Abstract
The objective of this study was to better understand the role of transforming growth factor-β (TGF-β) and its primary signaling protein Smad3 in the development of intimal hyperplasia. Male Sprague-Dawley rats underwent left carotid balloon injury followed by intra-arterial infection with adenovirus-expressing Smad3 (AdSmad3). In uninfected injured arteries, endogenous Smad3 was upregulated with the expression peaking at 14 days. Moreover, in arteries infected with AdSmad3, we observed an enhancement of intimal hyperplasia and increased vascular smooth muscle cell (VSMC) proliferation. The novel finding, that TGF-β/Smad3 stimulated rather than inhibited VSMC proliferation, was confirmed in cultured VSMCs infected with AdSmad3 and treated with TGF-β. To identify the mechanism underlying TGF-β/Smad3-mediated VSMC proliferation, we studied the cyclin-dependent kinase inhibitor p27. Although the upregulation of Smad3 in VSMCs had no significant effect on total p27 levels, Smad3 did stimulate the phosphorylation of p27 at serine-10 as well as the nuclear export of p27, events associated with cell proliferation. Furthermore, serine-10-phosphorylated p27 was also increased in AdSmad3-infected injured rat carotid arteries, demonstrating the existence of this same mechanism in vivo. In conclusion, our findings identify a novel mechanism for the effect of TGF-β on intimal hyperplasia. In the presence of elevated levels of Smad3 that develop in response to injury, TGF-β stimulates smooth muscle cell proliferation through a mechanism involving the phosphorylation and nuclear export of p27.
Keywords: serine-10-phosphorylated p27, rat carotid balloon injury
intimal hyperplasia is a complex process associated with abnormal behavior of vascular smooth muscle cells (VSMCs). In response to injury, VSMCs transform from a quiescent, differentiated state to a proliferative and synthetic phenotype (30). Although the biology of intimal hyperplasia has been studied in detail and many hypotheses exist, the molecular mechanisms that trigger VSMC proliferation after vascular injury remain unclear.
Transforming growth factor-β (TGF-β) is believed to be a critical factor in the formation of intimal hyperplasia. Both in vitro and in vivo studies have shown that TGF-β is upregulated at sites of vascular injury. Elevated levels of TGF-β have been detected within 6 h of arterial balloon injury and are sustained for up to 14 days (31, 40). In vivo, the overall effect of TGF-β appears to be the stimulation of intimal hyperplasia (19, 52).
TGF-β, however, is known to have a diverse array of effects on cultured VSMCs, many of which relate to processes critical to the development of intimal hyperplasia (26, 28, 41). As a profibrotic cytokine, the prevailing view is that TGF-β enhances neointimal formation by stimulating extracellular matrix (ECM) synthesis (35). Consistent with this view, we have previously shown that TGF-β, through Smad3 signaling, stimulates VSMC fibronectin synthesis, thus enhancing neointimal ECM accumulation (41). Moreover, TGF-β is a potent stimulus of collagen type I, which is also a primary constituent of the neointimal lesion (8, 21). However, TGF-β has also been shown by our group and others to be an in vitro inhibitor of both VSMC proliferation and migration (11, 28). These inhibitory effects of TGF-β would be expected to counter its profibrotic effects, therefore limiting the development of intimal hyperplasia. Thus, although the overall stimulatory effect of TGF-β on intimal hyperplasia has been clearly demonstrated, the underlying mechanisms remain unclear given the complex and contradictory effects of TGF-β on VSMC function.
The varying effects of TGF-β on VSMC function may be related to differential signaling through numerous downstream pathways. TGF-β has been shown to signal through members of the ERK, JNK, and p38 MAPK kinase pathways, the phosphatidylinositol 3-kinase (PI3-kinase) pathway, and most commonly the Smad family signaling pathway (7). The Smad proteins are classified into three groups: receptor-activated Smads (Smad2 and Smad3), common Smad (Smad4), or inhibitory Smads (Smad6 and Smad7). Briefly, the activation of the transmembrane TGF-β receptor leads to the phosphorylation and activation of Smad3, which then forms a complex with Smad4. This complex translocates into the nucleus where it can regulate the transcription of target genes by binding to DNA at specific Smad-binding elements. Inhibitory Smads antagonize TGF-β signaling by either inhibiting receptor-activated Smad activation or by mediating TGF-β receptor degradation (49).
The effect of TGF-β on cell growth has been shown to be mediated, at least in part, by the cyclin-dependent kinase inhibitor p27 (33, 34). Interestingly, p27 has also been shown to play an important role in regulating cell proliferation after vascular injury (4, 48). Tanner et al. (48) demonstrated that after balloon injury in a porcine model, total p27 levels varied inversely with cell proliferation, suggesting that the proliferative capabilities of VSMCs after injury are regulated in part by changes in p27 levels. Additionally, Chen et al. (4) have previously demonstrated that p27 overexpression inhibited VSMC growth and neointimal formation at late time points (2 wk) after injury.
