Abstract
Anabolic-androgenic steroids (AAS) are synthetic derivatives of testosterone that are illicitly self-administered for enhancement of performance and body image, but which also have significant effects on the brain and on behavior. While the stereotypical AAS user is an adult male, AAS abuse in women is rapidly increasing, yet few studies have examined AAS effects in female subjects. We have assessed the effects in female mice of a combination of commonly abused AAS on neuronal activity and neurotransmission mediated by γ-aminobutyric acid type A (GABAA) receptors in the medial preoptic nucleus (MPN); a nexus in the circuits of the hypothalamus and forebrain that are critical for the expression of social behaviors known to be altered in AAS abuse. Our data indicate that chronic exposure to AAS resulted in androgen receptor (AR)-dependent upregulation of α5, β3 and δ subunit mRNA. Acute application of the α5 subunit-selective inverse agonist, L-655,708, indicated that a significant fraction of the synaptic current is carried by α5-containing receptors and that AAS treatment may enhance expression of α5-containing receptors contributing to synaptic, but not tonic, currents in the MPN. AAS treatment also resulted in a significant decrease in action potential frequency in MPN neurons that was also correlated with an increased sensitivity to L655,708. Our data demonstrate that chronic exposure to multiple AAS elicits significant changes in GABAergic transmission and neuronal activity that are likely to reflect changes in the expression of α5-containing synaptic receptors within the MPN.
Keywords: real time PCR, patch clamp, medial preoptic area, L-655, 708, flutamide
Anabolic-androgenic steroids (AAS) are synthetic derivatives of testosterone originally devised for the treatment of hypogonadal dysfunction in men, initiation of delayed puberty, and growth promotion (Basaria et al., 2001; Shahidi, 2001), but are now predominantly self-administered to enhance performance or body image (for review, Kochakian and Yesalis, 2000; Llewellyn, 2007). Humans administer a myriad of AAS in complex regimes characterized by the concurrent and prolonged use of supraphysiological doses of multiple compounds that differ in their chemical properties and thus in their metabolic fate and signaling capacity (for review, Clark et al., 2006; Llewellyn, 2007). While both men and women abuse steroids, the long-term risks from AAS abuse may be greater in females than in males (Franke and Berendonk, 1997; for review Clark et al., 2006), and the most recently available data indicate that young women constitute the demographic with the most rapidly increasing AAS use (for review, Elliot and Goldberg, 2000). Importantly, the physiological outcomes of AAS administration will depend not only on the actions and interactions of the different drugs administered, but also on differences in cellular substrates and in the endogenous hormonal milieu, which vary significantly between males and females. Despite these facts, nearly all published studies on AAS effects have focused on men or on male nonhuman subjects.
In both human abusers and animal models, the most prominent behavioral effects associated with illicit AAS use encompass both positive mood symptoms, including euphoria, hypomania, increased libido, and negative symptoms, including increased anxiety, irritability, elevated levels of aggression and decreased libido (Su et al., 1993; Franke and Beredonk, 1997; Pope et al., 2000; Perry et al., 2003; for review, Gruber and Pope, 2000 ). The medial preoptic area (mPOA) is an integral node in a core of reciprocally connected regions of the hypothalamus and forebrain that form the neural networks underlying the regulation of these social behaviors, and neural transmission mediated by γ-aminobutyric acid type A (GABAA) receptors within this network plays a critical role in their expression (for review, Clark et al, 2006). The central region of the mPOA, the medial preoptic nucleus (MPN) is characterized by a dense population of neurons that release GABA (Gao and Moore; 1996; Sagrillo and Selmanoff, 1997) and express GABAA receptors (Penatti et al., 2005; this study), as well as high levels of androgen receptors (AR), estrogen receptors (ER) and aromatase, consistent with the exquisite steroid-sensitivity of this region (for review, Penatti and Henderson, in press).
The native GABAA receptor is a pentameric ionotropic transmembrane protein for which sixteen different receptor subunit genes (α1-α6, β1-β3, γ1-γ3, δ, ε, π, and θ) have been identified in mammals. Although the most common GABAA receptor subtype expressed throughout most regions of the adult brain consists of α1, β2 or β3, and γ2 subunits, regions in the forebrain/hypothalamus, including the mPOA, show a different pattern and continue to express high levels of α2 subunit, as well as appreciable levels of otherwise uncommon subunits, including α5, γ1 and ε, into adulthood. Alpha subunit composition imparts differences to the GABAA receptor with respect to GABA affinity, deactivation, desensitization, and sensitivity to allosteric modulators, including sensitivity to allosteric modulation by the AAS themselves (for review, Henderson, 2007), all of which may affect information transfer along the circuits that govern social behaviors. While the role of α5-containing receptors in the mPOA and hypothalamus has not been explored, receptors containing this α subunit have been implicated in hippocampal functions, including long-term potentiation (Atack et al., 2006), presynaptic inhibition (Glykys and Mody, 2006), spatial learning (Collinson et al., 2002; Dawson et al., 2006), and trace fear conditioning (Crestani et al., 2002; Yee et al., 2004), which also has an important α5-dependent amygdalar component (Heldt and Ressler, 2007).
Previous studies have shown that GABAA receptor expression and function within the mPOA is regulated by chronic exposure to the single AAS, 17α-methyltestosterone, in a sex-specific manner in adolescent mice (McIntyre et al., 2002; Penatti et al., 2005). The goal of the present study was to determine how chronic exposure to a cocktail of chemically distinct AAS with more complex signaling capability that more accurately reflects self-administration regimes of human abusers alters GABAergic signaling and neuronal activity within this brain region in adult female mice.
EXPERIMENTAL PROCEDURES
Animal care and use
C57Bl/6 mice (Charles River Laboratories, Wilmington, MA) were housed in a temperature controlled and 12 hr on:12 hr off light cycle facility with lights on starting at 0700. All animal care procedures and treatment paradigms were approved by Institutional Animal Care and Use Committee at Dartmouth and are in agreement to the guidelines and recommendations of the National Institutes of Health and American Veterinary Medical Association.
Drug Treatment Paradigms
On postnatal day (PN) 50, mice were injected intraperitoneally with a combination of three AAS that represent the three major chemical groups of AAS:
Testosterone esters, which are derived from esterification of the 17β-hydroxyl group of testosterone, can be hydrolyzed into free testosterone, reduced to 5α-dihydrotestosterone (DHT) (Martini, 1982; Winters, 1990; Kochakian and Yesalis, 2000) or aromatized to estrogens, including 17β-estradiol (Winters, 1990; Kochakian and Yesalis, 2000). Molecules that have been 5α-reduced cannot be metabolized into estrogens, but may be metabolized into other androgens, such as 3α-androstanediol (3α-diol).
19-nor-testosterone derivatives. These compounds have, in conjunction with the addition of long side chain moieties, substitution of a hydrogen for the methyl group at C19. Like the testosterone esters, 19-nor-testosterone can be aromatized to 17β-estradiol and other estrogens, albeit with less efficiency than free testosterone (Ryan, 1959; Winters, 1990).
C17-alkylated derivatives. The 17-methyl moiety precludes aromatization to 17β-estradiol or reduction to DHT (Quincey and Gray, 1967; Winters, 1990; Ryan, 1959), although production of other weak estrogens has been reported (Papaconstantinou et al., 2002; de Gooyer et al., 2003).
Each animal received a "cocktail" containing equal doses of one AAS from each class: 2.5 mg/kg testosterone cypionate, 2.5 mg/kg nandrolone decanoate, and 2.5 mg/kg 17α-methyltestosterone (17α-MeT) for a total of 7.5 mg AAS/kg/day in sesame oil. Animals were injected for 6 days/week for a period of 6 weeks. This total daily AAS concentration is comparable to high doses in human abusers (Pope and Katz, 1988; Perry et al., 1990; Kibble and Ross, 1987). Duration of treatment was chosen to provide chronic exposure and predicated on the results of prior studies that indicated that changes in GABAA receptor subunit expression and function that were trends after 3 weeks of treatment attained significance in animals treated for 6 weeks (Penatti et al., 2005). Control subjects were administered with the same injection paradigm and the same volume (~50 µL; based on body weight) with sesame oil alone. All oil-injected control animals used were in diestrus as assessed by vaginal smears (Long and Evans, 1922). To determine the role of the androgen receptor (AR) in mediating the effects of the AAS cocktail, flutamide (20 mg/kg/day; Blasberg et al., 1998) was injected into mice for 6 days per week for 6 weeks either alone or in conjunction with the AAS cocktail at concentrations indicated above. All control animals received equivalent volume injections of oil.
