Abstract
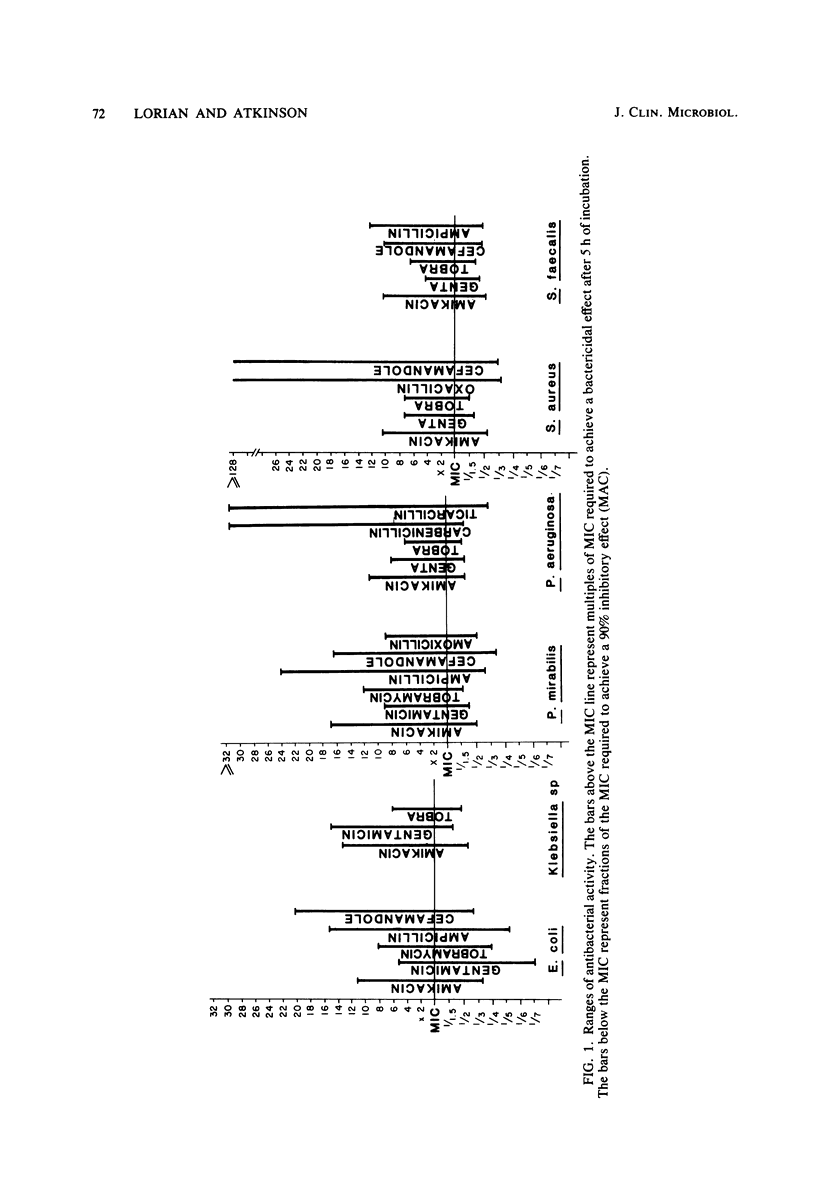
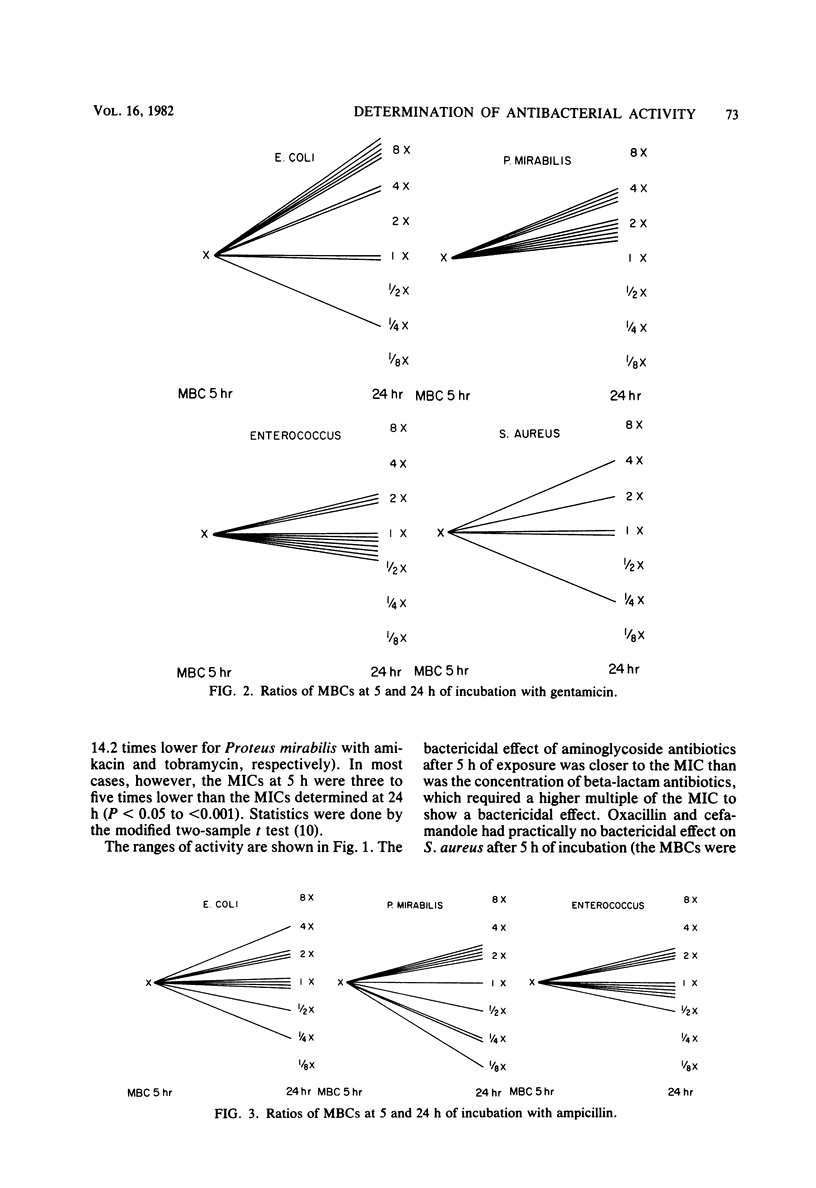
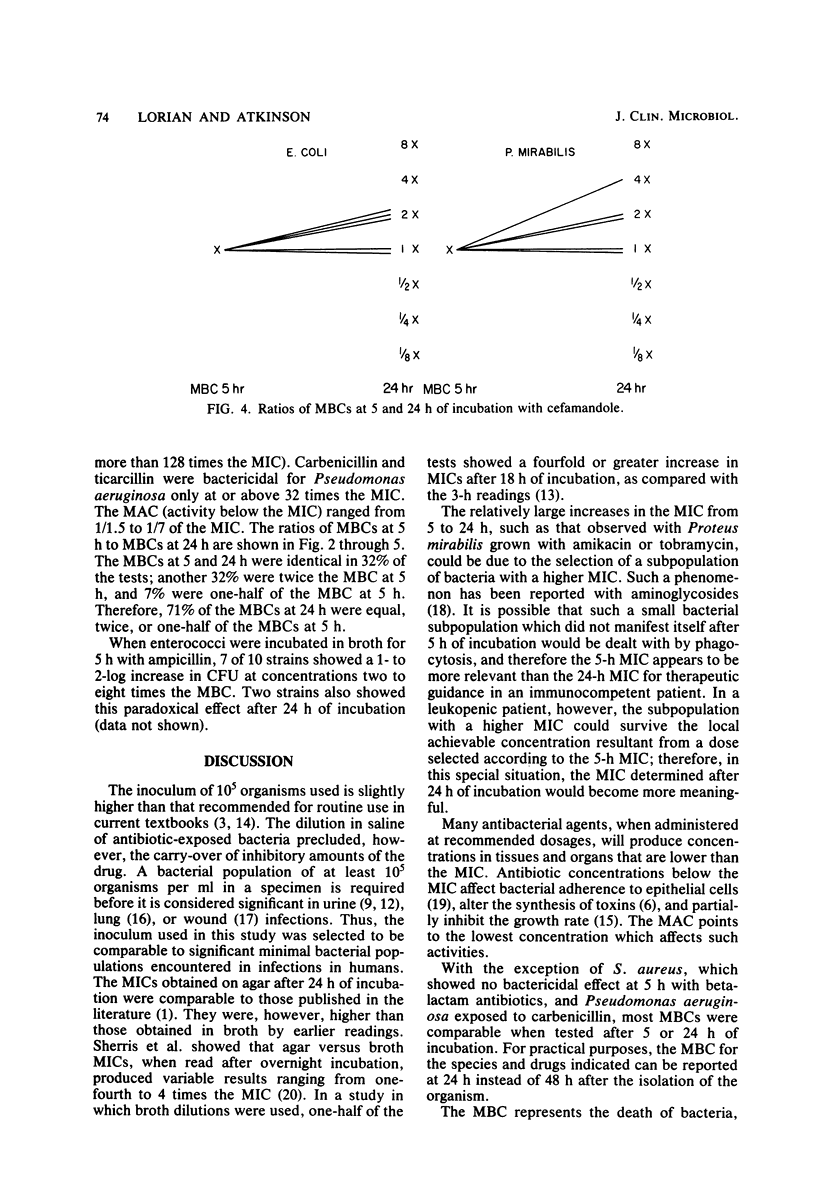
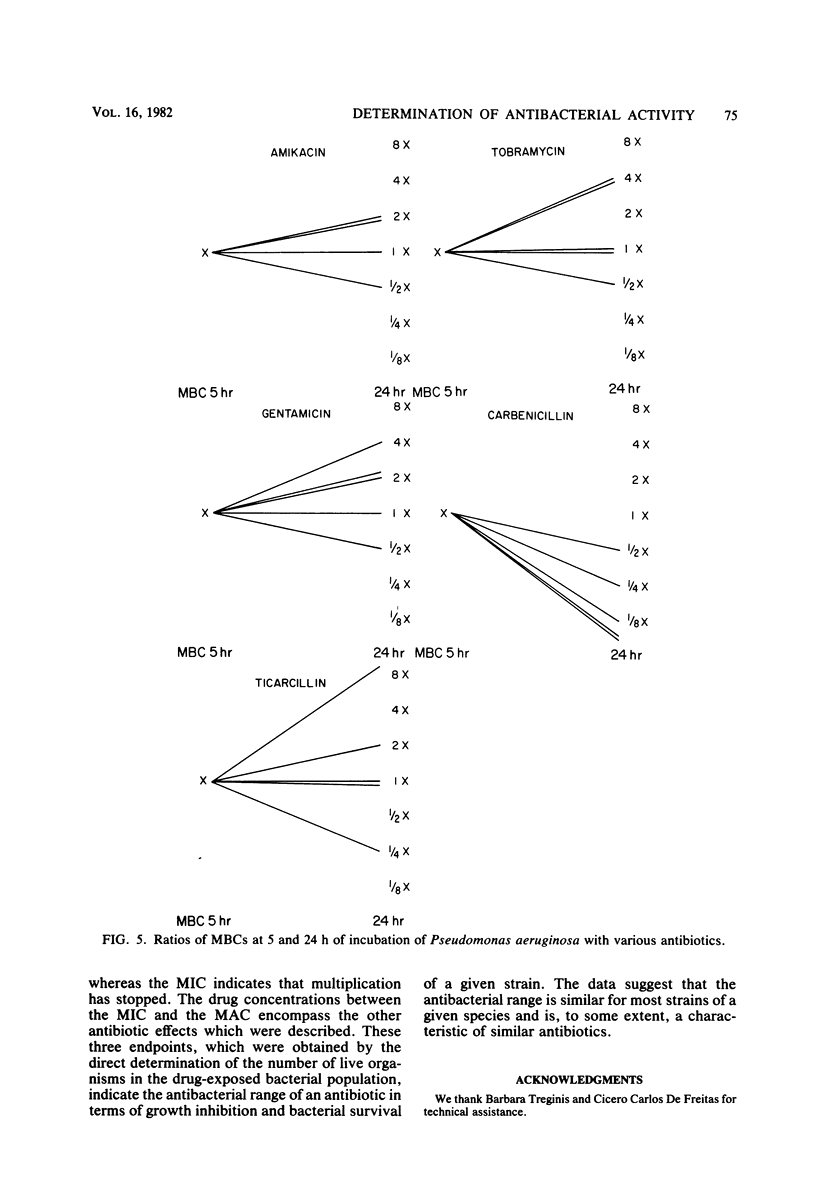
The activity of three aminoglycosides and six beta-lactam antibiotics on strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, and enterococci was studied. The minimal inhibitory concentrations (MICs), the minimal bactericidal concentrations (MBCs), and the minimal antibiotic concentrations (MACs) were determined after 5 h of incubation in broth cultures by colony-forming-unit counts. The MICs were also determined by agar dilution after 24 h of incubation. The MICs on agar after 24 h of incubation were higher than those in broth after 5 h of incubation. The differences ranged from 1.1- to 14.2-fold, but in most cases were only three- to fivefold (P less than 0.05 to less than 0.001). The MBCs at 5 and 24 h were comparable in 71% of tests. For current practice, the MBC of enterococci can be determined after 5 h of incubation with antibiotics. The aminoglycosides showed MBCs which were closer to the MICs than were those of the beta-lactam