Abstract
The dorsal striatum, which consists of the caudate and putamen, is the gateway to the basal ganglia. It receives convergent excitatory afferents from cortex and thalamus and forms the origin of the direct and indirect pathways—distinct basal ganglia circuits involved in motor control. It is also a major site of activity-dependent synaptic plasticity. Striatal plasticity alters the transfer of information throughout basal ganglia circuits and may represent a key neural substrate for adaptive motor control and procedural memory. Here, we review current understanding of synaptic plasticity in the striatum and its role in the physiology and pathophysiology of basal ganglia function.
Introduction
The basal ganglia consist of interconnected subcortical nuclei that serve critical motivation, motor planning, and procedural learning functions (Graybiel et al., 1994; Hikosaka et al., 2000; Nicola, 2007; Packard and Knowlton, 2002; Yin and Knowlton, 2006). Neural circuits involving the basal ganglia form a key part of the extrapyramidal motor system, and dysfunction of these circuits is associated with prominent neurological disorders including Parkinson's disease (PD) and Huntington's disease (HD) (Albin et al., 1989; DeLong, 1990; DeLong and Wichmann, 2007; Graybiel, 2000), as well as psychiatric disorders such as obsessive-compulsive disorder (OCD) (Aouizerate et al., 2004; Graybiel and Rauch, 2000). The primary input nucleus is the striatum, which receives excitatory afferents from the cortex and thalamus, as well as dense innervation from midbrain dopamine neurons, and represents a major site of synaptic plasticity in the basal ganglia (Bolam et al., 2000; Gerdeman et al., 2003; Gerfen, 2000; Wilson, 1998).
For such a large nucleus, the striatum is unique in its complete lack of glutamatergic neurons. Instead, most cells are GABAergic, including a large population of principal cells and small interneuron population. Striatal GABAergic interneurons can be divided into at least two classes, based on their physiological properties: (1) fast-spiking (corresponding to histochemically-identified parvalbumin-positive cells), and (2) low-threshold spiking (corresponding to histochemically-identified somatostatin-, nitric oxide synthase-, and neuropeptide Y-positive cells; also potentially calretinin-positive interneurons) (Kawaguchi et al., 1995; Tepper and Bolam, 2004). The striatum also contains a small population of giant cholinergic interneurons distinguished by their large cell bodies, tonic activity in vivo, and dense local axonal arborizations (Zhou et al., 2002). However, the vast majority of striatal neurons are medium spiny neurons (MSNs), characterized by their high spine density, negative resting potential, and low firing rates in vivo. They can be categorized into at least two different subtypes, based on their gene expression and axonal projections (Gerfen et al., 1990; Smith et al., 1998). Striatonigral MSNs exhibit high expression of dopamine D1 and muscarinic M4 receptors and project directly to basal ganglia output nuclei—the internal globus pallidus (GPi in primates, GPm in rodents) and substantia nigra pars reticulata (SNr). In contrast, striatopallidal MSNs exhibit high expression of dopamine D2 and adenosine A2A receptors and send axons to the external globus pallidus (GPe in primates, GP in rodents). Both types of MSN integrate vast numbers of inputs to generate spikes that represent the sole output of the striatum to downstream basal ganglia nuclei. Synaptic plasticity in the striatum is thus well-suited for regulating basal ganglia circuit activity.
The most influential model of basal ganglia circuit function is based on the segregation of information processing into direct and indirect pathways (Figure 1), which act in opposing ways to control movement (Albin et al., 1989; Alexander and Crutcher, 1990; DeLong, 1990). In its simplest anatomical form, it describes two parallel cortex-basal ganglia-thalamus-cortex loops that diverge within the striatum and are differentially modulated by dopamine. In more complex schemes, these circuits are integrated within an ascending hierarchy of open interconnected loops emerging from limbic cortex and ventral striatum into associative and motor regions (Haber et al., 2000; Joel and Weiner, 1994; Parent, 1990).
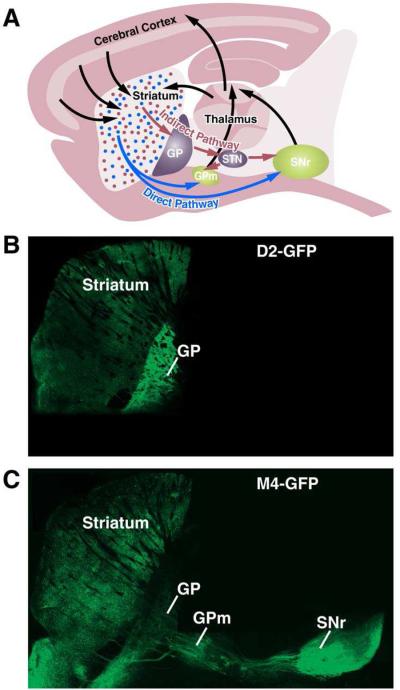
Figure 1. Direct and indirect pathway basal ganglia circuits.
Sagittal view of a mouse brain (A), depicting cortex-basal ganglia-thalamus-cortex circuits. Axons from the thalamus and striatum form excitatory synapses onto striatonigral/direct-pathway MSNs (blue) and striatopallidal/indirect-pathway MSNs (red). Direct pathway MSNs send axons directly to basal ganglia output nuclei (medial globus pallidus, GPm, and substantia nigra, pars reticulata, SNr), where they form inhibitory synapses. Indirect-pathway MSNs inhibit neurons in the globus pallidus (GP), which in turn make inhibitory connections with the subthalamic nucleus (STN). STN projections target the GPm and SNr, where they form excitatory synapses onto GABAergic basal ganglia output neurons. These inhibitory output neurons send axons to ventroposterior thalamic motor nuclei. Finally, glutamatergic neurons in the thalamus project back to cortex, completing the circuit. A sagittal slice from a BAC-transgenic mouse expressing GFP under the control of genomic regulatory elements for the dopamine D2 receptor (D2-GFP) (B), or the muscarinic M4 receptor (M4-GFP) (C), which labels indirect- and direct-pathway MSNs, respectively. Figure inspired by (Gerfen, 2006).
The direct-pathway circuit originates from striatonigral MSNs, which receive excitatory glutamatergic afferents from sensorimotor cortex and thalamus. Direct-pathway MSNs project to GABAergic neurons in the GPi and SNr, which in turn send axons to motor nuclei of the thalamus. In a simplified “rate model” of basal ganglia function, in which neural information is encoded by firing rate alone, the net effect of direct pathway activity is a disinhibition of excitatory thalamocortical projections, leading to activation of cortical premotor circuits and the selection or facilitation of movement.
