Abstract
Characteristics of voltage-dependent sodium current recorded from adult rat muscle fibers in loose patch mode were rapidly altered following nearby impalement with a microelectrode. Hyperpolarized shifts in the voltage dependence of activation and fast inactivation occurred within minutes. In addition, the amplitude of the maximal sodium current decreased within 30 min of impalement. Impalement triggered a sustained elevation of intracellular Ca2+. However, buffering Ca2+ by loading fibers with AM-BAPTA did not affect the hyperpolarized shifts in activation and inactivation, although it did prevent the reduction in current amplitude. Surprisingly, the rise in intracellular Ca2+ occurred even in the absence of extracellular Ca2+. This result indicated that the injury-induced Ca2+ increase came from an intracellular source, but it was not blocked by an inhibitor of release from the sarcoplasmic reticulum, which suggested involvement of mitochondria. Ca2+ release from mitochondria triggered by carbonyl cyanide 3-chlorophenylhydrazone was sufficient to cause a reduction in sodium current amplitude but had little effect of the voltage dependence of activation and fast inactivation. Our data suggest the effects of muscle injury can be separated into a Ca2+-dependent reduction in amplitude and a largely Ca2+-independent shift in activation and fast inactivation. Together, the impalement-induced changes in sodium current reduce the number of sodium channels available to open at the resting potential and may limit further depolarization and thus promote survival of muscle fibers following injury.
Keywords: sodium channels, ion channel gating, calcium
it has traditionally been thought that the voltage dependence of sodium channel isoforms is a relatively invariant property (18). The logical extension of this idea is that sodium channel behavior in a given cell type is primarily determined by the sodium channel isoform expressed (14). However, more recently it has become clear that gating of sodium channels is under intense modulation (5). Whereas there has been a great deal of work identifying mechanisms that can alter the voltage dependence of sodium channel gating, most of the work has been done in heterologous expression systems in vitro. Little is known about modulation of sodium channel gating in vivo.
We study modulation of gating of Nav1.4 in mature rat muscle ex vivo using the loose patch-clamp recording technique. During our studies of sodium currents, we impale muscle fibers to determine resting potential. Several minutes after muscle fibers are impaled, hyperpolarizing shifts occur in the voltage dependence of sodium current activation and fast inactivation. Large shifts in the voltage dependence of gating of Nav1.4 have not been reported ex vivo. We characterized the shifts and examined whether elevation of intracellular Ca2+ following injury was the trigger for the hyperpolarized shift in gating of Nav1.4. Our data suggest that injury of muscle fibers ex vivo triggers both Ca2+-dependent and Ca2+-independent regulation of the Nav1.4 sodium channel.
MATERIALS AND METHODS
Tissue preparation and perfusion.
Female Wistar rats 8 to 10 wk old (220–250 g body wt) were used for all experiments. Rats were euthanized by carbon dioxide inhalation in accordance with Wright State University laboratory animal care and use committee guidelines. All procedures involving animals were approved by the Wright State LACUC committee. The extensor digitorum longus muscle was dissected tendon to tendon and placed in a recording chamber continuously perfused with normal ringer containing (in mM) 118 NaCl, 3.5 KCl, 1.5 CaCl2, 0.7 MgSO4, 26.2 NaHCO3, 1.7 NaH2PO4, and 10.8 glucose (pH 7.3–7.4, 20–22°C) equilibrated with 95% O2-5% CO2. Except when Fluo4 NW was used for Ca2+ imaging, fibers were labeled with 10 μM 4-(4-diethylaminostyrl)-N-methylpyridinium iodide (4-Di-2-ASP) to allow visualization of fibers and with α-bungarotoxin to visualize end plates as previously described (29, 30).
Loose patch-voltage clamp.
Loose patch-voltage clamp recordings and analysis were performed as previously described (11, 29, 30). Briefly, patch electrodes (resistance 0.25 to 0.5 MΩ) were filled with external solution and were slowly pressed against fibers to allow seal formation. Leak current was compensated manually and shunt resistance was measured on-line immediately before the application of each voltage step and used to adjust the step amplitude. To minimize potassium currents, sodium currents were recorded near (within 100–200 μm) muscle fiber end plates.
Fast inactivation was measured by applying 50-ms conditioning prepulses to various holding potentials between −130 mV and −40 mV followed by a 10-ms test pulse to −30 mV to activate sodium current. Measurement of activation was performed using a 50-ms prepulse to −120 mV to relieve fast inactivation followed by a series of 10-ms test voltage steps from −80 to 0 mV. For calculation of sodium conductance, the sodium reversal potential was assumed to be +45 mV (1, 34). During most loose patch recordings, muscle fibers were also impaled with two sharp electrodes (resistance 10–20 MΩ, filled with 3 M KCl), and the internal potential was voltage clamped to −80 mV using a two electrode voltage clamp.
Manipulation and measurement of internal Ca2+ concentration.
To image changes in intracellular Ca2+, muscle was loaded with Fluo4 NW (Molecular Probes) in solution supplemented with 2.5 mM Probenecid (Molecular Probes) and 0.1% pluronic F-127 (Molecular Probes) for 30 min at 33°C followed by 30 min at room temperature. Images were captured using an MTI SIT 66 camera and custom software (M. Pinter, Emory University, Atlanta, GA). Images were analyzed for mean pixel intensity using Image Pro software (Media Cybernetics, Bethesda, MD). For each muscle imaged, we manually adjusted the camera settings such that the initial image gave a good signal that was not near saturation. Camera settings were left stable following impalement and repeat imaging was performed. The Fluo-4 calcium signal was followed and was normalized to the initial signal for each fiber. No attempt was made to determine absolute Ca2+ concentration.
