Abstract
The CDC14 family of multifunctional evolutionarily conserved phosphatases includes major regulators of mitosis in eukaryotes and of DNA damage response in humans. The CDC14 function is also crucial for accurate chromosome segregation, which is exemplified by its absolute requirement in yeast for the anaphase segregation of nucleolar organizers; however the nature of this essential pathway is not understood. Upon investigation of the rDNA nondisjunction phenomenon, it was found that cdc14 mutants fail to complete replication of this locus. Moreover, other late-replicating genomic regions (10% of the genome) are also underreplicated in cdc14 mutants undergoing anaphase. This selective genome-wide replication defect is due to dosage insufficiency of replication factors in the nucleus, which stems from two defects, both contingent on the reduced CDC14 function in the preceding mitosis. First, a constitutive nuclear import defect results in a drastic dosage decrease for those replication proteins that are regulated by nuclear transport. Particularly, essential RPA subunits display both lower mRNA and protein levels, as well as abnormal cytoplasmic localization. Second, the reduced transcription of MBF and SBF-controlled genes in G1 leads to the reduction in protein levels of many proteins involved in DNA replication. The failure to complete replication of late replicons is the primary reason for chromosome nondisjunction upon CDC14 dysfunction. As the genome-wide slow-down of DNA replication does not trigger checkpoints [Lengronne A, Schwob E (2002) Mol Cell 9:1067–1078], CDC14 mutations pose an overwhelming challenge to genome stability, both generating chromosome damage and undermining the checkpoint control mechanisms.
Keywords: DNA replication, genome, rDNA, RFA2, SWI6, importins
Successful chromosome segregation in mitosis requires the coordinated work of cellular regulatory circuits, which transmit cell cycle signals to chromatin and spindle proteins. One of such factors is the Cdc14 protein, an evolutionary conserved protein phosphatase (1–3). While in human cells a CDC14 ortholog has been recently shown to play a key role in DNA damage response (4), studies on S. cerevisiae were mostly focused on Cdc14p roles in anaphase regulation and in the exit from mitosis. The scope of Cdc14p activity in budding yeast is believed to be limited to anaphase, because Cdc14p is sequestered in the nucleolus (5) in apparently inactive form (6) at other cell cycle stages. Therefore, while Cdc14 can potentially dephosphorylate many substrates (7, 8), the most studied physiological pathways are the anaphase pathways (FEAR and MEN), which are both dependent on the two sequential bursts of Cdc14 release (1, 9). The cdc14 mutations cause a mitotic exit block, but also display defects in nucleolar (10) and telomeric (11) segregation. The mechanisms of chromosome segregation defects (11–15) in cdc14 mutants are generally poorly understood. While condensin mutations phenocopy the cdc14 rDNA nondisjunction (11, 16) and Cdc14p is required for condensin loading to rDNA (14), it is unlikely that condensin deficiency is the primary cause of chromosome missegregation in cdc14 mutants. Indeed the interference of rDNA transcription with condensin binding (17) and the elevated levels of mitotic rDNA transcription in cdc14 cells (18–20) suggest that the role of Cdc14 in condensin loading is indirect. Incidentally, some role of CDC14 in DNA replication was demonstrated genetically (21), and many replication factors could be direct substrates of Cdc14p (7). Cdc14 is also known to organize prereplication complex formation and the G1 transcriptional program, which controls expression of cyclins and replication factors. While bulk DNA replication is complete at cdc14 arrest (22), the rDNA locus is sensitive to collision of transcription with DNA replication (23, 24), which could be related to the specific increase of rDNA nondisjunction in cdc14 mutants.
Here we demonstrate that chromosome nondisjunction in cdc14 mutants is largely caused by stretches of unreplicated DNA. We show that the compounding deregulation of both G1 transcription and nuclear import of replication factors in cdc14 is the most probable mechanism responsible for the DNA underreplication in this mutant. This phenotype encompasses two cell cycles due to the constitutive (hypomorphic) defect of the mutant Cdc14 protein, which likely affects multiple targets relevant to DNA replication. However, because DNA replication is not stalled in the mutants, the DNA replication checkpoint is not triggered, demonstrating that a hypomorphic mutation in a single gene can significantly compromise genome stability by generating genome-wide chromosome lesions that are invisible to checkpoint control mechanisms.
Results
rDNA Is Underreplicated in cdc14.
As the nature of the linkage between nondisjoined sister chromatids in the cdc14 mutant anaphase remains unknown, we tested whether rDNA replication is defective in cdc14 mutants. Due to its extended replicon size (25) and largely unidirectional replication, the rDNA locus must be particularly sensitive to DNA underreplication, which might produce irresolvable sister chromatids links (Fig. 1A). First, we used a plasmid loss assay (21) to examine the activity of rDNA ARS in cdc14 mutants. The effect of cdc14 mutation was quite dramatic on plasmids carrying rDNA-derived origins: both colony size and transformation efficiency were markedly reduced, and the minichromosome stability was below 5% (Fig. 1B). This suggests that the cdc14-1 allele is a strong hypomorph for rDNA origin function. In contrast, the early-firing ARS1 or its weakened ars1′ derivative showed a comparable stability in wild type and cdc14 mutants (Fig. 1B). Thus cdc14-1 cells might replicate the native 1 Mb long rDNA locus more sluggishly, either due to inefficient/late origin firing or to slower fork progression along the rDNA array.
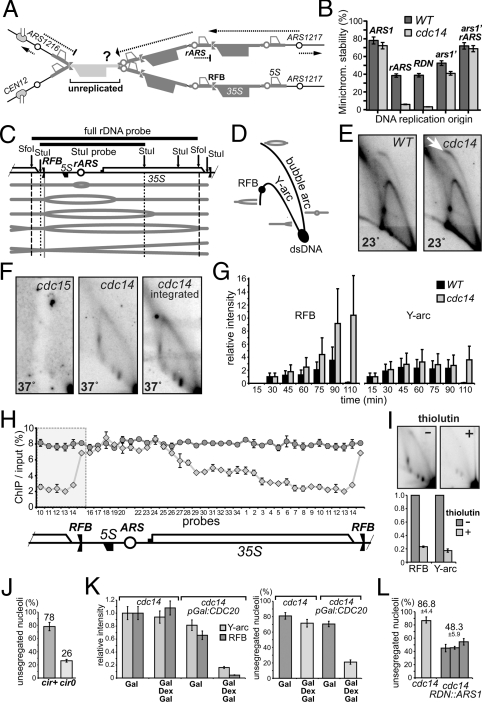
Fig. 1.