To better understand the role of TGF-β and its downstream signaling pathways in the formation of intimal hyperplasia, we overexpressed the signaling protein Smad3 at the time of arterial injury. We found that Smad3 overexpression enhanced intimal hyperplasia and that the mechanism for this effect was through the stimulation of VSMC proliferation. Using cultured VSMCs, we confirmed that in the presence of elevated levels of Smad3, TGF-β stimulated rather than inhibited cell proliferation. Furthermore, we report that the mechanism underlying TGF-β-induced VSMC proliferation involves Smad3-mediated p27 phosphorylation and nuclear export.
MATERIALS AND METHODS
Balloon injury model and in vivo gene delivery.
Male Sprague-Dawley rats (450–500 g) underwent balloon injury of the left common carotid artery as described elsewhere (13) in accordance with institutional guidelines and approval from the Institutional Animal Care and Use Committee at Weill Cornell Medical College. Animals were anesthetized with isoflurane-inhaled anesthetic. Recombinant adenoviral vectors were constructed to express Smad3 as previously described; an empty viral vector was used as a control (42). Following injury, the animals received an intraluminal administration of adenoviral vectors as indicated (200 μl of 2.5 × 109 plaque-forming units/ml over 20 min). In total, 20 rats received no virus (15 for time course and 5 for morphometric studies), 9 rats received empty virus (AdNull), and 9 rats received adenovirus-expressing Smad3 (AdSmad3). The external carotid artery was then ligated, and the flow was reestablished through the common carotid and internal carotid arteries.
At the time of euthanasia, the arteries were perfusion fixed in 4% paraformaldehyde for morphometric analysis or snap frozen for protein extraction.
Morphometric analysis and immunohistochemistry.
Morphometric analysis was carried out on elastin-stained arteries as previously described (20). Quantitative immunohistochemistry using mouse anti-α-smooth muscle actin (1:800; Sigma), mouse anti-ED-1 (1:50; Serotec), rabbit anti-Smad3 (1:50; Zymed), and mouse anti-proliferating cell nuclear antigen (PCNA; Santa Cruz) was performed as described elsewhere (13). The PCNA index was calculated as the number of PCNA positive cells/number of total nuclei per section. Immunofluorescent staining was performed with avidin-conjugated fluorescein or Texas red. Slides were visualized with a Nikon Eclipse E800 upright microscope. Digital images were acquired using a RetigaEXi charge-coupled device digital camera and processed and analyzed using IPLab software.
Smooth muscle cell culture.
VSMCs were isolated from the aorta of Sprague-Dawley rats based on a protocol described by Clowes et al. (6) and maintained in DMEM containing 10% FBS at 37°C with 5% CO2-95% room air.
Proliferation assay.
Primary rat aortic smooth muscle cells (RSMCs) (passages 2–6) were infected with either AdNull or AdSmad3 (3 × 104 particles/cell in 2% FBS for 4 h), followed by starvation in 0.5% FBS for 72 h. Tritiated thymidine incorporation, as a surrogate for DNA synthesis, was assessed as previously described after treatment with recombinant human TGF-β1 (5 ng/ml; R&D systems), PDGF-BB (5 ng/ml; Upstate Biotechnology), and TGF-β + PDGF (25). Recombinant TGF-β1 was dissolved in 4 mM HCl with 2% BSA.
Western blot analysis.
Western blot analysis was performed to determine the protein expression using antibodies to p27 (1:200; SC 528; Santa Cruz), phospho-p27 [serine-10 (S10); 1:200; SC 12939R, Santa Cruz], β-actin (1:1,000; Sigma), and Smad3 (2 μg/ml; Invitrogen). Western blot band intensity was determined using NIH image software (ImageJ 1.36b).
Immunocytochemistry.
RSMCs were infected with AdNull or AdSmad3 and then seeded onto chamber slides and stimulated with recombinant TGF-β1 for 24 h. Cells were then fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. The sections were incubated with mouse anti-p27kip1 (1:100; Transduction Laboratories) followed by Alexa 555 donkey anti-mouse IgG (Molecular Probes). Fluorescent staining was visualized on a Nikon Eclipse TE2000-inverted microscope and Nikon C1si confocal laser imaging system with the appropriate argon beam lasers. Digital images were acquired with a CoolSNAPcf Monochrome camera (Photometrics) and analyzed on Adobe Photoshop 7.0.
Statistical analysis.
Values are expressed as means ± SE. An unpaired Student's t-test was used to evaluate the statistical differences between the control and treated groups. Values of P < 0.05 were considered significant. In cases of multiple groups, differences were first analyzed with one-way ANOVA. Pairwise multiple comparisons were made using the Student-Newman-Keuls method. All experiments were repeated at least three times.
RESULTS
Endogenous Smad3 is upregulated after vascular injury.
