg and Garfinkel 1971).
Analysis of both the non-edited and edited 1H{13C} NMR spectra allowed direct determination of the isotopic enrichments. The glutamine enrichment was found to be higher than glutamate enrichment (44.3 ± 0.1% for glutamine C4 vs. 14.0 ± 0.9% for glutamate C4 during the last 30 min prior to the end of the experiment) in groups 2, 3 and 4, which is again consistent with the expected precursor-product relationship between glial glutamine and neuronal glutamate during 13C acetate infusion (Lebon et al. 2002; Tyson et al. 2003).
Rate of Acetate Utilization (CMRace)
The value of , , and was determined by simultaneously fitting the time course of S[brain] in group 3 and the relation [Sbrain] = f([Splasma]) at steady-state from all animals. An example of the fits obtained in one of the 4 animals studied in group 3 is shown in Figure 4. In this particular example, CMRace increased and reached steady-state value (0.54 μmol/g/min) in about 10 min following acetate infusion, reflecting the fact that acetate utilization is not saturated under normal physiological conditions. The non-linearity of the fit for the steady-state data (Figure 4) at low acetate concentration is consistent with that observed for glucose transport at low glucose concentration (de Graaf et al. 2001). For all four animals in group 3, the average fitted values were: , , , , and CMRace= 0.49 ± 0.08 μmol/g/min (mean ± SD; n = 4).
Figure 4.
Determination of , , and from acetate data in one animal in group 3. (A) Best fits (represented by solid line) obtained after simultaneously fitting the time course of brain acetate concentration for one animal in group 3 together with the curve [Sbrain] = f([Splasma]) at steady-state obtained from data from all animals in all four groups. In this particular example, the fitted values of transport parameters were , , and . The calculated CMRace as a function of time for the same animal showed a rapid increase before reaching its steady-state value (0.54 μmol/g/min) in about 10 min following [2-13C]acetate infusion. The blood acetate concentration which was used as input function for fitting the dynamic data is also shown.
Since high values of plasma acetate were used in the present study, the effect of diffusion through the BBB was also considered. A previous study with lactate showed that the diffusive component becomes significant at high levels of plasma lactate (LaManna et al. 1993). We estimated the diffusion constant for acetate to be 0.002 ml/g/min at steady-state (at least 10-fold lower than that reported for D-lactate (LaManna et al. 1993)) after fitting the data with an additional diffusion term in Equation 2. This value was roughly ∼5% of the total acetate net flux through the MCT transporter, implying that acetate diffusion through the BBB was negligible compared to facilitated transport. With the diffusion term used in the model, was 0.87 ± 0.14 μmol/g/min and was 2.94 ± 0.70 mM.
Changes in Total Concentrations of Glutamate and Glutamine During Acetate Infusion
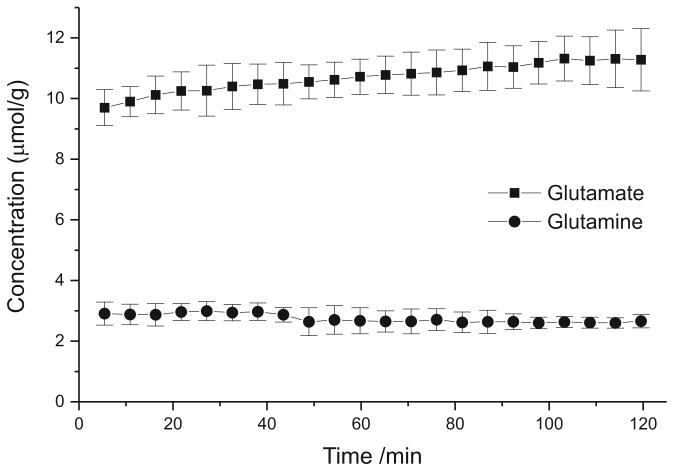
During metabolic modeling, it is usually assumed that total metabolite concentrations remain constant throughout the experiment (except for the infused substrate). This has been demonstrated to be true during glucose infusion (Rothman et al. 1992). However in the present study, during acetate infusion the total concentrations of metabolites (glutamate and glutamine) were found to change with time (See Figure 5). Glutamate concentration increased progressively by approximately 16 ± 6% (from 9.7 to 11.3 mM, paired t-test, P < 0.01) during 2 hours of acetate infusion while glutamine concentration decreased by approximately 9 ± 7% (from 2.9 to 2.6 mM) during the same time period (P < 0.02).