Nucleolar missegregation in cdc14 is consistent with rDNA under-replication. (A) Putative linking of rDNA sister chromatids due to under-replication, leading to the segregation failure. Only three repeats are shown for simplicity. Dotted lines - paths of replication forks. (B) rARS cannot maintain minichromosomes in cdc14 cells. The minichromosome stability was tested in isogenic strains under selective conditions at 23 °C. TRP1/CEN3 minichromosomes had various replication origins: full-length rDNA, short rARS-containing fragments, full-length ARS1 and a truncated (weakened) ARS1 (ars1′). Minichromosomes containing rARS (pAS1071) or full-length rDNA repeat (pAS1072) as a single replicator failed to propagate in cdc14 cells: all surviving Trp+ clones contained TRP1 integration. The rARS is not completely inactive in cdc14-1 cells, as indicated by the synergistic restoration of minichromosome stability when ars1′ and rARS are combined (last column). (C) Schematic of a single rDNA repeat progressing through replication stages. Southern hybridization probes and restriction sites used for 2D gel analyses (SfoI or StuI) are shown. (D) Replication intermediates distribution on a neutral-neutral two-dimensional gel. (E) Replication intermediates in asynchronous wild type and cdc14 mutant at permissive temperature. Wild type and cdc14-1 cells were grown at 23 °C. Purified DNA was digested with SfoI, enriched by ssDNA affinity chromatography, separated by 2D electrophoresis and analyzed by Southern blotting. The arrow: relative enrichment of the bubble arc. (F) cdc14 mutants arrested at 37 °C still contain rDNA replication intermediates. Cultures were arrested in G1 for 2 h at 23 °C, 1 h at 37 °C, and released at 37 °C for 3 h. Anaphase-arrested cells were analyzed as in E, except that rDNA was digested with StuI. To exclude the possibility that the observed retarded replication is a property of a hidden (non-CDC14) mutation, the cdc14-1 allele was cloned by PCR and reintroduced it into a wild-type strain by gene replacement (cdc14 integrated). (G) The cdc14 mutation delays progression of rDNA replication. Cultures were synchronously released from G1 arrest as in F, time-course cell samples were collected every 15 min and analyzed as in E. RFB and Y-arc signals were normalized to the 30 min and 15 min points, respectively, as signals at these time points were similar for wild type and cdc14. (H) BrdU incorporation in rDNA is progressively inhibited in cdc14 cells in the RNA PolI-transcribed region. Both strains were arrested as in G and BrdU was added to the media 30 min before the release from G1 into fresh YPD containing BrdU. BrdU incorporation was analyzed 2 h after release by ChIP/qPCR using rDNA probes as in (14). Shaded area is duplicated to show RFB surroundings. (I) Inhibition of transcription reduces underreplication in cdc14. The cdc14-1 mutant was arrested and released as in F in the absence or presence of thiolutin (87 μM), which was added in the mid-S-phase. The RFB and Y-arc signals were quantified and normalized to the corresponding values without thiolutin. (J) Curing cdc14 strains of 2-μm episomes significantly rescues nucleolar missegregation. Four independent cir0 clones were analyzed after arresting cells as in F. Minimum 200 nuclei were scored. (K) Metaphase delay suppresses both the rDNA replication delay and nucleolar segregation defects in cdc14 mutants. pGal:CDC20 and control cells were arrested in YPRG media in G1 and then arrested in metaphase (the Cdc20p depletion after addition of dextrose) with inactivated Cdc14p (37 °C). Cultures were grown in YPRG and split in half after the G1 arrest, one-half was released to YPRG at 37 °C for 5 h (Gal), while another half was released to YPD at 37 °C (to arrest cells in metaphase). Cells were harvested after 3 h, re-suspended in prewarmed YPRG media and incubated at 37 °C for 2 h (Gal Dex Gal). Continuous CDC20 expression (Gal) was used to control for possible overexpression effects unrelated to the mitotic arrest itself. Replication intermediates were analyzed as in E; the nucleolus segregation was scored by Sik1p-RFP, in 200 anaphase cells. (L) ARS1 integration in the rDNA locus partially rescues nucleolar nondisjunction in cdc14. Three control cdc14 cultures and three independent clones with ARS1 integration into rDNA were grown at 23 °C and arrested at 37 °C for 3 h. Cells were fixed and stained with DAPI, nucleolar segregation was monitored by Sik1p-RFP, with two sets of 100 cells scored per each strain.
Upon analysis of rDNA replication deficiency by 2D GEF (26) (Fig. 1 C and D) the asynchronous cdc14 cell population at 23 °C did not show any major abnormalities, except for a nearly 4-fold accumulation of the bubble arc signal, relatively to the Y-arc (Fig. 1E). This accumulation signified a slowing of replication fork progression, as digestion of rDNA with StuI (converting long replication bubbles into Y-shaped molecules) selectively enriched the Y-arcs only in wild type, indicating that replication bubbles in cdc14-1 were disproportionally small (Fig. S1A). Furthermore, Y-shaped rDNA molecules were persistent in cdc14 cells fully arrested in anaphase (Fig. 1F), and were observed even if the synchronized cell population was shifted to nonpermissive temperature well after the G1-S transition (Fig. S1B). This replication delay is not due to later onset of rDNA replication, as time-course 2D analysis of synchronized cdc14-1 cells showed that rDNA replication initiated at the same time in cdc14 and in wild type, but that cdc14 cells failed to complete it on time (1G). Because Cdc14p was supposedly inactive throughout this experiment, up until anaphase, this result suggests a dual reason for the cdc14-1 replication defect: first, replication must already be defective at 23 °C, generating one or more lagging forks; second, the replication delay rescue, which likely occurs at 23 °C when additional Cdc14p is released in anaphase, is not feasible at 37 °C.
Further analysis of replication completion in cdc14 anaphase by BrdU ChIP showed a gradual decrease of BrdU incorporation upon increased distance from the origin (Fig. 1H). This profile prompted us to test whether one of the anaphase-specific obstacles to rDNA replication might be RNA PolI transcription itself. When cdc14-1 cells synchronously released from G1 were treated with thiolutin (27), an RNA synthesis inhibitor (28), the rDNA underreplication was substantially reduced (Fig. 1I). This suggests that elevated transcription prevents the completion of rDNA replication in anaphase of cdc14 cells. Deletion of the FOB1gene, which removes the rDNA replication fork barrier (RFB), reduced the Y-arcs in cdc14-1 anaphase more than 5-fold, indicating that unidirectional replication of rDNA is an additional obstacle in cdc14.
