Abstract
Red clover isoflavones are increasingly used in dietary supplements for their purported estrogenic effects. However, little is known about their metabolism in animals due to a lack of commercially available isotopically-labeled tracers. The goal of this research was to establish red clover cell culturing methodology for 14C-biolabeling of isoflavones. When root, leaf, and petiole—derived suspension cultures were grown in darkness or light, dark-grown, petiole-derived solution cultures produced the highest concentrations of the two major red clover isoflavones, formononetin (0.67 mg/g FM inoculum) and biochanin A (0.13 mg/g FM inoculum). Varying levels and timing of copper chloride elicitor did not significantly affect isoflavone accumulation. Approximately 38% of the 14C-sucrose dose accumulated in the cells. Eighteen percent of the initial labeled dose was detected in the isoflavone-rich methanolic extract and of that, 22% accumulated in isoflavones.
Keywords: red clover, isoflavones, metabolic tracers, plant cell culture, copper chloride, radiolabeling, Trifolium pratense
Introduction
Isoflavones are a class of phytoestrogens believed to exhibit bioactivity in hormone-related diseases such as cardiovascular disease, cancer, and osteoporosis, as well as menopausal symptoms (Tham et al. 1998). These effects are likely associated with the estrogenic and anti-estrogenic actions, antioxidant, and tyrosine kinase inhibition properties of isoflavones. The phenolic ring structure and nearly equivalent hydroxyl groups of isoflavones and endogenous 17 β-estradiol allow isoflavones to weakly bind to the estrogen receptor-β, which is found in brain, bone, bladder, and vascular epithelia (Setchell et al. 1999). In humans, isoflavones can accumulate at 100-fold higher concentrations than endogenous estrogens, thus making a significant contribution to hormonal signaling as well as interactions with other cellular processes not mediated by the estrogen receptor (Tham et al. 1998).
Isoflavones are commonly found in leguminous plants, most notably soybean, but also accumulate in clover species (Tham et al. 1998). Red clover (Trifolium pratense) dietary supplements and herbal medicines typically consisting of dried floral blossoms in tablet or tea forms, are sold as “natural” alternatives to estrogen replacement therapy (National Center for Complementary and Alternative Medicine. 2008). Extensive epidemiological evidence indicates a substantially lower incidence of hormone-dependent diseases for populations practicing a phytoestrogen-rich traditional Asian diet versus a low phytoestrogen Western diet (Tham et al. 1998).
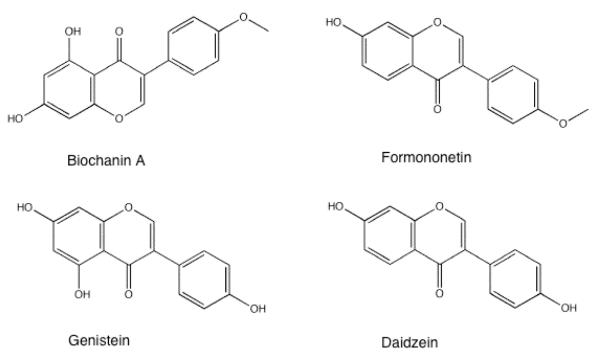
Thirty-one isoflavones in the leaves and stems, 25 in the roots, and 26 in flowers of red clover (Trifolium pratense) have been identified, with the highest concentrations of formononetin and biochanin A, the two major red clover isoflavone aglycones, found in the leaves (Wu et al. 2003). Biochanin A and formononetin are the 4′-O- methylated plant precursors to genistein and daidzein (Figure 1), respectively and are the predominant isoflavones in alfalfa, chickpea, and red clover (Tham et al. 1998; Tolleson et al. 2002). These prominent red clover isoflavone aglycones may have beneficial physiological effects (Beck et al. 2005). Red clover extracts and supplements have been shown to treat menopause and high blood lipids in clinical trials, and they have been used to slow osteoporosis in animal studies (Hidalgo et al. 2005; Nestel et al. 2004; Occhiuto et al. 2007).
Fig. 1.
Structures of prominent red clover isoflavones, biochanin A and formononetin, and their corresponding demethylated derivatives genistein and daidzein.
Limited information on red clover isoflavone metabolism and bioactivity exists. It is known that the O-methoxylation of these precursors reduces their antioxidant and estrogenic activities, however the gut bacterium E. limosum demethoxylates the compounds to yield the more potent estrogenic products (Hur and Rafii. 2000; Kuiper et al. 1998; Arora et al. 1998). Other metabolism studies have examined the effect of proportions of red clover isoflavones on in vitro enterocyte uptake, and the in vitro conversion of biochanin A and formononetin by hepatic cytochrome P450 isoforms (Tolleson et al. 2002; Wang et al. 2008). However, there is a paucity of research on in vivo human and animal bioconversions of red clover isoflavones.
In order to rigorously study red clover isoflavone metabolism in animal models and humans, a source of isotopically labeled red clover isoflavones is needed. For production of labeled isoflavones, the controlled conditions of in vitro cell cultures have a number of advantages. In vitro cell culture systems can be engineered for reproducible product accumulation independent of season because its physical and chemical environments can be controlled (Yousef et al. 2004b). Secondary metabolite production by in vitro plant systems can be stimulated by use of elicitors, which are biotic or abiotic compounds that initiate a defense mechanism such as a structural change, gene activation, or change in chemical composition, designed to defend a plant cell from bacterial or viral infection (Ebel et al. 1998). Plant secondary metabolites including tomato carotenoids; grape and berry flavonoids, proanthocyanins and anthocyanins; and kudzu isoflavones have been successfully radiolabeled using in vitro plant cell culture methodologies (Yousef et al. 2004b; Campbell et al. 2006; Vitrac et al. 2002; Reppert et al. 2008). Although currently needed for nutritional research, the full array of radiolabeled red clover isoflavones is not commercially available.
