Abstract
A disparity remains between graft survival of renal allografts from deceased donors and from living donors. A better understanding of the molecular mechanisms that underlie this disparity may allow the development of targeted therapies to enhance graft survival. Here, we used microarrays to examine whole genome expression profiles using tissue from 53 human renal allograft protocol biopsies obtained both at implantation and after transplantation. The gene expression profiles of living-donor kidneys and pristine deceased-donor kidneys (normal histology, young age) were significantly different before reperfusion at implantation. Deceased-donor kidneys exhibited a significant increase in renal expression of complement genes; posttransplantation biopsies from well-functioning, nonrejecting kidneys, regardless of donor source, also demonstrated a significant increase in complement expression. Peritransplantation phenomena, such as donor death and possibly cold ischemia time, contributed to differences in complement pathway gene expression. In addition, complement gene expression at the time of implantation was associated with both early and late graft function. These data suggest that complement-modulating therapy may improve graft outcomes in renal transplantation.
The persisting graft survival difference between living- and deceased-donor kidneys for renal transplantation1,2 suggests that a significant potential to improve graft survival further lies in new approaches to overcome the deleterious effects of often inevitable longer cold ischemia times3 and brain death.4 Apart from further refining storage solutions and machine perfusion techniques, targeted therapy and modulation of specific pathways before organ retrieval, during cold storage or after implantation, could have beneficial effects and improve the outcome of deceased-donor kidney transplantation.
To identify these specific pathways underlying the difference between pristine (normal histology, young donor age) deceased- and living-donor kidneys, we undertook this microarray study comparing implantation and posttransplantation protocol biopsies without rejection, in both deceased- and living-donor recipients. Previous studies using gene expression microarray experiments on implantation biopsies have demonstrated expression profile differences between living- and marginal deceased-donor kidneys,5–7 have not always been validated in independent data sets,5,6 and did not always provide in-depth analyses of the pathways involved.5–7 In addition to a transcriptional analysis of pristine implantation biopsies, we assessed the impact of donor origin on the evolution of posttransplantation expression of differently expressed genes at implantation.
Results
Clinical Parameters
Clinical and histologic information for the 53 allograft biopsy samples included in this study is provided in Table 1 and Supplemental Figure 1 (28 implantation biopsies and 25 posttransplantation biopsies). Full data are available in Supplemental Table 1. All of these biopsies were from adult-sized kidneys, which were transplanted into pediatric recipients, and none of these kidneys exhibited delayed graft function. There were no differences between the deceased- and living-donor kidney demographics, except that the deceased donors were younger compared with the living donors, reflecting the allocation protocol preferably attributing kidneys from young deceased donors to young recipients. Histologic evaluation of the implantation biopsies, reviewed according to the revised Banff criteria,8 confirmed the pristine quality of the kidneys at implantation (Table 1). The selection of the samples was based on RNA quality and matching of the different groups for clinical and histologic parameters.
Table 1.
Clinical data and histologic appearance of the 53 renal allograft protocol biopsies included in this studya
| Parameter | Baseline Biopsy Training Set |
Baseline Biopsy Test Set 1 |
||||
|---|---|---|---|---|---|---|
| Living Donor | Deceased Donor | P | Living Donor | Deceased Donor | P | |
| n | 9 | 9 | 5 | 5 | ||
| Cold ischemia time (h) | 3.0 (0.5 to 9.0) | 9.1 (5.0 to 28) | 0.001 | 2.0 (1.0 to 3.0) | 8.0 (2.0 to 21.6) | 0.04 |
| Donor age (yr) | 40 (22 to 58) | 25 (19 to 31) | 0.009 | 36 (21 to 54) | 14 (14 to 21) | 0.01 |
| Donor gender (M/F) | 3/6 | 3/6 | 1.0 | 3/2 | 4/1 | 1.0 |
| Recipient age (yr) | 9.2 (1.1 to 21.0) | 17.0 (6.6 to 18.0) | 0.085 | 17.0 (2.4 to 19.0) | 18.0 (2.6 to 19.0) | 0.75 |
| Recipient gender (M/F) | 8/1 | 8/1 | 1.0 | 3/2 | 2/3 | 1.0 |
| GFR at 3 mo (ml/min per 1.73 m2)d | 105 (62 to 146) | 92 (59 to 199) | 0.57 | 96 (79 to 124) | 84 (72 to 157) | 1.0 |
| Tubulitis scoreb | 0 (0 to 0) | 0 (0 to 0) | 1.0 | 0 (0 to 0) | 0 (0 to 0) | 1.0 |
| Interstitial inflammation scoreb | 0 (0 to 0) | 0 (0 to 0) | 1.0 | 0 (0 to 0) | 0 (0 to 0) | 1.0 |
| Vascular intimal thickening scoreb | 0 (0 to 1) | 0 (0 to 1) | 1.0 | 0 (0 to 1) | 0 (0 to 0) | 0.32 |
| Interstitial fibrosis scoreb | 0 (0 to 0) | 0 (0 to 0) | 1.0 | 0 (0 to 0) | 0 (0 to 0) | 1.0 |
| Tubular atrophy scoreb | 0 (0 to 1) | 0 (0 to 1) | 1.0 | 0 (0 to 1) | 0 (0 to 0) | 0.13 |
| Arteriolar hyalinosis scoreb | 0 (0 to 1) | 0 (0 to 0) | 0.32 | 0 (0 to 0) | 0 (0 to 0) | 1.0 |
| Global glomerulosclerosis score | 1 (0 to 1) | 0 (0 to 1) | 0.004 | 0 (0 to 1) | 0 (0 to 1) | 1.0 |
| Parameter | Posttransplantation Biopsy Test Set 2 |
Baseline, Posttransplantation Paired Set |
||||
|---|---|---|---|---|---|---|
| Living Donor | Deceased Donor | P | Baseline | Posttransplantation | P | |
| n | 9 | 9 | 8 | 8c | ||
| Cold ischemia time (h) | 4.0 (0.5 to 5.0) | 9.0 (4.0 to 14.0) | 0.001 | 3.0 (0.5 to 4.0) | ||
| Donor age (yr) | 24 (21 to 42) | 21 (14 to 31) | 0.046 | 37 (22 to 53) | ||
| Donor gender (M/F) | 7/2 | 7/2 | 1.0 | 3/5 | ||
| Recipient age (yr) | 11.0 (1.6 to 21.0) | 18.0 (2.3 to 19.0) | 0.31 | 8.9 (1.1 to 17.0) | ||
| Recipient gender (M/F) | 7/2 | 6/3 | 1.0 | 7/1 | ||
| GFR at 3 mo (ml/min per 1.73 m2)d | 105 (62 to 132) | 100 (65 to 159) | 0.57 | 114 (71 to 146) | ||
| Tubulitis scoreb | 0 (0 to 0) | 0 (0 to 0) | 1.0 | 0 (0 to 0) | 0 (0 to 0) | 1.0 |
| Interstitial inflammation scoreb | 0 (0 to 0) | 0 (0 to 0) | 1.0 | 0 (0 to 0) | 0 (0 to 0) | 1.0 |
| Vascular intimal thickening scoreb | 0 (0 to 2) | 0 (0 to 2) | 0.57 | 0 (0 to 1) | 0 (0 to 2) | 0.25 |
| Interstitial fibrosis scoreb | 1 (0 to 1) | 0 (0 to 2) | 0.46 | 0 (0 to 0) | 0 (0 to 1) | 0.50 |
| Tubular atrophy scoreb | 1 (0 to 1) | 1 (0 to 2) | 0.84 | 0 (0 to 1) | 1 (0 to 1) | 0.22 |
| Arteriolar hyalinosis scoreb | 0 (0 to 1) | 0 (0 to 1) | 1.0 | 0 (0 to 1) | 0 (0 to 0) | 1.0 |
| Global glomerulosclerosis score | 0 (0 to 1) | 0 (0 to 1) | 1.0 | 1 (0 to 1) | 0 (0 to 1) | 0.45 |
aData are median (range).
bHistologic score according to the Banff classification.8
cOne sample of the posttransplantation paired biopsy set was already included in the posttransplantation biopsy test set 2.
dSchwartz GFR at time of biopsy assessment. P values were calculated using Fisher exact test, Wilcoxon-Mann-Whitney U nonparametric ANOVA, or the signed rank test for paired data.