Except where indicated to the contrary, mRNA analyses reflect data from three separate cohorts of 8 mice for each treatment condition and physiological assays reflect data from 5 separate cohorts of 8 mice for each treatment condition.
Serum testosterone assays
Trunk blood was collected, allowed to clot overnight, centrifuged at 3,000 × g and assayed for testosterone by ELISA according to instructions supplied with the commercially available kit (Cayman Chemical, Ann Arbor, MI).
Tissue analyses
For electrophysiological analyses, coronal brain slices were prepared and all recordings were made from the central region of the mPOA corresponding to the dorsal aspect of the medial preoptic nucleus (MPN), MPN-medial and encompassing the MPN-central, as defined by Franklin and Paxinos (1997: Figures 29–32) (Penatti et al., 2005). For mRNA assessments, dissections were made from a region approximating the MPN, but likely to contain surrounding cells in the mPOA region.
RNA Extraction and Reverse Transcription coupled with Quantitative Real Time Polymerase Chain Reaction (Q-RT-PCR)
Tissue dissected from coronal slices as described above was stored in the RNA stabilization solution, RNAlater® (Ambion Inc., Austin TX) at −20° C. Total RNA was extracted according to manufacturer’s protocol for RNAqueous-4PCR kit (Ambion Inc., Austin TX). Briefly, tissue was added to 200 µl lysis/binding buffer and homogenized. An equal volume of 64% EtOH was added and the sample vortexed. The lysate/ethanol mix was applied to an RNAqueous filter (supplied in the kit) and centrifuged for 1 min at 12,000 rpm. The flow through was discarded, the cartridge washed several times and RNA eluted with 50 µl hot elution buffer. The RNA was treated with 2 units DNase1 (supplied in the kit) for 30 min at 37° C to remove any contaminating genomic DNA. DNase was inactivated by the addition of 0.1 volume DNase Inactivation Reagent, incubated at room temperature (20–22°C) for 2 min, centrifuged, and the DNase-free RNA supernatant collected. The concentration of the RNA was determined by measuring the optical density at 260 nm. Fifty nanograms of total RNA was then reverse transcribed using RETROscript First-Strand Synthesis Kit for RT-PCR (Ambion Inc., Austin TX) in a total reaction volume of 20 µl. RNA was denatured for 3 min at 75° C with 2 mM dNTPs and 5 µM random decamers. Ten units of RNase inhibitor, RT buffer to 1× and 100 units of M-MLV reverse transcriptase was added to this mixture, and the reaction was incubated for 1 hr at 42° C, followed by inactivation at 92° C for 10 min.
PCR primers and TaqMan® MGB probes specific for mouse GABAA receptor α subunit mRNAs were designed using the oligo primer design programs Primer Express Software (Applied Biosystems Inc (ABI), Foster City, CA) and OLIGO 6 (Molecular Biology Insights, Inc., Cascade, CO) (Penatti et al., 2005). The specificity of each primer/probe set sequence, for individual GABAA receptor subunits was confirmed by using the National Center for Biotechnology Information sequence alignment algorithm. Primers and probes for the β, γ, δ and ε subunit mRNA were obtained from ABI: Mm00433461_m1 (β1), Mm00549788_s1 (β2), Mm00433473-m1 (β3), Mm00433476_m1 (δ), Mm00489932_m1 (ε), Mm00439047_m1 (γ1), and Mm00433489_m1 (γ2), as were primer/probes for the aromatase mRNA (Mm00484049_m1). Amplification plots for primers corresponding to α1, α2 and α5 GABAA receptor subunit mRNAs had slopes of −3.36 (for α1 and α2) and −3.19 (for α5), giving efficiencies of 0.9844 for α1 and α2 and of 1.0581 for α5 (Mx4000TM Multiplex Quantitative PCR Systems; Stratagene). Slopes of the standard curves were used to assess efficiencies, which were between 0.984 and 1.0581 for all primers used.
Real time PCR was performed using an ABI 7500 Sequence Detection System. Validation experiments were conducted to show that subunit-specific primers sets amplified with equal efficiency as reported previously (Penatti et al., 2005). Each mPOA sample was analyzed in triplicate for each GABAA receptor subunit cDNA and the 18S rRNA as an internal standard. For each GABAA receptor subunit mRNA assessed, a PCR master mix was prepared, containing final concentrations of: 1× TaqMan Universal Master Mix (containing AmpliTaq Gold DNA Polymerase, AmpErase UNG, dNTPs with dUTP, Passive Reference 1, and optimized buffer components), 900 nM forward primer, 900 nM reverse primer, 250 nM probe in a total reaction volume of 25 µl. PCR for 18S rRNA was performed in a 25 µl reaction containing 1× TaqMan Universal Master Mix, 1× Eukaryotic 18S rRNA Endogenous Control (VIC/MGB Probe, Primer Limited; ABI), to which 1 µl cDNA was added. Thermocycling conditions included initial steps of 2 min at 50° C, 10 min at 95° C and 40 cycles of PCR at 95° C for 15 sec to denature cDNA and 60° C for 1 min for primer/probe annealing and extension. Samples with reverse transcriptase omitted were used to control for genomic DNA contamination and a no template control to control for any reagent contamination. Messenger RNA levels were determined as either 2−ΔCT or 2−ΔΔCT (Livak and Schmittgen, 2001; Peirson et al., 2003).
Electrophysiological Recordings
Electrophysiological recording and analyses were carried out as described previously (Yang et al., 2002; Penatti et al., 2005; Jones et al., 2006). For each experiment, whole brains were quickly removed and placed in ice-cold oxygenated sodium-free sucrose-supplemented dissection solution, in mM: 250 sucrose, 1.2 CaCl2, 10 glucose, 4 KCl, 7 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, and 1 ascorbic acid at pH 7.35. Using an Electron Microscopy Sciences OTS-4000® vibroslicer (Hatfield, PA). Coronal sections (250 µm) that included the mPOA were prepared and superfused with 95%O2/5%CO2 saturated artificial cerebrospinal fluid (aCSF), in mM: 125 NaCl, 1.2 CaCl2, 10 glucose, 4 KCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 1 ascorbic acid (pH 7.35). For recordings of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs), aCSF was supplemented with 2 mM kynurenic acid to block excitatory transmission, and recordings were made at room temperature (20–22°C) in the whole cell configuration of the patch clamp technique at a holding potential of −70 mV. The internal electrode solution consisted of (in mM): 153 CsCl, 1 MgCl2, 5 EGTA, and 10 HEPES, to which 2 MgATP was added for every experiment. To confirm that IPSCs were mediated by GABAA receptors, 10 µM bicuculline was used, in some experiments, to reversibly block the events. Spontaneous IPSCs were acquired and averaged from each neuron for a period of 4–12 minutes (with criteria imposed that seal resistances (> 1GΩ), access resistance (<25 MΩ) and holding current did not change more than ± ~10% during the recording) to determine peak current (Ipeak) and current decays (biphasic and fitted with two time constants, τ1 and τ2; Nett et al., 1999; Jorge et al., 2002; Penatti et al., 2005). Events were accepted for analysis only if they had rise-times of less than 2.5 ms and were isolated from other synaptic events (i.e., a synchronous, but concurrent release did not obscure either the rising or the decay phase of the measured response). All events were accepted for assessment of IPSC frequencies. Data were recorded to tape and analyzed using Mini Analysis (Synaptosoft, Decatur, GA). In addition to estimates of τ1 and τ2, overall synaptic current decay was also described by a single weighted time constant (τw) (Yang et al., 2002, 2005; Jones et al., 2006). The magnitude of tonic GABAA-receptor-mediated currents (Itonic) attributed to α5-containing receptors and the contribution of α5-containing receptors to synaptic currents were estimated by minor modifications of previously published procedures (Farrant and Nusser, 2005; Jones et al., 2006) as a change in the baseline holding current (Ibaseline) in the presence of 50 µM (0.1% DMSO) of the α5-selective inverse agonist, L-655,708 (L6; Quirk et al., 1996); a concentration that has been reported to be selective for α5-containing receptors in slice preparations (Caraiscos et al., 2004; Scimemi et al., 2005) and shown to be without effect in α5−/− mice (Caraiscos et al., 2004). Acquisition of data in the presence of L6 was initiated ~1 min after the drug was perfused into the bath. All control recordings were performed in 0.1% DMSO for comparison. This concentration of vehicle had no effects on GABAA receptor-mediated responses (i.e., no significant differences between aCSF alone and aCSF/0.1%DMSO), as we have reported previously (Jones et al., 2006).