Figure 5.
Change in brain glutamate and glutamine concentrations (mean from all four groups) observed during [2-13C]acetate infusion. Error bars represent SD.
Discussion
Saturation of Acetate Utilization
Our study demonstrates that rat brain acetate utilization saturates and reaches close to its maximum value for acetate plasma levels above 2-3 mM. In addition, our data show that this limitation of CMRace is not due to transport through the blood-brain barrier but is due to limitation of acetate utilization after entry of acetate in the brain. This is evidenced by the fact that when infusing higher doses of acetate, brain acetate concentration keeps increasing while acetate utilization (as reflected by the 13C labeling of amino acids) does not increase substantially. This is consistent with our finding that maximum transport rate is higher for the blood-brain barrier ( ) than for acetate utilization after entry of acetate in the brain ( ). This is also consistent with data at lower infusion rate. The animals in group 1 which had the lowest rate of acetate infusion in our study (0.5 g/kg/hr), acetate utilization started to drop at 40 min (as evidenced by the drop in 13C enrichment in glutamate and glutamine as shown in Figure 3), which corresponds to [acetate]brain ∼ 0.5 mM and [acetate]plasma ∼ 2-3 mM. This indicates that when acetate concentration drops below these values, brain acetate utilization is no longer saturated.
Since transport through the blood-brain barrier is not rate-limiting, the actual rate-limiting step(s) for brain acetate utilization may be either the transport of acetate across the highly charged mitochondrial inner membrane of astrocytes, or the synthesis of acetyl-CoA, or both. These possibilities were first suggested in 1990 based on acetate and glucose metabolism in the rat brain using radioisotope labeling studies (Lear and Ackermann 1990). Formation of acetyl-CoA from acetate is catalyzed by acetyl-CoA synthetase primarily located in the glial mitochondria (Nakamura and Cheng 1969; Szutowicz and Lysiak 1980) before acetyl-CoA is further metabolized. From the low incorporation of radioactivity in acetylcholine synthesis in mammalian brain slices in the presence of [2-14C]acetate, acetyl-CoA synthesis was proposed to have a relatively low activity in the brain (Tucek 1967) suggesting that the main rate-limiting step is the conversion of acetate to acetyl-CoA, consistent with previous measurements reporting a low KM (0.073 mM) for acetyl-CoA synthetase (Luong et al. 2000).
One limitation of our study is that the model for acetate transport and utilization doesn't distinguish between different tissue types such as gray matter and white matter. Partial volume effects could potentially introduce a bias in our results. However the amount of white matter in rat brain is small (less than 10% of our volume of interest). We would therefore expect that the impact of partial volume effects on the determination of kinetic transport parameters in our study to be relatively limited.
Acetate vs. Glucose Utilization in Astrocytes
Under normal physiological condition, plasma acetate concentration is low (typically 0.1-0.2 mM). In contrast the average plasma brain glucose concentration is typically 5 mM under physiological conditions. As a result the rate of acetate utilization under normal physiological conditions is one order of magnitude lower than the rate of glucose utilization in the brain under normal conditions (Kammula and Fong 1973; Dienel et al. 2001).
However upon i.v. infusion of large amounts of acetate, as is the case in 13C MRS studies with [2-13C]acetate, plasma acetate concentration is greatly increased thus leading to an increase in brain acetate concentration and a corresponding increase in the rate of acetate utilization. For plasma acetate concentration above 2-3 mM, the rate of acetate utilization (CMRace) approaches its maximum value which is .
The labeled 13C-acetate may compete with unlabeled glucose for the generation of acetyl-CoA in astrocytes. Under saturated CMRace conditions, our data show that the enrichment of glutamine and glutamate at the C4 position are 44 % and 14 % respectively at isotopic steady-state. In order to obtain such enrichment levels in glutamate and glutamine, we estimated that ∼80% of acetyl-CoA entering the glial TCA cycle is synthesized from [2-13C]acetate, and the remaining ∼20% is synthesized from unlabeled glucose under saturated CMRace conditions.