To gain insight into the nature of the cdc14 replication problem itself, we tested whether the increased dosage of factors available to replicate chromosomal DNA (via the elimination of the endogenous 2-μm plasmid replicons) would suppress rDNA missegregation in cdc14. Indeed, such curing of cdc14 strains significantly suppressed the nucleolar nondisjunction phenotype (Fig. 1J). Furthermore, if cdc14 replication forks were slowed down, but not stalled, DNA replication might be rescued by delaying anaphase entry. We therefore generated such a delay by the depletion of Cdc20p, which does not interfere with DNA replication machinery or spindle formation. Transient, but significantly longer than described previously (13), pGAL:CDC20 shut-off resulted in both significant reduction of replication intermediates and in prominent rescue of the cdc14 nucleolar segregation defect (Fig. 1K). These data are consistent with 2D gel analysis above and concur with the idea that delayed replication might be responsible, at least in part, for the cdc14 rDNA segregation phenotype. In rDNA, this delay may be exacerbated by sparse positioning of active origins, because integration of ARS1 into the rDNA locus partially rescues its segregation (Fig. 1L). Thus, DNA replication forks in CDC14-deficient cells are likely active, but have reduced speed and/or efficiency.
Genome-Wide Defects in Chromosome Replication in cdc14.
As Cdc14 mutations are known to interfere with the function of non-rDNA origins (21), we compared the cdc14 rDNA replication delay to the whole genome. A time-course analysis of replication by DNA molecular combing (25) (Fig. 2 A and B), revealed genome-wide underreplication in cdc14. In wild-type, the fraction of unreplicated DNA fibers dropped from 24% at 60 min to the background 2% at 90 min, as expected from the FACS data (Fig. 2A). In cdc14-1 cells however, there were still 18% unreplicated fibers at 120 min after release, that is, 60 min after the entire culture had apparently (by FACS) completed S phase. This indicated that DNA replication was significantly delayed in cdc14-1 mutant cells at 37 °C, although probably not fully blocked as this number fell from 18 to 10% during the following 30 min (Fig. 2D). Surprisingly, the replication completion delay was only marginally more pronounced for rDNA, where 12% of the fibers remained unreplicated 150 min after release from G1, that is, when cells are well arrested in anaphase (Fig. 2C). These data are consistent with Fig. 1 results and concur with the idea that delayed replication might be responsible, at least in part, for the chromosome segregation defects of cdc14-1 cells.
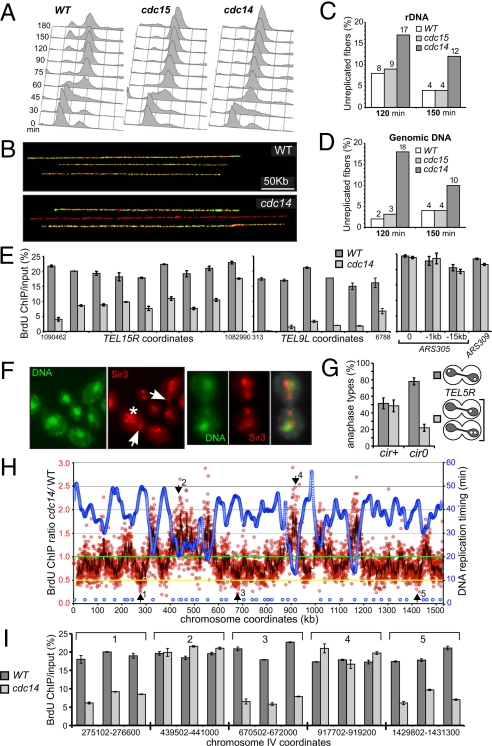
Fig. 2.
cdc14-specific DNA under-replication is not limited to rDNA. (A) DNA content (FACS) in a time-course experiment. Cultures were grown at 23 °C, arrested in G1 with α-factor, shifted to 37 °C for 30 min in YPD + BrdU and then released at 37 °C in YPD + BrdU for combing analyses in (B–D). (B) Representative image of combed DNA fibers showing fully replicated molecules and unreplicated molecules (red only) detected both with anti-ssDNA (red) and anti-BrdU (green) antibodies. Analysis was performed both for total genomic DNA and specifically for rDNA after cutting genomic DNA with restriction enzymes (BamHI and XhoI) that cleave every 5–10 kb, but leave the 1.5-Mb rDNA array intact. (C and D) Percent of DNA fibers (≥100 kb) largely unreplicated after DNA combing. DNA was analyzed at the indicated time for both total genomic DNA and rDNA. Plotting the percentage of unreplicated DNA fibers (lacking BrdU signal for longer than 100 kb) with respect to time after release shows the progression and completion of S phase. (E) BrdU incorporation is reduced at telomeres in cdc14. Strains, arrest and labeling procedures are as in A. BrdU incorporation into DNA was analyzed (2 h after release into 37 °C/nocodazole from G1) by ChIP/qPCR using the probes covering the telomeric regions of two chromosomes (15R and 9L). Neither probe set is unique for these locations, usually corresponding to 2–6 sites at other telomeres. The early-replicating regions of chromosome III were analyzed similarly, as a control. (F) Telomere segregation is impaired in cdc14. Strains carrying SIR3:mRFP gene replacement were arrested as in Fig. 1F, stained with DAPI (green), and scored for Sir3p-mRFP signal position (red). Unlike wild type, cdc14 had blocked telomere segregation (arrows and higher magnification), and prominent additional signs of asymmetric segregation (asterisk). (G) Telomere missegregation in cdc14-3 is suppressed by the loss of two-μm episomes. Four independent cir0 clones were analyzed after arresting cells as in Fig. 1F. Minimum 200 nuclei were scored. TEL5R-adjacent chromosomal tags were as in (11). (H) Late-replicating regions have reduced BrdU incorporation in cdc14. ChIP-chip analysis of BrdU incorporation is shown for chromosome IV using the arrest/labeling as in A. Four experiments (SI Microarray Hybridization Data) were averaged for each strain and plotted as a cdc14 to wild type ratio. Red circles (i.e., individual microarray features) show the relative BrdU incorporation in cdc14. The black curve: 10-feature running average. Blue circles: replication timing of microarray features and origin positions (Lower) from (63). Arrows: locations of validation regions (in H). (I) cdc14 cells delay BrdU incorporation in late-replicating regions. ChIP/qPCR validation of ChIP-chip results in G was done as in E, with five regions (numbered according to G) where three non-overlapping 300-bp PCR probes were selected.