Recordings of spontaneous action potentials were made in the on-cell configuration with aCSF in both the bath and the pipette. Data were recorded to tape and subsequently digitized using Acquire 4.0 software (Bruxton Corporation, Seattle WA). Action potential frequency was measured and autocorrelograms were constructed with bin-widths of 1 to 10 ms using software written locally by Brian L. Jones in MatLab 6.5 R13 (The Mathworks; Natick, MA), and rhythmicity was assessed over windows of 0.2 to 10 s. Larger bin-widths and windows were used for cells with lower firing rates. Classifications of firing patterns from autocorrelograms were made according to Bar-Gad et al. (2001). Categorization of each cell’s firing patterns as regular, irregular or bursty was also made from the raw data. This independent assessment of firing patterns was in universal agreement with autocorrelogram designations made independently by another observer.
Drugs
All chemicals were purchased from Sigma Chemical Corp. (St. Louis, MO) with the exception of L-655,708 (Tocris Bioscience, Ellisville, MO).
Statistical Analyses
Values for variables are presented as mean ± standard error or the mean. For electrophysiological experiments, non-normally distributed data (action potential frequency) were log-transformed. Statistical significance was assessed by one- or two-way analysis of variance (ANOVA) using the general linear model procedure of SAS or two-way repeated measures ANOVA (OriginLab, Northampton, MA) followed by means comparison by least significant means or the Student’s t-test, respectively, except for analysis of proportions/percentages, for which statistical significance was determined by the X2 test. For real-time PCR analysis, CT values were defined as outliers when ± 3 standard deviations from the mean. Results were qualitatively the same whether or not outliers were included in the final analysis. For all data, the alpha level was set at P ≤ 0.05. Throughout the text, n values indicate the number of mice analyzed per condition, except for electrophysiological analyses where n indicates the numbers of cells analyzed per condition.
RESULTS
I. AAS-dependent changes in GABAA receptor subunit mRNAs in the mPOA of female mice
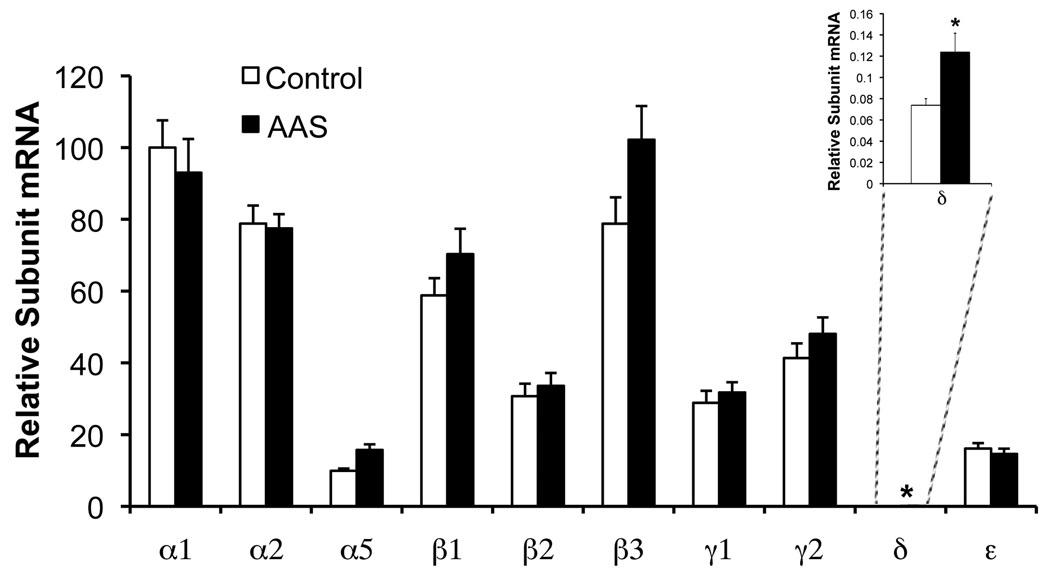
Changes in GABAA receptor subunit mRNA levels were assessed for those transcripts reported to be expressed mPOA (α1, α2, α5, β1, β2, β3, γ1, γ2, and ε (for review, Henderson, 2007), as well as the δ subunit which, while only weakly expressed in the mPOA, may contribute to receptors that mediate extrasynaptic tonic currents in this brain region, as it does in others (for review, Semyanov et al., 2004; Farrant and Nusser, 2005). Real-time PCR analysis revealed that AAS treatment significantly increased the levels of α5 (P = 3 × 10−4), β3 (P = 0.0370) and δ (P = 0.0018) subunit mRNAs versus control, with no significant changes observed for the α1, α2, β1, β2, γ1, γ2 or ε subunit mRNAs (n = 24 mice; see Methods). While the AAS-induced increase in δ subunit mRNA was significant (Figure 1 and Figure 2), the absolute level of this transcript, even in treated animals, was very low in comparison to other subunit mRNAs. AAS-induced expression of the α5 transcript was consistently observed in forebrain/hypothalamic regions we examined: the ventromedial nucleus of the hypothalamus (VMN) (P = 0.0025; n = 8) and the medial amygdala (MeA) (P = 0.0055; n = 8 mice) of adult female mice, as well as the mPOA of adolescent female mice (P = 0.0011; n = 8 mice).
Figure 1. Effects of AAS treatment on GABAA receptor subunit mRNAs.
Data indicating relative average levels of GABAA receptor subunit mRNAs in tissue isolated from the mPOA of control (white bars) or AAS-treated (AAS; black bars) female mice. Subunit mRNA levels were calculated as 2−ΔCT values (i.e., subunit mRNA levels normalized for 18S) and then expressed relative to this value for the α1 subunit from control mice (= 100). Inset: Levels of δ subunit mRNA, which was not abundant in the mPOA, shown on a more sensitive Y-axis to demonstrate AAS-induced change. Asterisks indicate values from AAS-treated subjects that were significantly different from control.
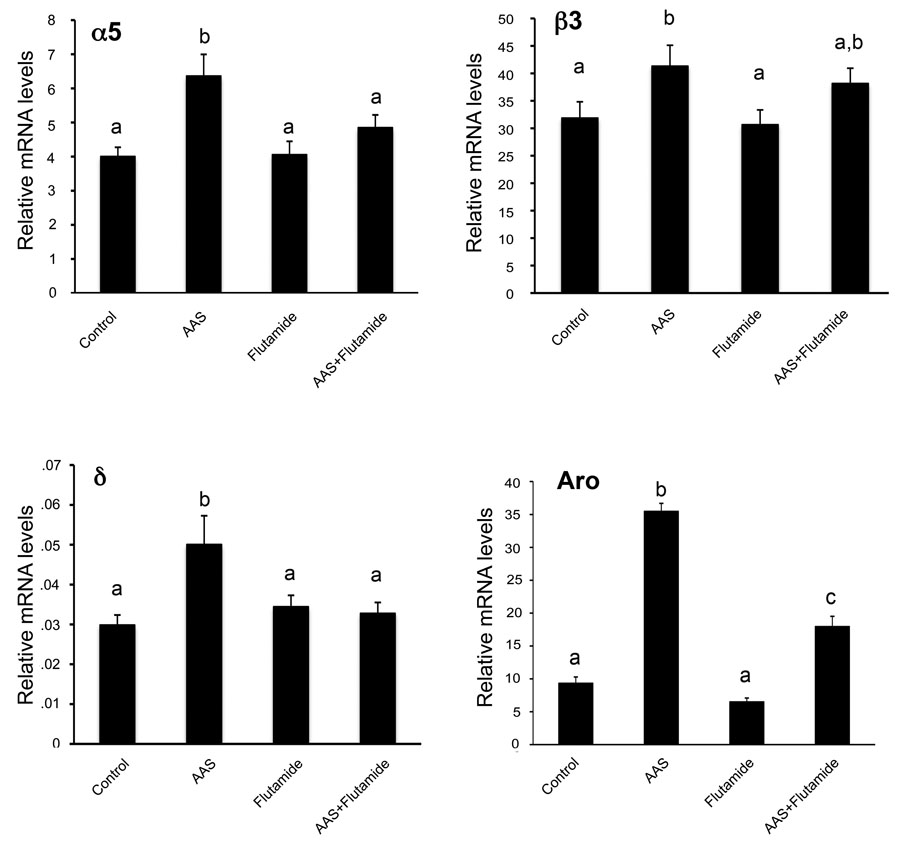
Figure 2. AR-dependent mediation of AAS effects on mRNA levels in the mPOA.