Before the experiments, 3–5 muscle fibers were impaled with two electrodes to measure resting potentials and to trigger muscle fiber action potentials by current injection. Muscles were discarded if mean resting potential was more positive than −75 mV or if increased Fluo4 NW signal was not triggered by muscle fiber action potentials. Sharp electrodes were filled with 3 M KCl and sulforhodamine B (excitation 565 nm, emission 586 nm) to allow visualization with a Leica filter module N2–1. In muscle injected with sulforhodamine, but not loaded with Fluo4 NW, no signal was observed using the Leica filter module I3 used to visualize Fluo4 NW. This indicated the sulforhodamine signal did not cross over to the Fluo4 NW signal (Fluo4 excitation 494 nm, emission 516 nm). To prevent contraction of muscle fibers during action potentials muscles were also loaded with 50 μM N-benzyl-p-toluenesulfonamide (BTS) (23) (Sigma-Aldrich) during loading of Fluo4 NW. In some muscles, fibers were loaded with 10 μM AM-BAPTA (Molecular Probes) during loading of Fluo4 NW. Dantrolene was dissolved in DMSO and added to the perfusate at a concentration of 12 μM.
In experiments where carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was applied to raise intracellular Ca2+ independent of impalement-induced injury, the amplitude and voltage dependence of sodium current activation and inactivation was measured in four to five muscle fibers before application of CCCP. For the initial loose patch recordings from these fibers, the muscle fibers were not impaled so as not to induce injury, and the resting potential was assumed to be −80 mV. Images were taken of the Fluo4 NW signal to get a baseline measurement. After recording from the fibers was obtained, 1 μM CCCP was added to the perfusate. The fibers were relocated after application of CCCP using the images taken before application of CCCP. Loose patch seals were reformed, sodium currents were measured, an image of the Fluo4 NW signal was taken, and then the fiber was impaled to determine the resting potential. After impalement, the measured resting potential was used to determine the true transpatch voltage for activation and fast inactivation pulse protocols. The voltage dependences of activation and inactivation measured before application of CCCP and impalement were corrected for the difference between the true resting potential and −80 mV.
Statistical analysis.
Boltzman fitting and statistical analysis were performed with Origin software (Origin Lab, Northampton, MA) as previously described (29). The Student's t-test was used for all comparisons of voltage dependence of gating, changes in Ca2+ signal as measured by Fluo-4 and current amplitudes. Means are ± SE.
RESULTS
Hyperpolarized shifts in activation and fast inactivation following impalement.
To determine the internal potential of muscle fibers during loose patch recordings, it was necessary to impale fibers with a sharp electrode. Fibers were also impaled with a second sharp electrode to allow use of two-electrode voltage clamp of the muscle fiber potential. This improves accuracy of loose patch recordings as it reduces depolarization of the muscle fiber by sodium currents activated via the patch electrode. We were surprised to find impaling and voltage clamping the internal potential of the fiber to −80 mV with two sharp electrodes induced hyperpolarized shifts in the voltage dependence of both sodium channel activation and fast inactivation. The sodium currents and voltage dependence of activation and fast inactivation for a single patch at various times after impalement and voltage clamp are shown in Fig. 1. In the example shown the voltage dependence of both activation and fast inactivation underwent hyperpolarized shifts of close to 25 mV during the hour following impalement. To determine whether voltage clamp with two sharp electrodes triggered the hyperpolarized shift, patches were followed in which the fiber was impaled but not voltage clamped. In those patches there was a similar shift in the voltage dependence of activation and fast inactivation (data not shown).
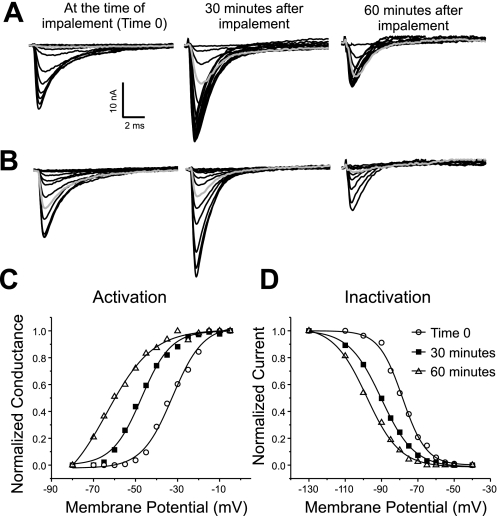
Fig. 1.