The indirect-pathway circuit originates from striatopallidal MSNs. Indirect-pathway MSNs form inhibitory synapses on GABAergic pallidal neurons, which in turn project to glutamatergic neurons in the subthalamic nucleus (STN). Subthalamic neurons send axons to basal ganglia output nuclei (GPi and SNr), where they form excitatory synapses on the inhibitory output neurons. The net effect of indirect pathway activity is thought to involve an inhibition of thalamocortical projection neurons, which would reduce cortical premotor drive and inhibit movement.
An important aspect of this model is the role of dopamine in regulating direct and indirect pathway activity through modulation of MSN firing in the striatum. Gs-coupled dopamine D1 receptors are proposed to facilitate MSN output, whereas Gi-coupled dopamine D2 receptors inhibit MSN firing. Thus, dopamine has been proposed to exert opposite effects on the direct and indirect pathway (Surmeier et al., 2007), but in both cases ultimately enhances movement (Albin et al., 1989).
While aspects of this scheme are oversimplified and not consistent with all the functional and anatomical data, it has nevertheless proven extremely valuable in guiding both basic and clinical investigations into basal ganglia function. However, despite its simplicity it has proven difficult to test empirically due to the anatomical complexity of the striatum. Recently the development of BAC transgenic mice has enabled the dissection of striatal circuitry with unprecedented specificity. In this review, we focus on the role of synaptic plasticity in regulating striatal output through the direct and indirect pathways. In addition to the well-characterized glutamatergic synapses on MSNs, we also consider plasticity at interneuronal synapses and dendritic integration of excitation and inhibition in MSNs. Although much remains speculative, we review potential roles for synaptic plasticity in striatal function and dysfunction. The ventral striatum (nucleus accumbens) and its role in motivation, reward, and addiction has been widely described elsewhere (Berridge, 2007; Hyman et al., 2006; Kalivas and Volkow, 2005; Kenny, 2007; Nicola, 2007) and will not be covered, except in instances where analogies or extrapolations to dorsal striatum can be drawn.
Synaptic plasticity in the striatum
Synaptic modification represents a fundamental mechanism for altering neural circuit function. In the striatum, MSN firing output to the direct and indirect pathways is influenced by fast excitatory and inhibitory synaptic inputs as well as slower modulation by dopamine and other signaling molecules. Whereas the spiking of glutamatergic afferents originates outside of the striatum, inhibitory inputs are controlled within the striatum (Gustafson et al., 2006; Tepper et al., 2004). Synaptic plasticity at multiple sites within the striatal microcircuit can therefore regulate striatal output (Figure 2).
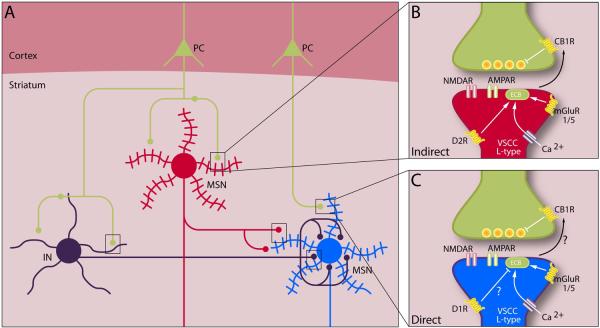
Figure 2. Synaptic plasticity in the striatum.
(A) Simplified schematic of striatal neurons and their interconnections. Cortical pyramidal neurons (green) project to striatal interneurons (INTs) and medium spiny neurons (MSNs) of the direct (blue) and indirect (red) pathways. Interneurons also form synapses on medium spiny neurons. Rectangles highlight potential sites of synaptic plasticity that could alter striatal output from MSNs. Corticostriatal synapses on direct and indirect pathway MSNs are expanded at right. (B) Indirect-pathway spines contain dopamine D2 receptors (D2R), group I mGluRs (mGluR1/5), and L-type voltage-sensitive calcium channels (VSCCs), which synergistically mobilize endocannabinoid (eCB) release that can induce presynaptic LTD by acting at cannabinoid receptors (CB1R). (C) Direct-pathway spines contain dopamine D1 receptors (D1R), group I mGluRs, and L-type VSCCs. Endocannabinoid-dependent LTD reportedly occurs at direct-pathway MSNs under conditions in which D1 receptors are not activated.
Cortico- and thalamostriatal synapses on MSNs
The majority of striatal physiology studies have examined synaptic transmission and plasticity at glutamatergic synapses on MSNs. Until recently, however, little attention was paid to the source of afferents (thalamus or cortex) or the MSN subtype targeted (direct- or indirect-pathway). Recent work has attempted to distinguish striatal afferents with mixed results. Paired-pulse and mean-variance analysis indicate that thalamic inputs exhibit higher release probability than cortical inputs at both direct- and indirect-pathway MSNs (Ding et al., 2008). However, a different study concluded that cortical synapses have a higher—not lower—release probability than thalamic synapses, based on analysis of paired-pulse ratios (Smeal et al., 2007). Moreover, the frequency of strontium-evoked asynchronous EPSCs was actually higher at cortical synapses than thalamic synapses (Ding et al., 2008). One factor confounding the interpretation of these experiments is that both cortical and thalamic projections are themselves heterogeneous (Lacey et al., 2007; Lei et al., 2004). Further study using more specific methods of stimulation will be required to characterize the properties of striatal afferents.
When the properties of excitatory synapses on direct- and indirect-pathway MSNs were examined using intrastriatal stimulation, synapses on indirect-pathway MSNs were found to exhibit lower paired-pulse ratios, indicative of a higher probability of glutamate release, relative to direct-pathway synapses (Ding et al., 2008; Kreitzer and Malenka, 2007). This finding suggests that synapses on indirect-pathway MSNs are more efficacious, a property that may partly underlie the relatively higher firing rates of indirect-pathway MSNs observed in vivo (Mallet et al., 2006). Interestingly, when stimulation was performed in either cortex or thalamus, no differences were observed in release probability between synapses on direct- and indirect-pathway MSNs (Ding et al., 2008), suggesting the possibility that different sets of afferents innervate direct- and indirect-pathway MSNs.