Copper chloride (CuCl2), a heavy metal salt, has been used in various leguminous species for elicitation of isoflavones and induction of other phytoalexin metabolism (Parry et al. 1994; Gagnon and Ibrahim. 1997; Mithofer et al. 2004; Preisig et al. 1990; Paiva et al. 1994; Maxwell and Phillips. 1990). An in vivo study of isoflavone metabolism in root tissue of 5-d-old red clover seedlings found that CuCl2 prompted the accumulation of both the free aglycones formononetin and maackiain and the loss of their respective conjugates. After 24 h incubation, maximal levels of free aglycones were reached and diminished levels of the metabolic precursors were noted (Tebayashi et al. 2001). In this report, uniform red clover cell cultures were developed and an elicitation protocol for in vitro red clover isoflavone radiolabeling was applied.
Materials and Methods
Plant material
Red clover (Trifolium pratense) seeds (Johnny’s Selected Seeds; Winslow, ME) were surface sterilized by wrapping in filter paper, submerging in 70% ethanol solution for 1 min, and then in a 1.2% sodium hypochlorite solution with polyoxyethylene sorbitan monolaurate (two drops/L, Tween 20, Sigma-Aldrich, St. Louis, MO) for 10 or 20 min. In separate trials, seeds were also surface sterilized using the same procedure except with 0.6% and 0.9% sodium hypochlorite solutions for 15 min each. After each procedure, seeds were thoroughly rinsed five times with sterile water over a slight vacuum using a sterile filter unit, and they were explanted onto 15 ml culture media consisting of half strength Murashige and Skoog salts (Murashige and Skoog. 1962), rose vitamins (Rogers et al. 1992), myoinositol (0.1 g/L), sucrose (30 g/L), and bacteriological grade agar (Phytotechnology Laboratories, Shawnee Mission, KS) (6 g/L) in 25 × 150 mm culture tubes.
Callus and suspension culture initiation
Six 17-d-old axenic seedlings were dissected into petiole, leaf, and root segments. Segments were placed onto 40 ml of solid red clover callus media as modified by Beach and Smith (Beach and Smith. 1979) consisting of Gamborg B5 salts (Gamborg et al. 1968), B5 vitamins, myoinositol (0.1 g/L), NAA (2 mg/L), 2,4-D (2.25 mg/L), kinetin (2.12 mg/L), sucrose (20 g/L), and bacteriological grade agar (6 g/L) in GA-7 cubes (Magenta Corporation, Chicago, IL). Callus cultures were maintained in the dark. During callus induction, explants were transferred to fresh solidified media after 34 d and then again after 42 days, and transferred every 28 d thereafter. When callus became friable, light yellow, and comparable across tissue types, 1 g callus tissue from each explant was separated from mother tissue and transferred to 40 ml fresh callus media in GA-7 cubes for a 28 d growth cycle. To induce suspension cultures, 2 g fresh mass (FM) of friable callus were transferred to 40 ml of the same media without agar in 125 ml Erlenmeyer flasks. Cell suspension cultures were maintained in darkness on a rotary shaker (150 rpm) at 27.5 +/- 1.5 °C. Cell suspension cultures were subcultured every 14 d by the transfer of 5 ml aliquots of established cultures, [1.5 ml settled cell volume (SCV) with 3.5 ml spent media] into 40 ml of fresh media. To determine the effect of irradiance on isoflavone production, cell suspension cultures were maintained under either cool white fluorescent light (100 μmol m-2 s-1), or in darkness.
Growth measurements and isoflavone extraction
Suspension and callus cultures were evaluated quantitatively for biomass and isoflavone content at the end of their respective growth cycles. Callus was harvested by careful separation from media using metal spatulas, and FM was promptly recorded. Suspension cultures were separated from spent media over a slight vacuum with #4 filter paper (Whatman, Springfield Mill, UK) and a Büchner funnel. Filtration ended when no more liquid was expressed for 30 s, and FM was recorded.
Cells were extracted for isoflavone analysis by first homogenizing 1-part cells with 5-parts 80% methanol for 20 s. Cell debris was separated from the methanolic fraction by vacuum filtration using a Büchner funnel and #4 filter paper and again extracted and separated from the extract as before. For a final extraction, cell debris was homogenized, stored overnight at 4 °C, and filtered the next day. All three methanolic fractions were combined and filtered with #1 filter paper. Methanol was removed under reduced pressure in a 40°C water bath using a Büchi Rotavapor R110 (CH-9230 Flawil, Switzerland). Extraction solutions were then frozen at -20 °C and completely lyophilized.
Copper chloride elicitation
To evaluate the effect of CuCl2 dose level and exposure duration on cell suspension cultures, liquid red clover callus media was adjusted to 0, 0.5, 0.05, and 0.005 mM CuCl2 by adding 40 μl of 0, 499.8, 49.98, and 4.998 mM aqueous CuCl2 elicitor solutions respectively on either day 9, 11, or 13 of the 14 d growth period. Cells were harvested on day 14 as described above. Spent media was reserved from each treatment, stored at -20 °C, and water was removed using a rotary evaporator and freeze-dryer.
[14C]-Labeling Red Clover Isoflavones
Uniformly labeled [14C]-sucrose with a specific activity of 10 mCi/mmol (374 MBq/mmol) in a crystalline solid form (ICN Biomedicals Inc., Irvine, CA) was used as the source of label for the red clover suspension cultures. The [14C]-sucrose stock solution was prepared in sterile double distilled H2O (pH 5.7), and was filter-sterilized prior to incorporation into the media. Concentrated medium containing all components except [14C]-sucrose was prepared by bringing the medium to 90% of the final volume. The medium was dispensed and autoclaved at 72 ml per 250 ml flask.