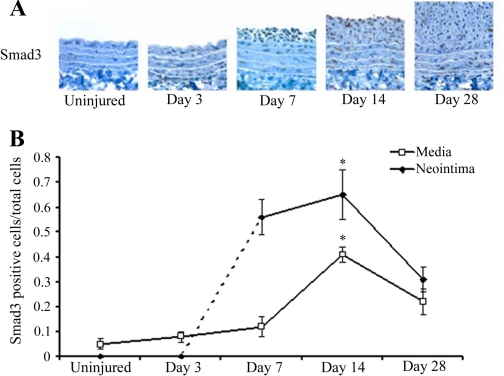
To dissect the effect of different TGF-β signaling pathways on intimal hyperplasia, we focused on the Smad3 signaling pathway and examined the expression of Smad3 after balloon injury in the rat carotid artery. While immunohistological staining for Smad3 was nearly undetectable in normal uninjured arteries, we found that after injury, endogenous Smad3 was significantly upregulated, with peak expression around 14 days (Fig. 1, A and B). This finding raises the possibility that the stimulatory effect of TGF-β on intimal hyperplasia is mediated by Smad3.
Fig. 1.
Time course of endogenous Smad3 expression after vascular injury. A: immunohistochemical staining with anti-Smad3 antibody in injured rat carotid arteries harvested 0, 3, 7, 14, or 28 days post-balloon angioplasty (magnification, ×200). B: quantification of Smad3 expression determined by semiquantitative immunohistochemistry as described in materials and methods (n = 3 rats per time point). *P < 0.05 compared with 0 days (uninjured control).
Manipulation of the Smad3 signaling pathway affects intimal hyperplasia and cell proliferation after vascular injury.
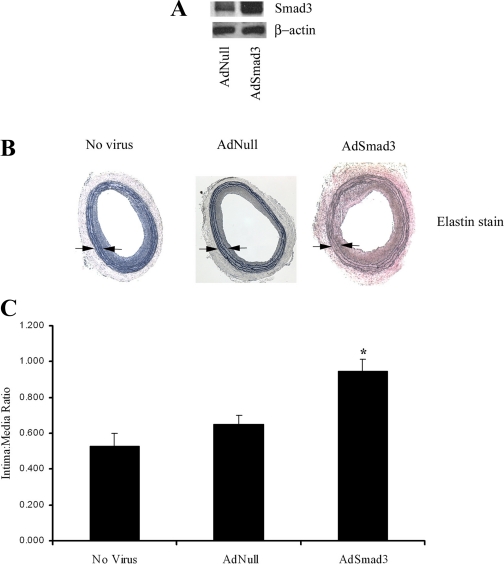
To further evaluate the role of Smad3 signaling in the development of intimal hyperplasia after vascular injury, we ectopically expressed Smad3 in the arterial wall in a vascular injury model. Male Sprague-Dawley rats underwent carotid balloon angioplasty followed by local adenovirus-mediated gene transfer with either AdNull (n = 6), AdSmad3 (n = 6), or no virus (n = 5). Since endogenous Smad3 expression peaked around 14 days after injury, we chose this as our time point for further evaluations. Adenovirus-mediated Smad3 overexpression at 14 days after injury was confirmed by Western blot analysis of protein derived from homogenized arterial wall (Fig. 2A). The quantification of Western blot band intensity confirmed a 26% increase in Smad3 expression in arteries treated with AdSmad3. A morphometric analysis of rat carotid arteries at this time point revealed a significant increase in intima-to-media ratio in rats treated with AdSmad3 (Fig. 2, B and C), suggesting that the upregulation of arterial Smad3 expression stimulated neointimal formation.
Fig. 2.
Smad3 overexpression stimulates intimal hyperplasia. A: Western blot of protein from homogenized arterial wall 14 days after injury and infection with empty virus (AdNull) or adenovirus-expressing Smad3 (AdSmad3). B: morphometric analysis of elastin-stained arteries 14 days after injury revealed a significant increase in intima-to-media (I/M) ratio in rats infected with AdSmad3 (magnification, ×40). C: graphical depiction of I/M ratio [no virus (n = 5 rats), 0.53 ± 0.08; AdNull (n = 6 rats), 0.65 ± 0.05; and AdSmad3 (n = 6 rats), 0.95 ± 0.07]. *P < 0.05 compared with AdNull and no virus. Arrows indicate medial borders.