Hyperpolarized shifts in the voltage dependence of sodium channel activation and fast inactivation following impalement. A: superimposed sodium current traces evoked by 10-ms pulses ranging from −80 to −5 mV. Shown are records from a muscle fiber 0, 30, and 60 min after impalement. Shaded traces, current evoked by a step to −60 mV. At time 0 no current is evoked. At 30 min the current evoked that is less than half the maximal amplitude. At 60 min the current evoked is near the maximal amplitude. The patch was held at −110 mV between voltage protocols. Maximal current amplitude was increased 30 min after impalement but was decreased by 60 min. B: superimposed sodium current inactivation records from the same fiber as in A and at the same times following impalement as in A. The currents were evoked by a step to −30 mV following prepulses ranging from −130 to −40 mV. Shaded traces, current evoked following a prepulse to −80 mV. At time 0 a prepulse to −80 mV inactivates less than 50% of channels, at 30 min it inactivates over 70% of channels, and by 60 min it inactivates 90% of channels. C: normalized sodium conductance calculated from the currents shown in A with an assumed sodium reversal potential of +45 mV (1, 34) plotted versus membrane potential. The data are fitted with a Boltzman distribution at each time point. There is a 24-mV hyperpolarized shift in the midpoint of activation during the recording. D: normalized current amplitude of the currents shown in B are plotted and fitted with a Boltzman distribution at each time point. There is a 23-mV hyperpolarized shift in the midpoint of inactivation during the recording.
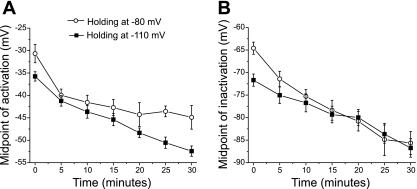
One possible cause of the hyperpolarized shift is the hyperpolarized holding potential applied to the patch during recordings. The sodium currents shown in Fig. 1 were recorded from a patch that was held at a potential of −110 mV to relieve slow inactivation. It has previously been reported that holding at hyperpolarized potentials induced a hyperpolarized shift in the voltage dependence of sodium channel gating (10, 35). However, the hyperpolarized shift that we previously reported when holding at −110 mV was smaller than the shift we observed after impaling fibers. To determine whether holding at −110 mV triggered the shifts in gating, sodium currents were recorded from a group of fibers that were impaled, and the transpatch potential was held at −80 mV (a potential near the resting potential of most muscle fibers). As previously reported, the initial voltage dependence of activation and inactivation were more depolarized when patches were held at a more positive holding potential [Fig. 2, A and B (10)]. However, hyperpolarized shifts in the voltage dependence of activation and inactivation occurred following impalement whether the patch was held at −110 mV or −80 mV (Fig. 2, A and B, P < 0.01 for both −80 mV and −110 mV by 15 min). As shown in Fig. 2A, for patches held at −110 mV the mean midpoint for activation shifted from −35.3 ± 1.0 mV to −53.9 ± 1.6 mV in 30 min (n = 8). The mean midpoint of inactivation shifted from −71.7 ± 1.4 mV to −86.7 ± 2.1 mV. The means shifts are similar in magnitude to the shifts shown in the example in Fig. 1 where activation shifted by 15 mV at 30 min and inactivation shifted by 12 mV at 30 min. These data suggest the holding potential of the patch does not trigger the hyperpolarized shift in activation and fast inactivation.
Fig. 2.
Time course of changes in sodium current following impalement. Mean midpoints of the Boltzman distribution are plotted versus time following impalement for sodium channel activation (A) and fast inactivation (B). Data are plotted from patches held at either −110 mV (solid squares, n = 8) or −80 mV (open circles, n = 7). At holding potentials of both −110 and −80 mV, there were hyperpolarized shifts in the mean voltage dependence of activation (P < 0.01 for both −80 mV and −110 mV by 15 min) and fast inactivation (P < 0.01 for both −80 mV and −110 mV by 15 min).
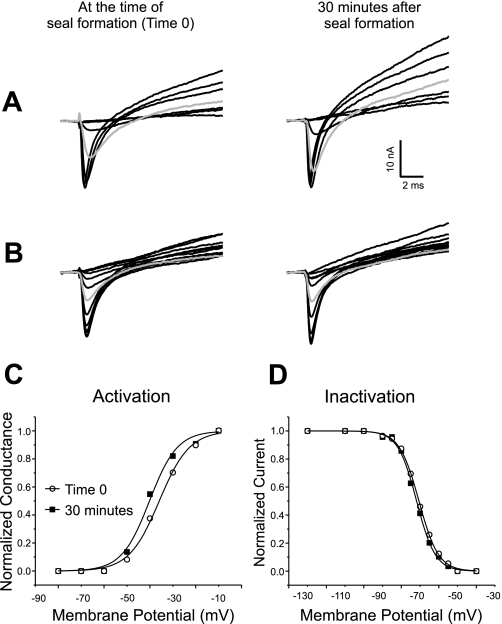
To determine whether placement of the patch electrode or impalement with sharp electrodes triggered the hyperpolarized shift, patches were formed on fibers where sharp electrodes were not used to voltage clamp the fibers during recordings. A single sharp electrode (20 to 30 MΩ, filled with 3 M KCl) was briefly inserted into the fiber before formation of a seal to measure the resting potential. The seal was formed after removal of the sharp electrode and sodium current measured for 30 min using the previously measured resting potential as the assumed intracellular potential. At the end of the recording the fiber was reimpaled and the resting potential measured again. If the resting potential had declined by more than 3 mV, the fiber was discarded. In fibers that were not impaled and voltage clamped for the duration of the recording there was no significant shift in the voltage dependence of either activation or fast inactivation (Fig. 3). For activation the mean midpoint went from −39.2 ± 1.7 mV to −40.8 ± 1.9 mV in 30 min (P = 0.54, n = 8). The mean midpoint of inactivation went from −74.6 ± 1.5 mV to −75.2 ± 1.4 mV (P = 0.79). It was possible to cause hyperpolarized shifts without impalement if the patch electrode was pushed very firmly against the fiber such that deformation of the fiber was visible (data not shown).