antibiotics, which required a higher multiple of the MIC to show a bactericidal effect. The MBCs of oxacillin and cefamandole for S. aureus after 5 h of incubation were greater than 128 times the respective MICs. The MACs ranged from 1/1.5 to 1/7 of the 5-h MICs. The three endpoints, MIC, MBC, and MAC, indicate the antibacterial range of an antibiotic in terms of inhibition of growth and bacterial survival. The data suggest that the antibacterial range of an antibiotic is similar for most strains of a given species and is, to some extent, a characteristic of similar antibiotics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Thornsberry C., Facklam R. R. Synergism, killing kinetics, and antimicrobial susceptibility of group A and B streptococci. Antimicrob Agents Chemother. 1981 May;19(5):716–725. doi: 10.1128/aac.19.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Gwynn M. N., Webb T. L., Rolinson G. N. Regrowth of Pseudomonas aeruginosa and other bacteria after the bactericidal action of carbenicillin and other beta-lactam antibiotics. J Infect Dis. 1981 Sep;144(3):263–269. doi: 10.1093/infdis/144.3.263. [DOI] [PubMed] [Google Scholar]
- HOEPRICH P. D. Culture of the urine. J Lab Clin Med. 1960 Dec;56:899–907. [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Inhibition of beta-lactamase: a continuing story. J Antimicrob Chemother. 1977 May;3(3):195–196. doi: 10.1093/jac/3.3.195. [DOI] [PubMed] [Google Scholar]
- Johnston H. H. Automation for sensitivity testing. J Antimicrob Chemother. 1979 May;5(3):246–247. doi: 10.1093/jac/5.3.246. [DOI] [PubMed] [Google Scholar]
- Kunin C. M. A ten-year study of bacteriuria in schoolgirls: final report of bacteriologic, urologic, and epidemiologic findings. J Infect Dis. 1970 Nov;122(5):382–393. doi: 10.1093/infdis/122.5.382. [DOI] [PubMed] [Google Scholar]
- Lampe M. F., Aitken C. L., Dennis P. G., Forsythe P. S., Patrick K. E., Schoenknecht F. D., Sherris J. C. Relationship of early readings of minimal inhibitory concentrations to the results of overnight tests. Antimicrob Agents Chemother. 1975 Oct;8(4):429–433. doi: 10.1128/aac.8.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V., De Freitas C. C. Minimal antibiotic concentrations of aminoglycosides and beta-lactam antibiotics for some gram-negative bacilli and gram-positive cocci. J Infect Dis. 1979 May;139(5):599–603. doi: 10.1093/infdis/139.5.599. [DOI] [PubMed] [Google Scholar]
- Lyman I. R., Tenery J. H., Basson R. P. Correlation between decrease in bacterial load and rate of wound healing. Surg Gynecol Obstet. 1970 Apr;130(4):616–621. [PubMed] [Google Scholar]
- Mawer S. L., Greenwood D. Specific and non-specific resistance to aminoglycosides in Escherichia coli. J Clin Pathol. 1978 Jan;31(1):12–15. doi: 10.1136/jcp.31.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg T., Stenqvist K., Svanborg-Edén C. Effects of subminimal inhibitory concentrations of ampicillin, chloramphenicol, and nitrofurantoin on the attachment of Escherichia coli to human uroepithelial cells in vitro. Rev Infect Dis. 1979 Sep-Oct;1(5):838–844. doi: 10.1093/clinids/1.5.838. [DOI] [PubMed] [Google Scholar]
- Sherris J. C., Rashad A. L., Lighthart G. A. Laboratory determination of antibiotic susceptibility to ampicillin and cephalothin. Ann N Y Acad Sci. 1967 Sep 27;145(2):248–267. doi: 10.1111/j.1749-6632.1967.tb50223.x. [DOI] [PubMed] [Google Scholar]