Unsupervised Hierarchical Clustering
Whole-genome expression data (54,000 probe sets encompassing 47,000 gene transcripts) were obtained by hybridizing each of the 53 samples onto an Affymetrix HG U133 Plus 2.0 Array. In a first orienting approach, the relatedness of genome-wide gene expression profiles of 18 implantation biopsies of the training set was assessed by unsupervised hierarchical clustering,9 using all gene probe sets with at least a three-fold difference from the median expression in at least five different samples. The samples clustered mainly according to donor gender and then according to donor origin (deceased versus living; Supplemental Figure 2). After exclusion of probe sets significantly associated with donor gender (selected with Significance Analysis of Microarrays [SAM]10 comparing female with male implantation biopsies, at a false discovery rate (FDR) <15%; n = 140 probe sets that were excluded), the samples segregated in two clearly distinct groups according to donor origin (Supplemental Figure 3). These results showed that donor origin (living versus deceased donor) was indeed an important determinant of the gene expression profile of biopsies obtained at implantation.
Gene Expression Differences between Deceased- and Living-Donor Kidney Implantation Biopsies
To assess which genes are differently expressed between living- and deceased-donor kidneys at implantation before reperfusion, we used supervised two-class unpaired SAM analysis,10 comparing gene expression of nine deceased- with that of nine living-donor kidney biopsies from the training set of 18 implantation biopsies. In total, 932 probe sets were significantly differently expressed with q <5% (Supplemental Table 2). A total of 479 probe sets (representing 319 unique identified genes) were higher expressed in deceased- compared with living-donor kidney biopsies at implantation, and 453 probe sets (representing 329 unique identified genes) were downregulated in deceased-donor kidney biopsies.
Furthermore, we validated these differences in expression profiles between deceased- and living-donor kidney implantation biopsies. Two-class Prediction Analysis of Microarrays11 on 54,000 probe sets was performed with the 18 training sets (the same biopsies as in the supervised two-class unpaired SAM analysis) and 10 other implantation biopsies (test set 1). As few as five gene probe sets could correctly classify the 10 test set biopsies with 100% sensitivity and 100% specificity. Supplemental Figure 4 shows the excellent prediction performance of a selected gene set (34 probe sets). This demonstrates that the gene expression differences between living- and deceased-donor kidney implantation biopsies are reproducible in an independent patient cohort.
Molecular Function and Pathway Analysis
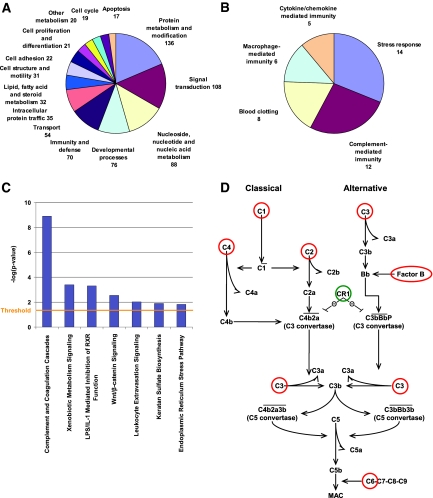
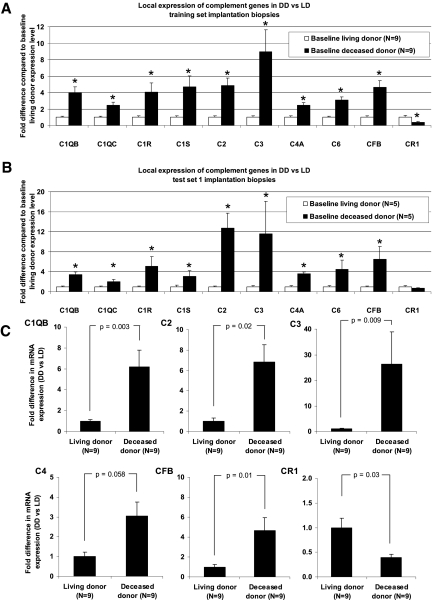
The distribution of the molecular functions of the genes differently expressed between living- and deceased-donor implantation kidneys (n = 932 probe sets), assessed using the PANTHER classification system,12 is shown in Figure 1, A and B. Canonical (generally accepted) pathways that are significantly overrepresented (assessed using the Ingenuity pathway program) in this gene set are presented in Figure 1C and Table 2. Most impressive, the complement system was significantly overenriched in the local upregulation of genes (P = 1.29 × 10−9), with significant increases in C1Q, C1R, C1S, C2, C3, C4, C6, and CFB in the deceased-donor kidneys at baseline. CR1 (CD35), which is a complement activation regulator, was expressed less in deceased-donor kidneys. Both molecules involved in the classical (C1Q, C1R, C1S, C2, C3, and C4) and alternative pathways (C3 and CFB) of complement and of the membrane attack complex (C6) were expressed more in deceased- versus living-donor kidneys (Figures 1D and 2A). Both test set 1 microarray results (Figure 2B) and quantitative reverse transcriptase–PCR (qRT-PCR) analysis (Figure 2C) confirmed the significant local overexpression of the complement cascade genes data for C1QB, C2, C3, C4, and CFB in the deceased-donor kidneys, with the most important difference in local C3 expression. Detailed information on other genes differently expressed between living- and deceased-donor kidneys at implantation is given in Table 2 and in Supplemental Table 2.
Figure 1.
(A) Gene ontology analysis of the biologic processes of the genes differently expressed between living- and deceased-donor implantation kidneys. Of the 932 significant probe sets, 648 mapped to different genes, which were assigned to 850 biologic processes. Genes for which no biologic process could be assigned were omitted from this display; only categories with more than 15 assigned genes are shown. The numbers represent the number of genes associated with a biologic process. Gene ontology analysis was done with the PANTHER program. (B) Subdivisions of the category immunity and defense responses in A. The 70 genes in this category in A were assigned with more detailed biologic processes. Categories with more than four assigned genes are shown. (C) Canonical pathways significantly overrepresented in this gene set consisting of 932 probe sets (648 unique identified genes). Significance values refer to the −log [P value], which is obtained by the Ingenuity pathway program, which is based on the Ingenuity Pathways Knowledge Base. (D) Significant overrepresentation of complement genes of both the classical and the alternative complement pathway in the difference between living- and deceased-donor kidneys at implantation. Complement genes expressed significantly higher in deceased- compared with living-donor kidneys are indicated in red. Complement activation inhibitor CR1 is expressed significantly lower in deceased-donor kidneys at implantation and is indicated in green.
Table 2.
