Abstract
Event-related potential (ERP) studies of affective picture processing have demonstrated an early posterior negativity (EPN) for emotionally arousing pictures that are embedded in a rapid visual stream. The present study examined the selective processing of emotional pictures while systematically varying picture presentation rates between 1 and 16 Hz. Previous results with presentation rates up to 5 Hz were replicated in that emotional compared to neutral pictures were associated with a greater EPN. Discrimination among emotional and neutral contents was maintained up to 12 Hz. To explore the notion of parallel processing, convolution analysis was used: EPNs generated by linear superposition of slow rate ERPs explained 70-93% of the variance of measured EPNs, giving evidence for an impressive capacity of parallel affective discrimination in rapid serial picture presentation.
Keywords: ERP, emotion, attention, rapid serial visual presentation
INTRODUCTION
Research in affective neuroscience supports the notion that emotional cues guide selective visual attention and receive enhanced processing (Lang, Bradley, & Cuthbert, 1997; Öhman, Flykt, & Lundqvist, 2000; Vuilleumier, 2005; Derryberry & Tucker, 1991). For instance, functional Magnetic Resonance Imaging (fMRI) revealed increased BOLD (Blood Oxygen Level Dependent) signals in associative visual regions and subcortical limbic structures when viewing emotionally arousing compared to neutral pictures (Junghöfer, Schupp, Stark, & Vaitl, 2005; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005). Furthermore, event-related brain potential studies detailed the temporal dynamics of emotion processing in the visual cortex. An early difference in processing emotional (pleasant and unpleasant) compared to neutral pictures is revealed by the early posterior negativity (EPN) developing around 120 – 150 ms after stimulus onset and lasting until about 300 ms (Junghöfer, Bradley, Elbert & Lang, 2001; Schupp, Junghöfer, Weike, & Hamm, 2003). Accordingly, the early differential ERP response may reflect a processing advantage of affective stimuli already at initial stages of perceptual processing (cf., Schupp et al., 2006).
A valuable insight into the mechanisms of the emotional guidance of visual attention has been gained by investigating the visual system in perceptually demanding conditions. Junghöfer and colleagues adopted the rapid serial visual presentation (RSVP; Potter, 1976) paradigm to study the speed of affective discrimination. Specifically, participants passively viewed a rapid and continuous stream of alternating emotional and neutral pictures. For both presentation rates (i.e., 200 and 333 ms), greater EPNs were observed for emotional compared to neutral materials. Interestingly, considering the 200 ms per picture rate, a spill-over effect was observed in that the EPN modulation appeared at a latency at which the next picture was already shown. These data showed the brain's capacity for affective discrimination of natural scenes presented approximately at the speed of saccadic eye movements.
The study of the speed of affective discrimination capitalized on cognitive studies revealing the remarkable efficiency and speed of the visual system in processing rapidly presented stimuli. For instance, when exposed to a rapid stream of pictures (i.e., 125 ms), participants can detect particular content, based on prior instruction (Potter, 1976). Similarly, studying briefly presented complex natural scenes, differential ERP responses to target compared to non-target stimuli developed around 150 ms poststimulus (Thorpe, Fize & Marlot, 1996; Delorme & Thorpe, 2001; Codispoti, Ferrari, Junghöfer & Schupp, 2006). Single cell recordings provided most remarkable evidence for the speed of visual processing showing that neurons in the temporal gyrus maintain their selective responding with stable latencies even with extremely fast rapid serial presentation times (Keysers, Xiao, Földiák & Perrett, 2001). In particular the latter findings raise the issue of temporal limits of affective discrimination, as indexed by the EPN component. Thus, increasing the speed of presentations of unrelated images promises insights into the boundary conditions of the selective processing of affectively salient stimuli.
In order to isolate the effects of time pressure on affective discrimination, sequence effects need to be considered as the processing of a currently presented picture is differentially affected whether preceded by emotional or neutral picture contents. A recent study explored this issue examining the nine possible pairs of preceding and current picture contents when participants view pleasant, neutral, and unpleasant pictures (Flaisch, Junghöfer, Bradley, Schupp & Lang, 2008). Results showed that the hedonic valence of the current (‘target’) and preceding (‘prime’) pictures independently modulated the ERPs in the time window from 150 – 300 ms poststimulus. Target effects replicated previous findings in that emotional pictures elicited an enlarged EPN. Moreover, prime effects on target processing were observed when contrasting emotional and neutral prime picture contents. Specifically, prime effects appeared as reduced EPN effects, i.e., relative positive potentials over posterior regions around 150 – 300 ms. These findings suggest that sequence effects may diminish or amplify affective discrimination, with alternating presentation of emotional and neutral picture contents at high presentation rates representing a reliable means to study electrocortical indices of affective discrimination.
