Abstract
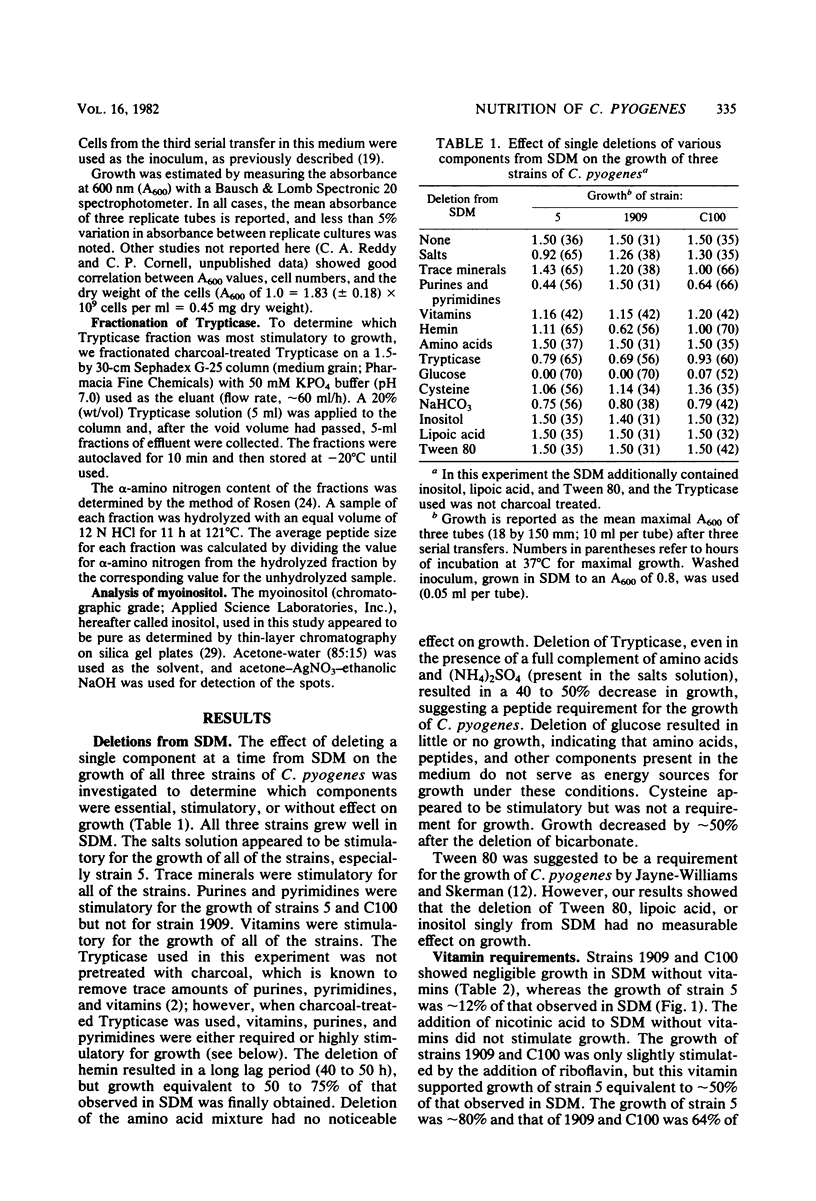
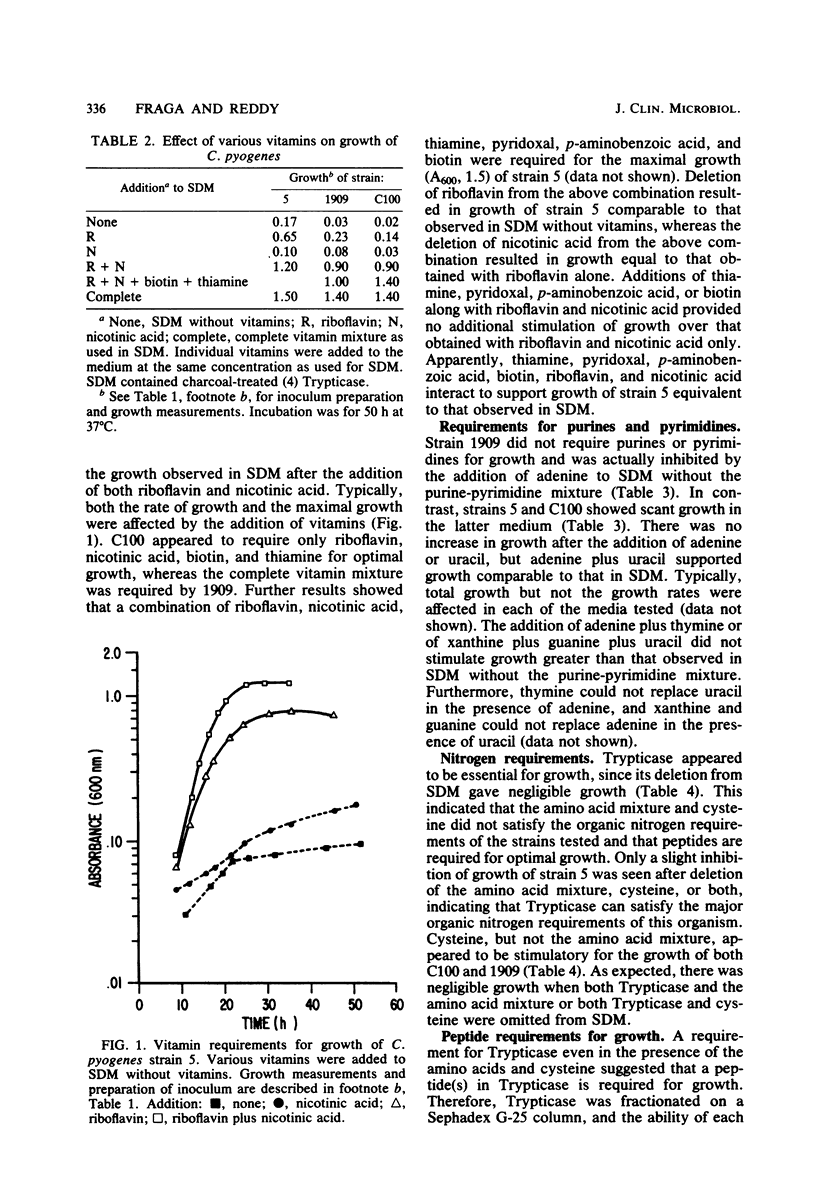
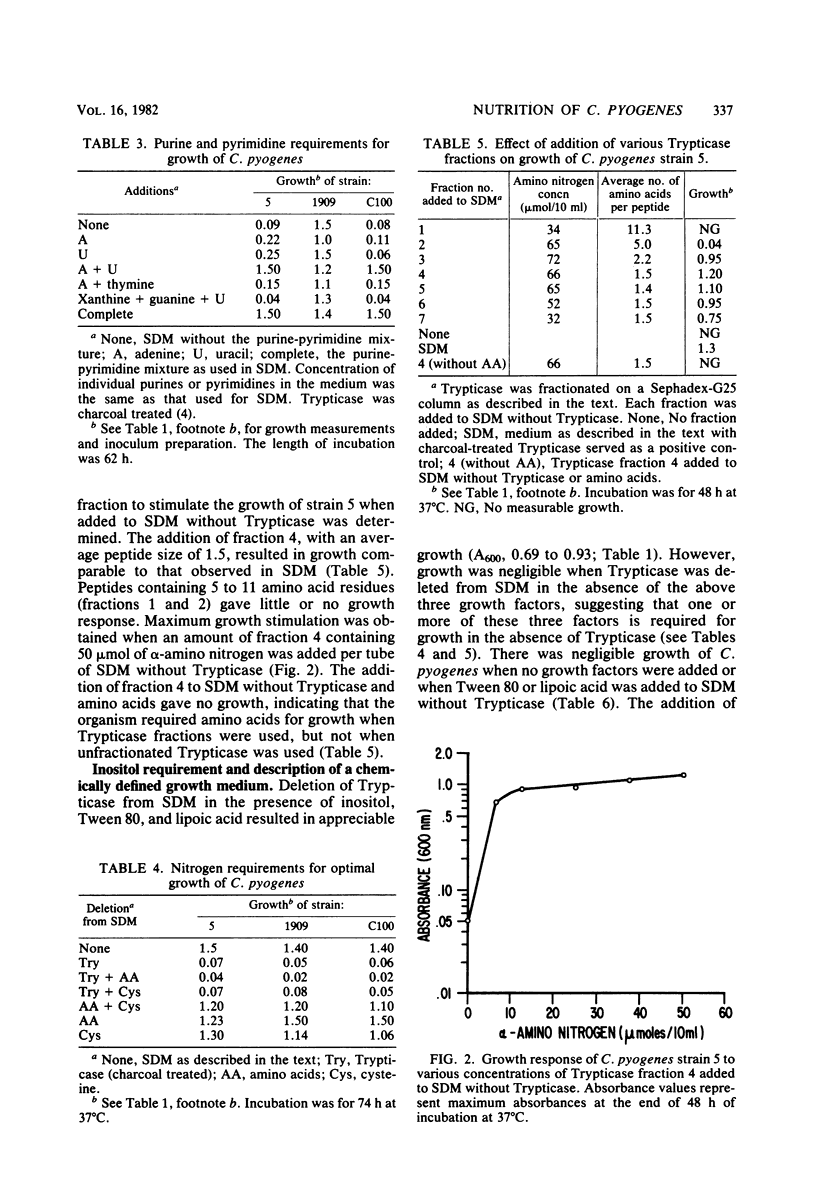
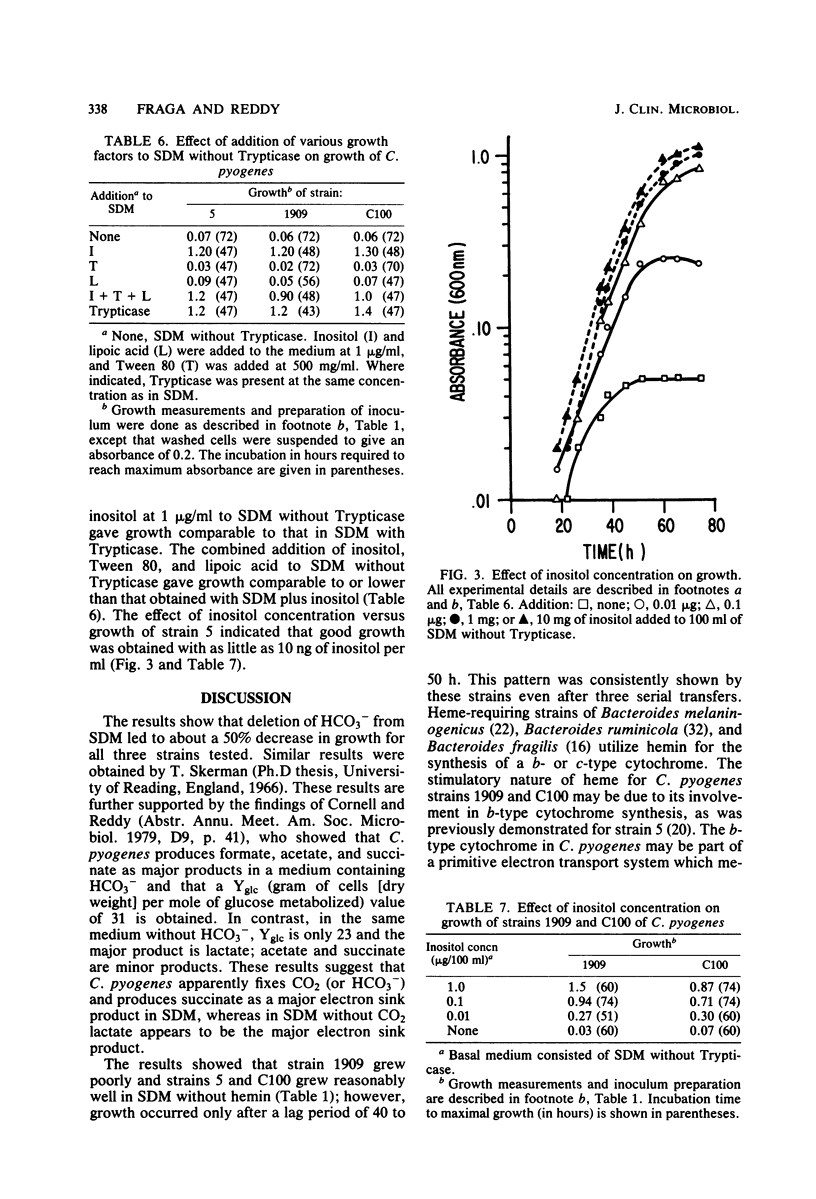
The nutritional requirements of Corynebacterium pyogenes (strains C100, 5, and 1909), a commonly encountered animal pathogen, were determined in this study. A semidefined medium (SDM) containing glucose, HCO3-, hemin, charcoal-treated Trypticase, and a defined mixture of purines and pyrimidines, amino acids, and minerals which supported optimal growth of C. pyogenes was employed in all nutritional studies. Adenine and uracil were required for optimal growth of strains 5 and C100 but were not required for strain 1909. Riboflavin and nicotinic acid were required for good growth of all three strains; biotin and thiamin were stimulatory but did not appear to be required for growth. Hemin and NaHCO3 were stimulatory for growth, whereas lipoic acid and Tween 80 were neither stimulatory nor required for growth. The replacement of Trypticase with a specific peptide fraction (obtained by fractionation of Trypticase on Sephadex G-25) rich in dipeptides gave growth comparable to that in SDM, indicating a peptide requirement for the growth of C. pyogenes. It was of considerable interest that growth comparable to that in SDM was obtained when Trypticase was replaced by inositol (1 microgram/ml of SDM).