Fig. 3.
Voltage dependence of sodium channel gating is stable during loose patch recording when muscle fibers are not impaled during the recording. A: superimposed sodium current traces evoked by 10-ms pulses ranging from −80 to −10 mV. Shown are records from a muscle fiber 0 and 30 min after formation of a seal. At time 0 the first current is evoked by a step to −50 mV. At 30 min the first current is still evoked by a step to −50 mV. At 30 min the current evoked by a step to −40 mV (shaded trace) is slightly larger than at time 0. The patch was held at −110 mV between voltage protocols. One difference between the current traces shown in this figure, and those shown in Fig. 1 is the amplitude of the outward potassium current. In Fig. 1 no potassium current is present, whereas in the traces shown here, large potassium currents are evoked. We have previously reported that potassium current amplitudes are highly variable from patch to patch (10) and this did not appear to be related to impalement. B: superimposed sodium current inactivation records from the same fiber as in A and at the same times following impalement as in A. The currents were evoked by a step to −30 mV following prepulses ranging from −130 to −40 mV. Both at time 0 and at 30 min, a prepulse to −70 mV (shaded trace) inactivates slightly more than half of the sodium channels. C: normalized sodium conductance calculated from the currents shown in A with an assumed sodium reversal potential of +45 mV plotted versus membrane potential. The data are fitted with a Boltzman distribution at each time point. In the example shown there is a 4-mV hyperpolarized shift in the midpoint of activation over the course of the recording. D: normalized current amplitude of the currents shown in B are plotted and fitted with a Boltzman distribution at each time point. There is no shift in the midpoint of inactivation during the recording.
Elevation of intracellular Ca2+ following injury.
It was recently reported that elevation of intracellular Ca2+ triggers greater than 30-mV hyperpolarized shifts in the voltage dependence of potassium channel activation in neurons (25, 27). The mechanism underlying the shifts is calcineurin-dependent dephosphorylation of channels (27). Phosphorylation of Nav 1.4 sodium channels has been shown to shift the voltage dependence of activation (3, 26). To determine whether Ca2+-dependent regulation of Nav 1.4 might underlie the hyperpolarized shift in gating following impalement, we examined whether impalement-induced injury triggered a sustained elevation of intracellular Ca2+. Muscle fibers were imaged before and at various times following impalement using Fluo4 NW as an indicator of intracellular Ca2+ levels (24).
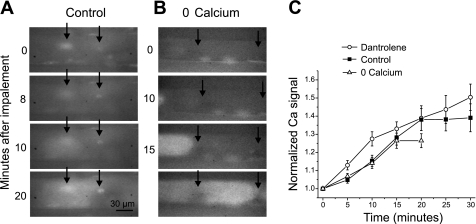
We found that impalement of fibers with two sharp electrodes caused an immediate increase in Ca2+ at the sites of impalement (Fig. 4A). At some impalement sites, the increase in intracellular Ca2+ remained localized throughout the 30-min recording. However, at other impalement sites, the increase in intracellular Ca2+ gradually spread throughout the fiber. Both the spatial and temporal rate of spread of the Ca2+ signal were variable as shown in Fig. 4, A and B. In the example shown in Fig. 4A, the Ca2+ signal at the right electrode remains relatively localized to the site of impalement throughout the recording, whereas the Ca2+ signal near the left electrode gradually spread throughout the fiber. In this example, the right electrode was used to pass current, whereas the left electrode was used to sense voltage. No consistent relationship between electrode function, and the spread of the Ca2+ signal was noted. Since areas of increased Ca2+ signal often occurred away from sites of impalement, it appeared likely that release of Ca2+ from intracellular stores played an important role in the increase in intracellular Ca2+. We measured the time course of the increase in global intracellular Ca2+ at 5-min intervals by averaging the pixel intensity throughout each fiber imaged. On average, the increase in global intracellular Ca2+ following impalement continued for 20 min and then stabilized (Fig. 4C, P < 0.01 Ca2+ signal at 0 vs. 20 min, n = 14).
Fig. 4.
Elevation of intracellular Ca2+ following impalement. A: images of the Ca2+ signal from a muscle fiber loaded with Fluo4 NW in control solution (containing 2 mM external Ca2+) at various times following impalement. At the time of impalement, two areas of increased intracellular Ca2+ are present in the muscle fiber at the sites of impalement (arrows). Over time, there is spread in the elevation of intracellular Ca2+ from the site of impalement on the left. At the site of impalement on the right, however, the elevation in intracellular Ca2+ remains localized. By 20 min, the elevation in intracellular Ca2+ is such that the upper and lower edges of the muscle fiber can be visualized. B: images of the Ca2+ signal from a muscle fiber in solution containing 0 Ca2+ (no added Ca2+ and 5 mM EGTA) at various times following impalement. At time 0 there is no increase of Ca2+ signal at the sites of impalement (arrows). At 10 min, the leading edge of a slow-moving Ca2+ wave is visible at the left side of the image. By 15 min the Ca2+ wave has reached the left electrode. By 20 min a different wave that started between the electrodes has brightened the fiber throughout the field of view. C: plots of the time course of the global increase in the normalized Ca2+ signal following impalement for muscle fibers loaded with Fluo4 NW. Shown are data for muscle fibers impaled in solution containing 2 mM external Ca2+ (control, solid squares, n = 14), muscle fibers impaled in solution with no added Ca2+ and 5 mM EGTA (0 Ca2+, open triangles, n = 13) and muscle fibers impaled in solution containing dantrolene (12 μM, open circles, n = 12). The plot for fibers in solution with no added Ca2+ only goes to 20 min due to the difficulty of maintaining prolonged impalements in solution containing no added Ca2+.