To better characterize which chromosomal regions are underreplicated, we first probed telomeric sites that were shown to missegregate in cdc14 mutants (11). BrdU incorporation followed by ChIP/qPCR indicated that the two tested telomeres (TEL15R, TEL9L) were still underreplicated in cdc14 mutants 2 h after release from G1 (nocodazole-arrested cells) (Fig. 2E). This underreplication was specific to cdc14-1 cells and not seen at early-replicating regions (around ARS305 or ARS309). Furthermore, using the Sir3p-mRFP telomeric marker, we showed that telomere segregation was severely disorganized in cdc14 mutants undergoing anaphase: more than 60% of cells had incomplete telomere separation and up to one-third of the total population showed clearly identifiable signs of asymmetric segregation (Fig. 2F). Next we analyzed the entire chromosomes III, IV, and V by BrdU ChIP-chip to get a more global picture of the cdc14 underrepplication defect. This revealed several distinct zones where the BrdU incorporation ratio (cdc14 to wild type) was less than 0.5 (Fig. 2H and Fig. S2). A sample of such zones was validated by qPCR/ChIP, which showed an average 70% reduction of BrdU incorporation in cdc14 compared to wild type (Fig. 2I). Furthermore, from the analysis of three chromosomes, it became evident that delayed BrdU incorporation coincides with late-replicating regions (Fig. 2H and Fig. S2). Thus, cdc14 underreplication affects the whole genome, specifically regions containing late-firing origins and/or replicating late during S phase.
DNA Checkpoint Is Not Activated by Underreplication in cdc14.
Strikingly, chromosome underreplication in cdc14-1 mutants does not activate the DNA replication checkpoint, since these cells arrest in anaphase/telophase. One possibility is that the checkpoint itself could be inactivated in cdc14 mutants, at least partially. This is unlikely, however, as cdc14 cells released from G1 at 37 °C in the presence of hydroxyurea (Fig. 3A) or MMS (Fig. 3B) showed the metaphase arrest kinetics similar to wild type. The phosphorylation levels of Rad53p (dependent on Mec1p or Tel1p) in cdc14 were comparable to cdc15 (control) in response to both stalled forks (hydroxyurea) and DNA damage (MMS) (Fig. 3C). The Mec1p/Tel1p-dependent phosphorylation of H2A at the serine residue 129 was also unaltered in cdc14 (Fig. 3D). Thus, an alternative explanation is likely, that is, slowed replication in cdc14 is carried out by replication forks that are structurally normal, and therefore cannot trigger checkpoints (29, 30). This appears to be different from clb5 mutants, which do not fire late origins (31) but do activate a Rad53 response (32).
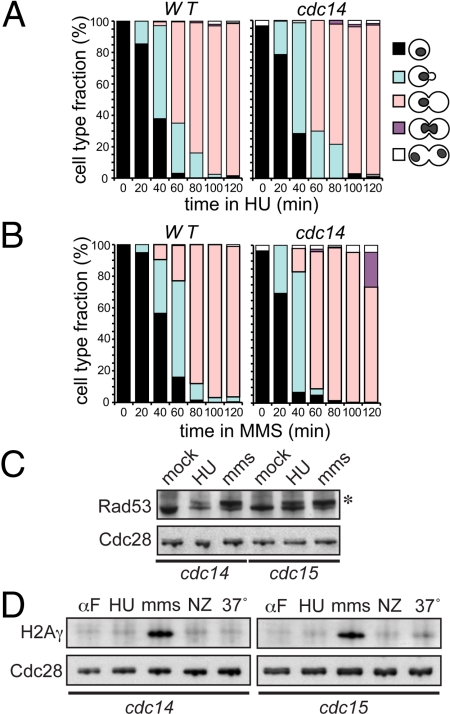
Fig. 3.
cdc14 mutants are proficient in DNA-damage checkpoints. (A and B) cdc14 mutants are properly arrested in response to DNA damage. Isogenic strains were grown to log phase at 23 °C, arrested in G1, and released at 37 °C for 2 h (A) with hydroxyurea (HU, 0.2 M) or (B) with MMS (0.01%). Cell were stained with DAPI and counted. (C) Phosphorylation of Rad53p is properly induced in cdc14 mutants in response to DNA damage. Cells were grown at 23 °C, arrested in G1 and released at 37 °C with either hydroxyurea (HU, 0.2 M) or MMS (0.01%), for 3 h. Checkpoint induction was monitored by western blotting with anti-Rad53 antibodies. (D) H2A is phosphorylated in cdc14 mutants in response to DSB. Cultures were grown in YPD to log phase at 23 °C, arrested in G1 and released in YPD at 37 °C for 3 h without any drug (37°), with hydroxyurea (HU, 0.2 M), with MMS (0.01%) or with nocodazole (Nz). The DSB presence was determined by western blotting with anti-phospho-S129 H2A antibody (64).
G1 Transcription and Nuclear Import Defects in cdc14 Impair Chromosome Replication.
The pathway(s) responsible for cdc14 replication insufficiency can be potentially revealed through the identification of Cdc14-dependent events in the previous cell cycle. Incidentally, dephosphorylation of Swi6p (Ser-160) by Cdc14 is needed for its nuclear import in telophase (33) and formation of the SBF and MBF transciption complexes (34), which activate the G1 expression of a wide range of genes essential for S phase. Indeed, we found that several key SWI6-controlled genes involved in DNA replication and other processes showed reproducibly lower transcript levels in cdc14 in G1 (at 23 °C), as well as 10 and 20 min into S phase (at 37 °C) (Fig. 4A, 10-min time point is shown). Swi6p overexpression (i.e., dosage increase of dephosphorylated Swi6p) also notably improved rDNA segregation in cdc14 (Fig. 4B). Thus, due to insufficient Swi6p amount in the nucleus (33), the replication machinery as a whole is expressed at a lower level in cdc14 mutants.
Fig. 4.