Data indicating average levels the α5, β3 and δ GABAA receptor subunit mRNAs and aromatase (Aro) mRNA in tissue isolated from the mPOA expressed as 2−ΔCT values (i.e., subunit mRNA levels normalized for 18S) for the 4 drug treatment groups. Bars with the same letter had means that were not statistically different from one another; bars with different letters had means that were significantly different from one another.
Testosterone levels are dramatically elevated (~14×) in AAS-treated (2037.4 ± pg/mL; n = 7) versus control (148.2 ± pg/mL; n = 7) mice (P < 0.0001). Although all AAS and AAS metabolites bind to the classical AR, many, upon aromatization, may also exert physiological effects via estrogen receptors (ER; for review, Basaria et al., 2001; Shahidi, 2001; Clark et al., 2006). Moreover, unlike gonadal steroids, the AAS allosterically modulate GABAA receptors, thus these drugs have the potential to alter GABAA receptor expression through allosteric changes in neural activity (for review, Clark and Henderson, 2003). To determine if AAS mediated changes in GABAA receptors require classical AR signaling, we assessed the ability of the AR-specific antagonist, flutamide, to abrogate AAS-dependent increases in receptor subunit mRNAs in the mPOA. Assessment of peripheral testosterone levels indicated that flutamide did not affect the augmented levels testosterone arising from the injected AAS: serum testosterone levels were not significantly different between those treated with AAS and flutamide (1732.4 ± pg/mL; n = 5 mice) versus AAS alone and were still greatly elevated in comparison to controls (~12×; P < 0.0001). Flutamide alone had no effect on plasma testosterone levels (98.87 ± pg/mL; n = 8 mice).
With respect to the effects of flutamide on AAS-induced changes in subunit mRNA expression within the mPOA, we found that the increases in α5 and δ observed with AAS treatment were reversed by co-treatment with flutamide (Figure 2). Values for these two subunit mRNAs in animals co-treated with flutamide and AAS were significantly lower than for AAS alone (P = 0.0163 and P = 0.0054, respectively) and were not significantly different from control. For the β3 subunit, while mRNA levels in animals co-treated with AAS and flutamide were not different than control, and levels in animals treated with AAS plus flutamide were lower than in those treated with AAS alone, this difference did not attain significance (Figure 2). There were no significant effects of any treatment (AAS, flutamide or AAS plus flutamide) on the levels of subunit mRNAs among any of the four treatment groups for the α1, α2, β1, β2, γ1, γ2 or ε subunit mRNAs (data not shown).
While our data support the hypothesis that flutamide antagonizes AAS-induced increases in GABAA receptor subunit mRNAs via a classical AR-dependent mechanism, flutamide has also been reported to have agonistic effects in the brain. To provide an additional assessment that flutamide, at the concentration and duration given here, is not acting as an agonist for classical AR mediated signaling, we also determined flutamide effects on the levels of aromatase mRNA, as this transcript is known to be regulated by classical AR signaling in the mPOA (Abdelgadir et al., 1994; Roselli et al., 1998). AAS treatment dramatically enhanced aromatase mRNA levels in the mPOA (P < 0.0001 versus control) of female mice. For females treated with flutamide alone, aromatase mRNA levels were not significantly different from those observed in control animals, indicating that flutamide did not have agonistic actions on levels of this AR-regulated transcript. While co-treatment with flutamide and AAS greatly reduced levels of aromatase mRNA below that observed in mice receiving AAS cocktail alone (P < 0.0011), the antagonism was not complete, and levels of this mRNA were significantly higher in mice receiving AAS and flutamide than in controls (P < 0.0011) (Figure 2).
II. AAS-dependent changes in GABAA receptor-mediated currents in the MPN of female mice
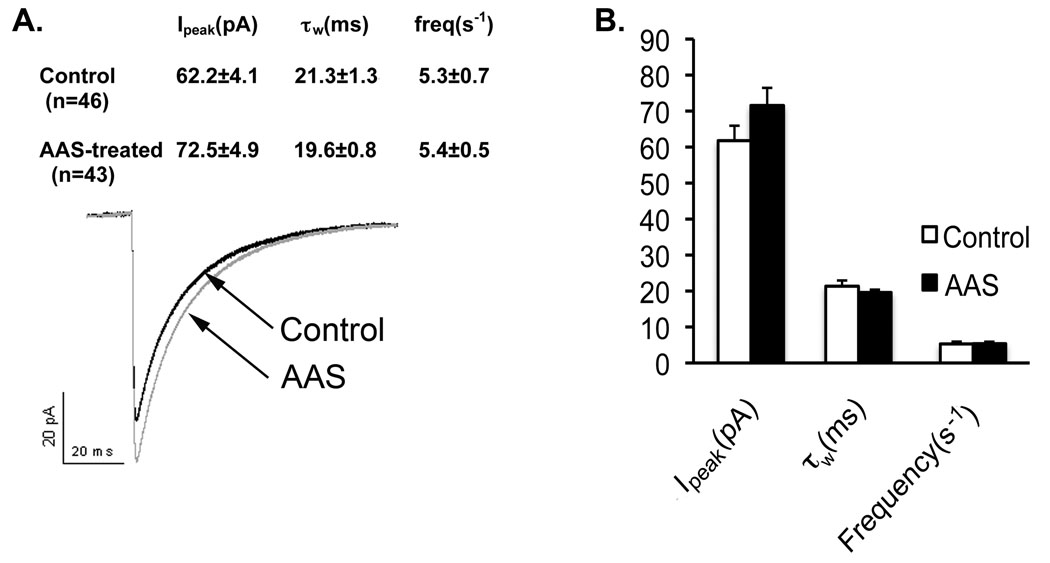
To determine if AAS-dependent changes in GABAA receptor subunit mRNA levels were correlated with changes in GABAergic transmission, we next assessed AAS-dependent changes in GABAA receptor-mediated currents within the central region of the mPOA (the MPN) of control and AAS-treated female mice. Spontaneous GABAA receptor-mediated synaptic currents (sIPSCs) recorded in aCSF from MPN neurons of control female mice were similar in magnitude and in kinetics of decay (biexponential decays with time constants, τ1 and τ2, of ~10 and ~35 ms), as described previously (Jorge et al., 2002; Penatti et al., 2005). AAS treatment elicited a trend towards larger averaged peak amplitudes (Ipeak), although this change did not attain significance. There was no significant effect of AAS treatment on sIPSC frequency or on the average sIPSC decay, as reflected in the single individual time constants (τ1 and τ2) or by the weighted time constant (τω) (Figure 3).
Figure 3. Effects of AAS treatment on synaptic currents.
A) Averaged sIPSCs from 46 MPN neurons in slices isolated from control mice (black line) and 43 neurons in slices from AAS-treated (grey line) mice. B) Graphic representation of the average peak current amplitude (Ipeak), the weighted time constant of current decay (τw) and the frequency of sIPSCs in MPN neurons from control (white bars) and AAS-treated (black bars) female mice.
III. Effects of an antagonist selective for α5-containing receptors on GABAA receptor-mediated currents in the MPN of female mice
As noted, the α5 subunit of the GABAA receptor is expressed at appreciable levels within the rodent mPOA (Nett et al., 1999; Penatti et al., 2005; this study), and levels of this transcript are significantly increased by AAS treatment. To determine the contribution of α5-containing receptors to GABAA receptor-mediated currents in the MPN, recordings of both tonic currents (Itonic) and sIPSCs were made first in aCSF alone and following acute addition of the α5-specific antagonist, L655,708 (L6) to the bath. Not surprisingly, there was heterogeneity within the MPN with respect to the responsiveness of neurons to this antagonist. Neurons were defined as L6-sensitive if GABAA-receptor-mediated currents were decreased by exposure to this antagonist.
Acute application of L6 decreased baseline currents (Itonic), which are likely to reflect activation of extrasynaptic receptors by ambient levels of micromolar GABA, in a comparable percentage of MPN neurons from control and AAS-treated mice: 73% (19/26) control; 71% (20/28) AAS-treated. We observed no significant differences in the magnitude of this current between control (10.4 ± 2.0 pA) and AAS-treated (9.9 ± 3.1 pA) mice.