We examined whether the global elevation in intracellular Ca2+ was triggered by entry of external Ca2+. When fibers were impaled while bathed in solution containing no added external Ca2+ and 5 mM EGTA, no elevation of intracellular Ca2+ occurred at sites of impalement (Fig. 4B), indicating that entry of external Ca2+ was responsible for the local increases in Ca2+ concentration. Despite the lack of local increase in intracellular Ca2+, the average global intracellular Ca2+ increased with a time course similar to the increase following impalement in solution containing normal extracellular Ca2+ (Fig. 4, B and C, Ca2+ signal P < 0.01 0 vs. 20 min, n = 13).
The two systems that regulate levels of intracellular Ca2+ in skeletal muscle are the sarcoplasmic reticulum and mitochondria (13). We inhibited ryanodine receptor-mediated Ca2+ release from the sarcoplasmic reticulum using 12 μM dantrolene, a dose previously used to block action potential-mediated muscle fiber contraction (21, 31). Despite the ability of dantrolene to block action potential-induced flashes of Fluo4 NW signal, it did not prevent global increase in intracellular Ca2+ following impalement of fibers (Fig. 4C, P < 0.01 at 20 min, n = 12). This argues against ryanodine receptor-mediated release from the sarcoplasmic reticulum as the source of the increase in intracellular Ca2+ (19, 21, 38). This suggests the intracellular Ca2+ store contributing to the global increase in intracellular Ca2+ following impalement-induced injury is mitochondrial Ca2+.
Role of elevation of intracellular Ca2+ in triggering changes in sodium current.
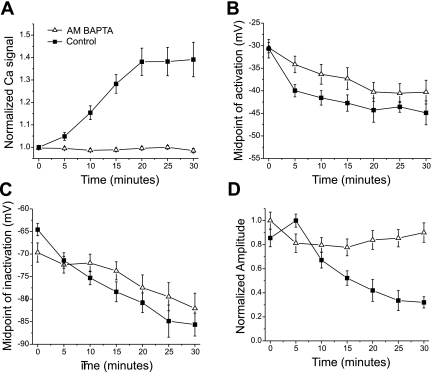
To determine whether elevation of intracellular Ca2+ triggered the hyperpolarized shift in sodium current gating following impalement, we prevented the increase by loading muscle with AM-BAPTA. Loading muscle fibers with AM-BAPTA did not prevent action potential-induced flashes in Fluo4 NW signal but did prevent impalement-induced increases in intracellular Ca2+ in 15/21 fibers. We discarded fibers in which intracellular Ca2+ increased following impalement despite loading with AM-BAPTA. In the selected fibers, no increase in Fluo4 NW signal occurred in the 30 min following impalement (Fig. 5A). Although loading with AM-BAPTA appeared to slow the initial rate of hyperpolarized shifts, after 30 min the voltage dependence of both activation and fast inactivation had shifted (Fig. 5, B and C, 9.0 ± 2.3 mV for activation, P < 0.01 and 10.9 ± 4.2 mV, P < 0.05 for fast inactivation, n = 15). Thus it appeared that whereas elevation of intracellular Ca2+ may speed the induction of hyperpolarized shifts, it is not essential.
Fig. 5.
Effect of buffering intracellular Ca2+ on impalement-induced changes in sodium current. A: plot of the time course of increase in the normalized Ca2+ signal following impalement for control fibers and selected fibers loaded with AM-BAPTA (n = 15). B: mean midpoints of the Boltzman distribution for sodium channel activation in control fibers and fibers loaded with AM-BAPTA plotted versus time following impalement. C: mean midpoints of sodium channel inactivation in control fibers and fibers loaded with AM-BAPTA plotted versus time following impalement. D: mean normalized sodium current amplitude versus time following impalement. When patches were held at −80 mV without loading with AM-BAPTA, there was a large reduction in sodium current amplitude following impalement (P < 0.01 vs. initial current amplitude). Loading fibers with AM-BAPTA lessened the reduction in sodium current amplitude following impalement (P < 0.01 vs. control at 30 min). Holding potential for all patches used to generate data shown in B–D was −80 mV. Control plot in A is from Fig. 4 and control plots in B and C are from Fig. 2.
In contrast, blocking elevation of intracellular Ca2+ prevented the reduction in current amplitude that occurred following impalement of muscle fibers (see Fig. 1 at 60 min). Studying current amplitude when holding at −110 mV is complicated by an initial increase in current amplitude due to relief of slow inactivation (17, 34, 40), which is later followed by a decrease in amplitude (Fig. 1). Thus to study changes in current amplitude, patches were held at −80 mV. When holding at −80 mV, impalement induced significant reductions in maximal sodium current amplitude within 30 min (Fig. 5D, P < 0.01). Blocking elevation of intracellular Ca2+ prevented the reduction of current amplitude during the 30-min recording period (Fig. 5D, P < 0.01 vs. control at 30 min).