Both transcription and nuclear import defects lead to replication deficiency in cdc14. (A) Genes under control of Swi6p have partially decreased transcription in cdc14 mutants. Cells were arrested in G1 at 23 °C for 3 h, shifted at 37 °C for 30 min, and then released from G1 for 10 min. Expression levels were determined by qRT-PCR. All signals are normalized to TUB2 transcript, rDNA NTS1 - negative control. (B) Swi6p overexpression partially rescues the nucleolus segregation defect in cdc14. The cdc14 strain with Sik1p-RFP was transformed with either the pRS426gal vector (mock) or the pGAL:GST:SWI6 plasmid [derived from the library in (65)]. The cells were grown at 23 °C in plasmid-selective raffinose media, arrested with alpha factor in YPRaf for 2 h at 23 °C, galactose was added for 1 h/37 °C in the presence of alpha factor, and the cells were the released into YPRG/37 °C for 3 h. (C) RPA subunit imbalance in cdc14 mutants. Cells were arrested in G1 at 23 °C and released at 37 °C for 3 h untreated (37°), with hydroxyurea (HU, 0.2 M), with MMS (0.01%) or with nocodazole (NZ). Alpha-factor arrested cells were incubated at 37° for 1 h. Rfa2p detection: anti-GFP antibodies, Rfa1p detection: by specific antibodies. (D) Rfa2p phosphorylation is altered in cdc14 mutants even when expression is induced to wild-type level. Strains were arrested in G1 at 23 °C and then released to 37 °C or, in the presence of hydroxyurea (HU, 0.2 M), to 23 °C. Rfa2p detection as in E. The Mec1-dependent Rfa2p Ser-122 phosphorylation was detected with a specific antibody. Cdc28p levels (anti-PSTAIRE antibody): the loading control. (E) Rfa2p-GFP is largely delocalized from the nucleus in arrested cdc14 cells. Cultures were grown in YPD to log phase at 23 °C and shifted to 37 °C for 3 h. (F) Rfa1p and Rfa2p accumulate in cytoplasm in cdc14 mutants. Both cultures were arrested at 23 °C for 3 h, shifted at 37 °C for 30 min and released at 37 °C, samples were collected at indicated time points. Localization of Rfa1p and Rfa2p in nuclear (nuc) and cytoplasmic (cyt) fractions was analyzed by western blotting with specific polyclonal antibodies. Hmo1: chromatin and loading control. (G) Rfa2p does not interact with Kap60p-Kap95p importin complex in cdc14. Cells were arrested at 23 °C for 3 h, shifted at 37 °C for 30 min and split in half, with one half released from G1 at 37 °C. Rfa2p was immunoprecipitated from both G1 and anaphase cells, and analyzed with anti-Rfa2, anti-Kap60, and anti-Kap95 antibodies.
Interestingly, the expression of RFA2, under direct MBF control (34), was reduced more (Fig. 4A) than RFA1, which is activated by the downstream factor Yhp1p (35). We investigated the RPA example further, and found that Rfa1 protein levels were comparable in cdc14 and cdc15 strains (Fig. 4C). However, the Rfa2p level was somewhat decreased in cdc14-1 in G1, and especially in mitosis (nocodazole and 37 °C arrests) (Fig. 4C). DNA damaging treatments, however, enabled the increase of Rfa2p levels even at 37 °C (Fig. 4C), probably due to the activation of the DNA-damage induced transcription of RFA2 (36, 37), further indicating that DNA checkpoints are functional but not triggered in cdc14 mutants.
As both Rfa1p and Rfa2p are known to be regulated by nuclear import (38, 39), similarly to Swi6p, we analyzed their localization in cdc14 cells. The RPA2 subunit is phosphorylated in mammals (40, 41) and, in response to DNA damage (42), in yeast (by the nuclear kinase Mec1). However, in cdc14 cells, Rfa2p is not phosphorylated at this site, even at 23 °C (Fig. 4D), indicating that Rfa2p may be constitutively mislocalized. Indeed, Rfa2p-GFP was abnormally enriched in the cytoplasm in arrested cdc14 cells, unlike in cdc15 (Fig. 4E) and wild type. This defect is also evident at 23 °C (Fig. S3A), but can be alleviated by the transient expression of wild type Cdc14p activity in anaphase (Fig. S3B).
To ensure that GFP does not interfere with Rfa2p import we separated nuclear and cytoplasmic fractions from untagged strains: both Rfa1p and Rfa2p were underrepresented in the nucleus and abundant in cytoplasm in cdc14 mutants (Fig. 4F). Furthermore, Rfa2p was not able to interact with the Kap60/Kap95 complex known to mediate the cell-cycle dependent import of Rfa2p into the nucleus (38, 39). As the RPA example in Fig. 4 shows, the mechanism of replication insufficiency in cdc14 mutants has a dual nature: the constitutively decreased G1-specific transcription of MBF/SBF-controlled genes and the insufficient/deregulated nuclear import of at least some replication proteins from anaphase through G1-S transition. Furthermore, additional data suggest that the replication protein insufficiency in the nucleus, as well as nuclear transport imbalance, associated with cdc14 lesions are global defects. First, when we overexpressed the RPA holocomplex (43), it did not suppress cdc14 phenotypes; second, nucleo-cytoplasmic distribution of many proteins is changed in cdc14 cells (Fig. S4 and Table S2).
Discussion
Underreplication Blocks rDNA Segregation in cdc14 Mutants.
Incomplete chromosome replication is rarely considered as a mechanism responsible for chromosome nondisjunction during cell division (44), because cells with underreplicated DNA should not enter anaphase due to the activity of DNA integrity checkpoints (45, 46). However, the rDNA locus in budding yeast stands out in this respect, as it has persistent DSBs (47), which apparently do not trigger a checkpoint response, because nucleolar chromatin is shielded from DNA damage checkpoint signaling (48–51). We show that in the cdc14 mutant the bulk of rDNA nondisjunction can be attributed to incomplete DNA replication (Fig. 1). The inability of cdc14 mutant to down-regulate transcription in mitosis (20) also contributes to underreplication specifically at rDNA (Fig. 1I).
However, the underreplication-related chromosome nondisjunction in cdc14 mutants is not rDNA-specific (Fig. 2) and is apparently due to a complex defect, which interferes with duplication of the late-replicating regions of the genome. Experiments in Fig. 1K indicate, however, that replication forks are still active during mitosis in cdc14 mutants, as an extended metaphase delay enables both the completion of rDNA replication and the improvement of nucleolar segregation.
Mechanisms of Underreplication in cdc14.