Acute application of L6 also decreased the average Ipeak in a comparable percentage of neurons from control and AAS-treated mice: 73% (22/30) control; 68% (19/28) AAS-treated. The average Ipeak of L6-sensitive neurons from control mice was larger (63.4 ± 5.7 pA; Table 1A) than the Ipeak in the other 8 cells (49.0 ± 6.3 pA), but the difference was not significant. However, Ipeak in the L6-sensitive cells of AAS-treated mice was significantly (P = 0.040) larger (78.9 ± 8.9 pA; Table 1A) that in the other 9 cells (54.5 ± 6.9 pA). Finally, there was a trend towards a larger average Ipeak of sIPSCs of MPN in AAS-treated than in control mice for these L6-sensitive cells (Table 1A), however, as with entire population of MPN neurons analyzed (Figure 3), this trend did not attain significance.
Table 1.
Properties of L6-sensitive sIPSCs in MPN neurons from control and AAS-treated (AAS) mice for recordings made in (A) aCSF alone (0 µM [GABA]o) or (B) in aCSF supplemented with 2 µM [GABA]o and in the absence (−L6) or presence (+L6) of the α5-selective GABAA receptor inverse agonist, L-655,708.
| (A) 0 µM GABA | ||||
|---|---|---|---|---|
| −L6 | +L6 | |||
| Ipeak(pA) | τw(ms) | Ipeak(pA) | τw(ms) | |
| Control | 63.4±5.7 | 20.4±1.7 | 52.8±5.4 | 20.7±1.5 |
| (n=22) | ||||
| AAS | 78.9±8.9 | 18.4±1.0 | 55.8±5.4 | 22.1±2.0 |
| (n=19) | ||||
| (B) 2 µM GABA | ||||
|---|---|---|---|---|
| −L6 | +L6 | |||
| Ipeak(pA) | τw(ms) | Ipeak(pA) | τw(ms) | |
| Control | 47.1±5.6 | 24.0±2.6 | 36.5±3.3 | 25.0±1.6 |
| (n=12) | ||||
| AAS | 56.4±10.1 | 21.2±2.6 | 48.5±8.0 | 23.6±3.2 |
| (n=10) | ||||
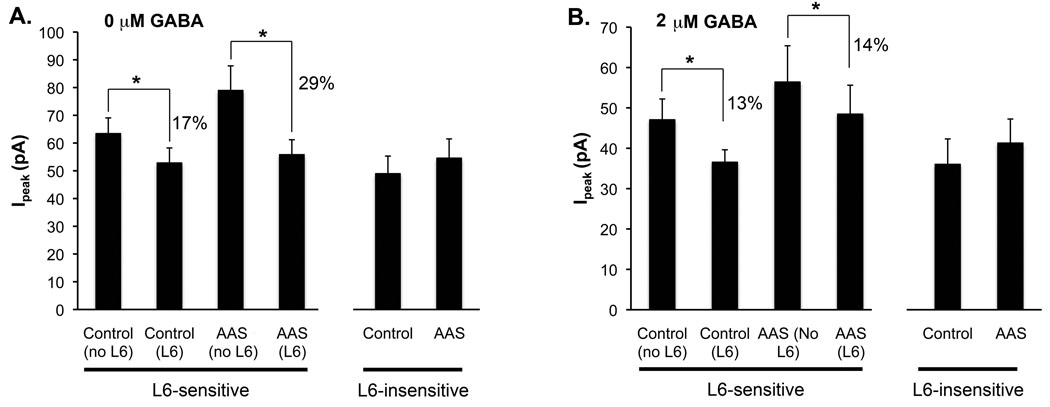
For L6-sensitive cells from control mice, acute application of L6 significantly (P = 7.2 × 10−8) decreased Ipeak by 17% of its initial value (Figure 4A; Table 1A) with no change in current decay kinetics. For L6-sensitive cells from AAS-treated mice, the decrease in Ipeak caused by acute application of L6 was also significant (P = 0.0066), but the magnitude of the decrease (29%) was significantly greater (P = 0.0500) than that observed for neurons from control mice (Figure 4A; Table 1A). In contrast to MPN neurons from control subjects where L6 did not alter synaptic current decay properties, L6 application to MPN neurons from AAS-treated mice elicited a modest, but nonetheless significant (P = 0.0156) increase in τw (Table 1A).
Figure 4. Spontaneous IPSC peak currents for L6-sensitive and -insensitive neurons in control and AAS-treated mice.
Bars represent average Ipeak for the indicated conditions for L6-sensitive and L6-insensitive MPN neurons from control and AAS-treated mice in recordings made in (A) aCSF alone or (B) aCSF supplemented with 2 µM GABA. Asterisks indicate significant differences in Ipeak elicited by exposure to L6; percentages indicate the decrease in Ipeak elicited by acute exposure to L6. Note the difference of scale on the vertical axes.
A growing body of work indicates low micromolar concentrations of ambient extracellular GABA present in the brain have critical actions on the properties of synaptic transmission and on action potential firing (for review, Semyanov et al., 2004; Farrant and Nusser, 2005). In particular, in the hippocampus, it has been proposed that α5-containing extracellular receptors that mediate Itonic make an appreciable contribution to the GABAA receptor-mediated conductances only under conditions of neuronal activity that result in elevated levels of ambient GABA (Scimemi et al., 2005). To further assess the role α5-containing receptors play in AAS-treated versus control subjects in solutions that may more faithfully mirror physiological conditions, recordings were also made in the presence of 2 µM GABA in the bath.
Neither the proportion of MPN neurons displaying an L6-sensitive Itonic nor the amplitude of the L6-induced decrease in Itonic in these neurons from either control or treated mice was different for recordings made in aCSF supplemented with 2 µM [GABA]o versus aCSF alone. Thus, experiments performed here with L6 indicate that α5-containing receptors carry a small (~ 10 pA) tonic current in ~70% of MPN neurons, but that AAS treatment did not result in a significant increase in the magnitude of this L6-sensitive tonic current under either standard recording conditions of aCSF alone or in the presence of elevated extracellular GABA.
For sIPSCs, the average Ipeak of L6-sensitive neurons from control mice was significantly smaller (P = 0.0486) for recordings made in 2 µM versus 0 µM [GABA]o (Table 1A and B). Peak sIPSC amplitudes for recordings made from MPN neurons of AAS-treated mice in the presence of 2 µM [GABA]o were also smaller than the average Ipeak from recordings made in aCSF alone, however the difference was not significant (Table 1A and B). Despite the lower average amplitude of events in elevated [GABA] o, which may reflect a general shunting of the membrane resistance, the overall profile of amplitudes for L6-sensitive and insensitive neurons in both control and AAS-treated mice was comparable in 2 µM and 0 µM [GABA]o (Figure 4B). Similarly, as with recordings made in 0 µM [GABA]o, acute addition of L6 to aCSF supplemented with 2 µM [GABA]o significantly (P = 0.0233 for control; P = 0.0128 for AAS-treated) reduced Ipeak for neurons from both treatment groups (Figure 4B).
There were no significant differences in the kinetics of synaptic current decay between recordings made in aCSF alone versus aCSF supplemented with 2 µM GABA. The frequency of sIPSCs for recordings from L6-sensitive neurons of control mice was also comparable in aCSF alone versus aCSF supplemented with 2 µM GABA (4.9 ± 0.8 s−1 vs. 5.8 ± 1.1 s−1). The frequency of sIPSCs for recordings from L6-sensitive neurons of AAS-treated mice was greater in the presence of 2 µM [GABA]o (9.3 ± 2.7 versus 5.0 ± 0.8 in aCSF alone), but the difference did not attain significance.