Several hypotheses regarding affect discrimination revealed by the EPN seem possible when the speed of the alternating sequence of emotional and neutral pictures is steadily increased. The most likely hypothesis may build upon the notion that each of the images evokes distinct large-scale neural representations, overlapping in time, and competing for processing resources (cf. Keysers & Perrett, 2002). Resources for parallel conscious processing are extremely limited as just a single emotionally arousing picture in a rapid stream of pictures receives conscious processing until it gets interrupted by a next popping up stimulus winning the rivalry due to its stronger emotional or behavioral salience (Anderson, 2005; Keil & Ihssen, 2004; Most, Scholl, Clifford & Simons, 2005). If the visual system capacity for parallel processing during mid-latency processing stages (< 300 ms) would be similarly limited, EPN effects would be expected to strongly decrease with increasing presentation rates, as not all but just few popping up stimuli would evoke preferential visual processing generating an EPN component. On the other hand, a high capacity for parallel processing at mid latency processing stages would predict that emotionally arousing pictures would receive preferential processing compared to neutral stimuli even if one or even several consecutive different emotionally arousing stimuli would have been presented before and during mid latency processing. In this case the topography, magnitude, and latency of the EPN would be expected not to change with increasing presentation rate.
Although simplified, in this model the limits of parallel processing resources can be estimated by linear superimposition (convolution) of electrocortical potentials evoked by stimuli shown at a fixed rate: In convolution analysis, ERPs measured for the slowest presentation time (1s/picture) can be used to generate ERP responses to the various presentation times by linear superimposition. As a result, a high correlation of convoluted and measured ERP would be consistent with the notion for undisturbed parallel processing at the level of the EPN component while weak correlations would indicate an excess of parallel processing resources.
In the present study, high-arousing pleasant and neutral picture contents from the International Affective Picture Series were presented as an alternating serial stream with no gap between successive picture presentations using different presentation rates. In ten separate experimental blocks the presentation rate was varied from 1 Hz (1000 ms per picture and longest exposure time) to 16 Hz (62.5 ms per picture and shortest presentation time). The identical pictures were shown in each condition since previous studies revealed that stimulus repetition does not modulate selective processing indicated by the early posterior negativity (Codispoti, Ferrari & Bradley, 2007; Schupp et al., 2006). Conditions with presentation rates of 1 Hz, 2 Hz, 3 Hz, 4 Hz and 5 Hz (1000 ms, 500 ms, 333 ms, 250 ms, and 200 ms) were included to provide a replication of previous findings in that pleasant stimuli were predicted to be associated with a pronounced relative negative potential over temporo-occipital sensor sites starting around 120 ms and peaking around 250 ms (EPN). Conditions with shorter presentation rates of 6 Hz, 8 Hz, 10 Hz, 12 Hz and 16 Hz (166 ms, 125 ms, 100 ms, 83 ms, 62.5 ms) were included to (i) determine the limits of affective discrimination, (ii) to investigate the appearance of expected interference effects in relation to the various presentation rates, and (iii) to provide insights into the capacity for parallel processing when extracting affective significance.
METHOD
Participants
Participants were fourteen (7 females) right-handed introductory psychology students from the Konstanz University, Germany. They received either course credits toward their research requirements or a monetary reward. Participants were between the ages of 21 and 35 years (M = 22.5). Prior to the experiment, a letters of consent conforming to institutional guidelines for human research was obtained from the participants (approved by the University's Ethic committee).
Stimulus Materials and Procedure
One hundred pleasant and one hundred neutral pictures were selected from the International Affective Picture series (Lang, Bradley, & Cuthbert, 2005). The pleasant picture contents comprised erotica and scenes of sports and adventure. The neutral contents included images depicting people, household objects and other neutral scenes (landscapes, buildings, mushrooms, animals, etc.). Pleasant and neutral picture contents differed significantly from each other in normative ratings of hedonic valence (1 to 9 range; pleasant: M = 7.2; neutral: M = 5.0) and mean emotional arousal ratings for the pleasant categories were significantly higher than for the neutral contents (pleasant: M = 6.5, neutral: M = 2.9). Brightness and contrast of all pictures was digitally adjusted and color distribution, horizontal and vertical spatial frequency distributions, perceptual complexity (measured by JPEG file size) and relation of simple figure/ground versus complex scene stimuli did not differ significantly between the emotional and neutral picture groups.
The experiment comprised 10 experimental conditions. In each condition pleasant and neutral pictures were shown only once and in an alternating fashion (…-emotional-neutral-emotional-neutral-…). Across conditions stimulus presentation time varied from 62.5 ms, 83.3 ms, 100 ms, 125 ms, 166.7 ms, 200 ms, 250 ms, 333 ms, 500 ms, and 1000 ms and pictures were presented as continuous stream without perceivable inter stimulus interval. Half of the participants viewed the conditions in increasing order of presentations rate (i.e. from 1 to 16 Hz) while the other half of participants were shown the conditions in decreasing order (i.e. from 16 Hz to 1 Hz).
Using Presentation (Neurobehavioral Systems, Inc., Albany, CA) software, the pictures were shown on a CRT-monitor (85 Hz refresh rate) with 28° maximum visual angle. Participants were instructed to simply view the pictures.