Despite the critical role of dopamine in striatal function, surprisingly there is no firm evidence for direct presynaptic modulation of glutamate release by dopamine in the dorsal striatum (Nicola et al., 2000). However, striatal dopamine D2 receptor activation mobilizes endocannabinoids (Giuffrida et al., 1999; Kreitzer and Malenka, 2005), which are released from MSNs and exert apparent presynaptic effects (Yin and Lovinger, 2006) that may account for some of the reported actions of dopamine on synaptic vesicle cycling (Bamford et al., 2004; Bamford et al., 2008).
LTD at excitatory synapses on MSNs
High-frequency stimulation (HFS) of excitatory striatal afferents in vitro leads to a long-lasting reduction in synaptic strength at MSN synapses (Calabresi et al., 1992a; Lovinger et al., 1993; Walsh, 1993) that is initiated postsynaptically, but expressed through a presynaptic reduction in neurotransmitter release (Choi and Lovinger, 1997). HFS-induced striatal LTD requires dopamine D2 receptors, Gq-coupled group I mGluRs, L-type calcium channels, and CB1 receptor activation, but notably, not NMDA receptors or mAChRs (Calabresi et al., 1992a; Calabresi et al., 1997; Choi and Lovinger, 1997; Gerdeman et al., 2002; Kreitzer and Malenka, 2005; Sung et al., 2001).
The basic model that has emerged from these results is that HFS in or near the dorsolateral striatum stimulates both glutamatergic and dopaminergic fibers. HFS-induced elevations of glutamate activate postsynaptic mGluRs, while increases in dopamine activate D2 receptors. In field recordings, HFS also depolarizes MSNs enough to activate L-type calcium channels, whereas in whole-cell voltage-clamp recordings, MSN depolarization using cesium-filled electrodes is required to trigger LTD. Activation of mGluRs and L-type channels leads to endocannabinoid production and release, possibly through phospholipase Cβ, which is both Gq-receptor-dependent and calcium-sensitive (Hashimotodani et al., 2005). Dopamine D2 receptor activation enhances the production of endocannabinoids (Giuffrida et al., 1999), which are released from MSNs and activate CB1 receptors on excitatory presynaptic terminals for several minutes (Ronesi et al., 2004), leading to the induction of presynaptic LTD. Some studies have also suggested a role for dopamine D1 receptors, nitric oxide release from interneurons, and DARPP-32 (Calabresi et al., 2000; Calabresi et al., 1999), although it is unclear how these signaling molecules relate to endocannabinoid signaling and presynaptic inhibition of neurotransmitter release.
One major question is whether LTD is expressed in both types of MSNs. The apparent requirement for D2 receptor activation in HFS-LTD might suggest that indirect-pathway MSNs selectively express LTD, given their high expression of D2 receptors. However, the first study to examine this issue found HFS-LTD at both direct- or indirect-pathway MSNs. In these experiments, higher-intensity macrostimulation was applied in the white matter separating cortex from striatum, which likely gives rise to broad and diffuse activation of striatal neurons. The requirement for D2 receptor activation was attributed to their inhibitory actions at cholinergic interneurons (Wang et al., 2006). In this model, D2 receptor activation transiently inhibits release of ACh (Maurice et al., 2004; Yan et al., 1997), which in turn reduces cholinergic tone and relieves muscarinic M1 receptor-mediated inhibition of L-type calcium channels present in both types of MSN (Howe and Surmeier, 1995), thus enabling endocannabinoid release. The same group also observed endocannabinoid-dependent LTD at both direct- and indirect-pathway MSNs, using intrastriatal microstimulation and a spike-timing-dependent plasticity (STDP) protocol (Shen et al., 2008). However, this STDP-LTD in direct-pathway MSNs could only be observed under conditions in which dopamine D1 receptors were blocked or not activated. Unlike HFS-LTD elicited using macrostimulation, STDP-LTD in direct-pathway MSNs did not require D2 receptor activation (Wang et al., 2006). STDP-LTD in indirect-pathway MSNs, on the other hand, was readily elicited under standard conditions and did require D2 receptors (Shen et al., 2008), as previously reported for HFS-LTD.
An independent study of HFS-LTD using intrastriatal microstimulation obtained different results in that HFS induced robust LTD at indirect-pathway MSNs, but no LTD at direct-pathway MSNs (Kreitzer and Malenka, 2007). Moreover, direct activation of group I mGluRs gave rise to endocannabinoid-mediated inhibition in indirect- but not direct-pathway MSNs (Kreitzer and Malenka, 2007), suggesting that, independent of the type of induction protocol,indirect-pathway MSNs more readily release endocannabinoids in response to mGluR activation. It is difficult to completely reconcile the sets of results from the independent studies and thus the prominence of endocannabinoid release and the expression of HFS-LTD at direct-pathway MSNs remains a controversial topic.
What is agreed upon is that D2 receptors serve to enhance endocannabinoid release at indirect-pathway MSNs (Kreitzer and Malenka, 2007). One role of postsynaptic Gi-coupled D2 receptors may be to potentiate and extend endocannabinoid signaling during the critical time window following HFS, possibly through Gβ/γ signaling. Indeed, Gβ/γ subunits liberated following D2 receptor activation bind and activate PLCβ (Hernandez-Lopez et al., 2000). In support of this model, LTD is not induced by depolarization or brief application of group I mGluR agonists alone, both of which only transiently elevate endocannabinoids. However, brief application of group I mGluR agonists in the presence of a D2 agonist is sufficient to elicit LTD (Kreitzer and Malenka, 2007). Thus, dopamine mediates a form of long-lasting inhibition at indirect-pathway synapses, consistent with: (1) dopamine as an inhibitor of indirect pathway function (Albin et al., 1989), and (2) dopamine as a learning signal that gates synaptic plasticity (Schultz, 2002). In contrast, LTD at direct-pathway MSNs is reportedly blocked by increased dopamine (Shen et al., 2008), consistent with its role as a potentiator of direct pathway function (Albin et al., 1989).
The slow kinetics of mGluR-driven endocannabinoid release at indirect-pathway MSNs provide a mechanism, at least in theory, for how dopamine could regulate changes in synaptic plasticity that were initiated prior to the onset of the dopamine signal. In this scheme, activation of a particular motor program would yield striatal mGluR activation and MSN depolarization that would drive endocannabinoid release for several seconds. Yet this alone would not induce sufficient endocannabinoid release to elicit LTD (Chevaleyre et al., 2006; Ronesi et al., 2004). However, if dopamine levels increase in the striatum within a few seconds of the initiation of endocannabinoid signaling (perhaps in response to a positive behavioral outcome), the ongoing release of endocannabinoids would be augmented, LTD induction would occur, and the motor program that led to the positive outcome would be reinforced.