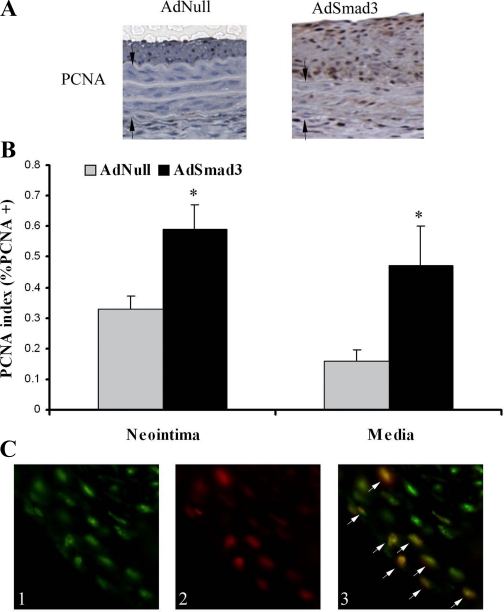
A further examination of the arterial wall 14 days after injury by immunohistochemistry revealed an increase in PCNA expression, a marker of cell proliferation, in rats treated with AdSmad3 (Fig. 3A). The quantification of PCNA staining using the PCNA index (as described in materials and methods) confirmed that the increase in cell proliferation was statistically significant in both cells of the neointima (AdNull, 0.33 ± 0.04; and AdSmad3, 0.59 ± 0.08, P < 0.05) and the media (AdNull, 0.16 ± 0.04; and AdSmad3, 0.47 ± 0.13, P < 0.05) (Fig. 3B). A similar increase in the PCNA index was also obtained from AdSmad3-treated arteries at 7 days after injury (neointima: AdNull, 0.53 ± 0.11, and AdSmad3, 0.70 ± 0.04; and media: AdNull, 0.21 ± 0.05, and AdSmad3, 0.45 ± 0.04).
Fig. 3.
Smad3 overexpression increases cell proliferation in vivo. A: rat carotid arteries were infected with AdSmad3 (n = 5 rats) or AdNull (n = 5 rats) and, 14 days after injury, were stained for proliferating cell nuclear antigen (PCNA). Arrows indicate medial borders. B: PCNA index, as described in materials and methods, increased in both the neointima (AdNull, 0.33 ± 0.04; and AdSmad3, 0.59 ± 0.08) and the media (AdNull, 0.16 ± 0.04; and AdSmad3, 0.47 ± 0.13). *P < 0.05 (magnification, ×200). C: double immunofluorescent labeling for Smad3 and PCNA in neointima of injured rat carotid arteries: 1) Smad3-positive cells stained with fluorescein, 2) PCNA-positive cells stained with Texas red, and 3) superimposing the red and green images reveals that PCNA-positive cells are also Smad3 positive (white arrows) (magnification, ×400).
To establish a relationship between Smad3 expression and the observed cell proliferation, we performed double immunofluorescent staining to colocalize the Smad3 and PCNA signals (Fig. 3C). Figure 3C shows that most of the proliferating cells (PCNA positive) are also Smad3 positive. Since TGF-β levels are known to be elevated after injury, these findings suggested that the enhancement of the TGF-β/Smad3 pathway, through Smad3 overexpression, is associated with an increased cell proliferation.
The characterization of the cells contributing to the neointima revealed that the majority of the cells were α-smooth muscle actin positive in arteries infected with AdNull, AdSmad3, and no virus (supplemental Fig. 1; note: all supplemental figures can be found with the online version of this article). Surprisingly, the infection with the adenovirus did not increase the number of inflammatory cells detected at 2 wk after injury. Only a few macrophages or CD68+ cells were indentified, and these were mostly found in the periadventitia. Our findings are consistent with previous reports describing the neointimal lesion after balloon injury as comprised predominantly of smooth muscle cells (SMCs) and rare inflammatory cells (5, 36, 39). Our data, in addition to the literature, suggest that intimal hyperplasia is a SMC pathology; therefore, the following in vitro studies are performed in cultured SMCs.
Inhibition of TGF-β/Smad3 signaling inhibits intimal hyperplasia and cell proliferation.
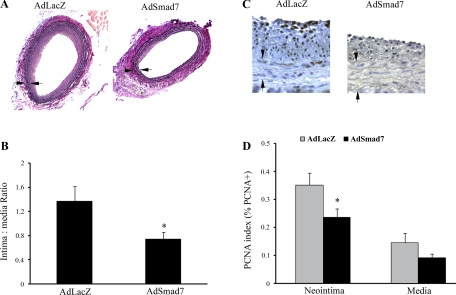
To confirm our findings that TGF-β through Smad3 signaling increased intimal hyperplasia by stimulating SMC proliferation, we next inhibited TGF-β/Smad3 signaling by overexpressing Smad7, the endogenous inhibitor of the TGF-β/Smad3 pathway. In a new set of experiments, male Sprague-Dawley rats underwent carotid balloon angioplasty followed by local adenovirus-mediated gene transfer with either AdSmad7 (n = 5) or the control adenovirus-expressing β-galactosidase (AdLacZ) (n = 4). As demonstrated in Fig. 4, A and B, 14 days after injury, rats, in which TGF-β/Smad3 signaling had been inhibited with AdSmad7, had significantly less intimal hyperplasia as reflected in a lower intima-to-media ratio. Further analysis revealed that Smad7 overexpression was also associated with a decreased cell proliferation (Fig. 4, C and D). This difference in the PCNA index was significant in cells of the neointima (AdLacZ, 0.35 ± 0.04 vs. AdSmad7, 0.24 ± 0.03, P < 0.05) and near significant in cells of the media (AdLacZ, 0.15 ± 0.03 vs. AdSmad7, 0.09 ± 0.01, P = 0.08).
Fig. 4.