The molecular combing experiments have shown that the cdc14 underreplication phenotype is only slightly more penetrant for rDNA than other parts of the genome (Fig. 2 C and D). This initial observation was extensively confirmed by other approaches (Fig. 2 E–I). Thus, chromosome loss (12) in cdc14 can be explained by the presence of unreplicated regions in chromosomes. The asymmetric and blocked segregation of telomeres (Fig. 2 F and G) is consistent with such an assessment.
The specific sensitivity of late replicating regions to the Cdc14 function (Fig. 2 H and I) let us to hypothesize that the late DNA replication “bottleneck” in cdc14 mutant cells is not a defect in a single specific replication protein, but is a cumulative quantitative insufficiency of replication machinery (Fig. 1J). This low dosage of replication machinery apparently enables initiation of early origins but prevents effective replication from the late origins, as replication factors are exhausted by the commitment to early forks. cdc14 mutants have at least two defects in the amount and balance of replication proteins (Fig. S5). First, the SBF/MBF-dependent production of the optimal supply of replication factors is constitutively decreased due to insufficient Swi6p import into the nucleus (Fig. 4 A–C). Second, cdc14 mutants show defects in the nuclear import both at 23 °C and 37 °C, which is illustrated with a particular case of Rfa1p and Rfa2p (Fig. 4 D–G). Thus, the impaired functions of Cdc14p likely most drastically affect replication proteins that are regulated by both Swi6p and nuclear entry. The execution point of this Cdc14p activity (i.e., activation of replication-specific nuclear import pathways) is likely in anaphase (Fig. S3B), but the resulting defect of the impaired nuclear import in cdc14 mutants has a long span: from anaphase (e.g., Rfa2p re-import) to G1-S transition (e.g., importing of the newly made pool of Rfa1p and Rfa2p, Fig. S3A and Fig. 4F).
The spectrum of Cdc14 phosphatase substrates in anaphase, which are relevant for the following G1 and S-phase, remains to be identified. It is conceivable that several cargo proteins regulated by nuclear import have to be directly dephosphorylated to enter the nucleus, by analogy with Swi6p (Fig. S5). Indeed, preliminary data from 2D-electrophoresis indicate that the spectrum of phosphorylated proteins in cdc14 cytoplasm (Pro-Q staining) is significantly distinct from cdc15 mutants. However, the prolonged cell-cycle span of the import defect, including the G1-S transition (when Cdc14p is inactive), suggests a possibility that the import machinery itself is also a substrate of the Cdc14 phosphatase. Indeed, a number of nuclear pore components interacting with Kap60p are CDK substrates (52), that is, potential Cdc14p targets; mutations in the Nup84 subcomplex are synthetically lethal with FEAR pathway mutations (53).
Dual Replication/Checkpoint Insufficiency in cdc14 Mutants.
The fact that DNA under-replication does not trigger DNA damage checkpoints in cdc14 mutants may either indicate that (a) cdc14 replication forks are not recognized as abnormal by checkpoints or that (b) the checkpoint system itself is impaired. However, a severe impairment of checkpoints is unlikely, because critical DNA damage-induced branches of the ATM/ATR signaling pathway are unaffected (Fig. 3). At the same time, the loss of Mec1p-mediated phosphorylation of S-122 in Rfa2p (Fig. 4D) indirectly indicates that some, more obscure, aspects of ATM/ATR activities may be compromised. However, the requirement of Mec1p for replication of late-replicating regions is attributed to Mec1's role in the fork progression itself (54, 55) (through direct phosphorylation of replication machinery), not due to a defect in ATR signaling. Thus, it is compelling to hypothesize that in cdc14 mutants failure to recognize underreplication is not due to a checkpoint system defect, but rather is a function of the slow replication speed itself, as was shown for DNA polymerase epsilon mutants (56, 57).
In conclusion, cdc14 mutants present a case of a lesion, which is extremely challenging to the cell and thus can potentially result in a rapid destabilization of the genome. Namely, a single point mutation creates profound chromosomal damage (underreplication), as well as eludes its detection by checkpoints. Genome stability mechanisms are found to be compromised in many cancer cell types, and thus are believed to contribute to multistep oncogenic transformations. Thus, hypomorphic mutations in CDC14 genes in mammals can potentially generate a wide spectrum of genome instability signatures seen in cancers. Strikingly, a recent study points out that expression of CDC14B is decreased in a variety of human tumors (4).
Methods
Additional methods, including DNA combing (58), are detailed in SI Methods. All yeast strains (Table S1) handling was done according to standard protocols. ARS variant plasmids were constructed based on the pAS231 backbone, which contained truncated ars1′ and was generated by inserting the AluI-SalI fragment of CEN3 into pRS404 digested with Ecl136 and XhoI. All other variants were constructed by replacing the HincII-PstI ars1′ fragment. Cloning of the cdc14-1 allele was done from a mutant strain by PCR. The TRP1 marker was inserted between the engineered SpeI and BglII restriction sites within the CDC14 terminator, giving the pAS1063 targeting construct.
For 2D DNA analysis, DNA was purified by CTAB method, as in (59), and digested with SfoI or StuI. In some experiments, ssDNA was enriched by affinity chromatography using BND-cellulose as in (23). Neutral-neutral 2D gel electrophoreses was as described by (47), with minor modifications.
For analysis of BrdU incorporation by ChIP, yeast strains were labeled essentially as in DNA combing experiment: after 2 h arrest at 37 °C, cells were fixed with 4 °C PBS with 0.1% sodium azide, and total DNA was extracted with QIAGEN genomic DNA kit. DNA was precipitated as in (60), except that replicated regions were captured using Protein G Dynabeads (Dynal) prebound with anti-BrdU antibody (Millipore). Immunoprecipitated material was analyzed by qPCR. For ChIP-CHIP experiments, precipitated DNA was further purified and amplified as in (61), 10 μg of DNA was sheared into 100-bp fragments by partial DNaseI digestion and end-labeled with biotin-N6-ddATP by recombinant calf terminal transferase. Probes were hybridized to the Affymetrix SC3456a pseudotiling array (chromosomes III, IV, V, and partial VI) (62). Samples were hybridized for 16 h at 42 °C in a GeneChip hybri-oven 320 (Affymetrix), washed and stained on a GeneChip fluidic station 450 (FS450_0001 protocol, Affymetrix).
Supplementary Material
Acknowledgments.