After transferring 10 ml of established cultures (3 ml SCV with 7 ml spent medium), 8 ml of stock [14C]-sucrose solution was added to cell suspension cultures to bring the final media volume to 80 ml. The final media [14C]-specific activity was 3.4 μCi/ml. Cultures were placed in a previously developed enclosed polyacrylic labeling chamber built atop a gyratory shaker, designed to provide for the safe containment of respired [14C]-labeled CO2 produced by the cell suspension cultures (Grusak et al. 2004). Red clover cell suspension cultures were incubated in the enclosed chamber for 14 d at 22 ± 2 °C in the dark, with the gyratory shaker set to 160 rpm. Sterilized CuCl2 was added to cultured cells on day 11 of a 14 day growth period for a final concentration of 0.05 mM, as previously described, to increase isoflavone production and [14C]-labeling efficiency.
Isoflavone screening and analysis
For isoflavone screening, dried media, cell extracts, and standards [formononetin, biochanin A (Indofine, Hillsborough, NJ), genistein, and daidzein (Sigma-Aldrich, St. Louis, MO)] were dissolved in methanol and applied to a silica gel plate (250 μm layer, Whatman Ltd., Maidstone, Kent, UK) for thin layer chromatography (TLC) using a methanol-chloroform solvent. Spots were visualized under UV light. All analyses were performed on cell suspension cultures harvested at 14 d after inoculation.
For quantitative HPLC analysis, dried methanolic extracts were weighed out in 20 mg portions, suspended in 4 ml of 80% aqueous methanol, and sonicated until fully dissolved. Isoflavone HPLC analysis was carried as previously described (Reppert et al. 2008). In short, isoflavones were separated using a Supelcosil LC18 column (250 mm × 4 mm × 5 μm) (Supelco, Bellefonte, PA) and analyzed using an 1100 HPLC series system (Agilent Technology Inc., New Castle, DE). Isoflavones were monitored at 262 nm using a photodiode-array UV-VIS detector. Standards for daidzein, genistein, formononetin, biochanin A (Sigma-Aldrich), genistin, malonyl-genistin (LC Laboratories, Woburn, MA), ononin, and biochanin A-7-glucoside (Indofine) were used for isoflavone identification and quantification in radiolabeled red clover extracts. Non-commercially available isoflavones were quantified with related standards using a previously described method (Chandra et al. 2001). A fraction collector (Agilent Technologies, Santa Clara, CA) was set to collect the radiolabeled isoflavone peaks separately. Analysis was performed with a quadratic pump (G1311A), degasser (G1322A), auto-sampler (G1313A), fraction collector (G1364C), and temperature controlled column compartment (G1316A) of 24 °C. The wavelength absorption was monitored at 262 nm using photodiode-array UV-VIS detector (PDA). Primary testing showed that major isoflavone compounds corresponded to peaks with retention times of 8.0, 9.0, 10.2, 11.3, 12.3, 13.9, 14.8, 15.9, 16.5, 18.4, 20.5, 22.5 min. In comparison with commercial references and HPLC-MS data described below, these peaks were associated with glycitin, genistin, calycosin glycosyl-malonate, malonyl-genistin, pratensein glycosyl-malonate, formononetin glycosyl-malonate, biochanin A 7-glucoside, genistein, pratensein, formononetin, irilone, and biochanin A, respectively. The injection volume was 25 μl for samples and standards with a flow rate of 1.5 ml/min. Solvent A was 90% H2O, 10% acetonitrile (ACN) with 0.1% trifluoroacetic acid (TFA) and solvent B was 90% ACN, 10% H2O, and 0.1% TFA. The mobile phase gradient system was 0, 70%, 100%, 0%, and 0% of solvent B at 0, 30, 35, 40, and 45 min, respectively. To quantify the isoflavones in red clover dry extract, three concentrations of external standards were used. The standards were prepared in 80% aqueous methanol at the following concentrations: daidzin at 50, 100, and 200 μg/ml, malonyl-genistin and genistin at 10, 20, and 40 μg/ml, genistein and daidzein at 3, 6, and 12 μg/ml. All data were processed using Chemstation Software for LC 3D systems (Rev. A.10.02, Agilent Technologies).
HPLC-ESI/MS Analysis of Radiolabeled Isoflavones
High performance liquid chromatography-electro spray ionization-mass spectrophotometer (HPLC-ESI-MS) methods were previously described (Reppert et al. 2008).
[14C]-Label Enrichment in Dry Extracts and Specific Activities of Isoflavones
Radioactivity in plant cells and isoflavone fractions were analyzed using a scintillation counter and scintillation cocktail as previously described (Reppert et al. 2008). In short, isoflavone 14C enrichment was measured using HPLC to separate individual isoflavones for scintillation analysis. Each isoflavone was collected upon elution from the separatory column. The concentrations of isoflavone compounds were estimated by using commercial standards as described previously. The collected mobile phase volume (0.8 ml) corresponding to each isoflavone HPLC peak was added to 20 ml of Biosafe II liquid scintillation cocktail (RPI, Mt. Prospect, IL) and read by a liquid scintillation counter. The specific activities of the isoflavone compounds were estimated by dividing the amount of 14C label in μCi by compound mass for each corresponding peak in g (specific activity = μCi/g).
Statistical analysis
The cell suspension culture experiments were conducted with 5 replicates per treatment for FM determinations. The callus culture experiments were conducted with 4-6 trials of 3 replicates/trial per tissue type for FM determinations. Replicates for each treatment were combined before extraction and isoflavone analysis. Significant differences between treatments for growth measurements were determined with a one-way analysis of variance followed by the Dunnett’s test at the 0.05 probability level using the SAS System for Windows v. 8.02 (SAS Institute, Inc., Cary, NC). Isoflavone concentrations in cultures and spent media were determined by combining repetitions and determining isoflavone concentrations per FM for callus cultures or per liter media for cell suspension cultures.