Inhibition of Smad3 signaling decreases intimal hyperplasia. A: morphometric analysis of elastin-stained arteries 14 days after injury revealed a significant decrease in I/M ratio in rats infected with AdSmad7 (magnification, ×40). B: graphical depiction of I/M ratio [AdLacZ (n = 6 rats), 1.36 ± 0.25; and AdSmad7 (n = 7 rats), 0.74 ± 0.12, *P < 0.05 compared with adenovirus-expressing β-galactosidase (AdLacZ)]. Arrows indicate medial borders. C: the above rat carotid arteries were stained for PCNA. D: infection with AdSmad7 significantly decreased PCNA index in the neointima (AdLacZ, 0.35 ± 0.04 vs. AdSmad7, 0.24 ± 0.03, P < 0.05) and near significantly in the media (AdLacZ, 0.15 ± 0.03 vs. AdSmad7, 0.09 ± 0.01, P = 0.08).
To confirm that the effects of Smad7 overexpression were limited to the inhibition of Smad3, we also examined Smad2 expression in the injured rat carotid artery. On immunohistochemical analysis, we found no evidence of Smad2 expression in either uninjured or injured rat carotid arteries (supplemental Fig. 2). Thus the effects of Smad7 that we observed are related specifically to the inhibitory effect of Smad7 on Smad3.
TGF-β stimulates cell proliferation in states of Smad3 overexpression.
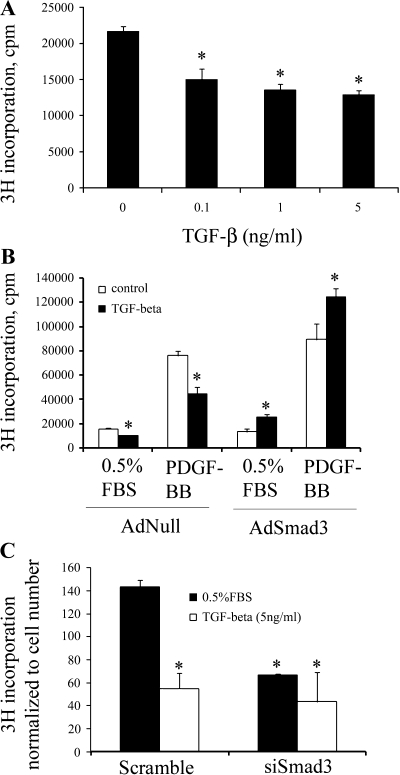
TGF-β has been shown to inhibit proliferation in most cell types, including VSMCs (11, 28). To confirm our in vivo observation that TGF-β through Smad3 signaling increases cell proliferation, we turned to cultured primary RSMCs and performed a tritiated thymidine incorporation assay. We first established that the inhibitory effect of TGF-β on VSMC proliferation was dose independent over a range from 0.1 to 5 ng/ml (Fig. 5A). Since there was no statistically significant difference between the doses, we chose to carry out the remaining experiments with 5 ng/ml TGF-β. We then tested the effect of Smad3 overexpression on RSMC proliferation (Fig. 5B). Consistent with our results, control RSMCs infected with AdNull responded to treatment with TGF-β1 (5 ng/ml for 24 h) with a 36% decrease in cell growth. This inhibitory effect of TGF-β was also observed in the presence of PDGF-BB, which is a known stimulant of cell proliferation (15). However, when we treated cultured RSMCs overexpressing Smad3 (by infection with AdSmad3) with TGF-β1, there was an 89% increase in basal cell growth. Furthermore, in the presence of PDGF-BB, the treatment with TGF-β of cells infected with AdSmad3 further enhanced cell proliferation by 40%.
Fig. 5.
Overexpression of Smad3 reverses the inhibitory effect of transforming growth factor-β (TGF-β) on smooth muscle cell (SMC) proliferation (tritiated thymidine incorporation assays). A: the effect of TGF-β dose on rat aortic SMC (RSMC) proliferation. *P < 0.05 compared with control (solvent). B: a representative result from 3 separate experiments is shown. RSMCs were infected with AdNull or AdSmad3 followed by stimulation with TGF-β1 in either low serum or in the presence of PDGF-BB. The inhibitory effect of TGF-β on SMC proliferation is reversed in cells infected with AdSmad3, both in low serum and in the presence of a growth stimulant. *P < 0.05 comparing treatment with TGF-β to treatment with solvent, n = 3 wells treated for each condition for each group in each experiment. C: a representative result from 3 separate experiments showing the effect of Smad3 small-interfering (si)RNA on RSMC proliferation. *P < 0.05 compared with cells treated with scrambled siRNA and 0.5% FBS. cpm, Counts/minute.
We next evaluated how SMCs with “downregulated” Smad3 expression respond to TGF-β. As shown in Fig. 5C, TGF-β inhibited cell proliferation in RSMCs that were treated with a scrambled small-interfering (si)RNA but not in cells treated with an siRNA specific to Smad3 (siSmad3). Of note, the downregulation of Smad3 by the siRNA significantly reduced baseline RSMC proliferation compared with cells transfected with the scrambled RNA control. It is therefore not surprising that an additional treatment with TGF-β had no further inhibitory effects on cell proliferation.