IV. AAS-dependent changes in action potential firing in MPN neurons of female mice
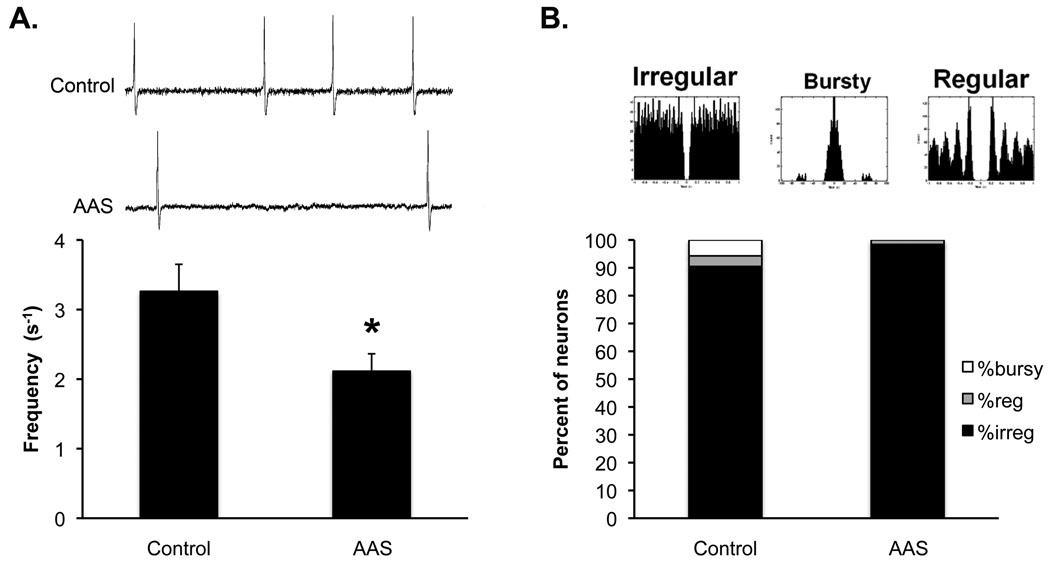
Analysis of sIPSCs and tonic currents suggested that AAS treatment had a negligible effect on the α5-sensitive tonic currents in MPN neurons, but had complex effects on sIPSCs in these neurons. Some of these individual, AAS-induced changes bordered on (but did not attain) significance, but additively may have acted to significantly change neuronal activity of MPN neurons. We next assessed action potential firing to determine if AAS-treatment resulted in changes in neuronal activity and if such changes could be correlated with GABAA receptor-mediated transmission through α5-containing receptors. For recordings made in aCSF alone, we observed a significant (P = 0.0257) decrease in action potential frequency in MPN neurons from 3.3 ± 0.4 s−1 (n= 53 cells) in control to 2.1 ± 0.3 s−1 (n = 62 cells) in AAS-treated mice (Figure 5A). AAS treatment also resulted in a trend (P = 0.0570) in which the percentage of neurons with irregular firing patterns was enhanced. In control mice, 90% of the MPN neurons were characterized by irregular firing, while 98% of the neurons from AAS-treated mice displayed this profile (Figure 5B).
Figure 5. Effects of AAS treatment on action potential frequency and firing patterns.
A) Representative on-cell recordings of action potentials from an MPN neuron of a control (top) and an AAS-treated (bottom) mouse and average action potential frequency in MPN neurons for control and AAS-treated (AAS) subjects in slices bathed in aCSF. B) Representative autocorrelograms corresponding to irregular, bursty and regular firing patterns and autocorrelational analysis demonstrating the distribution of MPN neurons with different firing patterns in control and AAS-treated animals. Asterisks indicate from AAS-treated subjects were significantly different from controls.
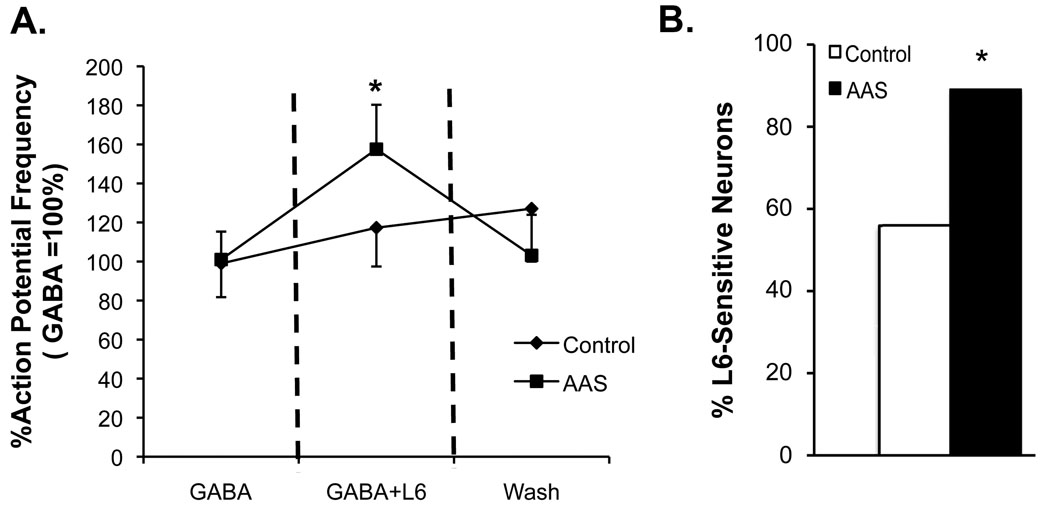
Analysis of action potential firing frequency in MPN neurons from recordings made in the presence of 2 µM [GABA]o also revealed significant differences between control and AAS-treated mice. Specifically, acute addition of L6 significantly increased action potential firing in MPN neurons in AAS-treated (P = 0.0094), but was without effect in these neurons from control mice (Figure 6A). In addition, there was a significantly (P = 0.0200) greater percentage of L6-sensitive MPN neurons in AAS-treated (89%; 17/19) versus in control (56%; 10/18) mice (Figure 6B).
Figure 6. Action potential firing in L6-sensitive neurons in recordings made in the presence of 2 µM [GABA]o.
A) Average action potential frequencies recorded in MPN neurons in aCSF supplemented with 2 µM [GABA]o (GABA); after acute application of L6 (GABA+L6), and following a 3 minute wash for MPN neurons from control and AAS-treated mice. Data have been normalized with the frequency of action potential firing in MPN neurons from control mice in the presence of aCSF and 2 µM [GABA]o being set at 100%. B) The percentage of L6-sensitive MPN neurons when recordings were made in aCSF supplemented with 2 µM [GABA]o for control (white bars) and AAS-treated (black bars) mice. Asterisk in (A) indicates that AP frequency was significantly higher in the presence of L6 than before its addition or following wash. Asterisk in (B) indicates that the percentage of L6-sensitive MPN neurons was significantly greater in AAS-treated than control mice.
DISCUSSION
In recent decades, the increased participation of women in elite athletics, as well as the social pressure to enhance body image has promoted increased use of AAS among women and girls (for review, Elliot and Goldberg, 2000). The preponderance of studies examining the effects of AAS effects on the brain and behavior have been carried out in male subjects (for review, Clark and Henderson, 2003). Our goal here was to examine the effects on neural signaling within the mPOA/MPN of female mice elicited by exposure to three commonly abused AAS taken concurrently in a manner that better mimics the multi-drug regimes self-administered by human abusers.
Exposure of gonadally-intact adult female mice to this AAS cocktail significantly enhanced expression of the α5, β3 and δ subunit mRNAs in the mPOA and the contribution of α5-containing receptors to the postsynaptic response in this brain region, while also diminishing action potential firing. AAS treatment did not alter the frequency of synaptic responses, suggesting that this treatment regime in these female subjects did not have an appreciable effect on presynaptic release. Previous studies have indicated that expression of α5 and β3 subunits are co-assembled in the hippocampus (Sur et al., 1998), and our data are consistent with a coordinate regulation of these two subunits in the mPOA (at the mRNA level) by AAS treatment. Despite the high levels of aromatase expressed in the mPOA (Abdelgadir et al., 1994; Roselli et al., 1998), and thus the potential for effects of this cocktail to be mediated by ER, increases in these GABAA receptor subunit mRNAs were shown to be AR-dependent: co-treatment with the AR-specific antagonist, flutamide, abrogated the AAS-induced increases in all three subunits such that levels in animals receiving both AAS and flutamide were not different from control.