Apparatus and Data Analysis
Electrophysiological data were collected from the scalp using a 129-channel system (EGI, Eugene, Oregon). Scalp impedance for each sensor was kept below 30 kΩ, which is appropriate for this type of electroencephalogram (EEG) amplifier. The EEG was collected continuously in the 0.1 to 100 frequency range with a sampling rate of 250 Hz. Continuous EEG data was low-pass filtered at 40 Hz and stimulus-locked epochs were extracted between 1000 ms pre- until 1000 ms post-onset of a given picture. A statistical approach was applied for artifact correction, including the transformation of the ERP data to an average reference (Junghöfer, Elbert, Tucker & Rockstroh, 2000).
With the alternating RSVP applied here, data epochs of the emotional condition were always preceded and followed by data from the neutral condition and vice versa. Thus extracting data epochs spanning the length of one stimulus pre and post picture onset resulted in mirrored condition differences in the pre and post trigger epoch with opposite sign. Baseline correction was calculated for the different presentation rates from 1000, 500, 333, 250 200, 167, 125, 100, 83 and 62 ms before to 1000, 500, 333, 250 200, 167, 125, 100, 83 and 62 ms after stimulus onset, thus comprising always the duration of one stimuli of both categories. In this way, and for all videos, baseline corrections were identical for both affective conditions allowing an optimal analysis of the EPN difference potential.
EPN analysis
Similar to previous research, the EPN appeared widespread over left and right temporo-occipital sensor sites, with right-hemispheric dominance, and a polarity reversal over anterior sites (see footnote 1). Consistent with the aim of the experiment, the EPN was scored in a sensor cluster across 36 bilateral occipito-temporal sensors (see footnote 2) as shown in the lower left corner of figure 1. To reveal the temporal characteristics of selective emotion processing as a function of exposure time, repeated-measures ANOVAs with the factor Emotion (pleasant vs. neutral) were calculated for each time point after picture onset using a significance criterion of α=0.01.
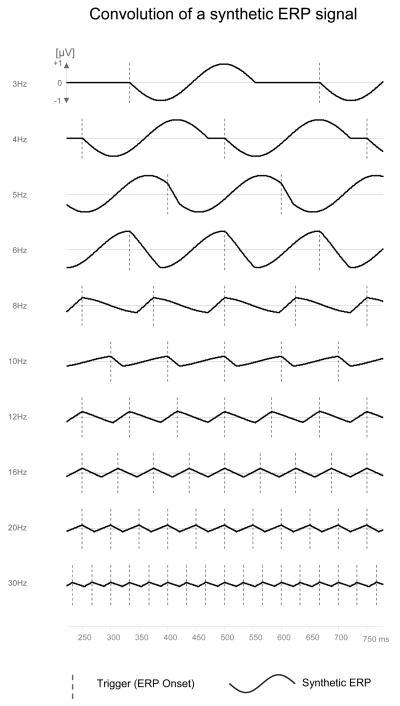
Figure 1.
Illustration of the convolution of a sinusoidal synthetic ERP. This synthetic ERP is assumed to get evoked by each trigger visualized by vertical dashed lines. With increasing presentation rates time courses assimilate in direction to oscillations with frequencies driven by the presentation rate. Moreover, increasing destructive interference of positive and negative ERP components increasingly reduces the signal amplitude.
Convolution analysis
For the investigation of processing interference, it was assumed that for the case of parallel processing at the levels of the visual sensory system, which are reflected by the early and mid latency ERP components (C1, P1, N1, P2), all incoming stimuli at high presentation rates are processed in the same manner as if presented slowly. Linear superposition (convolution) of slow rate ERPs in this case should allow a good estimation of the measured evoked potential of higher presentation rates. On the other hand, strong interference among successively presented stimuli and/or exhaustion of perceptual processing resources should strongly reduce the predictability of the high rate ERPs. Thus, “predicted” averaged ERPs for a 400 ms epoch of alternating emotional and neutral pictures were calculated by convolution of the 1 Hz ERPs for all faster f = 2, 3, 4, 5, 6, 8, 10, 12 and 16 Hz rates.
The convolution, which can be regarded as a moving average, was calculated based on the discrete form of the following convolution function:
.
With g(t,f1Hz) as measured ERP with 1Hz presentation rate and indicating the Dirac-like trigger function for each of the faster stimulus presentation rates the convoluted ERP was calculated as follows:
The difference potential (emotional – neutral) of these predicted (convoluted) ERPs across the above sensor cluster of interest were calculated and cross-correlated with the actually measured difference potentials in this sensor cluster and within the first 400 ms.