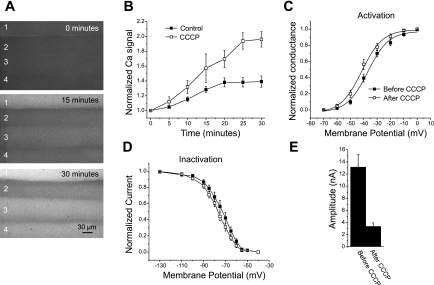
To test whether elevated intracellular Ca2+ was sufficient to trigger reduction in sodium current amplitude, intracellular Ca2+ was elevated in the absence of impalement-induced damage. We used the mitochondrial inhibitor CCCP (2) to raise intracellular Ca2+. The elevation of intracellular Ca2+ following application of CCCP was larger than that following impalement but had a similar time course (Fig. 6, A and B). Despite loading muscle with BTS to prevent muscle contraction (23), during application of CCCP muscle movement occurred that made it difficult to hold seals on muscle fibers to follow sodium currents over time. By 30 min after application of CCCP, muscle movement lessened, and it was possible to form seals and briefly record sodium currents. We recorded sodium currents from several fibers in each muscle at a holding potential of −110 mV before application of CCCP without impalement (assuming a resting potential of −80 mV). Each fiber was imaged so that it could be relocated, the patch pipette was removed, and CCCP was applied. After muscle movement decreased, fibers were relocated, seals were formed, sodium currents were recorded, and fibers were impaled to determine resting potential. Resting potentials following application of CCCP (−80.7 ± 1.1, n = 29) were similar to those we previously reported in rat extensor digitorum longus muscle (29, 31). There were small hyperpolarized shifts of about 4 mV in the average midpoints of activation and fast inactivation (Fig. 6, C and D, P < 0.05 for activation and fast inactivation, n = 16). The most dramatic effect of application of CCCP was reduction of sodium current amplitude (Fig. 6E). In 13 of 29 fibers, the current amplitude following application of CCCP was too small to determine the voltage dependence of either activation or fast inactivation. Currents averaged only 25% of the initial amplitude (Fig. 6E, P < 0.01).
Fig. 6.
Effect of elevation of intracellular Ca2+ on sodium current in the absence of injury. A: images of muscle fibers (1–4) loaded with Fluo4 NW at the time of application of CCCP (0 min) and 15 and 30 min later. The increase in Fluo4 NW signal is the most rapid and largest in fiber 1. However, all 4 fibers have significant elevation of intracellular Ca2+. B: plot of the time course of increase in the normalized Ca2+ signal in control fibers following impalement versus fibers treated with CCCP but not impaled (n = 28). The increase in Ca2+ level was much greater following application of CCCP but had a similar time course to the increase following impalement. C: voltage dependence of activation before (solid squares) and after (open squares) treatment with CCCP. A shift in the midpoint of activation from −35.4 ± 1.3 mV to −39.7 ± 1.5 mV was present following application of CCCP (P < 0.05, n = 16). D: steady-state voltage dependence of inactivation of fibers before and after application of CCCP. The mean midpoint of inactivation shifted from −74.1 ± 2.0 mV to −78.1 ± 1.3 mV following application of CCCP (P < 0.05, n = 16). E: sodium current amplitude before and after application of CCCP. The same patch pipette was used to record current before and after CCCP for each fiber. The mean current amplitude fell by close to 75% (P < 0.01).
DISCUSSION
Impalement-induced injury of muscle triggered hyperpolarized shifts in the voltage dependence of sodium channel activation and fast inactivation and caused reduction in maximal current amplitude. Elevation in intracellular Ca2+ following injury appears to trigger the reduction in current amplitude but not the hyperpolarized shifts in activation and fast inactivation. Our data are consistent with the existence of at least two pathways that reduce sodium current following muscle injury. One pathway is Ca2+ dependent and regulates current amplitude; the second pathway is largely Ca2+ independent and regulates the voltage dependence of activation and fast inactivation.
Potential mechanisms underlying the hyperpolarized shifts in gating of Nav1.4 sodium channels.
The only sodium channel present to a significant degree in mature skeletal muscle is Nav1.4 (45). The rapid time course of the hyperpolarized shift in gating of the sodium channels following impalement makes it unlikely that synthesis of a new sodium channel isoform or a change in glycosylation (which requires insertion of new channels in the membrane) underlie the shift. We thus favor the possibility that the hyperpolarized shift in gating of sodium channels is due to modulation of Nav1.4 channels that were already present in the sarcolemma before injury of muscle fibers.
There are three other situations in which gating of Nav1.4 is hyperpolarized. The first is in the muscle disease critical illness myopathy, in which reduced excitability of muscle results in paralysis (28, 32). The cause of reduced excitability is increased inactivation of sodium channels that is due, in part, to a 14-mV hyperpolarized shift in the voltage dependence of Nav 1.4 sodium channel inactivation that is accompanied by a similar shift in the voltage dependence in activation (11, 29, 30). The second situation in which gating of Nav1.4 (and other sodium channel isoforms) is hyperpolarized is following initiation of tight seal formation and whole cell patch-clamp recordings in vitro (9, 15, 22, 42). The third is following stretch of membrane in oocytes expressing Nav1.4 (37, 39). The magnitude of hyperpolarized shifts in activation and fast inactivation are similar in all three situations. However, stretch-induced shifts in gating of Nav 1.4 are accompanied by dramatic increase in the kinetics of inactivation. This suggests that the mechanism underlying stretch-induced shifts in oocytes differs from that underlying the shifts in other situations.