Results and Discussion
Surface disinfestation
Following surface disinfestation, no contamination was seen at any treatment (0.6, 0.9, nor 1.2% sodium hypochlorite). The short-duration/ high-concentration sodium hypochlorite treatment (10 min/1.2% sodium hypochlorite treatment) (Table 1) resulted in the highest rate of germination (76%) of axenic seedlings. Increasing durations of disinfestation to 15-20 min resulted in germination percentages that were 24-36% lower.
Table 1.
Percent germination of axenic red clover seedlings after various sodium hypochlorite disinfestation treatments.
| % Sodium hypochlorite | Immersion time (min) | % Germination |
|---|---|---|
| 1.2 | 10 | 76 |
| 1.2 | 20 | 52 |
| 0.6 | 15 | 45 |
| 0.9 | 15 | 40 |
Callus and cell suspension culture initiation
Callus growth was observed within 7-14 days on cut surfaces of root, petiole, and leaf explants. Callus became uniform in appearance within 3 months and was used to initiate suspension cultures, which were subcultured every 2 weeks. Suspensions initiated from friable leaf or petiole-derived callus became uniform enough to obtain 1.5 ml SCV by the 3rd cycle, whereas root callus-derived suspensions required 5 cycles after initiation.
Growth and isoflavone measurements of callus and suspension cultures
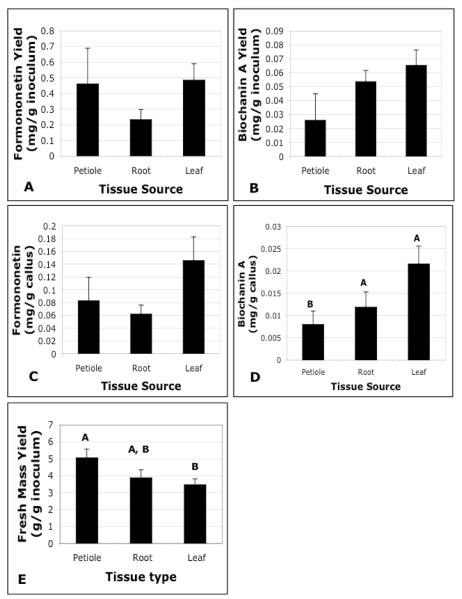
In solid callus culture, petiole-derived cells yielded significantly greater FM (5.06 g / g inoculum) compared to leaf (3.47 g/g inoculum) or root-derived callus (3.88 g/g inoculum). All three culture types produced similar formononetin and biochanin A yields (0.23-0.49 mg/g callus inoculum, and 0.03-0.07 mg/g callus inoculum, respectively), but concentrations were higher in root and leaf callus cultures for biochanin A (0.012 and 0.022 mg/g callus) than in petiole cultures (0.008 mg/g callus) (Figure 2). All three sources were adequate callus sources and were used to evaluate productivity for isoflavones in cell suspension culture.
Fig. 2.
A&B) Isoflavone concentrations, (C&D) isoflavone yields, and (E) mass yields of red clover solid callus cultures derived from petiole, root, or leaf tissues.
Error bars represent standard error of the mean. Letters denote significantly different means.
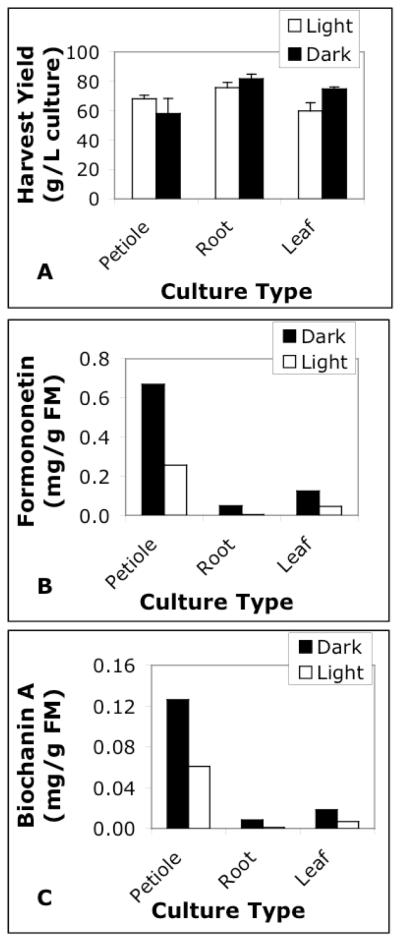
Dark-grown, petiole-derived cell suspension cultures produced the greatest concentrations of formononetin (0.67 mg/g FM inoculum) and biochanin A (0.13 mg/g FM inoculum) compared to other tissues grown in darkness and in light (Figure 3). Isoflavone concentrations were on average 9-fold greater in petiole-derived cell suspension cultures (0.7 mg/g FM) as compared to callus cultures (0.08 mg/g FM) (Figures 2 & 3). Previous reports indicated that field grown red clover had the highest levels of formononetin and biochanin A in leaf tissue (authors did not indicate if leaves included petiole tissue), and the lowest amounts in flowers, roots, and stems (Wu et al. 2003). Our results showed petiole-derived cell suspension cultures to have the highest concentration of isoflavones. Based on our findings in liquid media, petiole-derived cell suspension cultures were chosen for further elicitation study.
Fig. 3.
A) Harvest mass yields and (B&C) isoflavone concentrations of light and dark-grown petiole, leaf, and root solution cultures. Isoflavone analyses were performed on pooled samples (n=1) Error bars are standard error of the mean.