For a better understanding of the convolution procedure we would like to provide an example using a synthetic ERP with a simple sinusoidal signal of 222.2 ms wavelength corresponding to 4.5 Hz (see footnote 3) and zero potential at all other time points. This synthetic ERP function g(t) is assumed to get evoked by each trigger of the Dirac-like trigger function h(t) – visualized by vertical lines in figure 1. Given a 3 Hz stimulus rate (first row in figure 1) the ERP signal would appear every 333 ms – theoretically from minus infinite until plus infinite times – and thus for instance at 0, 333 and 666 ms. Due to the g(t) wavelength of 222.2 ms the ERPs at all onsets do not interfere. With 4 Hz (250 ms) presentation rate, there is still no superposition of ERPs. Superposition (convolution) starts with a stimulation rate exceeding the ERP driven frequencies (4.5 Hz). Thus 5 Hz stimulation rate leads to starting convolution with a slightly blurred sinusoidal signal. Importantly, this convolved signal now no longer oscillates with the 4.5 Hz frequency of the original ERP function but with the trigger h(t) frequency of 5 Hz (3rd row from top). With further increasing presentation rates convolution results in more and more temporal ‘blurring’ of the individual ERP components and time courses assimilate increasingly in direction to pure oscillations with frequencies driven by the presentation rate h(t). Moreover, as the overall integral of the synthetic ERP is zero (positive and negative potentials cancel out) increasing frequencies result in continuously increasing destructive interference of the positive and negative ERP components and thus continuously decreasing amplitudes. Real ERPs show more complex and longer lasting time courses and real trigger functions are of course not infinite. However, as long as the temporal extent of the trigger function is much longer than the maximal latency of relevant evoked potentials convolution results based on finite trigger functions are almost identical with the theoretical convolution based on indefinite functions.
Apart from identical stimulation frequencies this examples shares two important requirements with the study paradigm at hand: 1) the maximum latency of ERPs evoked by each single picture is much shorter than the complete block time; 2) the overall temporal integral of the ERP - the mean potential or the DC potential across the complete block - is zero at each sensor. Thus, although the real evoked ERP is more complex than the synthetic function g(t) the convolution with the trigger function should result in a roughly similar behavior.
It is, however, important to keep in mind that these calculated convolution results depend on the assumption of linear superposition. If the form of the ERP changed at faster presentation rates, the measured convolution function would significantly differ from the expected convolution function. Thus, if picture processing strongly depended on presentation rate, large discrepancies of expected and measured convolved signals should occur. However, due to the strong amplitude reduction with increasing frequencies the measure of discrepancy between expected and measures potentials gradually diminishes and eventually falls below the signal to noise level of the measured signals.
RESULTS
Figure 2 and 3 summarize the emotional modulation of the posterior ERP as a function of picture exposure time.
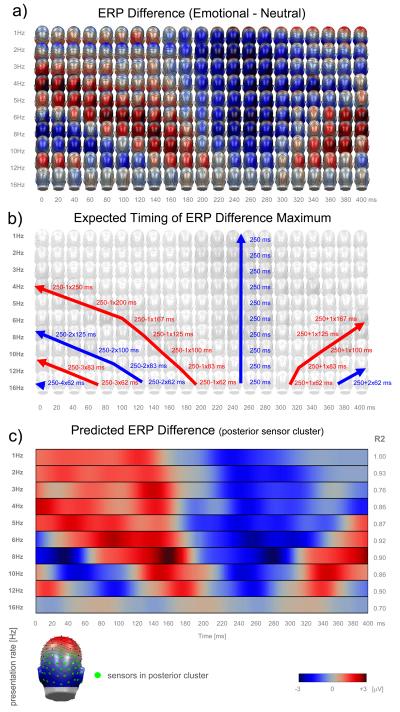
Figure 2.
Emotional modulation of the ERP at increasing presentation rates. Back view topographical maps (a) correspond to the ERP differences generated by the perception of pleasant and emotional arousing versus neutral pictures. The maximum difference effect appearing around 250ms is referred to as Early Posterior Negativity (EPN). Superimpositions of the EPN of preceding and subsequent stimulus pairs appear undisturbed at standard latencies relative to preceding and subsequent stimulus pairs (a, b). Expected latencies relative to preceding and subsequent stimulus pairs are illustrated schematically in (b), blue lines indicating regular EPNs (emotional minus neutral), red lines indicating “inverse” EPNs (neutral minus emotional; the occurrence of “inverse” EPNs is a consequence of the alternating presentation type). A colored representation of the predicted regional difference wave (posterior sensor cluster) based on convolution of the slowest 1Hz ERP effects is given in (c). Because only posterior sensors were in the analyzed cluster, the graph is very similar to the back view topographical maps in (a). Predicted ERP differences can explain most of the variance (R2) of measured ERP differences (c) at higher presentation rates, indicating little processing interference.
Figure 3.
Left side: Regional ERPs of the posterior sensor cluster at increasing presentation rates. Solid black lines correspond to the ERP differences generated by the perception of pleasant and emotional arousing versus neutral pictures. Right side: continuous F statistics of the factor EMOTION (pleasant vs. neutral) from the ANOVA of the regional ERPs of the posterior sensor cluster at increasing presentation rates.