Canonical pathways significantly overrepresented in the 932 probe sets differently expressed between living- and deceased-donor kidney biopsies at time of implantationa
| Pathway | Name | Description | UniGene ID | Fold Change | q (%) |
|---|---|---|---|---|---|
| Complement and coagulation cascades | |||||
| (P = 1.29 × 10−9) | A2M | α-2-macroglobulin | hs0.212838 | 1.87 | 3.22 |
| BDKRB1 | Bradykinin receptor B1 | hs0.525572 | −1.95 | 2.66 | |
| C2 | Complement component 2 | hs0.408903 | 5.01 | 0.64 | |
| C3 | Complement component 3 | hs0.529053 | 8.96 | 0.00 | |
| C6 | Complement component 6 | hs0.481992 | 3.11 | 0.00 | |
| C1QB | Complement component 1, q subcomponent, B chain | Hs0.8986 | 3.98 | 0.00 | |
| C1QC | Complement component 1, q subcomponent, C chain | hs0.467753 | 2.41 | 1.51 | |
| C1R | Complement component 1, r subcomponent | hs0.524224 | 3.98 | 0.00 | |
| C1S | Complement component 1, s subcomponent | hs0.458355 | 4.56 | 0.00 | |
| C4A | Complement component 4A (Rodgers blood group) | hs0.656152 | 2.49 | 1.96 | |
| CFB | Complement factor B | Hs0.69771 | 4.67 | 0.00 | |
| CR1 | Complement component (3b/4b) receptor 1 (Knops blood group) | hs0.334019 | −2.48 | 3.22 | |
| F13B | Coagulation factor XIII, B polypeptide | hs0.435782 | −2.14 | 4.08 | |
| KLKB1 | Kallikrein B, plasma (Fletcher factor) 1 | hs0.237642 | −2.45 | 4.08 | |
| SERPINA1 | Serpin peptidase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 1 | hs0.525557 | 2.45 | 1.51 | |
| Xenobiotic metabolism signaling | |||||
| (P = 4.07 × 10−4) | ABCC3 | ATP-binding cassette, sub-family C (CFTR/MRP), member 3 | hs0.463421 | 2.04 | 3.57 |
| ALDH1L2 | Aldehyde dehydrogenase 1 family, member L2 | Hs0.42572 | 2.41 | 1.96 | |
| CYP3A4 | Cytochrome P450, family 3, subfamily A, polypeptide 4 | hs0.654391 | −2.46 | 0.88 | |
| CYP3A7 | Cytochrome P450, family 3, subfamily A, polypeptide 7 | hs0.111944 | −5.05 | 0.00 | |
| FMO4 | Flavin containing monooxygenase 4 | hs0.386502 | −1.99 | 2.66 | |
| FMO5 | Flavin containing monooxygenase 5 | hs0.642706 | −1.86 | 4.08 | |
| GSTA3 | Glutathione S-transferase A3 | hs0.102484 | −2.84 | 1.04 | |
| MAOA | Monoamine oxidase A | hs0.183109 | 2.08 | 3.57 | |
| MAP2K3 | Mitogen-activated protein kinase kinase 3 | hs0.514012 | −2.77 | 1.04 | |
| NCOA1 | Nuclear receptor coactivator 1 | hs0.658005 | −1.72 | 4.08 | |
| PIK3R1 | Phosphoinositide-3-kinase, regulatory subunit 1 (p85 α) | hs0.132225 | 1.89 | 4.73 | |
| PPP2CB | Protein phosphatase 2 (formerly 2A), catalytic subunit, β isoform | hs0.491440 | −1.65 | 4.08 | |
| PPP2R1B | Protein phosphatase 2 (formerly 2A), regulatory subunit A, β isoform | hs0.584790 | −2.16 | 2.37 | |
| PPP2R2C | Protein phosphatase 2 (formerly 2A), regulatory subunit B, gamma isoform | hs0.479069 | −3.40 | 1.14 | |
| SULT1C2 | Sulfotransferase family, cytosolic, 1C, member 2 | hs0.436123 | 1.78 | 3.57 | |
| SULT1E1 | Sulfotransferase family 1E, estrogen-preferring, member 1 | hs0.479898 | 2.31 | 4.08 | |
| UGT1A6 | UDP glucuronosyltransferase 1 family, polypeptide A6 | hs0.654499 | 3.10 | 3.57 | |
| LPS/IL-1 mediated inhibition of RXR function | |||||
| (P = 4.90 × 10−4) | ABCC3 | ATP-binding cassette, sub-family C (CFTR/MRP), member 3 | hs0.463421 | 2.04 | 3.57 |
| ALDH1L2 | Aldehyde dehydrogenase 1 family, member L2 | Hs0.42572 | 2.41 | 1.96 | |
| CYP3A4 | Cytochrome P450, family 3, subfamily A, polypeptide 4 | hs0.654391 | −2.46 | 0.88 | |
| CYP3A7 | Cytochrome P450, family 3, subfamily A, polypeptide 7 | hs0.111944 | −5.05 | 0.00 | |
| FMO4 | Flavin containing monooxygenase 4 | hs0.386502 | −1.99 | 2.66 | |
| FMO5 | Flavin containing monooxygenase 5 | hs0.642706 | −1.86 | 4.08 | |
| GSTA3 | Glutathione S-transferase A3 | hs0.102484 | −2.84 | 1.04 | |
| LBP | LPS-binding protein | hs0.154078 | 2.86 | 4.73 | |
| MAOA | Monoamine oxidase A | hs0.183109 | 2.08 | 3.57 | |
| PLTP | Phospholipid transfer protein | hs0.439312 | 4.43 | 0.00 | |
| PPARA | Peroxisome proliferator–activated receptor α | hs0.103110 | 1.79 | 3.22 | |
| RARA | Retinoic acid receptor α | hs0.654583 | −1.91 | 3.57 | |
| SULT1C2 | Sulfotransferase family, cytosolic, 1C, member 2 | hs0.436123 | 1.78 | 3.57 | |
| SULT1E1 | Sulfotransferase family 1E, estrogen-preferring, member 1 | hs0.479898 | 2.31 | 4.08 | |
| Wnt/b-catenin signaling | |||||
| (P = 2.82 × 10−3) | CD44 | CD44 molecule (Indian blood group) | hs0.502328 | −3.65 | 0.00 |
| DKK4 | Dickkopf homolog 4 (Xenopus laevis) | hs0.159311 | −2.67 | 1.04 | |
| FZD1 | Frizzled homolog 1 (Drosophila) | Hs0.94234 | 1.82 | 1.96 | |
| FZD6 | Frizzled homolog 6 (Drosophila) | hs0.591863 | 2.06 | 2.66 | |
| MAP4K1 | Mitogen-activated protein kinase kinase kinase kinase 1 | Hs0.95424 | −1.92 | 4.08 | |
| PPP2CB | Protein phosphatase 2 (formerly 2A), catalytic subunit, β isoform | hs0.491440 | −1.65 | 4.08 | |
| PPP2R1B | Protein phosphatase 2 (formerly 2A), regulatory subunit A, β isoform | hs0.584790 | −2.16 | 2.37 | |
| PPP2R2C | Protein phosphatase 2 (formerly 2A), regulatory subunit B, gamma isoform | hs0.479069 | −3.40 | 1.14 | |
| RARA | Retinoic acid receptor α | hs0.654583 | −1.91 | 3.57 | |
| SOX1 | SRY (sex determining region Y)-box 1 | hs0.202526 | −2.51 | 0.88 | |
| SOX4 | SRY (sex determining region Y)-box 4 | hs0.643910 | −2.81 | 2.66 | |
| WIF1 | WNT inhibitory factor 1 | hs0.284122 | −2.15 | 2.66 | |
| Leukocyte extravasation signaling | |||||
| (P = 9.33 × 10−3) | ACTA2 | Actin, α 2, smooth muscle, aorta (α-SMA) | hs0.500483 | 2.40 | 3.57 |