LTP at excitatory synapses on MSNs
The induction and expression of LTP in the dorsal striatum is less well characterized. Early studies indicated that an NMDA receptor-dependent form of LTP at excitatory MSN afferents could be elicited by HFS in the absence of extracellular magnesium (Calabresi et al., 1992b; Kerr and Wickens, 2001). It is perplexing however, why depolarization of MSNs alone is not sufficient to unblock NMDA receptors and trigger LTP, as has been observed in the hippocampus and other brain regions. In studies performed in the dorsomedial striatum, HFS was reported to induce an NMDA receptor-dependent LTP without removal of magnesium (Partridge et al., 2000), and in vivo, LTP has been elicited by pairing cortical stimulation with MSN depolarization (Charpier and Deniau, 1997). Dopamine D1-like receptors have been implicated in striatal LTP (Calabresi et al., 2000; Kerr and Wickens, 2001), while dopamine depletion has been reported to block LTP (Centonze et al., 1999). However, other studies demonstrate that striatal LTP can be induced in indirect-pathway MSNs of dopamine-depleted mice (Kreitzer and Malenka, 2007; Shen et al., 2008), suggesting that LTP mechanisms may differ in direct- and indirect-pathway MSNs. Unlike more extensively studied forms of LTP in the hippocampus (Malenka and Bear, 2004), striatal LTP has proven difficult to reliably elicit and therefore, little is known about the molecular pathways underlying its induction and expression, although PKA signaling has been implicated (Centonze et al., 2003a). More recently, LTP was studied at direct-and indirect-pathway MSNs using a spike-timing dependent plasticity protocol (Shen et al., 2008). Under these conditions, LTP could be elicted at both direct- and indirect-pathway MSNs. However, D1 receptors were required only in direct-pathway MSNs. LTP in indirect-pathway MSNs was independent of dopamine, and instead required Gs-coupled adenosine A2A receptors, consistent with the presence of LTP at indirect-pathway MSNs in dopamine-depleted mice (Kreitzer and Malenka, 2007; Shen et al., 2008).
Cortico- and thalamostriatal synapses on GABAergic interneurons
Corticostriatal and thalamostriatal axons form en passant synapses with multiple neurons across large regions of striatum, including interneurons (Kemp and Powell, 1971; Lapper and Bolam, 1992; Parent and Parent, 2006). Some of these synapses occur at GABAergic interneurons, which despite their small numbers can significantly influence MSN firing properties (Koos and Tepper, 1999). Although the cellular physiology of these interneurons has been described (Kawaguchi, 1993), little is known about the properties of their glutamatergic inputs. However, it is clear that fast-spiking interneurons respond more rapidly and readily to stimulation of excitatory afferents both in vitro (Plotkin et al., 2005) and in vivo (Mallet et al., 2005), making them well-suited for mediating feedforward inhibition. Interestingly, dopamine inhibits GABAergic inputs onto fast spiking interneurons via D2 receptor activation (Bracci et al., 2002), although the precise location of the D2 receptors and the possible role of endocannabinoid signaling in this inhibition has not been determined. Glutamatergic synapses onto striatal fast-spiking interneurons also exhibit LTP and LTD (Fino et al., 2008), suggesting that this synapse is a potentially important site for activity-dependent synaptic plasticity.
In the hippocampus, plasticity of glutamatergic synapses on aspiny interneurons has been well described (Buzsaki and Eidelberg, 1982; Gibson et al., 2008; Lamsa et al., 2007; McMahon and Kauer, 1997; Ouardouz and Lacaille, 1995), and it is possible that similar mechanisms underlie plasticity in the striatum. Of particular interest is the role of calcium-permeable AMPARs, which underlie a form of anti-Hebbian LTP in hippocampal interneurons (Lamsa et al., 2007) and have also been linked to endocannabinoid release from cerebellar interneurons (Soler-Llavina and Sabatini, 2006).
Interneuron synapses on MSNs
Different classes of GABAergic interneurons have been shown to form synapses on MSNs with different properties. Synapses originating from fast-spiking interneurons display a relatively low failure rate and paired-pulse depression of IPSCs, consistent with a high-probability of neurotransmitter release (Koos et al., 2004; Narushima et al., 2006). Synapses from low-threshold spiking interneurons are less well characterized, but one anatomical study found evidence of a consistently small active zone area that was independent of bouton size (Kubota and Kawaguchi, 2000). This was in contrast to parvalbumin-positive fast-spiking interneurons that exhibited larger active zones with increasing bouton volumes. Given the correlation between active zone size and release probability (Schikorski and Stevens, 1997), this may suggest that low-threshold spiking interneurons exhibit a lower release probability than fast-spiking interneurons. Short-term synaptic plasticity mediated by depolarization-evoked endocannabinoid release from MSNs has been observed at synapses originating from fast-spiking interneurons (Narushima et al., 2006). It will be important to test whether endocannabinoids can mediate LTD at inhibitory synapses, as has been demonstrated in the hippocampus (Chevaleyre and Castillo, 2003). In particular, differences in synaptic plasticity at inhibitory synapses on direct- or indirect-pathway MSNs could alter the relative output of these circuits and influence motor behavior.
The function of dopamine at inhibitory synapses on direct- and indirect-pathway MSNs also remains an open question. Dopamine was found to inhibit GABAergic currents in MSNs in a subpopulation of neurons (Delgado et al., 2000), perhaps due to differential effects on MSN subtypes. According to the classical model, inhibitory synapses onto direct-pathway MSNs might be inhibited by dopamine, whereas inhibition onto indirect-pathway MSNs might be enhanced by dopamine; these are predictions that need to be tested experimentally.
MSN synapses on MSNs
The vast majority of striatal neurons are MSNs, and anatomical studies long ago identified MSN axon collaterals that appeared to contact other MSNs (Somogyi et al., 1981; Wilson and Groves, 1980). However, these connections were weak and difficult to detect physiologically (Jaeger et al., 1994). It was eventually determined that only a fraction of MSNs are interconnected. MSN to MSN synapses are typically unidirectional, distally localized, and exhibit higher failure rates than synapses from fast spiking interneurons (Koos et al., 2004; Plenz, 2003; Taverna et al., 2004; Tunstall et al., 2002). Direct-pathway MSNs preferentially innervate other direct-pathway MSNs, whereas indirect-pathway MSNs innervate both subtypes equally (Taverna et al., 2008). Functionally, this suggests that increased indirect-pathway activity, which is correlated with inhibition of movement, can influence direct-pathway function. Dopamine reportedly exerts both excitatory and inhibitory effects on MSN lateral connections. Enhancement has been reported via D1 receptors, whereas inhibition was found via D2 receptors (Guzman et al., 2003; Tecuapetla et al., 2007). The net effect of such modulation, as well as its possible MSN subtype specificity remains unclear.