Previous findings with presentation rates of 3 Hz (333 ms) and 5 Hz (200 ms) were replicated in the present study. The typical bilateral and right hemispheric dominant Early Posterior Negative (EPN) difference potential (ERP to emotional minus ERP to neutral pictures) was observed, arising around 150 ms with a maximum around 250 ms after stimulus onset (topographical plots in figure 2a and solid black lines in figure 3a). A new finding is that enhanced EPN amplitudes can not only be observed for the slower presentation rates of 2 Hz and 1 Hz but, more interestingly, also for the faster rates of 6 Hz, 8 Hz, 10 Hz, 12 Hz and, although distinctly smaller in amplitude, even at 16 Hz presentation rate.
EPN-based evidence of processing interference appeared minimal. Beginning with a picture presentation time of 250 ms (4 Hz) a similarly reliable but inversed EPN effect emerged, as the EPN of the preceding picture pair started to superimpose with the early visual ERP components of the subsequent stimulus pair. The respective difference potentials now appear as an inverse EPN with relative positive difference potentials (reddish colors in figure 2a) because it now reflects the subtraction of ERPs to neutral minus emotional pictures. With increasing presentation rates but a relatively constant EPN maximum latency of around 250 ms this superimposition would be expected to appear in subsequently later time windows (first left tilted red line in Fig. 2b). In fact, the strong correspondence of measured (Fig.2a) and predicted (Fig. 2b) latencies of the EPN maximum support the hypothesis of a practically constant EPN maximum latency independent of presentation rate.
With 4 Hz presentation rate, the maximum inverse EPN appeared around 0 ms (250 ms - 250 ms) (footnote 4), with 5 Hz around 50 ms (250 ms - 200 ms), with 6 Hz around 90 ms (250 ms - 167ms), with 8 Hz around 125 ms (250 ms – 125 ms), with 10 Hz around 150 ms (250 ms - 100ms), with 12 Hz around 170 ms (250 ms – 83 ms) and a residual inverse EPN is even visible around 190 ms (250 ms -62 ms) at 16 Hz presentation rate. Thus, these latencies were in line with the predictions made by linear superimposition. In the same vein an inverse EPN was observed for the subsequent picture pairs (again neutral minus emotional), with EPN peaks measured at the latencies at 375 ms (250 ms + 125 ms) in the 8 Hz, at 350 ms (250 ms + 100 ms) in the 10 Hz, around 330 ms (250 ms + 83 ms) in the 12 Hz and around 310 ms (250 ms + 62 ms) in the 16 Hz video (first right tilted red line in Fig. 2b).
With presentation rates of 8 Hz or faster (<= 125 ms per picture) and a still unchanged stable EPN latency of 250 ms, a further ‘regular EPN’ (emotional minus neutral) would be predicted belonging to the pair of pictures shown two onsets before (first left tilted blue line in Fig. 2b). In fact, this effect appears visible in increasingly later time windows with increasing presentation rates, at 0 ms (250 ms − 2 × 125 ms) in the 8 Hz, at 50 ms (250 ms − 2 × 100 ms) in the 10 Hz, around 85 ms (250 ms − 2 × 83 ms) in the 12 Hz, and around 125 ms (250 ms − 2 × 62 ms) in the 16 Hz video. Again, and consistently, a second regular EPN starts to appear at the end of the 400 ms interval (250 ms + 2 × 83 ms) in the 12 Hz video as well as around 375 ms (250 ms − 2 × 62 ms) in the 16 Hz video (Fig. 2a and right tilted blue line in Fig. 2b).
Pairs of pictures shown three onsets before were predicted to generate a further inverse EPN (neutral minus emotional) around 0 ms (250 ms − 3 × 83 ms) in the 12Hz and around 65 ms (250 ms − 3 × 62 ms) in the 16 Hz condition (second left tilted red line in Fig. 2b) and these maximum latencies are in fact visible in figure 2a.
Even pairs of pictures shown four onsets before, still generate an expected residual regular EPN around 0 ms (250 ms − 4 × 62 ms) in the fastest 16 Hz condition (second left tilted blue arrow in Fig. 2b).
The grand mean potentials across all subjects and all sensors in the posterior EPN sensor cluster defined above, for all presentation rates, are visualized in figure 3a. In the lower rate conditions (1 - 3 Hz) the typical middle latency visual evoked components such as C1, P1, N1 and P2 remain visible but the increase of presentation rates results in more and more temporal ‘blurring’ of the individual ERP components. In fact, time courses assimilate increasingly in direction to pure oscillations with frequencies driven by the presentation rate, while residual individual ERP components only appear as smaller waves with continuously decreasing amplitude ‘riding’ on the dominant oscillation.
In order to allow a rough estimation which part of this temporal ‘blurring’ could possibly be explained by convolution (linear superposition) of visual early and middle latency components, convolutions of the averaged difference potentials measured for the slowest 1 Hz stimulation rate were calculated for all faster rates. These convolved time courses (Fig. 2c) represent the difference ERPs which would have been measured, if the cortical visual processing would have been completely unaffected by increasing presentation rates. Differences between the synthetic convolved and the actually measured data will thus indicate modulation of cortical visual processing with presentation rate.