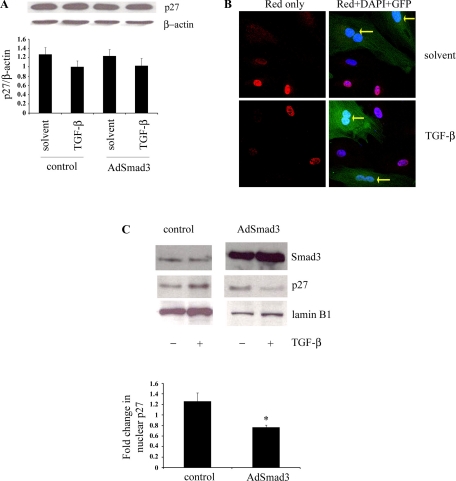
Smad3 overexpression decreases nuclear p27 activity through p27 nuclear export.
To understand the mechanism whereby Smad3 overexpression stimulates cell proliferation, we turned our attention to cell cycle regulators. Western blot analysis of a panel of cell cycle regulators revealed no significant changes in total protein levels in response to TGF-β and/or Smad3 overexpression (supplement Fig. 3). This was also the case for p27, as shown in Fig. 6A. However, both total p27 levels as well as subcellular localization have been shown to be important in regulating p27 activity and cell cycle progression (51). We therefore focused on the effect of Smad3 on p27 subcellular localization in RSMCs (Fig. 6B). RSMCs were infected with AdSmad3, which also contains a green fluorescent protein cDNA controlled by a separate promoter, and stimulated with TGF-β1. Infected cells were distinguished from uninfected cells by green fluorescent protein expression. Figure 5B shows that in uninfected cells stimulated with TGF-β, p27 was predominantly nuclear. However, an enhanced TGF-β/Smad3 signaling through the overexpression of Smad3 decreased nuclear p27.
Fig. 6.
Smad3 overexpression changes p27 subcellular localization without changing total p27 levels. RSMCs were infected with AdSmad3 followed by stimulation with TGF-β1 or solvent for 24 h. A: representative Western blot of total cell lysates for p27, with quantification of p27 band intensity normalized to β-actin (ANOVA, P = 0.48, n = 4 iterations of the experiment). B: RSMCs were infected with AdSmad3 and stimulated with TGF-β1 or solvent. Immunocytochemistry was performed for p27 expression. p27 staining was visualized with Alexa 555, and nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Uninfected cells showed strong nuclear p27 staining, whereas cells infected with AdSmad3 [green fluorescent protein + (GFP+)] showed weaker p27 nuclear staining (yellow arrows) (magnification, ×600). C: Western blot of nuclear protein from RSMCs with graphical representation of the fold change in nuclear p27 after treatment with TGF-β (5 ng/ml). *P < 0.05; n = 4 iterations of the experiment.
To confirm the observations made on immunocytochemistry, nuclear fractions of RSMC protein were isolated and immunoblotted for p27. As shown in Fig. 6C, in the setting of Smad3 overexpression, the effect of TGF-β on nuclear p27 is reversed: in control cells, the treatment with TGF-β increases nuclear p27, whereas in cells overexpressing Smad3, the treatment with TGF-β decreases nuclear p27.
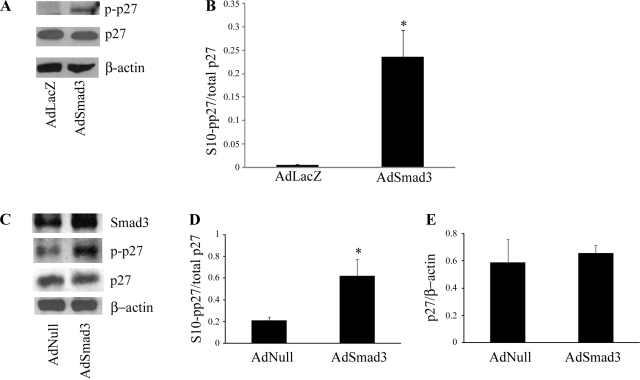
Increased S10-phosphorylated p27 (S10-pp27) accounts for the exaggerated neointimal lesion observed in rats overexpressing Smad3.
p27 nuclear export has been shown to be dependent on S10 phosphorylation (14). We therefore sought to use S10 phosphorylation of p27 as a marker for p27 cytoplasmic localization. Primary RSMCs were infected with either control virus or AdSmad3 followed by the stimulation with TGF-β1. Using an antibody to S10-pp27, we observed that TGF-β stimulation in the setting of Smad3 overexpression was associated with increased S10-pp27 within 4 h and that phosphorylation was sustained for up to 24 h (Fig. 7A). Figure 7B shows quantitatively that the ratio of S10-pp27 to total p27 was significantly higher in cells infected with AdSmad3 when compared with cells infected with control virus. Taken together with the results of our subcellular localization studies, these data reveal that in our model, there is an association between the increased levels of S10-pp27 and p27 nuclear export.
Fig. 7.