Synaptic Integration in MSNs
MSNs exhibit negative resting potentials due to their high expression of inwardly-rectifying potassium channels (Nisenbaum and Wilson, 1995). However, as MSNs are depolarized, these inwardly-rectifying channels close and MSNs are more readily depolarized to reach spike threshold. Thus, a barrage of excitatory synaptic inputs can depolarize MSNs and yield a spike, whereas less coherent input is ineffective (Wilson and Kawaguchi, 1996). Interestingly, the pattern of activation at individual synapses may also be critical. When individual spines are repetitively activated, glutamate receptors desensitize and reduce synaptic efficacy (Carter and Sabatini, 2007). If multiple spines are activated, the resulting postsynaptic potentials can summate more effectively, particularly when MSNs are more depolarized. These results suggest that MSNs are optimized for integrating multiple distinct inputs.
The influence of GABAergic synapses on MSN firing is more complicated. When MSNs are at rest, the reversal potential for chloride is significantly more positive than the typical MSN resting potential. GABAergic synaptic activation is therefore depolarizing under these conditions (Bracci and Panzeri, 2006; Misgeld et al., 1982). However, once MSNs become more depolarized than the chloride reversal potential, activation of GABAergic synapses inhibits further depolarization and limits MSN firing. Thus, GABAergic synapses may function to actually facilitate MSN excitability by slightly depolarizing MSN dendrites, thus blocking inwardly-rectifying potassium channels and priming MSNs to respond to subsequent synaptic excitation (Wilson, 2007).
The output from the striatum to downstream basal ganglia nuclei thus reflects a complex interplay between intrinsic properties of MSNs and their excitatory and inhibitory synaptic inputs. Neuromodulators such as dopamine can alter both cellular and synaptic function to adjust output through the direct and indirect pathways and regulate motor function. However, elucidating the specific relation between changes in cellular or synaptic function in the striatum and behavior remains a challenging endeavor that is likely to keep investigators busy for many years to come.
Basal ganglia circuit function and dysfunction
Goal-directed learning
A primary function of the basal ganglia may be the selection of appropriate actions (Balleine et al., 2007). Properly performed actions lead to successful and rewarding results, which reinforce the choice of actions that led to that outcome. Over time, sequences of actions can be associated with each other enabling the rapid selection of motor routines, which are no longer dependent on reward values and can thus be considered as habits. An accumulating body of evidence suggests that rapid, goal-directed learning primarily involves the dorsomedial striatum, whereas the slower acquistion of habits, which are insensitive to changes in the reward value of the outcome, involves the dorsolateral striatum (Balleine et al., 2007; Yin and Knowlton, 2006). Importantly, goal-directed learning of new motor routines appears to be initiated in the dorsomedial striatum, whereas the long-term motor memory required to execute previously learned sequences may be stored in the dorsolateral striatum. Within this general context, activation of direct-pathway circuits has been proposed to facilitate or select appropriate movements, whereas activity in the indirect pathway may inhibit unwanted or inappropriate movements (Albin et al., 1989; DeLong, 1990). As learning progresses, a motor routine can be acquired as wanted movements are maintained and unwanted movements are eliminated in a precise temporal sequence. In the striatum, the primary instructive signal for learning is thought to be dopamine release, which, according to one prominent theory, can signal a “reward prediction error” (Montague et al., 2004; Schultz, 2007). At the cellular and synaptic level, such learning is thought to occur through long-term changes in synaptic strength at striatal synapses.
A major goal of current research is to understand how changes at the molecular and cellular level translate into altered neural circuit function and behavior. Thus, for example, injection of a NMDA receptor antagonist into dorsomedial striatum, which is predicted to disrupt LTP, also impairs goal-directed learning (Yin et al., 2005). Although NMDA receptors are important for other aspects of striatal information processing, this finding is nevertheless suggestive of an important role for LTP in motor learning. Another in vivo study found that the magnitude of LTP induced in vivo can be correlated with the acquisition speed of a lever-pressing task for intracranial self-stimulation (Reynolds et al., 2001). Thus, LTP in the dorsomedial striatum may partially underlie increases in striatal neuron firing observed during goal-directed learning. Blocking D1 receptors in the dorsomedial striatum, which may be required for LTP, also reduces reward-dependent learning in a saccadic eye movement task (Nakamura and Hikosaka, 2006), implicating a role for direct-pathway MSNs in this process. Together, these results suggest that LTP in the dorsomedial striatum is a key cellular mechanism for goal-directed learning. Far less is known about the in vivo role of LTD, although recent findings in Parkinson's disease models suggest that LTD at indirect-pathway synapses is critical for maintaining normal movement (Kreitzer and Malenka, 2007).
Parkinson's disease
The progressive loss of midbrain dopamine neurons leads to reduced dopamine levels in the striatum and the severe hypokinetic motor deficits characteristic of Parkinson disease (PD). Thus, PD provides powerful insight into the normal functions of dopamine in the striatum and its role in basal ganglia circuit function. Studies in both animal models of PD (Filion and Tremblay, 1991; Mallet et al., 2006) and in human PD patients (Obeso et al., 2000) have indicated that striatal dopamine depletion leads to enhanced indirect-pathway MSN output and decreased direct-pathway MSN output, and a consequent decrease in activity in GPe and increase in GPi. These in vivo results are generally consistent with cellular studies of the excitatory effects of dopamine D1 receptor activation on direct-pathway MSNs and the inhibitory actions of dopamine D2 receptors on indirect-pathway MSNs. Because the indirect pathway normally inhibits unwanted movements, its overactivity may lead to the inhibition of wanted movements and the disruption of learned motor routines. On the other hand, accepting that direct pathway activity normally selects appropriate movements, its underactivity may additionally contribute to difficulties in initiating and performing movements in PD.