Effect of CuCl2 treatments on harvest mass and isoflavone yields
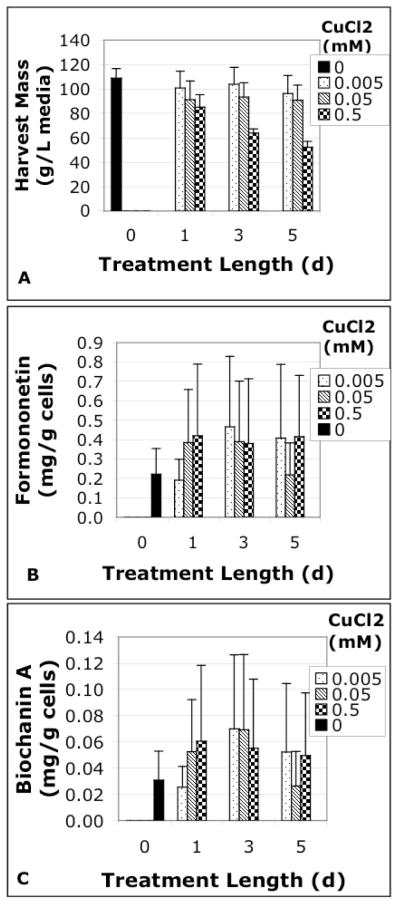
Copper chloride had a small effect on petiole-derived cell suspension culture harvest mass, where the greatest yields for 1, 3, and 5 d treatments resulted from the 0.005 mM CuCl2 treatment (101, 104, and 96 g/L, respectively). While no treatments were significantly different, the lowest overall yield resulted from the longest, highest copper chloride treatment (52 g/L) (Figure 4). A preliminary study of non-elicited petiole-derived cell suspension cultures showed an average yield of 75 g/ L, which is 31% less than the control groups (109 g/L) in the elicitation experiments. These results may indicate that growth rates rise as cell suspension cultures adapt to the in vitro environment.
Fig. 4.
A) Harvest yield and (B&C) isoflavone concentrations of petiole—derived cell suspension cultures treated with different levels of CuCl2 for different durations of time. Data represent the average of 3 trials, and error bars are standard error of the mean.
Based on TLC analysis of the first trial of CuCl2-elicited cell and media extracts, it was determined that cell extracts from suspension cultures contained formononetin, biochanin A, genistein and very low amounts of genistin, and daidzein (data not shown). Media extracts did not contain any detectable amounts of isoflavones, indicating the isoflavones were not released from the cells in significant amounts. Based on this data, our analyses focused on biochanin A and formononetin from cell samples.
HPLC analysis of elicited petiole cultures showed most CuCl2 treatments caused increased biochanin A and formononetin accumulation compared to control (Figure 4), with the exception of 1 d, 0.005 mM and 5 d, 0.05 mM treatments. Based on preliminary results, the 3 d, 0.05 mM CuCl2 treatment was chosen for radiolabeling experiments detailed hereafter.
[14C]-Isoflavone Radiolabeling Efficiency and Isoflavone Analysis
Red clover cell suspension cultures grown for 14 d in the labeling chamber reached a final average fresh mass yield of 83.8 g/L, an average increase of 335% in weight from inoculum. The cultures accumulated 458.4 μCi (17.0 MBq) (38.2% of the administered [14C]-sucrose label), in an average of 26.9 g per run of cultured biomass (17.0 μCi/g or 0.6 MBq/g fresh weight), whereas 27.3% of the [14C]-label remained in the filtered media (Table 2). Scintillation counts of samples from CO2 traps revealed that loss of 14C in the form of CO2 from respiration accounted for 303.6 μCi (11.2 MBq) (25.3%) of the label. The final recovery of the [14C]-label from petiole-derived cell suspension cultures into the isoflavone-rich dried extract was 214.8 μCi (7.9 MBq), which indicated an overall labeling efficiency of 17.9%. Of the label recovered in the dry extract, actual incorporation into the isoflavones was found to be 22.7% of the total amount of label recovered in the isoflavone-rich extract; the remainder was incorporated into various other compounds in the extract. The percentage of label present in the various isoflavones was proportional to their concentrations in the dry extract (Table 3) with a correlation coefficient of 97% (r2= 0.97).
Table 2.
Percent distribution of 14C in cells, media, and dried extract of petiole-derived red clover cell suspension cultures.
| Sample | % of 14C-sucrose dose final 14C distribution * |
|---|---|
| Cells | 38.2 ± 5.7 |
| Media | 27.3 ± 2.7 |
| Respiration (CO2) | 25.3 ± 4.8 |
| Dried isoflavone extract | 17.9 ± 4.5 |
Values are mean percentages ± SD of total administered radioactivity in three runs (1,200 μCi 14C dose per run), n=4 per run. Cultures were incubated with 14C label for 14 d.
Table 3.
Concentrations and specific activities of isoflavones found in red clover petiole-derived cell suspension culture extract as measured by HPLC and scintillation counting.
| Isoflavone | Concentration (mg/g extract)* |
Specific Activity (μCi/g extract)* |
Label Recovery (% label in extract) |
|---|---|---|---|
| Glycitin | 1.5 ± 0.6 | 68.1 ± 13.7 | 1.1 ± 0.2 |
| Genistin | 1.3 ± 0.8 | 44.4 ± 5.6 | 1.3 ± 0.2 |
| Calycosin GM | 7.4 ± 1.6 | 150.7 ± 44.7 | 2.9 ± 0.6 |
| Malonyl-genistin | 7.8 ± 1.8 | 162.8 ± 39.0 | 2.8 ± 0.5 |
| Ononin | 4.3 ± 0.7 | 81.7 ± 20.1 | 1.6 ± 0.3 |
| Pratensein GM | 5.3 ± 0.7 | 108.6 ± 20.3 | 1.5 ± 0.3 |
| Formononetin GM | 21.4 ± 2.8 | 167.6 ± 18.7 | 5.2 ± 0.6 |
| Biochanin A 7-G | 1.3 ± 0.6 | 47.1 ± 8.3 | 1.1 ± 0.4 |
| Genistein | 1.8 ± 0.6 | 110.3 ± 29.0 | 1.2 ± 0.4 |
| Pratensein | 2.6 ± 1.0 | 100 ± 9.1 | 1.4 ± 0.2 |
| Formononetin | 5.2 ± 2.4 | 83.8 ± 28.2 | 1.6 ± 0.5 |
| Irilone | 0.6 ± 0.1 | 51.1 ± 13.2 | 0.4 ± 0.1 |
| Biochanin A | 1.2 ± 1.0 | 58.1 ± 18.7 | 0.8 ± 0.2 |
| Total | 61.7 ± 3.4 | 1234.2 ± 148.7 | 22.7 ± 1.2 |
Values are means ± SD of isoflavone content, specific activity, and label recovery across three runs, n=4 for each. GM, glycosyl-malonate. G, glucoside.