Smad3 overexpression is associated with increased serine-10-phosphorylated p27 (S10-pp27). RSMCs were infected with AdSmad3 followed by stimulation with TGF-β1 for 24 h. A: representative Western blot for p27 and S10-pp27. B: quantification of Western blot band intensity depicting ratio of S10-pp27 to total p27 after 24 h (*P < 0.05, n = 3 iterations of the experiment). C: injured rat carotid arteries infected with AdSmad3 or AdNull were harvested 14 days after injury, and protein was extracted for Western blot for p27, S10-pp27, and Smad3. A representative example from 3 separate Western blots shows that S10-pp27 levels are increased in arteries infected with AdSmad3 compared with control. Quantification of S10-pp27-to-total p27 (D) and total p27-to-β-actin (E) ratios in protein extracts from injured rat carotid arteries are shown (*P < 0.05, n = 3 rats).
We then turned to the rat carotid injury model to test whether Smad3 expression produced a similar increase in S10-pp27. Both total p27 and S10-pp27 expression were examined by Western blot analysis in injured rat carotid arteries that had been treated with the local administration of AdNull (n = 3) or AdSmad3 (n = 3). As shown in Fig. 7, C and D, we found that while total p27 levels did not differ between control and AdSmad3-treated rats, the addition of exogenous Smad3 was associated with increased S10-pp27 without a significant change in levels of total p27 (Fig. 7E). These findings imply that in vivo, Smad3 leads to the phosphorylation of p27, which may, in turn, lead to p27 nuclear export and the stimulation of SMC proliferation.
DISCUSSION
Our data are significant for three novel findings. First, we have shown that endogenous Smad3 is upregulated in both medial and neointimal SMCs after vascular injury. Second, we have found that further overexpression of Smad3 in an animal model of vascular injury is associated with an exacerbation of intimal hyperplasia and increased cell proliferation. We attribute the proliferative response in neointimal cells overexpressing Smad3 to our third novel finding, which is that Smad3 overexpression stimulates the nuclear export of p27 and the consequent VSMC proliferation.
TGF-β has been repeatedly demonstrated to be a stimulant of intimal hyperplasia (19, 40, 52). However, the mechanism through which TGF-β produces this effect is not clear. The dilemma arises from the fact that although TGF-β is a stimulant of fibrosis, which could contribute to intimal hyperplasia, TGF-β is also a potent inhibitor of cell growth and migration, effects that would inhibit the development of restenosis (11, 28, 35, 41). Of note, TGF-β signaling through Smad2 and p38 has been shown to inhibit VSMC proliferation in vitro (43). Although Smad2 and Smad3 are closely related, they are believed to have some important functional differences. This is most dramatically demonstrated by the fact that in mice, Smad2 gene deletion is embryonically lethal; Smad3 null mice, however, develop normally but are more susceptible to the development of neoplasms. TGF-β signaling through different downstream pathways may result in various effects on cell proliferation. Our studies with Smad3 are novel and allow us to reconcile the discrepancy between TGF-β as a stimulant of intimal hyperplasia and an inhibitor of cell growth. In the basal state, there is minimal Smad3 expression in arterial wall VSMCs, and TGF-β signaling through Smad3-independent pathways may inhibit cell growth, as suggested by Seay et al. (43). However, we have shown that Smad3 expression increases with injury. In the presence of upregulated Smad3, VSMCs undergo a transformation so that the response to TGF-β is a very robust proliferative response. Thus the injury-induced Smad3 expression, and the associated switch to Smad3-dependent signaling, appears to be pivotal in determining the effect of TGF-β on the arterial wall.
Surprisingly and contrary to our results, Kobayashi et al. (20), using a Smad3 knockout, have shown that a deficiency in the Smad3 gene is also associated with an exaggerated neointimal response (20). They also reported that the neointimal lesion observed in Smad3 null arteries was comprised of proliferating SMCs; the proliferation index was nearly two times greater than in wild-type arteries. Although these findings and the results of our study appear contradictory, several experimental differences could contribute to discrepancies between our findings. First, the arterial injury in the study by Kobayashi et al. (20) was produced via photochemically induced thrombosis, and the pathophysiology of this process may differ from that produced by balloon angioplasty. Varying forms of arterial injury elicit differing effects on the vessel wall and trigger different cellular responses (47). Second, in the Smad3 null mouse, all cells are deficient in Smad3. Therefore, the enhanced neointimal lesion may result from the loss of Smad3 in other cell types besides VSMCs, such as inflammatory cells or progenitor cells. In support of this theory, Feinberg et al. (9) have reported that the loss of Smad3 in macrophages is associated with an enhanced inflammatory response (9). These data suggest that the larger neointimal lesion observed by Kobayashi et al. (20) in Smad3-deficient mice may result, at least in part, from an enhancement in inflammation related to macrophages deficient in Smad3. Lastly, although Kobayashi's group showed that VSMCs derived from Smad3 null mice had a slightly increased basal growth rate, TGF-β still exhibited an inhibitory effect on cell proliferation. This finding suggests that the hyperplastic response observed in Smad3 knockout mice is unlikely related to an effect on VSMC proliferation and that factors outside the arterial wall may have accounted for the intimal hyperplasia observed in these animals.