Given that the striatum is a primary target of dopamine innervation, it is likely that most of the pathophysiology observed in PD patients arises from striatal dysfunction, which can propagate throughout basal ganglia circuits. In response to the loss of dopamine innervation, a host of cellular and synaptic changes occur in the striatum. A compensatory increase in dopamine D1 and D2 receptor responses is observed (Mishra et al., 1974), as well as increased firing at indirect-pathway MSNs (Mallet et al., 2006). An increase in membrane resistance of MSNs has also been reported (Fino et al., 2007), along with a compensatory loss of spines specifically on indirect-pathway MSNs (Day et al., 2006), suggesting a reduced synaptic convergence onto indirect pathway MSNs.
Among the most significant changes that occur following dopamine-depletion is the loss of indirect-pathway LTD (Kreitzer and Malenka, 2007). Instead, LTP is observed with the original LTD-inducing stimulus protocol (Kreitzer and Malenka, 2007; Shen et al., 2008). This shift from LTD to LTP may importantly contribute to the increased activity in the indirect pathway, ultimately resulting in excessive inhibition of movement. In fact, dopamine D2 receptor agonists are routinely prescribed as a first-line treatment for PD, and a recent study in mice showed that the efficacy of D2 receptor agonists in restoring locomotor activity could be significantly enhanced, even in severely dopamine-depleted animals, by co-administration of an endocannabinoid degradation inhibitor (Kreitzer and Malenka, 2007). Together these results indicate that striatal LTD may be critical for regulating indirect-pathway activity and motor control. An additional consequence of dopamine depletion and enhanced transmission at indirect-pathway MSNs could be increased entrainment to cortical and thalamic oscillations (Costa et al., 2006). In this scheme, synchronous firing of indirect-pathway projections to the GPe would be amplified by the recurrent GPe-STN circuit (Bevan et al., 2002), leading to abnormal oscillations that interfere with normal basal ganglia output, and which could also be associated with resting tremor in PD (Bevan et al., 2006).
Direct pathway signaling may also be altered in PD (Albin et al., 1989; DeLong, 1990). In vivo evidence indicates that direct-pathway MSNs exhibit lower firing rates following dopamine depletion (Mallet et al., 2006). Consistent with this finding, recent work suggests that direct-pathway MSNs exhibit LTD instead of LTP following dopamine-depletion (Shen et al., 2008). Similarly, as discussed previously, antagonists of D1 receptors reportedly block STDP-LTP and unmask STDP-LTD in direct-pathway MSNs (Shen et al., 2008). Further work will be required to confirm and extend these findings.
Of course, dopamine acts in numerous additional ways beyond effects on synaptic plasticity to shape the transfer of information through the striatum. In particular, the rapid and reversible actions of dopamine need to be distinguished from persistent changes that long outlast the dopamine signal itself. In the former case, dopamine can transiently alter synaptic integration, state transitions, and microcircuit function, which may enhance the transfer of certain kinds of information through the striatum. In the latter case, dopamine is gating changes in network function that may correspond to long-term motor memory itself.
Huntington's disease
Huntington's disease (HD) is a progressive neurological disorder resulting from a polyglutamine expansion in the ubiquitously-expressed huntingtin (htt) protein, which for unknown reasons leads to the degeneration of certain types of neurons, including striatal MSNs. In particular, indirect-pathway MSNs appear to be selectively vulnerable to this disease process (Reiner et al., 1988). Their preferential loss is thought to reduce the amount of inhibitory control over unwanted movements, leading to the chorea and hyperkinesia typically associated with HD. However, neuronal death in HD may not be a purely cell-autonomous process (Gu et al., 2005). Additional evidence suggests that altered cellular and synaptic properties may result in aberrant overexcitation and eventual glutamate excitotoxicity (Cepeda et al., 2007; Gu et al., 2005).
Early physiological changes in MSNs observed in animal models of HD are reminiscent of those observed in dopamine-depleted animals, including an increase in input resistance and a decrease in spine density (Klapstein et al., 2001), as well as increased expression of calcium binding proteins (Huang et al., 1995; Sun et al., 2005). While these changes may arise as a direct consequence of mutant htt expression in MSNs, another possibility is that they represent compensatory responses to increased excitatory drive from cortex. Indeed, in vivo experiments in mouse models of HD indicate an increase in MSN firing rate (Rebec et al., 2006). Expanded htt is also associated with abnormal trafficking and increased surface expression of NR2B-containing NMDA receptors (Fan and Raymond, 2007), which could contribute to excitotoxicity, particularly in indirect-pathway MSNs that already exhibit larger NMDA currents (Kreitzer and Malenka, 2007). Mutant htt may also enhance IP3-mediated release of calcium from intracellular stores that could additionally contribute to dysregulation of calcium signaling (Tang et al., 2003).
Unfortunately, less is known about the role of striatal plasticity in the degeneration of MSNs in HD. In a pharmacological model of HD, field recordings in the striatum failed to show LTD, while LTP was readily observed (Dalbem et al., 2005). Another study found a loss of depotentiation following the induction of LTP at corticostriatal synapses (Picconi et al., 2006). Together, these studies suggest that a net enhancement of excitatory synaptic transmission in the striatum may contribute to the observed changes in MSN properties and their eventual cell death in HD.
Obsessive-compulsive disorder
In contrast to PD and HD, obsessive-compulsive disorder (OCD) and other OC-spectrum disorders such as Tourette's syndrome (TS) and trichotillomania (hair-pulling) are psychiatric disorders characterized by repetitive thought patterns (obsessions) and uncontrollable urges (compulsions) to perform motor routines (rituals), often directed at easing obsessions (Graybiel and Rauch, 2000). For example, someone with OCD may repeatedly verify that a door is locked or repeatedly wash their hands, displaying an addict-like need to perform these actions despite evidence that the appropriate outcome has been achieved. The neural circuits underlying OCD are thought to involve frontal and cingulate cortices, as well as the caudate nucleus and thalamus (Aouizerate et al., 2004), implicating basal ganglia circuitry required for reward-dependent learning. However, it remains unclear whether OCD represents a gain- or loss-of-function in basal ganglia circuits. It is tempting to speculate that it may represent an enhanced propensity to form both cognitive and motor habits. In the case of TS, dopamine D2 antagonists are an effective treatment, suggesting perhaps that TS involves excessive D2 receptor-mediated striatal plasticity.