CMRace vs. Astrocytic TCA Cycle Rate
Assuming that all acetate consumed in the brain is used for synthesis of acetyl-CoA, the rate of synthesis of acetyl-CoA from acetate would be equal to CMRace (0.49 ± 0.08 μmol/g/min from group 3). When adding 20% of acetyl-CoA synthesized from unlabeled glucose (see previous section), the total rate of acetyl-CoA synthesis would be 0.59 ± 0.1 μmol/g/min. In comparison with previous published studies in rat brain using 13C-glucose, the rate of acetyl-CoA synthesis in astrocytes (equal to the rate of pyruvate dehydrogenase since glucose is used as a substrate) ranged from 0.15 μmol/g/min in halothane anesthetized rats (Patel et al. 2004) to 0.54 μmol/g/min in awake rats (Oz et al. 2004). The rate of acetyl-CoA synthesis estimated in the present study (0.59 ± 0.1 μmol/g/min) is close to the value found by Oz et al. in awake brain. High metabolic activity is expected under our experimental conditions with morphine + pancuronium anesthesia (Sibson et al. 1998).
Alternatively, acetate may be used in other pathways than in the glial TCA cycle so that the above estimate is only an upper limit. In addition to producing acetyl-CoA which enters the astrocytic TCA cycle, acetate may also be used for the synthesis of N-acetylaspartate (NAA) and/or lipids. However, it has been reported that the turnover rate of NAA (Moreno et al. 2001; Choi and Gruetter 2004) and the rate of incorporation of acetyl-CoA into lipids (Dienel et al. 2001) are both very slow in the brain. Therefore, a large fraction of acetate taken up by the brain is most likely converted into glial acetyl-CoA.
Upregulation of Pyruvate Carboxylase
The increase in total (glutamate + glutamine) pool size observed in this study implies that there is net synthesis of new carbon skeletons through anaplerosis. The main anaplerotic pathway in the brain is through pyruvate carboxylase (PC) (Patel 1974). In the present study, an elevated amount of acetyl-CoA may be expected as a result of the large increase in brain acetate concentration. This, in turn, may upregulate PC activity as it has been shown that, in most tissues (except in muscles), PC is activated by high level of acetyl-CoA.
Physiological Effects of Acetate
Infusion of acetate has known physiological effects. For instance, it has been shown that acetate affects both metabolic and respiratory functions in normal human subjects (Burnier et al. 1992). Furthermore, acetate can produce behavioral effects such as headache and fatigue (Diamond and Henrich 1987). In animals, high levels of acetate can reduce the motor activity (Carmichael et al. 1991) and lower the requirement for general inhalation anesthetics (Campisi et al. 1997). These effects of acetate have been shown to be mediated by central nervous system adenosine receptors, and can be reversed with adenosine receptor antagonists (Campisi et al. 1997; Haberg et al. 2000). Since adenosine is a by-product of adenosine monophosphate resulting from acetate metabolism, it was suggested that its production should increase in the brain following acetate administration as previously found in both liver and heart (Kiviluoma et al. 1989; Campisi et al. 1997). However it was recently shown that no adenosine could be detected after cerebral acetate metabolism (Haberg et al. 2000; Hammer et al. 2001). Potential short-term effects of acetate infusion on brain metabolism remain to be investigated in more detail.
Acetate Studies in Humans
13C-acetate studies in humans are generally done using a lower dose of acetate infusion compared to animal studies for safety reasons. All 13C-acetate studies performed in humans to date have used a constant infusion rate of 3 mg/kg/min (Bluml et al. 2002; Lebon et al. 2002; Mason et al. 2006) with corresponding plasma acetate levels ∼1 mM at steady-state. Assuming that transport parameters are similar in humans and rats, we would expect that acetate utilization would not be saturated under these conditions, and that glutamate and glutamine enrichment would be reduced (due to the fact that the fraction of acetyl-CoA coming from 13C-acetate would be reduced compare to acetyl-CoA coming from glucose). Indeed the enrichment levels obtained in glutamate and glutamine reported in human studies (typically 14% and 4% for glutamine and glutamate C4 respectively (Lebon et al. 2002)) are about three times lower than isotopic enrichments obtained under saturated conditions with higher infusion rates of acetate in rats in our study (44% and 14% for glutamine and glutamate C4 respectively).