The role of the α5 subunit has been most extensively studied with respect to its contribution to extrasynaptic GABAA receptors that mediate tonic conductances in the hippocampus, but receptors containing this subunit have also been shown to contribute to synaptic responses in the hippocampus and the cortex (Dunning et al., 1999; Collinson et al., 2002; Serwanski et al., 2006; Ali and Thomson, 2008; Zarnowska et al., 2008). Several of the experiments performed here converge to suggest that the increase in α5 mRNA expression observed in AAS-treated mice resulted in augmented synaptic responses through α5-containing receptors and concomitant changes in action potential firing and patterning. Treatment with L6 indicated that ~70% of MPN neurons express α5-containing receptors at synaptic sites. The average Ipeak in L6-sensitive neurons was significantly larger than that observed in L6-insensitive neurons for AAS-treated, but not control animals. In addition, the decrease elicited in Ipeak by L6 application was greater for these L6-sensitive neurons from AAS-treated versus control subjects (29 vs. 17%). With respect to action potential firing, AAS treatment resulted in a significant decrease in action potential frequency. Moreover, under conditions of elevated ambient GABA, acute application of L6 promoted a significant increase in action potential frequency in MPN neurons from AAS-treated, but not control mice, and the percentage of L6-sensitive MPN neurons was significantly greater in AAS-treated subjects. These observations also suggest that AAS treatment results in augmented inhibitory synaptic transmission and that inhibition mediated by α5-containing receptors contributes significantly to that enhanced inhibition in AAS-treated mice. Finally, previous studies in the cerebellum have demonstrated that GABAergic synaptic input from inhibitory interneurons transforms the firing patterns of their postsynaptic targets from regular to irregular firing patterns (Haüsser and Clark, 1997). The increase in irregular firing observed in MPN neurons with AAS-treatment would thus also be consistent with enhanced inhibitory synaptic input. While all of these data are consistent with the hypothesis that AAS treatment enhanced synaptic transmission through α5-containing receptors in MPN neurons, it was nonetheless the case that the increases in sIPSC Ipeak observed with treatment was a trend that did not attain significance. That such increases may have a significant effect on action potential generation, but not attain significance in somatic whole-cell recordings, may reflect complexities of the location of these synapses with respect to excitatory inputs and how such signals are shaped and integrated by the morphological properties of the MPN neurons (for review, Gulledge et al., 2005).
The most common behaviors altered in AAS abuse are sexual/reproductive behaviors, aggression and anxiety (for review, Clark and Henderson, 2003). While the mPOA is involved in forebrain circuitry that underlies the generation of aggression and anxiety, the relationship between hormone-sensitive cellular events in the mPOA and behavioral output has been most extensively characterized for the expression of reproductive and sexual behaviors. Specifically, it has been shown that infusion of GABA into the mPOA inhibits the expression of behavioral receptivity in female rats (McCarthy et. al., 1990), and that decreases in neuronal activity within selective subsets of mPOA neurons are positively correlated with exposure to physiological levels of 17β-estradiol and with the expression of female proceptive and receptive sexual behaviors (Bueno and Pfaff, 1976; Sakuma, 1994; Takeo and Sakuma, 1995; Kato and Sakuma, 2000; c.f., Kubo et al., 1975; Weick and Dyer, 1982). Our results suggest a qualitative change in GABAergic signaling (an increase in the representation of α5-containing synaptic receptors), a diminished level of firing of MPN neurons in AAS-treated versus diestrous females and a disruption of peripheral cyclicity, as indicated by a constant state of vaginal diestrus.
Caution must be taken in drawing parallels between changes in neuronal activity in the mPOA and the expression of sexual behaviors in female rodents exposed to physiological concentrations and durations of estrogens and progestins versus female mice exposed for prolonged durations to supraphysiological levels of synthetic androgens, which have the capacity to activate quite disparate signaling mechanisms than normal levels of gonadal hormones (for review, Clark et al., 2006). These caveats notwithstanding, it is interesting to note that while sexual behavior has not been assessed in rats or mice treated concurrently with the AAS used here for this duration, chronic treatment with each of the three AAS of this cocktail administered individually has been determined in gonadally-intact rats. Treatment with a high dose of either 17α-methyltestosterone or nandrolone decanoate for two weeks suppressed both vaginal cyclicity and sexual receptivity in female rats (Blasberg et al., 1997), and co-administration of flutamide with 17α-methyltestosterone antagonized these effects (Blasberg et al., 1998). However, while testosterone cypionate given at comparable duration and dose also suppressed vaginal cyclicity, this AAS enhanced sexual receptivity (Blasberg et al., 1997; Clark et al., 1998), demonstrating divergent effects of chemically distinct AAS on these two reproductive endpoints. This diversity in the effects of the AAS on reproductive and sexual behaviors underscores the fact that these drugs are likely to have variable effects on the brain and resulting behaviors that will depend not only upon the regime (dose, duration and chemical signature) of the different AAS taken, but also on the balance of disparate signaling mechanisms through which each compound acts. These mechanisms, in turn, will vary not only across different brain regions, but also with the age, sex and hormonal state of the subject.
Beyond the effects on sexual behaviors, what are other potential behavioral correlates of these AAS-induced changes in expression of α5-containing receptors within the MPN? Chronic treatment with this AAS cocktail promoted enhanced levels of α5 subunit mRNA not only in the mPOA of adult female mice, but also in the MeA and VMN of adult female mice and the mPOA of adolescent female mice, as well as the mPOA and lateral septum of adult male mice (Penatti CAA, Costine, BA, Porter DM, and Henderson LP, unpublished data), suggesting that enhanced expression of α5-containing receptors is a widely elicited response in forebrain/hypothalamic regions that contribute not only to the expression of sexual behaviors, but also anxiety and aggression (for review, Newman, 1999; Blaustein and Erskine, 2002; Hull et al., 2002; Sewards and Sewards, 2002; Gammie, 2005; Goodson, 2005; Veening et al., 2005). In this regard, it is interesting to note that Pibiri et al. (2006) report that a three week treatment of adult female Swiss Webster mice with the AAS, testosterone propionate, elicited increased levels of the α5 subunit mRNA in frontal cortex in animals subjected to social isolation conditions that also promoted inter-female offensive aggression. With respect to anxiety, Navarro et al. (2002) have shown that L6 has anxiogenic effects on male mice on the elevated plus maze (Navarro et al., 2002), although other investigators have made contradictory findings. Specifically, Collinson et al. (2002) report no differences between wild type and α5−/− female or male mice on either the elevated plus maze or the two-way active avoidance task and Dawson et al. (2006) found no effects on anxiety in male rats upon treatment with the α5-selective inverse agonist, α5IA. These discrepancies in the role of α5-containing receptors in mediating anxiety may arise reflect differences in sex or species of the subjects or in methodology (pharmacological blockade versus knockout mice). Interestingly, Yee et al. (2004) report that female mice exhibit a more intense freezing response in a trace conditioning paradigm and that this sex-specific difference is augmented in α5−/− mice. In addition, Heldt and Ressler (2007) report a significant decrease in α5 subunit mRNA in the central amygdala of C57Bl/6 male mice during trace fear conditioning (as assessed by freezing behavior to paired acoustic stimulus), while we find significantly enhanced acoustic startle response amplitude in female C57Bl/6 mice treated with this AAS cocktail (Beth Costine, Matthew Davis, Robert Leaton, and Leslie Henderson, unpublished findings). Taken together, these data suggest that an expanded analysis of AAS effects on α5 expression and GABAergic function throughout forebrain structures including the “extended amygdala” (for review, Davis and Whalen, 2001), hippocampus and septum, by the use of α5-selective pharmacological agents and α5−/− mice may provide important new insights into the neurobiological underpinnings of the effects of these abused steroids on social behaviors in female subjects.
Acknowledgements
This work was supported by the NIH (DA18255 and DA14137).