Although little is known about the causes of OCD, deletions in two genes have been shown to give rise to OCD-like behaviors in mice. Hoxb8 is a transcription factor involved in early development that is expressed widely in the brain, including the cortex and striatum. Hoxb8 knock-out mice exhibit excessive grooming, leading to hair removal and self-inflicted wounds (Greer and Capecchi, 2002). More recently, a postsynaptic scaffolding protein expressed in the striatum, Sapap3, has been implicated in OCD behaviors including pathological overgrooming and anxiety (Welch et al., 2007). Remarkably, these behaviors are reversed by prolonged administration of a selective serotonin reuptake inhibitor, the most commonly used treatment for OCD. Furthermore, selective postnatal expression of Sapap3 in the striatum eliminates the behavioral abnormalities. Consistent with a critical role for striatal circuitry in mediating the OCD-like behaviors, the Sapap3 knockouts displayed complex abnormalities in excitatory synaptic function and structure in the striatum including an apparent enhancement of NMDA receptor-mediated synaptic responses. Further study is clearly warranted to elucidate the detailed synaptic abnormalities in the striatum caused by the loss of Sapap3 and whether they are restricted to specific striatal neuron subtypes.
Conclusions and future directions
Over the past three decades, a vast amount of anatomical, experimental, and clinical data on the striatum and basal ganglia function has accumulated. However, the complex anatomy and difficulty in isolating specific subpopulations for experimental analysis has hampered progress in understanding the mechanisms and function of basal ganglia circuits. The development of transgenic mice that express proteins in specific neuronal populations represents a major step forward that should help unravel the complexity of striatal circuit function (Gong et al., 2003). Targeted whole-cell recording and analysis of direct- and indirect-pathway MSNs, as well as patch and matrix MSNs, and different classes of striatal interneurons has become possible for the first time. Indeed, it is now possible to generate transgenic mice that simultaneously have different fluorophores in D1 versus D2 expressing MSNs (Shuen et al., 2008). By gaining a precise understanding of the cellular and synaptic mechanisms that give rise to the functional properties of these neurons and their connections, novel approaches can be developed for selectively altering cellular and synaptic function in vivo, with the goal of both understanding their role in basal ganglia related behaviors, as well as their potential as therapeutic targets for neurological disorders.
Expression of proteins enabling optical stimulation, such as channelrhodopsin and halorhodopsin (Boyden et al., 2005; Zhang et al., 2007), may also accelerate our understanding of basal ganglia function. It may soon be possible to control spiking of direct or indirect pathway MSNs, cholinergic interneurons, or midbrain dopamine neurons with millisecond precision both in vitro and in vivo. In vitro, this may allow the creation of arbitrary activity patterns across entire networks, thus enabling an examination of firing synchrony, synaptic integration, and striatal microcircuits in basal ganglia function with high resolution. In vivo, fiber optics implanted into specific brain regions may be able to excite or silence activity within distinct neuronal cell types (Adamantidis et al., 2007; Aravanis et al., 2007). In addition, molecular genetic manipulations in mice may allow alternative strategies for precise, drug-induced control over neuronal and circuit activity (Arenkiel et al., 2008; Conklin et al., 2008; Karpova et al., 2005; Lerchner et al., 2007). By applying these novel technologies to the striatum and associated basal ganglia circuitry, investigators may be able to make the critical leap from activity in specific striatal neurons and circuits to the role of those circuits in behaving animals.
Table 1.
Striatal cell types
| cell type: | abundance1: | Rinput: | Vrest2: | axonal targets: | key metabotropic receptors: |
references: |
|---|---|---|---|---|---|---|
| striatonigral MSN | 49 % | <100 MΩ | −80 to −90 mV |
GPi, SNr | D1, M1, M4, mGluR1/5 |
(Cepeda et al., 2008; Hersch et al., 1994; Ince et al., 1997; Kreitzer and Malenka, 2007) |
| striatopallidal MSN | 49 % | <100 MΩ | −80 to −90 mV |
GPe | D2, A2A, M1, mGluR1/5 |
(Cepeda et al., 2008; Hersch et al., 1994; Kreitzer and Malenka, 2007; Pollack et al., 1993) |
| FS interneuron | 0.5 % | <100 MΩ | −80 mV | MSNs | D5 | (Centonze et al., 2003b; Kawaguchi, 1993) |
| LTS interneuron | 1.5 % | >200 MΩ | −60 mV | MSNs | D1-like | (Centonze et al., 2002; Kawaguchi, 1993) |
| cholinergic interneuron | 0.5 % | >200 MΩ | −60 mV | MSNs, FS interneurons |
D2, D5, M2, M4 | (Kawaguchi, 1993; Koos and Tepper, 2002; Wilson et al., 1990; Yan and Surmeier, 1996) |
Abundance based on discussion in (Rymar et al., 2004), rounded to the nearest half-percent. Fifty percent of MSNs were assumed to be striatonigral (Huang et al., 1992). LTS interneurons were assumed to include both somatostatin- and calretinin-positive subtypes.