Chemical Composition of Isoflavonoid-Rich Fractions
Isoflavones present in radiolabeled petiole-derived suspension cell culture extracts were identified by HPLC-PDA and HPLC-ESI/MS using several criteria, including comparison of their UV spectra, retention time (tR) and major ion molecular weight (MW) values with those of commercial standards and isoflavones previously reported in extracts of red clover (Wu et al. 2003; Lin et al. 2000).
The predominant isoflavones accumulated in petiole-derived, red clover suspension cultures were glycosides and glycosyl-malonate conjugates of the aglycones formononetin, calycosin, pratensein, genistein, and biochanin A (Figure 5). Peak identities were assigned based on the tR values of known standards, the MW values of the major ion for each peak, and the order and identity of peaks reported in previous studies of red clover tissue extracts; MS data were consistent with these reports (Wu et al. 2003; Lin et al. 2000). Additional isoflavones found in the labeled petiole-derived cell suspension cultures included the aglycones irilone, formononetin, and biochanin A, in smaller quantities (Table 3).
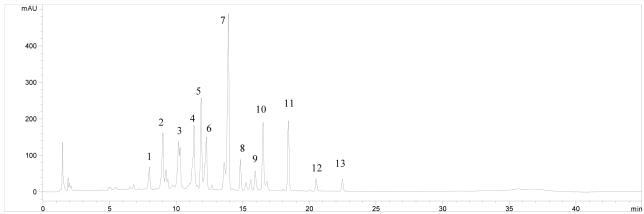
Fig. 5.
Typical RP-HPLC-PDA/UV chromatogram for isoflavones in radiolabeled, red clover, (Trifolium pratense) petiole-derived, cell suspension culture, 80% MeOH extract monitored at UV 262 nm. Peak identification: (1) glycitin, (2) genistin, (3) calycosin GM, (4) malonyl-genistin, (5) ononin, (6) pratensein GM, (7) formononetin GM, (8) biochanin A 7-G, (9) genistein, (10) pratensein, (11) formononetin, (12) irilone, (13) biochanin A.
The isoflavones in the dried extracts of red clover petiole-derived cell suspension cultures were quantified by HPLC-PDA to estimate the concentration of each isoflavone. This step was crucial in determining the efficiency of radiolabeled petiole-derived cell suspension cultures as a source of labeled, bioactive isoflavones. The most abundant isoflavone accumulated in the [14C]-labeled red clover petiole-derived cell suspension cultures was formononetin glycosyl-malonate, with an average concentration of 21.4 mg/g extract (equivalent to 1.0 mg/g FM) (Table 3). Glycosides of genistein and calycosin also were abundant in the petiole-derived cell suspension cultures, specifically malonyl-genistin and calycosin glycosyl-malonate, at average concentrations of 7.8 mg/g (0.4 mg/g FM) and 7.4 mg/g dried extract (0.3 mg/g FM), respectively. Concentrations for other isoflavones detected in red clover petiole-derived cell suspension cultures and the levels of 14C-incorporation are presented in Table 3.
Formononetin glycosyl-malonate also was found to have the highest 14C-enrichment as measured by specific activity (167.6 μCi/g or 6.2 MBq/g). The other two most predominant red clover isoflavones, malonyl-genistin and calycosin glycosyl-malonate, also had the next greatest specific activities of 162.8 μCi/g (6.0 MBq/g) and 150.7 μCi/g (5.6 MBq/g), respectively. The similarity between rankings by concentration and specific activity, however, did not continue beyond the three most predominant isoflavones. When measured by label recovery in the extract (% label in extract per isoflavone), the similarity was also limited between rankings by concentration and label recovery. However, the percentage of label present in the various isoflavones was proportional to the concentrations at which those isoflavones were present (Table 3). Formononetin glycosyl-malonate, for example, had the highest overall label incorporation at 5.2% of the label recovered in the dried extract, while the other two predominant red clover isoflavones, malonyl-genistin and calycosin glycosyl-malonate, had the next highest total label incorporation at 2.8% and 2.9%, respectively. Biochanin A and irilone, which had the lowest concentrations, accounted for only 0.8 and 0.4% of the recovered label, respectively.
Red clover petiole-derived cell suspension culture label incorporation (38.2%) (Table 2) was comparable to recently reported label incorporation in kudzu (Pueraria lobata), grape (Vitis vinifera), ohelo (Vaccinium pahalae), and tomato (Lycopersicon esculentum) cell cultures (Campbell et al. 2006; Vitrac et al. 2002; Reppert et al. 2008; Grusak et al. 2004) (17-34% radiolabel incorporation). Moreover, the amount of label recovered in the dried isoflavone-rich red clover extract was similar to that recovered in flavonoid-rich fractions of labeled grape and ohelo cell cultures (Yousef et al. 2004a).