Predicted and synthetically generated (convoluted) fast rate ERP differences appear as strongly correlated with the actually measured ERP differences (comparison of Fig. 2a and 2c). As based on correlation analysis, the convolved time course could explain between 70 – 93% of the measured signal variance (R2; right row in figure 2c). Interestingly, convolution does not only predict main frequencies and main phase shifts of the measured signal but also the distinct breakdown of difference amplitude with the fastest 16 Hz presentation rate.
Results of the point wise ANOVA – time course of F-values - for the main effect of emotional arousal are shown in figure 3b. It can be seen that the measured difference potentials reach significance with an alpha level of 0.01 (dashed lines in Fig. 3b) at the time intervals of standard and inverse EPNs of preceding and subsequent pictures (red and blue tilted lines in Fig. 2b) as predicted by the hypothesis of linear superposition. However, although a clear alternation of regular and inverted EPNs would have been predicted (Fig. 2c) and remains visible in the grand mean with the fastest 16 Hz condition (lowest column in figure 2a and 3a) this effect could not reach significance (lowest row in figure 3b).
DISCUSSION
What are the temporal limits of affective discrimination when viewing rapid sequences of emotional scenes? Previous studies utilizing rapid presentations of emotional pictures at rates up to 5 Hz have shown that emotionally significant stimuli are discriminated from neutral pictures, with both pleasant and unpleasant pictures prompting increased occipital negativity in a 150 - 300 ms time window (e.g. Junghöfer et al., 2001; Schupp, et al., 2003). These results were replicated in the present study and were extended to rates beyond 5 Hz, which involve presentation of one or even several subsequent pictures before the EPN to the preceding picture has fully evolved. As a main result, we found that affective discrimination can be demonstrated in rapid serial visual presentation with exposure times as short as 83 ms (12 Hz). Although a neutral and a further emotional picture appeared on the screen before the EPN to the preceding emotional picture reached its maximum, we observed enlarged EPN amplitudes for emotionally arousing stimuli, with unchanged latencies around 250 ms . Thus, affectively arousing scenes were discriminated despite high perceptual demands invoked by fast presentation rates. With regard to temporal interference effects, these findings suggest that picture viewing in a very rapid stream does not exhaust processing resources to an amount that would preclude the differential processing of emotional compared to neutral pictures. Furthermore, these data are inconsistent with notions predicting strong interference effects exerted by neutral contents in the picture stream that would act to impair processing of emotional stimuli. Overall, these findings provide evidence for a high capacity of parallel processing and early discrimination in visual processing.
Importantly, the measured ERP activity at rates between 5 and 12 Hz could be predicted with reasonable accuracy according to a parallel processing model, which assumes the linear superposition of ERP activity associated with each picture presentation, irrespective of the proximity of overlapping, competing processes. Thus, these results indicate that pictures can differentially activate the successive steps of hierarchical cortical processing as a function of their emotional content, even when these different levels of processing are engaged in parallel, i.e. one level is processing stimulus ‘n’, while the other is still occupied with processing the preceding stimulus “n-1”. Given that “n” and “n-1” may have different levels of emotional arousal and valence, this result does not indicate a close exchange of emotionally driven amplification among the different levels of visual processing, neither would it be consistent with an overall reentrant amplification by the amygdala. Rather, the outcome argues for a rapid and specific intensification according to affective significance along the visual processing streams. These findings are consistent with behavioral (Öhman, 2001), neuroimaging (Bradley et al., 2003), and electrophysiological studies (Stolarova, Keil & Moratti, 2006; Pourtois, Thut, Grave de Peralta, Michel & Vuilleumier, 2005) suggesting that stimulus features associated with affective content may be processed in a prioritized manner. Experimental designs involving rapid sequences of stimuli have attracted attention as a means to study the electrocortical correlates of such prioritized processing because they allow researchers to (i) deliver many trials in a short amount of time and (ii) to manipulate the temporal distance between stimuli. In particular, studies capitalizing on the so-called Attentional Blink design have suggested that affective cues embedded in a temporal stream may attract attentional resources at the cost of competing neutral stimuli (Anderson, 2005). This process may act to protect affective cues against interference exerted by neutral stimuli (Keil & Ihssen, 2004) but also may increase interference exerted by non-attended, task-irrelevant, affective cues on competing neutral stimuli (Most et al., 2005).
In the present study, we focused on the protection of affective pictures against rapidly following neutral and affective stimuli and found that affective stimuli were associated with enhanced electrocortical activation even in situations where subsequent stimuli were increasingly close in time. When the timing of the electrocortical response was considered, processing did not accelerate with increasing presentation rates, as indicated by unchanged EPN latencies. The distinct breakdown of the EPN effects with a presentation rate of 16 Hz (62.5 ms) compared to all slower rates is predicted based on a linear model. With rates above 12 Hz, negative and positive EPN difference components of consecutive picture pairs (arousing-neutral-arousing-neutral…) strongly superpose, which eventually results in strong destructive interference. As described in the convolution example, this interference as a mere consequence of component convolution will increase with even higher presentation rates. Thus, with 16Hz or even faster presentation rates differences of expected and measured convolved signals fall below the resolution of ERPs in this study design. Accordingly, this study is not conclusive regarding temporal limits of affective discrimination when viewing rapid sequences of emotional scenes above 12 Hz.