| CD44 | CD44 molecule (Indian blood group) | hs0.502328 | −3.65 | 0.00 | |
| CLDN11 | Claudin 11 (oligodendrocyte transmembrane protein) | Hs0.31595 | −2.78 | 0.88 | |
| CYBB | Cytochrome b-245, β polypeptide (chronic granulomatous disease) | hs0.292356 | 3.28 | 0.00 | |
| ITGB2 | Integrin, β 2 (complement component 3 receptor 3 and 4 subunit) | hs0.375957 | 2.31 | 2.66 | |
| MMP24 | Matrix metallopeptidase 24 (membrane-inserted) | hs0.567417 | −2.79 | 0.88 | |
| PIK3R1 | Phosphoinositide-3-kinase, regulatory subunit 1 (p85 α) | hs0.132225 | 1.89 | 4.73 | |
| PTK2 | PTK2 protein tyrosine kinase 2 | hs0.395482 | 1.65 | 4.08 | |
| RAP1GAP | RAP1 GTPase activating protein | hs0.148178 | 2.21 | 1.51 | |
| THY1 | Thy-1 cell surface antigen | hs0.653181 | −2.93 | 0.88 | |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | hs0.644633 | 3.73 | 0.64 | |
| VIL2 | Villin 2 (ezrin) | hs0.660646 | −1.78 | 4.73 | |
| Keratan sulfate biosynthesis | |||||
| (P = 1.29 × 10−2) | B3GNT1 | UDP-GlcNAc:βGal β-1,3-N-acetylglucosaminyltransferase 1 | Hs0.8526 | −1.80 | 4.08 |
| FUT8 | Fucosyltransferase 8 (α (1,6) fucosyltransferase) | hs0.654961 | 2.42 | 3.22 | |
| SULT1C2 | Sulfotransferase family, cytosolic, 1C, member 2 | hs0.436123 | 1.78 | 3.57 | |
| SULT1E1 | Sulfotransferase family 1E, estrogen-preferring, member 1 | hs0.479898 | 2.31 | 4.08 | |
| Endoplasmic reticulum stress pathway | |||||
| (P = 1.51 × 10−2)b | ATF6 | Activating transcription factor 6 | hs0.663951 | −1.93 | 2.66 |
| ERN1 | Endoplasmic reticulum to nucleus signalling 1 (IRE1) | hs0.133982 | −2.94 | 0.00 | |
| HSPA5 | Heat shock 70-kD protein 5 (glucose-regulated protein, 78 kD; Grp78; BiP) | hs0.605502 | 2.33 | 0.64 | |
| HSP90B1b | Heat-shock protein 90-kD β (Grp94), member 1 | hs0.192374 | 1.69 | 4.08 | |
| HYOU1b | Hypoxia upregulated 1 (ORP150; Grp170) | hs. 277704 | 2.17 | 3.57 | |
| PON2b | Paraoxonase 2 | hs0.530077 | 2.06 | 3.22 |
aCanonical pathways and P values were obtained using Ingenuity pathway program (Ingenuity Systems, Redwood City, CA) based on the Ingenuity Pathways Knowledge Base.
bGenes that were present in the 932 probe set list but not included in the calculation of the P values.
Figure 2.
(A) Complement gene expression in microarrays on the training set biopsies from deceased-donor baseline biopsies (n = 9), relative to gene expression in living-donor baseline biopsies (n = 9). P values were obtained using Wilcoxon-Mann-Whitney U nonparametric ANOVA. *P < 0.05. (B) Complement gene expression in microarrays on the test set 1 biopsies from deceased-donor baseline biopsies (n = 5), relative to gene expression in living-donor baseline biopsies (n = 5). P values were obtained using Wilcoxon-Mann-Whitney U nonparametric ANOVA. *P < 0.05. (C) qRT-PCR validation of the microarray results for different complement cascade genes in the training set (n = 9 living-donor [LD] and 9 diseased-donor [DD] samples). P values were obtained using Wilcoxon-Mann-Whitney U nonparametric ANOVA.
Gene Expression Evolution after Transplantation
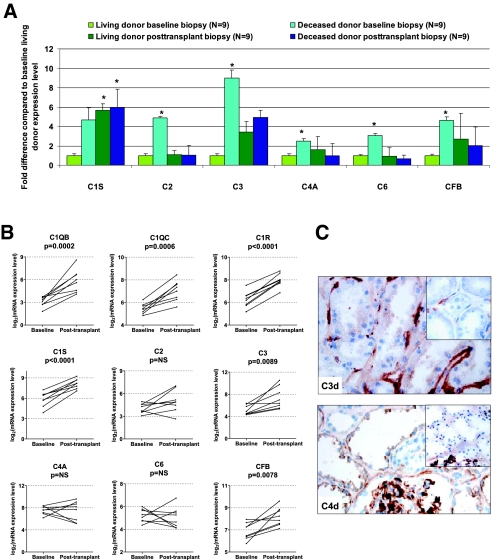
Posttransplantation biopsies were obtained from 3 up to 24 mo after transplantation (Supplemental Table 1). No global expression differences were noted between posttransplantation samples from living- and deceased-donor kidneys (n = 9 living and 9 deceased-donor posttransplantation kidney biopsies in test set 2) by SAM analysis providing stringent FDRs with q <5%. In a detailed analysis of the posttransplantation evolution of the complement genes differently expressed between living- and deceased-donor implantation biopsies (n = 18 of the training set), a significant (q <5%) increase in the expression of specific complement genes (C1 and C3) was noted in the posttransplantation living-donor kidney biopsies (n = 9) compared with the implantation biopsies from living donors (n = 9) using SAM analysis, whereas the posttransplantation expression levels of C1 and C3 genes were not different from pretransplantation levels in deceased donors (n = 9 posttransplantation versus n = 9 implantation biopsies). The other complement genes (C2, C4, and C6) had comparable expression levels posttransplantation compared with living-donor pretransplantation levels (Figure 3A).
Figure 3.
(A) Evolution of complement gene expression in implantation and posttransplantation biopsies according to donor source, expressed as fold difference from the expression level in implantation biopsies from living-donor kidneys. *ANOVA P value compared with baseline living-donor biopsy expression <0.05, after Bonferroni correction. (B) Paired evolution of complement gene expression in implantation and posttransplantation biopsies of living-donor kidneys (n = 8, obtained from 3 up to 24 mo after transplantation). P values were obtained using paired t test. This figure demonstrates that enhanced local complement gene expression in renal allografts not only is an immediate peritransplantation phenomenon but also is observed in well-functioning, nonrejecting kidneys from living donors. (C, top) C3d positivity apically in the tubular epithelium. Absence of C3d staining apically in the tubular epithelium is shown in the inset. Positivity of the tubular basement membrane is used as internal control. (Bottom) C4d positivity in the cytoplasm of the tubular epithelium. Absence of C4d staining in the cytoplasm of the tubular epithelium is shown in the inset. Mesangial positivity is used as internal control. Magnification, ×400.