The major isoflavones identified in unhydrolyzed extracts from red clover cell suspension cultures were the glycosides formononetin glycosyl-malonate, malonyl-genistin, and calycosin glycosyl-malonate with retention times of 13.9, 11.3, and 10.2 min, respectively (Figure 5). These isoflavones were present in large quantities compared to the other identified compounds, as determined by both HPLC-PDA/UV and HPLC-ESI/MS analyses. A different report analyzing hydrolyzed red clover extracts found the aglycones glycitein, genistein, irilone, and prunetin to be the major isoflavones in in vivo stem and leaf tissues. The isoflavone aglycones daidzein, genistein, formononetin, and biochanin A were identified as the primary isoflavones in the red clover formulations used in these studies, and these isoflavones have been linked to physiological benefits in other reports. For these reasons, a primary aim of this work was to produce a source of labeled bioactive red clover isoflavones formononetin and biochanin A, which could be used subsequently to study these purported health effects in vivo. The radiolabeled red clover extract produced in these experiments contained mostly glycosides and glycosyl-malonate conjugates of these aglycones. When consumed, these glycosides are cleaved upon digestion and absorption to the implicated bioactive free aglycones (Setchell et al. 2001). While the abundance of glycosylated isoflavones in the labeled red clover extract suggests that the majority of the [14C]-label was incorporated into those glycosides, their natural hydrolysis in vivo will allow us to track the hydrolysis and metabolism of these glycosides in vivo. This will be investigated in animal models aimed at using the radiolabeled red clover suspension culture extract to track the metabolism and physiological effects of red clover isoflavones.
The abundant compounds formononetin glycosyl-malonate, malonyl-genistin, and calycosin glycosyl-malonate in the labeled red clover petiole-derived cell suspension culture extract had the greatest specific activities compared to other isoflavones examined (167.6, 162.8 , and 150.7 μCi/g, respectively). The specific activities of these isoflavones are comparable to another study, which examined kudzu root cultures as a source of isoflavones, where puerarin, daidzin, and malonyl-daidzin had specific activities of 63.5, 112.8, and 85.4 μCi/g, respectively (Reppert et al. 2008). In contrast, stilbenes, as well as anthocyanins and catechins were produced with greater specific activities of 875 μCi/g and 260-350 μCi/g, respectively (Vitrac et al. 2002), and phytoene was found to have a much lower specific activity of 407 Bq/μg, or 0.11 μCi/g, when labeled (Campbell et al. 2006).
It should be noticed that the source of the label in this work, sucrose, has diverse potential metabolic fates, and this was a limitation of the present study with respect to the degree of enrichment produced. Sucrose is used in many biosynthetic pathways in plant cells, and therefore the label was incorporated randomly into a variety of compounds and cell constituents other than isoflavones. Nonetheless, this method is more cost-effective than using precursors specific to the isoflavone synthetic pathway, and the total label actually incorporated into each isoflavone was commensurate with the concentration of that isoflavone. That is, a larger amount of label was incorporated into the more abundant isoflavones, with the greatest label recovery in formononetin glycosyl-malonate, malonyl-genistin, and calycosin glycosyl-malonate.
In conclusion, [14C]-labeled isoflavones were biosynthesized and isolated from red clover using a novel tissue culture system. Red clover cell suspension cultures provide a renewable source of bioactive isoflavones. This study is the first, to our knowledge, to report the accumulation of a diverse profile of biolabeled isoflavones from red clover petiole-derived cell suspension cultures after introducing uniformly labeled [14C]-sucrose to the medium. Major isoflavones including formononetin glycosyl-malonate, malonyl-genistin, and calycosin glycosyl-malonate were isolated and quantified in this tissue culture system. These biolabeled isoflavones will be employed in studies utilizing an animal model and isotope-tracking methodology to assess the in vivo bioactivity and metabolism of the red clover extracts and their purified isoflavones, for a better understanding of their beneficial physiological effects.
Acknowledgments
This research was supported by the National Center for Complementary and Alternative Medicine sponsored Purdue-Universtiy of Alabama-Birmingham Botanicals Center for Dietary Supplement Research (NIH, 2 P50 AT000477-06). The authors would like to thank the University of Illinois James Scholar Program (College of Liberal Arts and Sciences). Nicola Lancki and Aaron McKerracher assisted with tissue culture maintenance and cell extractions. William Helferich also made contributions by assisting in TLC analysis, Tristan Kraft through helpful discussions regarding experimental design, and Jeevan Prasain with preliminary isoflavone analyses.
References
- Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch.Biochem.Biophys. 1998;356:133–141. doi: 10.1006/abbi.1998.0783. 10.1006/abbi.1998.0783. [DOI] [PubMed] [Google Scholar]
- Beach K, Smith R. Plant regeneration from callus of red and crimson clover. 1979.
- Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: an alternative to estrogen replacement therapy? J.Steroid Biochem.Mol.Biol. 2005;94:499–518. doi: 10.1016/j.jsbmb.2004.12.038. 10.1016/j.jsbmb.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Campbell JK, Rogers RB, Lila MA, Erdman JW., Jr. Biosynthesis of 14C-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. J.Agric.Food Chem. 2006;54:747–755. doi: 10.1021/jf0581269. 10.1021/jf0581269 [doi] [DOI] [PubMed] [Google Scholar]
- Chandra A, Rana J, Li Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J.Agric.Food Chem. 2001;49:3515–3521. doi: 10.1021/jf010389p. [DOI] [PubMed] [Google Scholar]
- Ebel J, Mithofer A, PLANAB Early events in the elicitation of plant defence. Planta. 1998 [Google Scholar]
- Gagnon H, Ibrahim RK. Effects of various elicitors on the accumulation and secretion of isoflavonoids in white lupin. Phytochemistry. 1997;44:1463–1467. [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Grusak MA, Rogers RB, Yousef GG, Erdman JWJ, Lila MA. An enclosed-chamber labeling system for the safe 14C-enrichment of phytochemicals in plant cell suspension cultures. In Vitro Cell Dev Biol Plant. 2004;40:80–85. [Google Scholar]
- Hidalgo LA, Chedraui PA, Morocho N, Ross S, Miguel G. San. The effect of red clover isoflavones on menopausal symptoms, lipids and vaginal cytology in menopausal women: a randomized, double-blind, placebo-controlled study. Gynecol.Endocrinol. 2005;21:257–264. doi: 10.1080/09513590500361192. 10.1080/09513590500361192. [DOI] [PubMed] [Google Scholar]
- Hur H, Rafii F. Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol.Lett. 2000;192:21–25. doi: 10.1111/j.1574-6968.2000.tb09353.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lin LZ, He XG, Lindenmaier M, Yang J, Cleary M, Qiu SX, Cordell GA. LC-ESI-MS study of the flavonoid glycoside malonates of red clover (Trifolium pratense) J.Agric.Food Chem. 2000;48:354–365. doi: 10.1021/jf991002+. [DOI] [PubMed] [Google Scholar]
- Maxwell CA, Phillips DA. Concurrent Synthesis and Release of Nod-Gene-Inducing Flavonoids from Alfalfa Roots. Plant Physiol. 1990;93:1552–1558. doi: 10.1104/pp.93.4.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithofer A, Schulze B, Boland W. Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett. 2004;566:1–5. doi: 10.1016/j.febslet.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol.Plantarum. 1962;15:473–497. 10.1111/j.1399-3054.1962.tb08052.x. [Google Scholar]
- National Center for Complementary and Alternative Medicine Herbs at a Glance: Red Clover. Jul 28, 2008.