It is possible that a single mechanism underlies the hyperpolarized gating of Nav1.4 following impalement in critical illness myopathy and during whole cell recording. There are little data to guide speculation about the mechanism underlying the shift of gating of Nav1.4 sodium channels in critical illness myopathy. However, there are data following impalement and during whole cell recording to suggest they could be due to the same mechanism. Our data suggest that the hyperpolarized shift in gating of sodium channels following impalement is Ca2+ independent. The hyperpolarized shift in gating of sodium channels in the whole cell mode is also Ca2+ independent (15, 42). The finding that the shifts in these cases are Ca2+ independent suggests that regulation of sodium channels by calmodulin (8, 16, 46), Ca2+-dependent proteolysis of sodium channels (20, 44), and direct binding of Ca2+ to sodium channels (43) are not causes of the shifts.
After establishment of a whole cell recording there is significant dialysis of intracellular molecules. After impalement of muscle fibers, there is little, if any, dialysis of internal solutes. Thus if the same mechanism underlies the shifts, it is unlikely to involve dialysis of soluble signaling molecules. There is no signaling cascade we are aware of that has been shown to alter the voltage dependence of sodium channel activation and fast inactivation, which does not involve soluble signaling cascades and is Ca2+ independent. This suggests that either a novel mechanism is involved or that more than one pathway combine to trigger the shifts in both activation and fast inactivation. Our work raises the possibility that determining the mechanism underlying the hyperpolarized shift in gating in the whole cell configuration may have in vivo implications for sodium channel function.
Reduced sodium current amplitude following impalement.
Two findings suggest the reduction in sodium current amplitude following injury is triggered by elevation of intracellular Ca2+. First, loading fibers with AM-BAPTA prevented the reduction in current amplitude. Second, elevation of intracellular Ca2+ by application of CCCP in the absence of injury triggered a large reduction in current amplitude. One mechanism that might underlie reduced current amplitude is an increase in slow inactivation (5–7, 36). However, in patches held at −110 mV the decrease in current amplitude still occurred (Fig. 1 and data not shown). The finding that holding at more hyperpolarized potentials did not prevent the decrease in current amplitude suggests either removal of channels from the membrane or a secondary modification that shuts down channel function underlies the decrease. Phosphorylation of Nav 1.4 sodium channels in vitro via protein kinase C has been shown to reduce sodium current amplitude (3, 26, 33) and might underlie the changes we see ex vivo.
Functional consequences of injury-induced changes in Nav 1.4 sodium current.
A hyperpolarized shift in the voltage dependence of fast inactivation together with a reduction in current amplitude following injury may serve to promote survival of muscle fibers. It has recently been shown that muscle injury following either prolonged exercise or osmotic stress leads to increased intracellular Ca2+ (12, 24). Our model of muscle injury also triggered a sustained increase in intracellular Ca2+. Increases in intracellular Ca2+ may trigger apoptosis or necrosis of muscle fibers so to promote survival it is likely necessary for muscle to limit the rise in intracellular Ca2+ (13). The hyperpolarized shift in fast inactivation and the reduction in current amplitude reduce the number of functional sodium channels available to open at the resting potential of injured fibers. Reducing the number of functional sodium channels limits sodium entry, reduces further depolarization, and thus reduces both the opening of surface membrane Ca2+ channels and Ca2+ release from intracellular stores (4). In this way, shutting down sodium channels may help injured muscle to survive. Whereas the reduction in number of sodium channels available for opening at the resting potential of an injured muscle fiber may promote survival of muscle, it also reduces excitability. There is evidence of reduced excitability in amphibian muscle following fatiguing stimulation or alterations of intracellular Ca2+ (41). Thus reduction in the density of sodium channels available to open at the resting potential following injury of muscle may promote survival of fibers, but at the cost of reducing excitability.
In summary, we report that following injury of muscle there are rapid hyperpolarized shifts in the voltage dependence of sodium channel activation and fast inactivation as well as reduction in current amplitude. Our data are the first to show that gating of Nav 1.4 can be rapidly modulated ex vivo. If other sodium channel isoforms undergo similar modulation in vivo, it could have significant effects on excitability following injury and in various disease states.
GRANTS
This work was supported by National Institutes of Health Grant NS-040826 (to M. M. Rich).