As an important methodological consideration, the current study included only pleasant and neutral stimulus materials. Thus it would be informative to extend these findings to unpleasant picture contents. Furthermore, pleasant and neutral stimulus contents were presented in an alternating sequence. This procedure was chosen to maximize the EPN as correlate of affective modulation because a neutral picture preceding an emotional cue has comparatively less detrimental effect on the posterior negativity compared to an emotional cue preceding a neutral image (Flaisch et al., 2008). Future studies may extend these findings systematically varying picture valence of the preceding and subsequent images. Finally, the contribution of perceptual stimulus characteristics may need to be considered. When pictures greatly differ with regard to simple figure/ground on the one hand or complex scene characteristics on the other hand, differences in the ERP are observed between 150 and 300 ms (Bradley et al., 2007). Although pleasant and neutral stimulus materials did not differ with regard to known simple/complex characteristics and were furthermore matched according to physical stimulus variables such as brightness, contrast and JPEG complexity, ERP differences related to residual uncontrolled perceptual stimulus characteristics were not ruled out. With this respect, stimulus materials allowing to improve the control of perceptual stimulus characteristics appear informative, i.e., affective words, hand gestures or faces with different facial expressions (Kissler, Herbert, Peyk & Junghöfer, 2007; Schupp et al., 2004). Moreover, conditioning approaches are desirable because these procedures are capable of controlling the structural features of conditioned stimuli. Previous ERP studies have suggested that differential sensitivity to fear-conditioned visual stimuli can result from associative learning already, and can affect early stages of visual analysis (Pizzagalli, Greischar & Davidson, 2003; Stolarova et al., 2006). Systematically varying the features of to-be-conditioned stimuli may help to elucidate perceptual features signaling emotionality in more complex media such as affective pictures.
CONCLUSION
Rapid serial presentation of pictures has been suggested as a laboratory tool to probe demanding conditions in natural scene perception. To explore the parallel processing of affective stimuli, alternating sequences of pleasant and neutral pictures were presented at increasing presentation rates. Results show that up to presentation rates of 12 Hz, pleasant pictures were discriminated from neutral contents as revealed by the modulation of the EPN component. The present findings suggest that the emotion discrimination at the level of perceptual processing is a robust phenomenon, obtained under high demanding conditions including subsequent and even multiple stimulus masking and the need for parallel processing.
Footnotes
With complete electrode coverage of the volume conductor and average reference the overall evoked potentials have to sum up to zero. Thus, the strong posterior relative negative potentials of the EPN have to have a weaker but more widespread anterior positive counterpart.
EGI sensor numbers: 56, 58, 59, 60, 67, 68, 73, 78, 86, 85, 77, 72, 66, 57, 64, 65, 71, 76, 84, 91, 96, 90, 83, 75, 70, 63, 69, 74, 82, 89, 95, 97, 92, 100, 101 and 108.
With 4.5 Hz we chose an ERP frequency which is not a multiple of the trigger function, as such relations of g(t) and h(t) reveal a special oscillating behavior of the convolution function.
The values in parentheses refer to the latencies of preceding and subsequent EPNs. Given the general latency of the ‘standard’ (1 Hz) EPN at 250ms, subsequent latencies are given as shifts from 250 ms in multiples of the presentations durations. For instance, “250 ms – 2 × 125 ms” of the 8 Hz condition refers to the latency of 250ms minus twice the picture duration of the 8 Hz condition (8Hz <=> 125ms per picture).