These unpaired results were then cross-validated in a last data set of paired implantation and posttransplantation samples (baseline–posttransplantation paired set). In paired SAM analysis comparing the global gene expression differences between implantation (n = 8) and posttransplantation rejection-free biopsies (n = 8) of living-donor kidneys, again, C1 components (q = 0%), C3 (q = 3.3%), and CFB (q = 5.4%) genes were expressed significantly higher in the posttransplantation samples compared with at time of engraftment, together with a significantly lower expression of CR1 in posttransplantation samples (q = 4.8%). The other complement genes (C2, C4, and C6) remained at baseline levels in the posttransplantation biopsies (Supplemental Table 3 and Figure 3B). In addition, using the Ingenuity pathway program, we observed that other genes that are associated with inflammation were significantly overrepresented in this gene set list (e.g., HLA-DQ, HLA-DR, ILs and IL receptors, chemokines, β-2 integrin, lactotransferrin (LTF), decay-accelerating factor for complement [CD55] and many others; P = 1.78 × 10−15), as well as genes associated with fibrosis (e.g., collagens 1A1/1A2/3A1/4A2/5A1/6A3, elastin, fibrillin, intercellular adhesion molecule 1, IGFB1, IGFB5, matrix metalloproteinase 1 [MMP1], MMP14, MMP15, MMP19, tissue inhibitor of metalloproteinase 1 and 3, vascular endothelial growth factor A, PDGFA, PDGFB; P = 1.12 × 10−9).
Immunohistochemistry
Immunohistochemical analysis of implantation biopsies was performed to assess whether increased local complement gene expression associates with local complement activation (deposition of complement C3 and C4 split products C3d and C4d). Donor demographics and results for the biopsies used for immunohistochemistry are given in Supplemental Table 4. In both living- and deceased-donor kidneys, there was variability in the deposition of C3d in the tubular epithelial cells (Figure 3C), but no association was seen between C3d deposition in any of the renal compartments and donor source or cold ischemia times. With regard to C4d deposition, there was no tubular epithelial deposition in any living-donor engraftment biopsy (zero of four), but C4d deposition in the tubular epithelial cells was present in 50% (three of six) of deceased-donor kidney biopsies (Figure 3C); all C4d-positive biopsies were seen in association with death from cerebrovascular hemorrhage.
Cold Ischemia Time versus Brain Death
To elucidate the relative impact of cold ischemia time versus brain death on local renal complement gene expression, we performed correlation analysis between cold ischemia time and complement gene expression in the subgroup of implantation biopsies from deceased donors (n = 14). There was a significant correlation between longer cold ischemia time and higher expression of complement gene expression of C1QB (P = 0.003), C1QC (P = 0.01), C1R (P = 0.004), C1RL (P = 0.02), C1S (0.003), and C3 (P = 0.02) and a trend for CFB (P = 0.08) in the microarray results of the 14 deceased-donor baseline biopsies, whereas the expression levels of the deceased-donor kidneys with the shortest cold ischemia times (<10 h) were still higher compared with the expression in kidneys from living-donor kidneys (Supplemental Figure 5). This suggests that the longer cold ischemia time of deceased-donor kidneys is contributing to the overexpression of complement genes in these kidneys, together with the effects of the deceased state of the donor in itself.
Donor Age and Baseline Gene Expression Analyses
Because donors in the deceased group were significantly younger than living donors, it cannot be excluded that the difference in donor age between living- and deceased-donor groups affected the results of this study. It should be noted, however, that the overall donor age range was relatively small, and no pediatric or geriatric donors were used in this study (mean 32.1 ± 11.2 yr; range 19 to 57.7 yr in the training set of 18 implantation biopsies). In a quantitative SAM analysis using donor age as a continuous variable in these 18 training set implantation biopsies, 270 of the 54,000 probe sets were significantly associated with donor age using an FDR cutoff of <5%, all lower expressed with higher donor age. Twenty-six probe sets overlapped with the differentially expressed gene set, as expected lower expressed in living-donor kidney biopsies, because the donors of these kidneys were older (Supplemental Table 5). In gene set enrichment analysis with another previously published gene set associated with kidney age,13 there was no enrichment of the difference between deceased and living donors, adding to the robustness of our findings.
Association with Graft Function
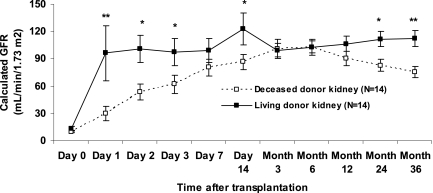
Transplanted kidneys in this study did not have any delayed graft function (defined as need for dialysis in the first posttransplantation week), which allowed investigation only of transcriptional profiles in pristine deceased-donor kidneys. This is also more representative of the donor quality and transplant outcomes in pediatric recipients in our program, as was recently described in detail.14 Despite immediate graft function in all cases, there was a significant difference in the evolution of early (first 14 d after transplantation) graft function between living-donor (n = 14) and deceased-donor (n = 14) kidneys (Figure 4), with a slower onset of graft function in the deceased-donor kidneys. Complement gene expression (C1QB, C1QC, C1R, C1RL, C1S, C2, C3, C4A, C6, and CFB) of the biopsies obtained at implantation correlated significantly with early graft function, most important at days 2 and 3 after transplantation, and additionally with late graft function at 2 and 3 yr after transplantation (Supplemental Table 6).
Figure 4.
Graft function evolution in living-donor (n = 14) and deceased-donor (n = 14) kidneys included in this study. *Wilcoxon-Mann-Whitney U P < 0.05; **Wilcoxon-Mann-Whitney U P < 0.01. Overall mixed model repeated measures P value for Schwartz GFR according to donor origin, P = 0.04. Data are means ± SEM.
Discussion
This study demonstrates that mRNA expression profiles are highly significantly different between living- and pristine deceased-donor kidney biopsies at implantation before reperfusion, with a marked overrepresentation of locally expressed complement genes, even in the complete absence of delayed graft function. Interestingly, the increased local renal overexpression of complement genes is not restricted to deceased-donor kidneys at time of transplantation. For the first time, we demonstrate that there is also a significant increase in local renal expression of complement components C1, C3, and CFB, other immune genes, and genes involved in tissue fibrosis in posttransplantation biopsies from well-functioning, nonrejecting kidneys, compared with engraftment biopsies, irrespective of donor source. The gene expression differences between deceased- and living-donor kidneys are not related to the intrinsic histologic quality of the grafts but relate to immediate peritransplantation phenomena such as differences in the length of cold ischemia time and donor death. This local complement gene expression in the implantation biopsies correlates significantly with early and, most important, late renal allograft function.
In this study, the data were obtained from a well-matched set of biopsies of pristine quality, were validated in a completely independent data set, and were cross-validated using qRT-PCR. These extensive validation studies add to the robustness of our findings, together with extensive literature on the role of local complement gene expression obtained in animal models.
Indeed, the consistent regulation of local renal expression of complement genes in human kidneys observed in this study is in accordance with previous animal studies, demonstrating an important role of intrinsic renal synthesis of complement genes.15 Also in a human setting, the importance of the local renal complement gene expression in transplanted kidneys was previously suggested.16,17 The latter study, earlier studies,18 and an in vitro study19 demonstrated that the renal tubular epithelial cells are the major source of local C3 and also C2, C4, and CFB gene expression in disease conditions, with local translation into protein and intracellular localization of the complement proteins. In addition, when previous microarray studies on the difference between deceased- and living-donor kidneys are closely reviewed, it seems that also in these studies, local renal complement genes were differently expressed,5,6 which adds to the robustness and independent reproducibility of this study.