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIE A. O., PORTEOUS J. W. The cultivation of a single strain of actinomyces israelii in a simplified and chemically defined medium. J Gen Microbiol. 1962 Jul;28:443–454. doi: 10.1099/00221287-28-3-443. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- Charalampous F. C. Metabolic functions of myo-inositol. VII. Role of inositol in the transport of alpha-aminoisobutyric acid in KB cells. J Biol Chem. 1969 Apr 10;244(7):1705–1710. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- HALLIDAY J. W., ANDERSON L. The synthesis of myo-inositol in the rat. J Biol Chem. 1955 Dec;217(2):797–802. [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Yanagawa R. Nutritional requriements of Corynebacterium renale. Jpn J Vet Res. 1967 Sep;15(3):121–134. [PubMed] [Google Scholar]
- KIHARA H., SNELL E. E. Peptides and bacterial growth. II. L-alanine peptides and growth of Lactobacillus casei. J Biol Chem. 1952 May;197(2):791–805. [PubMed] [Google Scholar]
- Lembach K., Charalampous F. C. Metabolic functions of myo-inositol. VI. Impairment of amino acid transport in KB cells caused by inositol deficiency. J Biol Chem. 1967 Jun 10;242(11):2606–2614. [PubMed] [Google Scholar]
- Macy J., Probst I., Gottschalk G. Evidence for cytochrome involvement in fumarate reduction and adenosine 5'-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol. 1975 Aug;123(2):436–442. doi: 10.1128/jb.123.2.436-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A. W., GIBBS P. A. Techniques for the fractionation of microbiologically active peptides derived from casein. Biochem J. 1961 Dec;81:551–556. doi: 10.1042/bj0810551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTMAN K. A., BRYANT M. P. PEPTIDES AND OTHER NITROGEN SOURCES FOR GROWTH OF BACTEROIDES RUMINICOLA. J Bacteriol. 1964 Aug;88:401–410. doi: 10.1128/jb.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Reddy C. A., Cornell C. P., Fraga A. M. Chemically defined growth medium for Corynebacterium pyogenes. Am J Vet Res. 1980 May;41(5):843–845. [PubMed] [Google Scholar]
- Reddy C. A., Cornell C. P., Kao M. Hemin-dependent growth stimulation and cytochrome synthesis in Corynebacterium pyogenes. J Bacteriol. 1977 May;130(2):965–967. doi: 10.1128/jb.130.2.965-967.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. A., Kao M. Value of acid metabolic products in identification of certain corynebacteria. J Clin Microbiol. 