Experimental variability due to different extracellular potassium concentrations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, Burbaud P. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog. Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat. Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J. Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, Andre VM, Cohen R, Cepeda C, Levine MS, et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berlin) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Atherton JF, Baufreton J. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr. Opin. Neurobiol. 2006;16:621–628. doi: 10.1016/j.conb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J. Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J. Neurophysiol. 2002;87:2190–2194. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- Bracci E, Panzeri S. Excitatory GABAergic effects in striatal projection neurons. J. Neurophysiol. 2006;95:1285–1290. doi: 10.1152/jn.00598.2005. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J. Neurophysiol. 1982;48:597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J. Neurosci. 1999;19:2489–2499. doi: 10.1523/JNEUROSCI.19-07-02489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J. Neurosci. 1992a;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur. J. Neurosci. 1992b;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J. Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J. Neurosci. 2007;27:8967–8977. doi: 10.1523/JNEUROSCI.2798-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur. J. Neurosci. 2002;15:2049–2052. doi: 10.1046/j.1460-9568.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J. Neurosci. 2003a;23:8506–8512. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, et al. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J. Neurosci. 2003b;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G. Unilateral dopamine denervation blocks corticostriatal LTP. J. Neurophysiol. 1999;82:3575–3579. doi: 10.1152/jn.1999.82.6.3575. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur. J. Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, Andre VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington's disease. Prog. Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc. Natl. Acad. Sci. U. S. A. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR, Guettier JM, Chang WC, Pei Y, McCarthy KD, et al. Engineering GPCR signaling pathways with RASSLs. Nat. Methods. 2008;5:673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Dalbem A, Silveira CV, Pedroso MF, Breda RV, Werne Baes CV, Bartmann AP, da Costa JC. Altered distribution of striatal activity-dependent synaptic plasticity in the 3-nitropropionic acid model of Huntington's disease. Brain Res. 2005;1047:148–158. doi: 10.1016/j.brainres.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Delgado A, Sierra A, Querejeta E, Valdiosera RF, Aceves J. Inhibitory control of the GABAergic transmission in the rat neostriatum by D2 dopamine receptors. Neuroscience. 2000;95:1043–1048. doi: 10.1016/s0306-4522(99)00495-9. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J. Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-Methyl-d-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Prog. Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- Fino E, Deniau JM, Venance L. Cell-specific spike-timing-dependent plasticity in GABAergic and cholinergic interneurons in corticostriatal rat brain slices. J. Physiol. 2008;586:265–282. doi: 10.1113/jphysiol.2007.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Effects of acute dopamine depletion on the electrophysiological properties of striatal neurons. Neurosci. Res. 2007;58:305–316. doi: 10.1016/j.neures.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Indirect-pathway neurons lose their spines in Parkinson disease. Nat. Neurosci. 2006;9:157–158. doi: 10.1038/nn0206-157. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr., Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr. Biol. 2000;10:R509–511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- Gu X, Li C, Wei W, Lo V, Gong S, Li SH, Iwasato T, Itohara S, Li XJ, Mody I, et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Gustafson N, Gireesh-Dharmaraj E, Czubayko U, Blackwell KT, Plenz D. A comparative voltage and current-clamp analysis of feedback and feedforward synaptic transmission in the striatal microcircuit in vitro. J. Neurophysiol. 2006;95:737–752. doi: 10.1152/jn.00802.2005. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J. Neurosci. 2003;23:8931–8940. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J. Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J. Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J. Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Sapp E, Aizawa H, Ge P, Bird ED, Vonsattel JP, DiFiglia M. Quinolinic acid-induced increases in calbindin D28k immunoreactivity in rat striatal neurons in vivo and in vitro mimic the pattern seen in Huntington's disease. Neuroscience. 1995;65:397–407. doi: 10.1016/0306-4522(94)00494-p. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. J. Neurophysiol. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karpova AY, Tervo DG, Gray NW, Svoboda K. Rapid and reversible chemical inactivation of synaptic transmission in genetically targeted neurons. Neuron. 2005;48:727–735. doi: 10.1016/j.neuron.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1971;262:429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol. Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J. Neurophysiol. 2001;86:2667–2677. doi: 10.1152/jn.2001.86.6.2667. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J. Neurosci. 2002;22:529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J. Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J. Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J. Neurosci. 2000;20:375–386. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J. Neurosci. 2007;27:4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–545. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J. Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J. Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J. Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J. Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Wagner A, Ohno T. Depolarizing IPSPs and Depolarization by GABA of rat neostriatum cells in vitro. Exp. Brain Res. 1982;45:108–114. doi: 10.1007/BF00235769. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Gardner EL, Katzman R, Makman MH. Enhancement of dopamine-stimulated adenylate cyclase activity in rat caudate after lesions in substantia nigra: evidence for denervation supersensitivity. Proc. Natl. Acad. Sci. U. S. A. 1974;71:3883–3887. doi: 10.1073/pnas.71.10.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J. Neurosci. 2006;26:5360–5369. doi: 10.1523/JNEUROSCI.4853-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur. J. Neurosci. 2006;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berlin) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR. Pathophysiologic basis of surgery for Parkinson's disease. Neurology. 2000;55:S7–12. [PubMed] [Google Scholar]
- Ouardouz M, Lacaille JC. Mechanisms of selective long-term potentiation of excitatory synapses in stratum oriens/alveus interneurons of rat hippocampal slices. J. Neurophysiol. 1995;73:810–819. doi: 10.1152/jn.1995.73.2.810. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J. Comp. Neurol. 2006;496:202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J. Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Picconi B, Passino E, Sgobio C, Bonsi P, Barone I, Ghiglieri V, Pisani A, Bernardi G, Ammassari-Teule M, Calabresi P. Plastic and behavioral abnormalities in experimental Huntington's disease: a crucial role for cholinergic interneurons. Neurobiol. Dis. 2006;22:143–152. doi: 10.1016/j.nbd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Plotkin JL, Wu N, Chesselet MF, Levine MS. Functional and molecular development of striatal fast-spiking GABAergic interneurons and their cortical inputs. Eur. J. Neurosci. 2005;22:1097–1108. doi: 10.1111/j.1460-9568.2005.04303.x. [DOI] [PubMed] [Google Scholar]
- Pollack AE, Harrison MB, Wooten GF, Fink JS. Differential localization of A2a adenosine receptor mRNA with D1 and D2 dopamine receptor mRNA in striatal output pathways following a selective lesion of striatonigral neurons. Brain Res. 1993;631:161–166. doi: 10.1016/0006-8993(93)91204-6. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience. 2006;137:327–336. doi: 10.1016/j.neuroscience.2005.08.062. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J. Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J. Comp. Neurol. 2004;469:325–339. doi: 10.1002/cne.11008. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J. Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal RM, Gaspar RC, Keefe KA, Wilcox KS. A rat brain slice preparation for characterizing both thalamostriatal and corticostriatal afferents. J. Neurosci. Methods. 2007;159:224–235. doi: 10.1016/j.jneumeth.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat. Neurosci. 2006;9:798–806. doi: 10.1038/nn1698. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Bolam JP, Smith AD. Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscopic study using the Golgi-peroxidase transport-degeneration procedure. J. Comp. Neurol. 1981;195:567–584. doi: 10.1002/cne.901950403. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang HB, Deng YP, Lei WL, Xie JP, Meade CA, Del Mar N, Goldowitz D, Reiner A. Increased calbindin-D28k immunoreactivity in striatal projection neurons of R6/2 Huntington's disease transgenic mice. Neurobiol. Dis. 2005;20:907–917. doi: 10.1016/j.nbd.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J. Neurophysiol. 2001;86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J. Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CM. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. J. Neurophysiol. 2004;91:1111–1121. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Carrillo-Reid L, Bargas J, Galarraga E. Dopaminergic modulation of short-term synaptic plasticity at striatal inhibitory synapses. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10258–10263. doi: 10.1073/pnas.0703813104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J. Neurophysiol. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Walsh JP. Depression of excitatory synaptic input in rat striatal neurons. Brain Res. 1993;608:123–128. doi: 10.1016/0006-8993(93)90782-i. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. Basal Ganglia. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; New York: 1998. pp. 329–375. [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog. Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J. Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J. Comp. Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J. Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur. J. Neurosci. 2005;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc. Natl. Acad. Sci. U. S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J. Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]