References
- 1.Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology. 2005;134(2):258–81. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 2.Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117(2):369–80. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- 3.Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44(3):364–73. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 4.Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19(4):577–86. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- 5.Codispoti M, Ferrari V, Junghöfer M, Schupp HT. The categorization of natural scenes: brain attention networks revealed by dense sensor ERPs. Neuroimage. 2006;32(2):583–91. doi: 10.1016/j.neuroimage.2006.04.180. [DOI] [PubMed] [Google Scholar]
- 6.Delorme A, Thorpe SJ. Face identification using one spike per neuron: resistance to image degradations. Neural Networks. 2001;14(6-7):795–803. doi: 10.1016/s0893-6080(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 7.Flaisch T, Junghöfer M, Bradley MM, Schupp HT, Lang PJ. Rapid picture processing: Affective primes and targets. Psychophysiology. 2008;45(1):1–10. doi: 10.1111/j.1469-8986.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 8.Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans: a framework for defining “early” visual processing. Experimental Brain Research. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- 9.Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38:175–178. [PubMed] [Google Scholar]
- 10.Junghöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artefacts in dense array EEG/MEG studies. Psychophysiology. 2000;37(4):523–32. [PubMed] [Google Scholar]
- 11.Junghöfer M, Sabatinelli D, Bradley MM, Schupp HT, Elbert TR, Lang PJ. Fleeting images: Rapid affect discrimination in visual cortex. Neuroreport. 2006;17:225–229. doi: 10.1097/01.wnr.0000198437.59883.bb. [DOI] [PubMed] [Google Scholar]
- 12.Junghöfer M, Schupp HT, Stark R, Vaitl D. Neuroimaging of emotion: empirical effects of proportional global signal scaling in fMRI data analysis. Neuroimage. 2005;25(2):520–6. doi: 10.1016/j.neuroimage.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Keil A, Gruber T, Müller MM, Moratti S, Stolarova M, Bradley MM, Lang PJ. Early modulation of visual perception by emotional arousal: evidence from steady-state visual evoked brain potentials. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(3):195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- 14.Keil A, Ihssen N. Identification facilitation for emotionally arousing verbs during the attentional blink. Emotion. 2004;4(1):23–35. doi: 10.1037/1528-3542.4.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Keil A, Ihssen N, Heim S. Early cortical facilitation for emotionally arousing targets during the attentional blink. BMC Biology. 2006:4–23. doi: 10.1186/1741-7007-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keysers C, Perrett DI. Visual masking and RSVP reveal neural competition. Trends in cognitive sciences. 2002;6(3):120–125. doi: 10.1016/s1364-6613(00)01852-0. [DOI] [PubMed] [Google Scholar]
- 17.Keysers C, Xiao DK, Földiák P, Perrett DI. The speed of sight. Journal of Cognitive Neuroscience. 2001;13(1):90–101. doi: 10.1162/089892901564199. [DOI] [PubMed] [Google Scholar]
- 18.Kissler J, Herbert C, Peyk P, Junghöfer M. Buzzwords: early cortical responses to emotional words during reading. Psychological Science. 2007;18(6):475–80. doi: 10.1111/j.1467-9280.2007.01924.x. [DOI] [PubMed] [Google Scholar]
- 19.Lang PJ, Bradley MM, Cuthbert BN. Emotion and motivation: measuring affective perception. Journal of Clinical Neurophysiology. 1998;15(5):397–408. doi: 10.1097/00004691-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; Gainesville, FL: 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- 21.Moratti S, Keil A, Stolarova M. Motivated attention in emotional picture processing is reflected by activity modulation in cortical attention networks. Neuroimage. 2004;21(3):954–64. doi: 10.1016/j.neuroimage.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Most SB, Scholl BJ, Clifford ER, Simons DJ. What you see is what you set: sustained inattentional blindness and the capture of awareness. Psychological Review. 2005;112(1):217–42. doi: 10.1037/0033-295X.112.1.217. [DOI] [PubMed] [Google Scholar]
- 23.Müller MM, Hillyard S. Concurrent recording of steady-state and transient event-related potentials as indices of visual-spatial selective attention. Clinical Neurophysiology. 2000;111(9):1544–52. doi: 10.1016/s1388-2457(00)00371-0. [DOI] [PubMed] [Google Scholar]
- 24.Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psycholpgy. 2001;130(3):466–78. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- 25.Pizzagalli DA, Greischar LL, Davidson RJ. Spatio-temporal dynamics of brain mechanisms in aversive classical conditioning: high-density event-related potential and brain electrical tomography analyses. Neuropsychologia. 2003;41(2):184–94. doi: 10.1016/s0028-3932(02)00148-3. [DOI] [PubMed] [Google Scholar]
- 26.Potter MC. Very short-term conceptual memory. Memory & Cognition. 1993;21(2):156–61. doi: 10.3758/bf03202727. [DOI] [PubMed] [Google Scholar]
- 27.Pourtois G, Thut G, Grave de Peralta R, Michel C, Vuilleumier P. Two electrophysiological stages of spatial orienting towards fearful faces: early temporo-parietal activation preceding gain control in extrastriate visual cortex. Neuroimage. 2005;26(1):149–63. doi: 10.1016/j.neuroimage.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24(4):1265–70. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(2):257–61. [PubMed] [Google Scholar]
- 30.Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Anders, Ende, Junghöfer, Kissler, Wildgruber, editors. Emotion and Attention: Event-related brain potential studies; in “Understanding Emotions”. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- 31.Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14(8):1107–10. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- 32.Schupp HT, Öhman A, Junghöfer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4(2):189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- 33.Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Stimulus novelty and emotion perception: the near absence of habituation in the visual cortex. Neuroreport. 2006;17(4):365–9. doi: 10.1097/01.wnr.0000203355.88061.c6. [DOI] [PubMed] [Google Scholar]
- 34.Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Selective visual attention to emotion. Journal of Neuroscience. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: evidence for sensory plasticity in early affective perception. Cerebral Cortex. 2006;16(6):876–87. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381(6582):520–2. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- 37.Tucker DM, Derryberry D, Luu P, Phan KL. Anatomy and physiology of human emotion: Vertical integration of brainstem, limbic, and cortical systems. In: Borod JC, editor. The neuropsychology of emotion. Oxford University Press; New York: 2000. pp. 56–79. [Google Scholar]
- 38.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]