LIST OF ABREVATIONS
- AAS
anabolic-androgenic steroids
- aCSF
artificial cerebral spinal fluid
- ANOVA
analysis of variance
- AR
androgen receptor
- CT
threshold cycle
- GABAA
γ-aminobutyric acid type A receptor
- Ipeak
peak current amplitude of synaptic responses
- L6
the α5-selective GABAA receptor inverse agonist, L-655,708
- MPN
medial preoptic nucleus
- mPOA
medial preoptic area
- PBS
phosphate buffered saline
- PN
postnatal day
- sIPSC
spontaneous inhibitory postsynaptic current
- τ
time constant of decay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Synaptic α5 subunit-containing GABAA receptors mediate IPSCs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bar-Gad I, Ritov Y, Bergman H. The neuronal refractory period causes a short-term peak in the autocorrelation function. J Neurosci Methods. 2001;104:155–163. doi: 10.1016/s0165-0270(00)00335-6. [DOI] [PubMed] [Google Scholar]
- Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–5117. doi: 10.1210/jcem.86.11.7983. [DOI] [PubMed] [Google Scholar]
- Blasberg ME, Langan CJ, Clark AS. The effects of 17αmethyltestosterone, methandrostenolone, and nandrolone decanoate on the rat estrous cycle. Physiol Behav. 1997;61:65–72. doi: 10.1016/s0031-9384(96)00409-x. [DOI] [PubMed] [Google Scholar]
- Blasberg ME, Robinson S, Henderson LP, Clark AS. Inhibition of estrogen-induced sexual receptivity by androgens: role of the androgen receptor. Horm Behav. 1998;34:283–293. doi: 10.1006/hbeh.1998.1484. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 1. Orlando: Academic Press; 2002. pp. 139–213. [Google Scholar]
- Bueno J, Pfaff DW. Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res. 1976;101:67–78. doi: 10.1016/0006-8993(76)90988-4. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampus CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Blasberg ME, Brandling-Bennett EM. Stanozolol, oxymetholone, and testosterone cypionate effects on the rat estrous cycle. Physiol Behav. 1998;63:287–295. doi: 10.1016/s0031-9384(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CAA, Porter DM, Yang P, Henderson LP. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126:122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27:413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani FR, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exper Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiat. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Gooyer ME, Oppers-Tiemissen HM, Leysen D, Verheul HAM, Kloosterboer HJ. Tibolone is not converted by human aromatase to 7α-methyl-17α-ethynylestradiol (7α-MEE): Analyses with sensitive bioassays for estrogens and androgens and with LC-MSMS. Steroids. 2003;68:235–243. doi: 10.1016/s0039-128x(02)00184-8. [DOI] [PubMed] [Google Scholar]
- Dunning DD, Hoover CL, Soltesz I, Smith MA, O'Dowd DK. GABAA receptor-mediated miniature postsynaptic currents and α-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Elliot DL, Goldberg L. Women and Anabolic Steroids. In: Yesalis CE, editor. Anabolic Steroids in Sport and Exercise. Champaign: Human Kinetics; 2000. pp. 225–246. [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Franke WW, Berendonk B. Hormonal doping and androgenization of athletes: a secret program of the German Democratic Republic government. Clin Chem. 1997;43:1262–1279. [PubMed]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego CA: Academic Press; 1997. [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behav Cogn Neurosci Rev. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gao B, Moore RY. The sexually dimorphic nucleus of the hypothalamus contains GABA neurons in rat and man. Brain Res. 1996;742:163–171. doi: 10.1016/s0006-8993(96)01005-0. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit–deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG. Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychother Psychosom. 2000;69:19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Haüsser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Heldt AS, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: Effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 1. Orlando: Academic Press; 2002. pp. 3–137. [Google Scholar]
- Jones BL, Whiting PJ, Henderson LP. Mechanisms of anabolic androgenic steroid inhibition of mammalian ε-subunit-containing GABAA receptors. J Physiol (Lond) 2006;573(3):571–593. doi: 10.1113/jphysiol.2006.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge JC, McIntyre KL, Henderson LP. The function and the expression of forebrain GABAA receptors change with hormonal state in the adult mouse. J Neurobiol. 2002;50:137–149. doi: 10.1002/neu.10021. [DOI] [PubMed] [Google Scholar]
- Kato A, Sakuma Y. Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Res. 2000;862:90–102. doi: 10.1016/s0006-8993(00)02076-x. [DOI] [PubMed] [Google Scholar]
- Kibble MW, Ross MB. Adverse effects of anabolic steroids in athletes. Clin Pharm. 1987;6:686–692. [PubMed] [Google Scholar]
- Kochakian C, Yesalis CE. Anabolic-androgenic steroids: a historical perspective and definition. In: Yesalis CE, editor. Anabolic Steroids in Sport and Exercise. Champaign: Human Kinetics; 2000. pp. 4–33. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−Δ ΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Llewellyn W. Anabolics. 6th Edition. Jupiter FL: Body of Science; 2007. [Google Scholar]
- Long JA, Evans HM. The estrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;6:1–137. [Google Scholar]
- Martini L. The 5 alpha-reduction of testosterone in the neuroendocrine structures. Biochemical and physiological implications. Endocr Rev. 1982;3:1–25. doi: 10.1210/edrv-3-1-1. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Malik KF, Feder HH. Increased GABAergic transmission in the medial hypothalamus facilitates lordosis but has the opposite effect in preoptic area. Brain Res. 1990;507:40–44. doi: 10.1016/0006-8993(90)90519-h. [DOI] [PubMed] [Google Scholar]
- McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABAA receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43:634–654. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- Nett ST, Jorge-Rivera J-C, Myers M, Clark AS, Henderson LP. Properties and sex-specific differences of GABAA receptors in neurons expressing γ1 subunit mRNA in the preoptic area of the rat. J Neurophysiol. 1999;81:192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Burón E, Martín-López M. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABAA receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1389–1392. doi: 10.1016/s0278-5846(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavioral network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou AD, Umbreit TH, Goering PL, Brown KM. Effects of 17α-methyltestosterone on uterine morphology and heat shock protein expression are mediated through estrogen and androgen receptors. J Steroid Biochem Mol Biol. 2002;82:305–314. doi: 10.1016/s0960-0760(02)00221-2. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Henderson LP. Androgen Actions on Receptors and Channels: Regulation of Electrical Excitability and Synaptic Transmission. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. second edition. Elsevier; in press. [Google Scholar]
- Penatti CA, Porter DM, Jones BL, Henderson LP. Sex-specific effects of chronic anabolic androgenic steroid treatment on GABAA receptor expression and function in adolescent mice. Neuroscience. 2005;135:533–543. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Andersen KH, Yates WR. Illicit anabolic steroid use in athletes. Am. J. Sports Med. 1990;18:422–428. doi: 10.1177/036354659001800416. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J Forensic Sci. 2003;48:1–6. [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Carboni G, Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 2006;17:1537–1541. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Affective and psychotic symptoms associated with anabolic steroid use. Am J Psychiatry. 1988;145:487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. Arch Gen Psychiatry. 2000;57:133–140. doi: 10.1001/archpsyc.57.2.133. [DOI] [PubMed] [Google Scholar]
- Quincey RV, Gray CH. The metabolism of [1,2-3H]17α-methyltestosterone in human subjects. J Endocrinol. 1967;37:37–55. doi: 10.1677/joe.0.0370037. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]-L655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Abdelgadir SE, Rønekleiv OK, Klosterman Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- Ryan KJ. Biological aromatization of steroids. J Biol Chem. 1959;234:268–272. [PubMed] [Google Scholar]
- Sagrillo CA, Selmanoff M. Castration decreases single cell levels of mRNA encoding glutamic acid decarboxylase in the diagonal band of Broca and the sexually dimorphic nucleus of the preoptic area. J Neuroendocrinol. 1997;9:699–706. doi: 10.1046/j.1365-2826.1997.00630.x. [DOI] [PubMed] [Google Scholar]
- Sakuma Y. Estrogen-induced changes in the neural impulse flow from the female rat preoptic area. Horm Behav. 1994;28:438–444. doi: 10.1006/hbeh.1994.1041. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the α5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Fear and power-dominance drive motivation: neural representations and pathways mediating sensory and mnemonic inputs, and outputs to premotor structures. Neurosci Biobehav Rev. 2002;26:553–579. doi: 10.1016/s0149-7634(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23:1355–1390. doi: 10.1016/s0149-2918(01)80114-4. [DOI] [PubMed] [Google Scholar]
- Su T-P, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269:2760–2764. [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal α5 subunit-containing γ-aminobutyric acidA receptors have α5β3γ2 pharmacological characteristics. Molec Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- Takeo T, Sakuma Y. Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neurosci Res. 1995;22:73–80. doi: 10.1016/0168-0102(95)00885-w. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Weick RF, Dyer RG. Stimulation of single unit activity in the preoptic area and anterior hypothalamus, and secretion of luteinizing hormone, by ovarian steroids in ovariectomized rats with diencephalic islands. Proc R Soc Lond B Biol Sci. 1982;216:461–473. doi: 10.1098/rspb.1982.0086. [DOI] [PubMed] [Google Scholar]
- Winters SJ. Androgens: endocrine physiology and pharmacology. NIDA Res Monogr. 1990;102:113–130. [PubMed] [Google Scholar]
- Yang P, Jones B, Henderson LP. Mechanisms of anabolic androgenic steroid modulation of α1β3γ2L GABAA receptors. Neuropharmacology. 2002;43:619–633. doi: 10.1016/s0028-3908(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Role of the α subunit in the modulation of GABAA receptors by anabolic androgenic steroids. Neuropharmacology. 2005;49:300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Möhler H, Rudolph U, Feldon J. GABAA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor α5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus. J Neurophysiol. 2008 doi: 10.1152/jn.91203.2008. ePub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]