- Nestel P, Cehun M, Chronopoulos A, DaSilva L, Teede H, McGrath B. A biochanin-enriched isoflavone from red clover lowers LDL cholesterol in men. Eur.J.Clin.Nutr. 2004;58:403–408. doi: 10.1038/sj.ejcn.1601796. 10.1038/sj.ejcn.1601796. [DOI] [PubMed] [Google Scholar]
- Occhiuto F, Pasquale RD, Guglielmo G, Palumbo DR, Zangla G, Samperi S, Renzo A, Circosta C. Effects of phytoestrogenic isoflavones from red clover (Trifolium pratense L.) on experimental osteoporosis. Phytother.Res. 2007;21:130–134. doi: 10.1002/ptr.2037. 10.1002/ptr.2037. [DOI] [PubMed] [Google Scholar]
- Paiva NL, Sun YJ, Dixon RA, Vanetten HD, Hrazdina G. Molecular-Cloning of Isoflavone Reductase from Pea (Pisum-Sativum L) - Evidence for a 3r-Isoflavanone Intermediate in (+)-Pisatin Biosynthesis. Arch.Biochem.Biophys. 1994;312:501–510. doi: 10.1006/abbi.1994.1338. [DOI] [PubMed] [Google Scholar]
- Parry A, Tiller S, Edwards R, PLPHAY The effects of heavy metals and root immersion on isoflavonoid metabolism in alfalfa (Medicago sativa L.) Plant Physiol. 1994 doi: 10.1104/pp.106.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig CL, Bell JN, Sun YJ, Hrazdina G, Matthews DE, Vanetten HD. Biosynthesis of the Phytoalexin Pisatin - Isoflavone Reduction and further Metabolism of the Product Sophorol by Extracts of Pisum-Sativum. Plant Physiol. 1990;94:1444–1448. doi: 10.1104/pp.94.3.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert A, Yousef GG, Rogers RB, Lila MA. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. J.Agric.Food Chem. 2008;56:7860–7865. doi: 10.1021/jf801413z. 10.1021/jf801413z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R, Smith M, JHSCA Consequences of in vitro and ex vitro root initiation for miniature rose production. J.Hortic.Sci. 1992 [Google Scholar]
- Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J.Nutr. 2001;131:1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- Setchell K, Cassidy A, JONUAI Dietary isoflavones: biological effects and relevance to human health. J.Nutr. 1999 doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Tebayashi S, Ishihara A, Iwamura H. Elicitor-induced changes in isoflavonoid metabolism in red clover roots. J.Exp.Bot. 2001;52:681–689. doi: 10.1093/jexbot/52.357.681. [DOI] [PubMed] [Google Scholar]
- Tham DM, Gardner CD, Haskell WL. Clinical review 97 - Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J.Clin.Endocrinol.Metab. 1998;83:2223–2235. doi: 10.1210/jcem.83.7.4752. [DOI] [PubMed] [Google Scholar]
- Tolleson WH, Doerge DR, Churchwell MI, Marques MM, Roberts DW. Metabolism of biochanin A and formononetin by human liver microsomes in vitro. J.Agric.Food Chem. 2002;50:4783–4790. doi: 10.1021/jf025549r. [DOI] [PubMed] [Google Scholar]
- Vitrac X, Krisa S, Decendit A, Vercauteren J, Nuhrich A, Monti JP, Deffieux G, Merillon JM. Carbon-14 biolabelling of wine polyphenols in Vitis vinifera cell suspension cultures. J.Biotechnol. 2002;95:49–56. doi: 10.1016/s0168-1656(01)00441-2. [DOI] [PubMed] [Google Scholar]
- Wang SW, Chen Y, Joseph T, Hu M. Variable isoflavone content of red clover products affects intestinal disposition of biochanin A, formononetin, genistein, and daidzein. J.Altern.Complement.Med. 2008;14:287–297. doi: 10.1089/acm.2007.0617. 10.1089/acm.2007.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wang M, Simon JE. Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J.Chromatogr.A. 2003;1016:195–209. doi: 10.1016/j.chroma.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Yousef GG, Seigler DS, Grusak MA, Rogers RB, Knight CT, Kraft TF, Erdman JW, Jr, Lila MA. Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. J.Agric.Food Chem. 2004a;52:1138–1145. doi: 10.1021/jf035371o. 10.1021/jf035371o. [DOI] [PubMed] [Google Scholar]
- Yousef G, Seigler D, Grusak M, Rogers R, Knight C, Kraft T, Erdman J, Jr, Lila M. Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. Journal of agricultural and food chemistry. 2004b;52:1138–1145. doi: 10.1021/jf035371o. [DOI] [PubMed] [Google Scholar]