Conclusion
We investigated the rates of acetate transport and utilization in rat brain in vivo using 1H{13C} NMR spectroscopy. We conclude that brain acetate utilization is saturated when plasma acetate concentrations increase above 2-3 mM. At high acetate concentration, the rate-limiting step for acetate utilization is not the blood-brain barrier, but occurs after entry of acetate into the brain. This study provides new insights into the kinetics of acetate uptake and utilization in the brain, and opens the way to dynamic metabolic modeling of glutamate and glutamine 13C labeling time courses obtained during 13C-labeled acetate infusion.
Acknowledgments
Support Sources: This work was supported by NIH grants P41RR008079, P30NS057091, R01NS038672 (P.G.H.) and by the W. M. Keck Foundation.
Abbreviations/Footnote
- BBB
blood-brain barrier
- Gln
glutamine
- Glu
glutamate
- PC
pyruvate carboxylase
- POCE
proton-observed carbon-edited
- TCA
tricarboxylic acid
References
- Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Turkalj I, Seelig J, Keller U. 13C NMR for the assessment of human brain glucose metabolism in vivo. Biochemistry. 1991;30:6362–6366. doi: 10.1021/bi00240a002. [DOI] [PubMed] [Google Scholar]
- Behar KL, Petroff OA, Prichard JW, Alger JR, Shulman RG. Detection of metabolites in rabbit brain by 13C NMR spectroscopy following administration of [1-13C]glucose. Magn Reson Med. 1986;3:911–920. doi: 10.1002/mrm.1910030611. [DOI] [PubMed] [Google Scholar]
- Berl S, Lajtha A, Waelsch H. Amino acid and protein metabolism - VI Cerebral compartments of glutamic acid metabolism. Journal of Neurochemistry. 1961;7:186–197. doi: 10.1111/j.1471-4159.1959.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 2002;15:1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- Burnier P, Tappy L, Jequier E, Schneeberger D, Chiolero R. Metabolic and respiratory effects of infused sodium acetate in healthy human subjects. Am J Physiol. 1992;263:R1271–1276. doi: 10.1152/ajpregu.1992.263.6.R1271. [DOI] [PubMed] [Google Scholar]
- Campisi P, Carmichael FJ, Crawford M, Orrego H, Khanna JM. Role of adenosine in the ethanol-induced potentiation of the effects of general anesthetics in rats. Eur J Pharmacol. 1997;325:165–172. doi: 10.1016/s0014-2999(97)00124-6. [DOI] [PubMed] [Google Scholar]
- Carmichael FJ, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, Campisi P, Orrego H. Central nervous system effects of acetate: contribution to the central effects of ethanol. J Pharmacol Exp Ther. 1991;259:403–408. [PubMed] [Google Scholar]
- Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem. 1990;265:12916–12926. [PubMed] [Google Scholar]
- Chassain C, Bielicki G, Donnat JP, Renou JP, Eschalier A, Durif F. Cerebral glutamate metabolism in Parkinson's disease: an in vivo dynamic (13)C NMS study in the rat. Exp Neurol. 2005;191:276–284. doi: 10.1016/j.expneurol.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1-13C]glucose in the rat brain in vivo. J Neurochem. 2004;91:778–787. doi: 10.1111/j.1471-4159.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92:934–947. doi: 10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Behar KL. Quantitative 1H NMR spectroscopy of blood plasma metabolites. Anal Chem. 2003;75:2100–2104. doi: 10.1021/ac020782+. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Brown PB, Mason GF, Rothman DL, Behar KL. Detection of [1,6-13C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn Reson Med. 2003;49:37–46. doi: 10.1002/mrm.10348. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Pan JW, Telang F, Lee JH, Brown P, Novotny EJ, Hetherington HP, Rothman DL. Differentiation of glucose transport in human brain gray and white matter. J Cereb Blood Flow Metab. 2001;21:483–492. doi: 10.1097/00004647-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Ugurbil K, Henry PG. Investigating brain metabolism at high fields using localized 13C NMR spectroscopy without 1H decoupling. Magn Reson Med. 2006;55:279–286. doi: 10.1002/mrm.20756. [DOI] [PubMed] [Google Scholar]