It is not possible to distinguish clearly the effects of prolonged ischemia time from the direct effects of brain death in a heart-beating human deceased donor; however, the increased expression of complement genes with increasing cold ischemia times in this study and the finding that the expression levels of the deceased-donor kidneys with the shortest cold ischemia times were still higher compared with the expression in kidneys from living-donor kidneys suggest that both the length of cold ischemia and brain death individually contribute to the baseline injury response in deceased-donor kidneys in humans. This direct relationship between local complement factor expression at time of transplantation and cold ischemia duration was previously observed in mouse renal isografts.20 We could not confirm, however, the association between cold ischemia time and tubular C3d deposition, a marker of local tubular complement pathway activation, as was described in a previous animal study.20 The deposition of C3d and C4d in renal tubules was described previously under various conditions21,22 and could be an important pathogenic mechanism in tubulointerstitial damage.23 That we did not observe a clear association of C3d deposition and cold ischemia time in this study could be related to differences in the timing of the histologic sampling of the engraftment biopsy, because all biopsies were obtained before reperfusion in our study. This is supported by animal studies that, although showing that upregulation of complement gene expression is related to the duration of cold ischemia time, found that altered complement gene expression associated with cold ischemia does not occur until after the donor kidney is reconnected to the recipient circulation.20 From this, it could be hypothesized that brain injury and other contributors to donor death are the more likely cause of enhanced complement gene expression in prereperfusion biopsies in our study, rather than longer cold ischemia times.
In addition, we found only tubular epithelial deposition of C3d and C4d in implantation biopsies. It should be noted that tubular C3d or C4d staining was not related to deposition of these complement split products at the peritubular capillaries, which is regarded as a marker of antibody-mediated rejection after kidney transplantation.24 In implantation biopsies, C4d deposition at peritubular capillaries has been described secondary to disease conditions in the donor.25 Although several 5,17–19,21–23 studies suggested that complement is mainly expressed in tubular epithelial cells, it is not possible to determine which renal compartment is responsible for local complement gene expression in this study, because the gene expression studies were performed on whole-core biopsies. Further study is therefore necessary to elucidate the timing and localization of complement cascade expression and activation in human kidneys.
What could be the consequences of the local overexpression of complement genes in implantation biopsies of deceased donors? Deficiency of complement components, of complement activators, or of complement receptors, and anti-complement treatment in experimental animals protect against ischemia-reperfusion injury, whereas deficiency of complement control proteins exacerbates ischemia-reperfusion injury of kidneys.15,20,26 Furthermore, there is extensive evidence that the innate immune system interacts with the adaptive immune system.27 In renal transplantation, it has been demonstrated that local renal C3 production and activation can markedly augment the immune responses against the renal allograft, ultimately leading to more rapid allograft rejection in animal models.28,29 In this study on adult-sized kidneys transplanted into pediatric recipients, even in the absence of delayed graft function, we observed a significant association of complement gene expression with the onset of graft function. This is in agreement with the study of Hauser et al.,6 who demonstrated in adult recipients upregulation of complement genes in donor kidneys that subsequently developed delayed graft function; therefore, the subtle and clinically relevant association we observed with early graft function in children will be clinically important in adult transplantation. To confirm the previously observed28 causal relation between local renal complement gene expression and graft outcome in a human setting, much larger prospective studies need to be undertaken in representative donor and recipient cohorts with a wider age range and against the background of various degrees of kidney graft quality at implantation. Finally, it should be emphasized that the association between complement gene expression and graft function does not prove an independent causal role of local complement expression in this study; however, taken together with previous animal studies demonstrating a benefit of complement inhibition on ischemia-reperfusion injury30–34 and given the association between complement gene polymorphisms and renal allograft outcome in a human setting,16 it could be extrapolated that local complement gene expression in transplanted kidneys plays an important causal role also in humans.
Most interesting, this study highlights for the first time that C1, C3, and CFB gene expression continues to be upregulated after transplantation, regardless of donor source and in the absence of graft rejection or functional impairment. This suggests that the injury response of complement overexpression is not restricted to the earliest period of transplantation but that other triggers also drive persistent local complement production in transplants, away from the immediate peritransplantation period and in the absence of any histologic injury. The mechanisms underlying the persistent upregulation of complement genes C1, C3, and CFB later after transplantation remain unclear, however.
Putative triggers of this complement overexpression in posttransplantation biopsy samples include chronic ischemia and the use of immunosuppressive drugs. In addition, there is evidence in humans that acute rejection is associated with increased local expression of complement C3.17 Likewise, in a comparison of 32 acute rejection biopsy samples with 20 rejection-free samples obtained in pediatric kidney recipients at Stanford University, whole-genome expression analysis using Affymetrix HG U133 Plus 2.0 Arrays showed a significant overrepresentation of complement cascade genes (P = 0.001), including overexpression of C1, C2, C3, and CFB in acute rejection samples (unpublished data); however, it is not clear from these human studies whether rejection phenomena are the cause or the result of local renal complement in transplanted kidneys. In animal experiments, however, local renal complement was shown to be a major determinant of rejection risk28; therefore, these animal studies strongly suggest a pivotal role of local renal complement for rejection phenomena.
The clinical meaning of the persistent upregulation of complement genes C1, C3, and CFB later after transplantation is unclear. It can be hypothesized that complement overexpression in stable grafts is associated with subclinical but in the long term deleterious complement activation, contributing to chronic histologic damage, graft dysfunction, and ultimately graft loss. This is supported by previous animal studies35–37 and by the recent observation that the complement C3 allotype of the donor was associated with graft survival as a result of “chronic allograft nephropathy, ” interstitial fibrosis, and tubular atrophy but not with differences in the prevalence or severity of rejection episodes,16 which reinforces this hypothesis also in a human setting. The overexpression of genes involved in immunity and fibrosis in posttransplantation samples included in this study fits well with this hypothesis. Long-time follow-up of the patients included in this study will be needed to confirm the association of local complement expression with the histologic evolution and ultimately survival of renal allografts.
Next to the increased local overexpression of complement genes, other genes involved in immune activation were also differently expressed between deceased- and living-donor kidney biopsies, although the overrepresentation was much less significant than the complement gene overrepresentation. Molecules possibly involved in leukocyte trafficking and extravasation were especially significantly overrepresented in deceased-donor kidneys, such as β-2 integrin (ITGB2; CD18; forms LFA-1),38 osteopontin (SPP1),39,40 and transglutaminase 2 (implicated in osteopontin polymerization41), chemokine CCL23 (also known as CKβ8, MPIF-1, or MIP-3)42 and Rap1 inhibitor RAP1GAP.43 SPP1 was also found to be upregulated in deceased-donor kidneys in the study of Kainz et al.5 In addition, the markedly higher expression in deceased-donor kidneys of CD163, a monocyte-macrophage lineage-specific scavenger receptor regulated by IL-10, acting as an acute phase protein, points in the direction of increased “alternative ” macrophage responses.44 Next to CD163, haptoglobin expression was significantly higher in deceased-donor kidneys compared with living-donor kidneys, further strengthening a role of the hemoglobin scavenging process.44 In addition, LTF was the top gene expressed higher in deceased- versus living-donor kidney biopsies and represents a multifunctional protein involved in many different processes and upregulated in inflammatory conditions.45 This overexpression of LTF in deceased-donor kidneys is in accordance with the study of Hauser et al.,6 who also demonstrated overexpression of LTF in the deceased-donor kidneys. SERPINA3 mRNA (serine protease inhibitor, α-1 antichymotrypsin), another acute phase protein heavily modulated by cytokines, was also expressed significantly higher in deceased-donor kidneys compared with living-donor kidneys, both in our study and the study of Kainz et al.5 Other genes that were previously reported to be involved in the difference between living- and deceased-donor kidneys5,6 were not differently expressed in this study. These differences may relate to the relatively pristine nature of our deceased-donor kidneys and the use of different array platforms.
Together with previous animal studies demonstrating beneficial effects of complement inhibition on ischemia-reperfusion injury,30–34 rejection phenomena,46,47 tubulointerstitial injury, and renal dysfunction,48,49 our study sets the stage to design approaches to inhibit complement activation in a clinical setting in transplant recipients, for prevention or treatment of ischemia and reperfusion injury, in the prevention or treatment of rejection episodes, and for long-term maintenance of graft function and graft survival.