REFERENCES
- 1.Almers W, Roberts WM, Ruff RL. Voltage clamp of rat and human skeletal muscle: measurements with an improved loose-patch technique. J Physiol 347: 751–768, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balemba OB, Bartoo AC, Nelson MT, Mawe GM. Role of mitochondria in spontaneous rhythmic activity and intracellular calcium waves in the guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol 294: G467–G476, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bendahhou S, Cummins TR, Potts JF, Tong J, Agnew WS. Serine-1321-independent regulation of the mu 1 adult skeletal muscle Na+ channel by protein kinase C. Proc Natl Acad Sci USA 92: 12003–12007, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80: 1215–1265, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci 2: 397–407, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron 39: 793–806, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron 49: 409–420, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF. Isoform-specific modulation of voltage-gated Na(+) channels by calmodulin. Circ Res 90: E49–57, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol 331: 599–635, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filatov GN, Pinter MJ, Rich MM. Resting potential-dependent regulation of the voltage sensitivity of sodium channel gating in rat skeletal muscle in vivo. J Gen Physiol 126: 161–172, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filatov GN, Rich MM. Hyperpolarized shifts in the voltage dependence of fast inactivation of Nav1.4 and Nav15 in a rat model of critical illness myopathy. J Physiol 559: 813–820, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredsted A, Gissel H, Madsen K, Clausen T. Causes of excitation-induced muscle cell damage in isometric contractions: mechanical stress or calcium overload? Am J Physiol Regul Integr Comp Physiol 292: R2249–R2258, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gissel H The role of Ca2+ in muscle cell damage. Ann NY Acad Sci 1066: 166–180, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Goldin AL Resurgence of sodium channel research. Annu Rev Physiol 63: 871–894, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Gonoi T, Sherman SJ, Catterall WA. Voltage clamp analysis of tetrodotoxin-sensitive and -insensitive sodium channels in rat muscle cells developing in vitro. J Neurosci 5: 2559–2564, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzog RI, Liu C, Waxman SG, Cummins TR. Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav16 and differentially modulates their functional properties. J Neurosci 23: 8261–8270, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilber K, Sandtner W, Kudlacek O, Glaaser IW, Weisz E, Kyle JW, French RJ, Fozzard HA, Dudley SC, Todt H. The selectivity filter of the voltage-gated sodium channel is involved in channel activation. J Biol Chem 276: 27831–27839, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Hille B Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer, 1992.
- 19.Ikemoto T, Hosoya T, Aoyama H, Kihara Y, Suzuki M, Endo M. Effects of dantrolene and its derivatives on Ca(2+) release from the sarcoplasmic reticulum of mouse skeletal muscle fibres. Br J Pharmacol 134: 729–736, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF, Smith DH. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci 24: 4605–4613, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene–a review of its pharmacology, therapeutic use and new developments. Anaesthesia 59: 364–373, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kunze DL, Lacerda AE, Wilson DL, Brown AM. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol 86: 691–719, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macdonald WA, Pedersen TH, Clausen T, Nielsen OB. N-Benzyl-p-toluene sulphonamide allows the recording of trains of intracellular action potentials from nerve-stimulated intact fast-twitch skeletal muscle of the rat. Exp Physiol 90: 815–825, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol 586: 197–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci 7: 711–718, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Numann R, Hauschka SD, Catterall WA, Scheuer T. Modulation of skeletal muscle sodium channels in a satellite cell line by protein kinase C. J Neurosci 14: 4226–4236, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science 313: 976–979, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Rich MM, Bird SJ, Raps EC, McCluskey LF, Teener JW. Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve 20: 665–673, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Rich MM, Pinter MJ. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol 547: 555–566, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich MM, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol 50: 26–33, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Rich MM, Pinter MJ, Kraner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy [see comments]. Ann Neurol 43: 171–179, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Rich MM, Teener JW, Raps EC, Schotland DL, Bird SJ. Muscle is electrically inexcitable in acute quadriplegic myopathy [see comments]. Neurology 46: 731–736, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 60: 145–153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruff RL Effects of temperature on slow and fast inactivation of rat skeletal muscle Na(+) channels. Am J Physiol Cell Physiol 277: C937–C947, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Ruff RL, Simoncini L, Stuhmer W. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve 11: 502–510, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Scheuer T, Catterall WA. Control of neuronal excitability by phosphorylation and dephosphorylation of sodium channels. Biochem Soc Trans 34: 1299–1302, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Shcherbatko A, Ono F, Mandel G, Brehm P. Voltage-dependent sodium channel function is regulated through membrane mechanics. Biophys J 77: 1945–1959, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szentesi P, Collet C, Sarkozi S, Szegedi C, Jona I, Jacquemond V, Kovacs L, Csernoch L. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol 118: 355–375, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabarean IV, Juranka P, Morris CE. Membrane stretch affects gating modes of a skeletal muscle sodium channel. Biophys J 77: 758–774, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todt H, Dudley SC Jr, Kyle JW, French RJ, and Fozzard HA. Ultra-slow inactivation in mu1 Na+ channels is produced by a structural rearrangement of the outer vestibule. Biophys J 76: 1335–1345, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usher-Smith JA, Xu W, Fraser JA, Huang CL. Alterations in calcium homeostasis reduce membrane excitability in amphibian skeletal muscle. Pflügers Arch 453: 211–221, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Wang DW, George AL Jr, and Bennett PB. Comparison of heterologously expressed human cardiac and skeletal muscle sodium channels. Biophys J 70: 238–245, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wingo TL, Shah VN, Anderson ME, Lybrand TP, Chazin WJ, Balser JR. An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nat Struct Mol Biol 11: 219–225, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci 21: 1923–1930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang JS, Sladky JT, Kallen RG, Barchi RL. TTX-sensitive and TTX-insensitive sodium channel mRNA transcripts are independently regulated in adult skeletal muscle after denervation. Neuron 7: 421–427, 1991. [DOI] [PubMed] [Google Scholar]
- 46.Young KA, Caldwell JH. Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin. J Physiol 565: 349–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]