- Diamond SM, Henrich WL. Acetate dialysate versus bicarbonate dialysate: a continuing controversy. Am J Kidney Dis. 1987;9:3–11. doi: 10.1016/s0272-6386(87)80155-5. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Popp D, Drew PD, Ball K, Krisht A, Cruz NF. Preferential labeling of glial and meningial brain tumors with [2-(14)C]acetate. J Nucl Med. 2001;42:1243–1250. [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Adriany G, Choi IY, Henry PG, Lei H, Oz G. Localized in vivo 13C NMR spectroscopy of the brain. NMR Biomed. 2003;16:313–338. doi: 10.1002/nbm.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter R, Novotny EJ, Boulware SD, Rothman DL, Mason GF, Shulman GI, Shulman RG, Tamborlane WV. Direct measurement of brain glucose concentrations in humans by 13C NMR spectroscopy. Proc Natl Acad Sci U S A. 1992;89:1109–1112. doi: 10.1073/pnas.89.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberg A, Qu H, Haraldseth O, Unsgard G, Sonnewald U. In vivo effects of adenosine A1 receptor agonist and antagonist on neuronal and astrocytic intermediary metabolism studied with ex vivo 13C NMR spectroscopy. J Neurochem. 2000;74:327–333. doi: 10.1046/j.1471-4159.2000.0740327.x. [DOI] [PubMed] [Google Scholar]
- Hammer J, Qu H, Haberg A, Sonnewald U. In vivo effects of adenosine A(2) receptor agonist and antagonist on neuronal and astrocytic intermediary metabolism studied with ex vivo (13)C MR spectroscopy. J Neurochem. 2001;79:885–892. doi: 10.1046/j.1471-4159.2001.00622.x. [DOI] [PubMed] [Google Scholar]
- Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem. 1995;64:2773–2782. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- Henry PG, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn Reson Med. 2006a;55:250–257. doi: 10.1002/mrm.20764. [DOI] [PubMed] [Google Scholar]
- Henry PG, Adriany G, Deelchand D, Gruetter R, Marjanska M, Oz G, Seaquist ER, Shestov A, Ugurbil K. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn Reson Imaging. 2006b;24:527–539. doi: 10.1016/j.mri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kammula RG, Fong BC. Metabolism of glucose and acetate by the ovine brain in vivo. Am J Physiol. 1973;225:110–113. doi: 10.1152/ajplegacy.1973.225.1.110. [DOI] [PubMed] [Google Scholar]
- Kiviluoma KT, Peuhkurinen KJ, Hassinen IE. Adenine nucleotide transport and adenosine production in isolated rat heart mitochondria during acetate metabolism. Biochim Biophys Acta. 1989;974:274–281. doi: 10.1016/s0005-2728(89)80244-0. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Harrington JF, Vendel LM, Abi-Saleh K, Lust WD, Harik SI. Regional blood-brain lactate influx. Brain Res. 1993;614:164–170. doi: 10.1016/0006-8993(93)91030-v. [DOI] [PubMed] [Google Scholar]
- Lear JL, Ackermann RF. Evaluation of radiolabeled acetate and fluoroacetate as potential tracers of cerebral oxidative metabolism. Metabolic Brain Disease. 1990;V5:45–56. doi: 10.1007/BF00996977. [DOI] [PubMed] [Google Scholar]
- Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong A, Hannah VC, Brown MS, Goldstein JL. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2000;275:26458–26466. doi: 10.1074/jbc.M004160200. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes. 2006;55:929–934. doi: 10.2337/diabetes.55.04.06.db05-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Ross BD, Bluml S. Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by (13)C MRS and [1-(13)C]glucose infusion. J Neurochem. 2001;77:347–350. doi: 10.1046/j.1471-4159.2001.t01-1-00282.x. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Cheng SC. Evidence for the metabolic compartmentalization of acetyl-coenzyme A in rat brain slices and its relation to the syntheses of acetylcholine and glutamate. Life Sci. 1969;8:657–662. doi: 10.1016/0024-3205(69)90223-9. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Clarke DD. Decarboxylation studies of glutamate, glutamine, and aspartate from brain labelled with [1-14C]acetate, L-[U-14C]-aspartate, and L-[U-14C]glutamate. J Neurochem. 1969;16:549–558. doi: 10.1111/j.1471-4159.1969.tb06854.x. [DOI] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, Shulman RG, Behar KL. Glutamatergic Neurotransmission and Neuronal Glucose Oxidation Are Coupled During Intense Neuronal Activation. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- Patel MS. The relative significance of CO2-fixing enzymes in the metabolism of rat brain. J Neurochem. 1974;22:717–724. doi: 10.1111/j.1471-4159.1974.tb04285.x. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Tkac I, Choi IY, Merkle H, Ugurbil K, Garwood M, Gruetter R. Localized in vivo 1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C] D-glucose. Magn Reson Med. 1999;41:1077–1083. doi: 10.1002/(sici)1522-2594(199906)41:6<1077::aid-mrm1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Ross BD, Higgins RJ, Boggan JE, Willis JA, Knittel B, Unger SW. Carbohydrate metabolism of the rat C6 glioma. An in vivo 13C and in vitro 1H magnetic resonance spectroscopy study. NMR Biomed. 1988;1:20–26. doi: 10.1002/nbm.1940010105. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Novotny EJ, Shulman GI, Howseman AM, Petroff OA, Mason G, Nixon T, Hanstock CC, Prichard JW, Shulman RG. 1H-[13C] NMR measurements of [4-13C]glutamate turnover in human brain. Proc Natl Acad Sci U S A. 1992;89:9603–9606. doi: 10.1073/pnas.89.20.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestov AA, Valette J, Ugurbil K, Henry PG. On the reliability of (13)C metabolic modeling with two-compartment neuronal-glial models. J Neurosci Res. 2007;85:3294–3303. doi: 10.1002/jnr.21269. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Koehler RC, Wilson DA, Brusilow SW, Traystman RJ. Methionine Sulfoximine, a Glutamine Synthetase Inhibitor, Attenuates Increased Extracellular Potassium Activity During Acute Hyperammonemia. 1997;17:44–49. doi: 10.1097/00004647-199701000-00006. [DOI] [PubMed] [Google Scholar]
- Szutowicz A, Lysiak W. Regional and subcellular distribution of ATP-citrate lyase and other enzymes of acetyl-CoA metabolism in rat brain. J Neurochem. 1980;35:775–785. doi: 10.1111/j.1471-4159.1980.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Tucek S. Subcellular distribution of acetyl-CoA synthetase, ATP citrate lyase, citrate synthase, choline acetyltransferase, fumarate hydratase and lactate dehydrogenase in mammalian brain tissue. J Neurochem. 1967;14:531–545. doi: 10.1111/j.1471-4159.1967.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Tyson RL, Gallagher C, Sutherland GR. 13C-Labeled substrates and the cerebral metabolic compartmentalization of acetate and lactate. Brain Res. 2003;992:43–52. doi: 10.1016/j.brainres.2003.08.027. [DOI] [PubMed] [Google Scholar]
- van den Berg CJ, Garfinkel D. A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J. 1971;123:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet D, Voet JG. Biochemistry. 3rd. Vol. 481. John Wiley & Sons; 2004. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RA, Wathen RL, Harding GB, Thompson LC. Comparative metabolic effects of acetate and dichloroacetate infusion in the anesthetized dog. Metabolism. 1985;34:680–687. doi: 10.1016/0026-0495(85)90098-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Li SS, Bacher J, Shen J. Quantification of cortical GABA-glutamine cycling rate using in vivo magnetic resonance signal of [2-13C]GABA derived from glia-specific substrate [2-13C]acetate. Neurochemistry International. 2007;50:371–378. doi: 10.1016/j.neuint.2006.09.011. [DOI] [PubMed] [Google Scholar]