In conclusion, our study provides an in-depth analysis of the gene expression differences between kidneys from living and pristine deceased donors. Significant renal overexpression of many components of the complement pathway are seen in deceased-donor kidneys before reperfusion. Complement gene expression in deceased-donor kidneys relates to donor death and correlates with the length of cold ischemic injury and with early and late graft function. After transplantation, in the absence of any delayed graft function or histologic injury, putative triggers of injury such as ischemia, immunosuppressive drugs, infections, and low-grade alloimmune activation further drive complement gene expression irrespective of donor source. Given the previously established association of complement gene expression with acute graft rejection, it can be hypothesized that the local milieu of the renal allograft is now primed for immune activation and targeted injury. The impact of this finding on the evolution of chronic graft injury would be a critical question to explore in future studies. Targeted therapy interfering with complement activation before organ recovery, during organ storage, or in the posttransplantation period is an attractive therapeutic axis that deserves further investigation.
Concise Methods
Patients and Biopsies
A total of 53 renal allograft biopsies obtained at Stanford University were included in this study. Renal allograft protocol biopsies were obtained as part of the routine clinical follow-up of pediatric and adolescent kidney recipients (1 to 21 yr of age) at implantation (before reperfusion) and at prescheduled time points after transplantation: 3, 6, 12, and 24 mo.50 Because the kidneys were used for transplantation in pediatric or adolescent recipients, no expanded-criteria-donor kidneys were used. The organs were stored in University of Wisconsin cold storage solution.
From the available biopsies, a training set of 18 matched implantation biopsies were selected, with nine biopsies from brain death–deceased donors and nine biopsies from living donors (training set), according to availability of RNA of adequate quality (see next section) and to match both groups for relevant clinical and histologic parameters. To allow for validation of the gene expression pattern associated with deceased-donor kidney transplantation, we selected an independent, blinded test group (test set 1) of baseline protocol biopsy samples on the basis of the same criteria (n = 10; five deceased- and five living-donor kidneys). Also, 18 posttransplantation protocol biopsies in patients with stable graft function were selected (nine deceased- and nine matched living-donor kidneys) on the basis of RNA quality and matching for relevant clinical and histologic factors (test set 2). Finally, eight paired posttransplantation biopsies, obtained in eight of the nine living-donor kidneys from the training set, were included in the study. For one of these nine living-donor kidney recipients of the engraftment biopsy training set, no posttransplantation samples were available, and one posttransplantation biopsy was already included in the posttransplantation test set of nine living-donor kidneys (test set 2). None of the posttransplantation samples was obtained from patients with a history of cellular or humoral rejection episodes or donor-specific antibodies, and no biopsy had cellular infiltrates or a positive staining for C4d. The posttransplantation biopsies were obtained from patients who were treated with a steroid-free immunosuppressive protocol.50 All patients gave written informed consent, and the study was approved by the institutional review board of Stanford University.
RNA Extraction, Quality Control, Amplification, Hybridization, and qRT-PCR Confirmation
For each kidney allograft biopsy, a separate biopsy core was placed in RNAlater (Ambion, Austin, TX) and stored at −20°C until RNA extraction. Total RNA was extracted from each biopsy using TRIzol Reagent (Invitrogen, Carlsbad, CA). RNA integrity was ensured using the RNA 6000 Nano LabChip Kit (Agilent Technologies, Waldbronn, Germany) on a 2100 Bioanalyzer (Agilent Technologies). RNA was amplified to cDNA and biotin labeled using the Ovation Biotin System (NuGEN Technologies, San Carlos, CA). The cDNA fragments were hybridized onto Affymetrix GeneChip Human Genome U133 Plus 2.0 Arrays comprising more than 54,000 probe sets, covering more than 47,000 transcripts and variants, including 38,500 well-characterized human genes (Affymetrix, Santa Clara, CA). The microarrays were scanned using GeneChip Scanner 3000 (Affymetrix).
For independent confirmation of the differential expression of genes from the microarray analyses, two-step qRT-PCR was used. After reverse transcription of the RNA samples with Superscript III Reverse Transcriptase (Invitrogen), Taqman Gene Expression assays (Applied Biosystems, Foster City, CA; for specific assays, see Supplemental Table 7) were used on a 7900HT Fast Real-Time PCR System (Applied Biosystems). Relative expression values were obtained using the ΔΔCt method after normalization against 18s. The good agreement between qRT-PCR results and the microarray data are shown in Supplemental Table 7.
Histologic Evaluation and Immunohistochemistry
All slides were stained with hematoxylin and eosin and with the periodic acid-Schiff method. All biopsies were reviewed according to the revised Banff criteria.8 Because no frozen tissue was available from patients who underwent transplantation at Stanford University, baseline biopsy tissue was used from the protocol biopsy program at Leuven (Belgium). Immunohistochemistry with antibodies against, respectively, C3d (DK-2600, dilution 1:400; Dako A/S, Glostrup, Denmark) and C4d (mAb, dilution 1:500; Quidel Corp., Santa Clara, CA) was performed on frozen sections, as described previously.51 The intensity of C3d and C4d deposition was scored from 0 to 3 (0 = absent, 1 = mild, 2 = moderate, 3 = severe), separately for the different renal compartments.
Data Processing and Analyses
For processing and normalization of the scanned images, dChip 2006 software was used, with perfect match/mismatch difference modeling and invariant set normalization.52 Unsupervised and supervised average-linkage hierarchical clustering and visualization were performed using Cluster and Treeview.9 SAM 3.0 for two-class unpaired and quantitative data was performed to detect expression differences based on q values (FDRs),10 and Predictive Analysis of Microarrays was used to predict phenotypes classification of the independent test group samples.11 Significance levels were set at 5%. After identification and validation of the differentially expressed genes, these probe sets were analyzed using the PANTHER classification system12 and Ingenuity pathway program (Ingenuity Systems, Redwood City, CA) to assess their biologic functions and examine canonical pathways based on the Ingenuity Pathways Knowledge Base. Clinical variables were compared using Fisher exact test, χ2 test, Wilcoxon-Mann-Whitney U nonparametric ANOVA, one-way ANOVA with Bonferroni correction, and the t test or the signed rank test for paired data. Microarray data were compared with qRT-PCR data and graft function using Pearson correlation. These analyses were performed with SAS 9.1.3 (SAS Institute, Cary, NC). The raw data sets for the 53 biopsies included are deposited at the Gene Expression Omnibus under GSE11166. Supplemental material is available at http://sarwal.stanford.edu/Complement/.
Disclosures
None.
Supplementary Material
Acknowledgments
M.N. receives a grant from the Fund for Scientific Research–Flanders (Belgium) and is the holder of the Emile Boulpaep Fellowship of the Belgian American Educational Foundation. M.M.S. and L.L. are funded by grant RO1AI061739 from the National Institutes of Health (National Institute of Allergy and Infectious Diseases).