We thank A. Amon, S. Brill, M. Nomura, E. Schiebel, I. Dawid, and G. Brush for research materials; R. Woodgate, M. Lichten, and D. Gordenin for critical comments; and N. Sghir for help on DNA combing experiments. E.S. acknowledges Association pour la Recherche sur le Cancer (ARC) and Institut National du Cancer (INCa) for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900190106/DCSupplemental.
References
- 1.Stegmeier F, Amon A. Closing mitosis: The functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet. 2004;38:203–232. doi: 10.1146/annurev.genet.38.072902.093051. [DOI] [PubMed] [Google Scholar]
- 2.Trautmann S, et al. The S. pombe Cdc14-like phosphatase Clp1p regulates chromosome biorientation and interacts with Aurora kinase. Dev Cell. 2004;7:755–762. doi: 10.1016/j.devcel.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Krasinska L, et al. Regulation of multiple cell cycle events by Cdc14 homologues in vertebrates. Exp Cell Res. 2007;313:1225–1239. doi: 10.1016/j.yexcr.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Bassermann F, et al. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visintin R, et al. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- 6.Traverso EE, et al. Characterization of the Net1 cell cycle-dependent regulator of the Cdc14 phosphatase from budding yeast. J Biol Chem. 2001;276:21924–21931. doi: 10.1074/jbc.M011689200. [DOI] [PubMed] [Google Scholar]
- 7.Bloom J, Cross FR. Novel role for Cdc14 sequestration: Cdc14 dephosphorylates factors that promote DNA replication. Mol Cell Biol. 2007;27:842–853. doi: 10.1128/MCB.01069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt LJ, et al. Evolution of Ime2 phosphorylation sites on Cdk1 substrates provides a mechanism to limit the effects of the phosphatase Cdc14 in meiosis. Mol Cell. 2007;25:689–702. doi: 10.1016/j.molcel.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan M, Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat Cell Biol. 2003;5:249–254. doi: 10.1038/ncb940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granot D, Snyder M. Segregation of the nucleolus during mitosis in budding and fission yeast. Cell Motil Cytoskeleton. 1991;20:47–54. doi: 10.1002/cm.970200106. [DOI] [PubMed] [Google Scholar]
- 11.D'Amours D, et al. Cdc14 and condensin control the dissolution of cohesin-independent chromosome linkages at repeated DNA. Cell. 2004;117:455–469. doi: 10.1016/s0092-8674(04)00413-1. [DOI] [PubMed] [Google Scholar]
- 12.Palmer R, et al. Mitotic transmission of artificial chromosomes in cdc mutants of the yeast, S cerevisiae. Genetics. 1990;125:763–774. doi: 10.1093/genetics/125.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan M, et al. Cdc14 phosphatase induces rDNA condensation and resolves cohesin-independent cohesion during budding yeast anaphase. Cell. 2004;117:471–482. doi: 10.1016/s0092-8674(04)00415-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang BD, et al. Cdc14p/FEAR pathway controls segregation of nucleolus in S. cerevisiae by facilitating condensin targeting to rDNA chromatin in anaphase. Cell Cycle. 2004;3:960–967. doi: 10.4161/cc.3.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Rosell J, et al. Nucleolar segregation lags behind the rest of the genome and requires Cdc14p activation by the FEAR network. Cell Cycle. 2004;3:496–502. [PubMed] [Google Scholar]
- 16.Freeman L, et al. The condensin complex governs chromosome condensation and mitotic transmission of rDNA. J Cell Biol. 2000;149:811–824. doi: 10.1083/jcb.149.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang BD, et al. Condensin function in mitotic nucleolar segregation is regulated by rDNA transcription. Cell Cycle. 2006;5:2260–2267. doi: 10.4161/cc.5.19.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomson BN, et al. Ribosomal DNA transcription-dependent processes interfere with chromosome segregation. Mol Cell Biol. 2006;26:6239–6247. doi: 10.1128/MCB.00693-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machin F, et al. Transcription of ribosomal genes can cause nondisjunction. J Cell Biol. 2006;173:893–903. doi: 10.1083/jcb.200511129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemente-Blanco A, et al. Cdc14 inhibits transcription by RNA polymerase I during anaphase. Nature. 2009;458:219–222. doi: 10.1038/nature07652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan E, Koshland D. Addition of extra origins of replication to a minichromosome suppresses its mitotic loss in cdc6 and cdc14 mutants of S. cerevisiae. Proc Natl Acad Sci USA. 1992:3098–3102. doi: 10.1073/pnas.89.7.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzpatrick PJ, et al. DNA replication is completed in S. cerevisiae cells that lack functional Cdc14, a dual-specificity protein phosphatase. Mol Gen Genet. 1998;258:437–441. doi: 10.1007/s004380050753. [DOI] [PubMed] [Google Scholar]
- 23.Muller M, et al. Replication of yeast rDNA initiates downstream of transcriptionally active genes. Mol Cell. 2000;5:767–777. doi: 10.1016/s1097-2765(00)80317-2. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi Y, et al. Transcription-dependent recombination and the role of fork collision in yeast rDNA. Genes Dev. 2003;17:1497–1506. doi: 10.1101/gad.1085403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasero P, et al. Single-molecule analysis reveals clustering and epigenetic regulation of replication origins at the yeast rDNA locus. Genes Dev. 2002;16:2479–2484. doi: 10.1101/gad.232902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewer BJ, et al. Analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 1988;6:229–234. doi: 10.1016/0076-6879(95)62048-6. [DOI] [PubMed] [Google Scholar]
- 27.Khachatourians GG, Tipper DJ. Inhibition of messenger ribonucleic acid synthesis in Escherichia coli by thiolutin. J Bacteriol. 1974;119:795–804. doi: 10.1128/jb.119.3.795-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigull J, et al. Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Mol Cell Biol. 2004;24:5534–5547. doi: 10.1128/MCB.24.12.5534-5547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lengronne A, Schwob E. The yeast CDK inhibitor Sic1 prevents genomic instability by promoting replication origin licensing in late G(1) Mol Cell. 2002;9:1067–1078. doi: 10.1016/s1097-2765(02)00513-0. [DOI] [PubMed] [Google Scholar]