1978 May;7(5):428–433. doi: 10.1128/jcm.7.5.428-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza V., Sinclair P. R., White D. C., Cuorant P. R. Electron transport system of the protoheme-requiring anaerobe Bacteroides melaninogenicus. J Bacteriol. 1968 Sep;96(3):665–671. doi: 10.1128/jb.96.3.665-671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEseltine W. P., Cox W. M., Kadis S. Minimal nitrogen requirements of Corynebacterium renal strains. Am J Vet Res. 1978 Jan;39(1):123–127. [PubMed] [Google Scholar]
- WHITE D. C., BRYANT M. P., CALDWELL D. R. Cytochromelinked fermentation in Bacteroides ruminicola. J Bacteriol. 1962 Oct;84:822–828. doi: 10.1128/jb.84.4.822-828.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARBROUGH H. F., Jr, CLARK F. M. Utilization of inositol, an essential metabolite for Schizosaccharomyces pombe. J Bacteriol. 1957 Mar;73(3):318–323. doi: 10.1128/jb.73.3.318-323.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., van Wyck-Kapteyn W. M., Stouthamer A. H. Generation of ATP during cytochrome-linked anaerobic electron transport in propionic acid bacteria. J Gen Microbiol. 1973 May;76(1):31–41. doi: 10.1099/00221287-76-1-31. [DOI] [PubMed] [Google Scholar]