We acknowledge Jonathan Martin, Snehal Mohile, Amery C. Chen, and Shiquan Wu for help. We gratefully acknowledge the invaluable and persisting efforts of all people involved in the pediatric kidney transplant program at Stanford University, the clinicians, renal nurses, transplant coordinators, and children and their families.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Port FK, Merion RM, Finley MP, Goodrich NP, Wolfe RA: Trends in organ donation and transplantation in the United States, 1996–2005. Am J Transplant 7: 1319–1326, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Salahudeen AK, Haider N, May W: Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int 65: 713–718, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bos EM, Leuvenink HG, van Goor H, Ploeg RJ: Kidney grafts from brain dead donors: Inferior quality or opportunity for improvement? Kidney Int 72: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kainz A, Mitterbauer C, Hauser P, Schwarz C, Regele HM, Berlakovich G, Mayer G, Perco P, Mayer B, Meyer TW, Oberbauer R: Alterations in gene expression in cadaveric vs. live donor kidneys suggest impaired tubular counterbalance of oxidative stress at implantation. Am J Transplant 4: 1595–1604, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hauser P, Schwarz C, Mitterbauer C, Regele HM, Muhlbacher F, Mayer G, Perco P, Mayer B, Meyer TW, Oberbauer R: Genome-wide gene-expression patterns of donor kidney biopsies distinguish primary allograft function. Lab Invest 84: 353–361, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Mueller TF, Reeve J, Jhangri GS, Mengel M, Jacaj Z, Cairo L, Obeidat M, Todd G, Moore R, Famulski KS, Cruz J, Wishart D, Meng C, Sis B, Solez K, Kaplan B, Halloran PF: The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant 8: 78–85, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ′05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (′CAN′). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Eisen MB, Spellman PT, Brown PO, Botstein D: Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95: 14863–14868, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibshirani R, Hastie T, Narasimhan B, Chu G: Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A 99: 6567–6572, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A: PANTHER: A library of protein families and subfamilies indexed by function. Genome Res 13: 2129–2141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodwell GE, Sonu R, Zahn JM, Lund J, Wilhelmy J, Wang L, Xiao W, Mindrinos M, Crane E, Segal E, Myers BD, Brooks JD, Davis RW, Higgins J, Owen AB, Kim SK: A transcriptional profile of aging in the human kidney. PLoS Biol 2: e427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naesens M, Kambham N, Concepcion W, Salvatierra O, Jr, Sarwal M: The evolution of non-immune histological injury and its clinical relevance in adult-sized kidney grafts in pediatric recipients. Am J Transplant 7: 2504–2514, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Li K, Sacks SH, Zhou W: The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol Immunol 44: 3866–3874, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Brown KM, Kondeatis E, Vaughan RW, Kon SP, Farmer CK, Taylor JD, He X, Johnston A, Horsfield C, Janssen BJ, Gros P, Zhou W, Sacks SH, Sheerin NS: Influence of donor C3 allotype on late renal-transplantation outcome. N Engl J Med 354: 2014–2023, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Serinsoz E, Bock O, Gwinner W, Schwarz A, Haller H, Kreipe H, Mengel M: Local complement C3 expression is upregulated in humoral and cellular rejection of renal allografts. Am J Transplant 5: 1490–1494, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Welch TR, Beischel LS, Witte DP: Differential expression of complement C3 and C4 in the human kidney. J Clin Invest 92: 1451–1458, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellano G, Cappiello V, Fiore N, Pontrelli P, Gesualdo L, Schena FP, Montinaro V: CD40 ligand increases complement C3 secretion by proximal tubular epithelial cells. J Am Soc Nephrol 16: 2003–2011, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Farrar CA, Zhou W, Lin T, Sacks SH: Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J 20: 217–226, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Khan TN, Sinniah R: Role of complement in renal tubular damage. Histopathology 26: 351–356, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Vakeva A, Meri S, Lehto T, Laurila P: Activation of the terminal complement cascade in renal infarction. Kidney Int 47: 918–926, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH: Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105: 1363–1371, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lerut E, Kuypers D, Van Damme B: C4d deposition in the peritubular capillaries of native renal biopsies. Histopathology 47: 430–432, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Thurman JM, Ljubanovic D, Royer PA, Kraus DM, Molina H, Barry NP, Proctor G, Levi M, Holers VM: Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest 116: 357–368, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carroll MC: The complement system in regulation of adaptive immunity. Nat Immunol 5: 981–986, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Pratt JR, Basheer SA, Sacks SH: Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med 8: 582–587, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Li K, Patel H, Farrar CA, Hargreaves RE, Sacks SH, Zhou W: Complement activation regulates the capacity of proximal tubular epithelial cell to stimulate alloreactive T cell response. J Am Soc Nephrol 15: 2414–2422, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Pratt JR, Jones ME, Dong J, Zhou W, Chowdhury P, Smith RA, Sacks SH: Nontransgenic hyperexpression of a complement regulator in donor kidney modulates transplant ischemia/reperfusion damage, acute rejection, and chronic nephropathy. Am J Pathol 163: 1457–1465, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel H, Smith RA, Sacks SH, Zhou W: Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol 17: 1102–1111, 2006 [DOI] [PubMed] [Google Scholar]
- 32.De Vries B, Matthijsen RA, Wolfs TG, Van Bijnen AA, Heeringa P, Buurman WA: Inhibition of complement factor C5 protects against renal ischemia-reperfusion injury: Inhibition of late apoptosis and inflammation. Transplantation 75: 375–382, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM: A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int 63: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Thurman JM, Royer PA, Ljubanovic D, Dursun B, Lenderink AM, Edelstein CL, Holers VM: Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol 17: 707–715, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Nangaku M, Pippin J, Couser WG: C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol 13: 928–936, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT: Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol 177: 4094–4102, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Sheerin NS, Risley P, Abe K, Tang Z, Wong W, Lin T, Sacks SH: Synthesis of complement protein C3 in the kidney is an important mediator of local tissue injury. FASEB J 22: 1065–1072, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Friedewald JJ, Rabb H: Inflammatory cells in ischemic acute renal failure. Kidney Int 66: 486–491, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H: Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science 287: 860–864, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J: Osteopontin: A molecule for all seasons. QJM 95: 3–13, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Higashikawa F, Eboshida A, Yokosaki Y: Enhanced biological activity of polymeric osteopontin. FEBS Lett 581: 2697–2701, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Forssmann U, Delgado MB, Uguccioni M, Loetscher P, Garotta G, Baggiolini M: CKbeta8, a novel CC chemokine that predominantly acts on monocytes. FEBS Lett 408: 211–216, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K: Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280: 11675–11682, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Moestrup SK, Moller HJ: CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med 36: 347–354, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Ward PP, Paz E, Conneely OM: Multifunctional roles of lactoferrin: A critical overview. Cell Mol Life Sci 62: 2540–2548, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, Ramcharran S, Garcia B, Zhong R, Rother RP: Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol 179: 4451–4463, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH: Effects of complement inhibition with soluble complement receptor-1 on vascular injury and inflammation during renal allograft rejection in the rat. Am J Pathol 149: 2055–2066, 1996 [PMC free article] [PubMed] [Google Scholar]
- 48.Bao L, Zhou J, Holers VM, Quigg RJ: Excessive matrix accumulation in the kidneys of MRL/lpr lupus mice is dependent on complement activation. J Am Soc Nephrol 14: 2516–2525, 2003 [DOI] [PubMed] [Google Scholar]
- 49.He C, Imai M, Song H, Quigg RJ, Tomlinson S: Complement inhibitors targeted to the proximal tubule prevent injury in experimental nephrotic syndrome and demonstrate a key role for C5b-9. J Immunol 174: 5750–5757, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Sarwal MM, Vidhun JR, Alexander SR, Satterwhite T, Millan M, Salvatierra O, Jr: Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation 76: 1331–1339, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Herman J, Lerut E, Van Damme-Lombaerts R, Emonds MP, Van Damme B: Capillary deposition of complement C4d and C3d in pediatric renal allograft biopsies. Transplantation 79: 1435–1440, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Li C, Wong WH: Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci U S A 98: 31–36, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.