- 30.Shimada K, et al. ORC and the intra-S-phase checkpoint: A threshold regulates Rad53p activation in S phase. Genes Dev. 2002;16:3236–3252. doi: 10.1101/gad.239802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCune HJ, et al. The temporal program of chromosome replication: Genomewide rReplication in clb5{Delta} S. cerevisiae. Genetics. 2008;180:1833–1847. doi: 10.1534/genetics.108.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson DG, et al. Diminished S-phase cyclin-dependent kinase function elicits vital Rad53-dependent checkpoint responses in S. cerevisiae. Mol Cell Biol. 2004;24:10208–10222. doi: 10.1128/MCB.24.23.10208-10222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geymonat M, et al. Clb6/Cdc28 and Cdc14 regulate phosphorylation status and cellular localization of Swi6. Mol Cell Biol. 2004;24:2277–2285. doi: 10.1128/MCB.24.6.2277-2285.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer VR, et al. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature. 2001;409:533–538. doi: 10.1038/35054095. [DOI] [PubMed] [Google Scholar]
- 35.Horak CE, et al. Complex transcriptional circuitry at the G1/S transition in S. cerevisiae. Genes Dev. 2002;16:3017–3033. doi: 10.1101/gad.1039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benton MG, et al. Analyzing the dose-dependence of the S. cerevisiae global transcriptional response to methyl methanesulfonate and ionizing radiation. BMC Genomics. 2006;7:305. doi: 10.1186/1471-2164-7-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fry RC, et al. The DNA-damage signature in S. cerevisiae is associated with single-strand breaks in DNA. BMC Genomics. 2006;7:313. doi: 10.1186/1471-2164-7-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida K, Blobel G. The karyopherin Kap142p/Msn5p mediates nuclear import and nuclear export of different cargo proteins. J Cell Biol. 2001;152:729–740. doi: 10.1083/jcb.152.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belanger KD, et al. The karyopherin Msn5/Kap142 requires Nup82 for nuclear export and performs a function distinct from translocation in RPA protein import. J Biol Chem. 2004;279:43530–43539. doi: 10.1074/jbc.M407641200. [DOI] [PubMed] [Google Scholar]
- 40.Bartrand AJ, et al. DNA stimulates Mec1-mediated phosphorylation of replication protein A. J Biol Chem. 2004;279:26762–26767. doi: 10.1074/jbc.M312353200. [DOI] [PubMed] [Google Scholar]
- 41.Brush GS, Kelly TJ. Phosphorylation of the replication protein A large subunit in the S. cerevisiae checkpoint response. Nucleic Acids Res. 2000;28:3725–3732. doi: 10.1093/nar/28.19.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallory JC, et al. Amino acid changes in Xrs2p, Dun1p, and Rfa2p that remove the preferred targets of the ATM family of protein kinases do not affect DNA repair or telomere length in S cerevisiae. DNA Repair (Amst) 2003;2:1041–1064. doi: 10.1016/s1568-7864(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 43.Maniar HS, et al. Roles of replication protein-A subunits 2 and 3 in DNA replication fork movement in S. cerevisiae. Genetics. 1997;145:891–902. doi: 10.1093/genetics/145.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belyaeva ES, et al. DNA underreplication in intercalary heterochromatin regions in polytene chromosomes of Drosophila melanogaster correlates with the formation of partial chromosomal aberrations and ectopic pairing. Chromosoma. 2006;115:355–366. doi: 10.1007/s00412-006-0063-7. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez Y, et al. Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan V, et al. DNA replication checkpoint prevents precocious chromosome segregation by regulating spindle behavior. Mol Cell. 2004;16:687–700. doi: 10.1016/j.molcel.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Burkhalter MD, Sogo JM. rDNA enhancer affects replication initiation and mitotic recombination: Fob1 mediates nucleolytic processing independently of replication. Mol Cell. 2004;15:409–421. doi: 10.1016/j.molcel.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Torres-Rosell J, et al. Anaphase onset before complete DNA replication with intact checkpoint responses. Science. 2007;315:1411–1415. doi: 10.1126/science.1134025. [DOI] [PubMed] [Google Scholar]
- 49.Ozenberger BA, Roeder GS. A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol Cell Biol. 1991;11:1222–1231. doi: 10.1128/mcb.11.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gangloff S, et al. Gene conversion plays the major role in controlling the stability of large tandem repeats in yeast. EMBO J. 1996;15:1715–1725. [PMC free article] [PubMed] [Google Scholar]
- 51.Torres-Rosell J, et al. The Smc5-Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat Cell Biol. 2007;9:923–931. doi: 10.1038/ncb1619. [DOI] [PubMed] [Google Scholar]
- 52.Ubersax JA, et al. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- 53.Ye P, et al. Gene function prediction from congruent synthetic lethal interactions in yeast. Mol Syst Biol. 2005;1 doi: 10.1038/msb4100034. 2005 0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osborn AJ, Elledge SJ. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 2003;17:1755–1767. doi: 10.1101/gad.1098303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cha RS, Kleckner N. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science. 2002;297:602–606. doi: 10.1126/science.1071398. [DOI] [PubMed] [Google Scholar]
- 56.Navas TA, et al. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 57.Dua R, et al. Role of the putative zinc finger domain of S. cerevisiae DNA polymerase epsilon in DNA replication and the S/M checkpoint pathway. J Biol Chem. 1998;273:30046–30055. doi: 10.1074/jbc.273.45.30046. [DOI] [PubMed] [Google Scholar]
- 58.Lengronne A, et al. Monitoring S phase progression globally and locally using BrdU incorporation in TK(+) yeast strains. Nucleic Acids Res. 2001;29:1433–1442. doi: 10.1093/nar/29.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allers T, Lichten M. A method for preparing genomic DNA that restrains branch migration of Holliday junctions. Nucleic Acids Res. 2000;28:e6. doi: 10.1093/nar/28.2.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katou Y, et al. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424:1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- 61.Winzeler EA, et al. Direct allelic variation scanning of the yeast genome. Science. 1998;281:1194–1197. doi: 10.1126/science.281.5380.1194. [DOI] [PubMed] [Google Scholar]
- 62.Lengronne A, et al. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature. 2004;430:573–578. doi: 10.1038/nature02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raghuraman MK, et al. Replication dynamics of the yeast genome. Science. 2001;294:115–121. doi: 10.1126/science.294.5540.115. [DOI] [PubMed] [Google Scholar]
- 64.Redon C, et al. Yeast histone 2A serine 129 is essential for the efficient repair of checkpoint-blind DNA damage. EMBO Rep. 2003;4:678–684. doi: 10.1038/sj.embor.embor871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ptacek J, et al. Global analysis of protein phosphorylation in yeast. Nature. 2005;438:679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.