In fact, our own in vitro and in vivo studies using AdSmad7 to inhibit Smad3 function suggest that the growth inhibitory effects of TGF-β are mediated, at least in part, by a Smad-independent pathway. Contrary to the findings of Kobayashi et al. (20), our data show that a local inhibition of Smad3 function decreases SMC proliferation and intimal hyperplasia and is consistent with our hypothesis that TGF-β, through Smad3 signaling, induces SMC proliferation and consequently intimal hyperplasia. Importantly, however, we acknowledge that Smad7 inhibits both Smad3 and Smad2 and that Smad7 overexpression itself may have independent effects on cell proliferation and the development of intimal hyperplasia.
Consistent with our in vivo findings, the overexpression of Smad3 in isolated VSMCs resulted in the stimulation rather than the inhibition of proliferation in response to TGF-β. We have previously shown that in normal, uninjured VSMCs, TGF-β inhibits cell proliferation through a reduction of cyclin A (17). Specifically, we reported that TGF-β-induced cAMP response element-binding protein phosphorylation resulted in a decreased cyclin A gene transcription and consequently cell cycle arrest (17). However, our current in vivo and in vitro findings demonstrate that VSMCs with high levels of Smad3 exhibit a proliferative response to TGF-β. It has been previously shown that “transformed cells” respond to TGF-β with an enhancement rather than an inhibition of proliferation. McCaffrey's group (27), for example, demonstrated that VSMCs grown from carotid endarterectomy plaques are resistant to TGF-β-induced apoptosis. Furthermore, these cells were shown to proliferate after treatment with TGF-β, due to the decreased expression of type II TGF-β receptor (26). Our present results in combination with McCaffrey's findings imply that in response to inflammation or vascular injury, changes in protein expression, such as the overexpression of Smad3 or the downregulation of type II TGF-β receptor, can alter the response of VSMCs to cytokines such as TGF-β.
We propose that the mechanism underlying the proliferative effect of TGF-β mediated by Smad3 involves p27. p27 is a cyclin-dependent kinase inhibitor that has been shown to be essential in the regulation of cell proliferation, particularly in the transitions from the G0 phase to S phase (12, 51). It primarily inhibits the cyclin E-cyclin-dependent kinase-2 (Cdk2) complex, which is necessary for progression through the G1 checkpoint (29, 33, 34). Normal cells in the G0 phase have high levels of nuclear p27, although upon reentry into the cell cycle, p27 is exported into the cytoplasm and, in many cases, total p27 levels decrease (23, 32, 38, 51).
The importance of p27 in regulating VSMC proliferation in animal models of vascular injury has been demonstrated by two previous investigators. Tanner et al. (48) showed that endogenous p27 levels varied inversely with VSMC proliferation after arterial injury, whereas Chen et al. (4) showed that the overexpression of p27 via adenovirus-mediated gene transfer inhibited VSMC proliferation and consequent neointimal formation. Of note, these studies demonstrate the importance of p27 upregulation as an inhibitor of cell proliferation at late time points after injury (2 wk). Our data suggest that a sustained Smad3 overexpression and its effect on p27 subcellular localization lead to an abnormal persistence of cell proliferation at later time points after injury, when p27 is believed to play a pivotal role in arresting cell growth.
There are two pertinent factors that influence p27 function: intracellular concentration and subcellular localization (51). The regulation of p27 levels is primarily posttranscriptional, through ubiquitination and proteolysis (33, 34, 51). The subcellular compartmentalization of p27 has also been shown to be essential in regulating p27 activity, since p27 must be nuclear to inhibit cyclin E-Cdk2 (51). Accordingly, it has been found that in response to mitogenic stimulation, a fraction of p27 is exported to the cytoplasm. The nuclear export of p27 has been shown to be dependent on the S10 phosphorylation of p27, which is mediated by at least two kinases: the serine-threonine kinase human kinase-interacting stathmin and the PI3-kinase pathway (2, 3, 10, 23). Furthermore, studies of cell cycle kinetics have suggested that the S10 phosphorylation of p27 regulates the reentry of quiescent cells in G0 into the cell cycle (12, 23).
The significance of subcellular localization of p27 in regulating cell proliferation has been well described in the cancer literature (51). Since 1998, cytoplasmic sequestration of p27 has been reported in several tumors, including prostate, esophageal, thyroid, ovarian, and breast carcinomas (51). Baldassarre et al. (1) showed that high Cdk2 activity in thyroid carcinomas can be attributed to cytoplasmic localization of p27; in this disease state, the total p27 levels were unchanged. Thus it appears that the nuclear export of p27 is able to regulate proliferation even in the absence of changes in total p27. It has also been observed that the cytoplasmic localization of p27 is associated with well-differentiated tumors, whereas the complete loss of p27 expression occurs in more advanced cancer (44, 46). These results support our finding that Smad3 overexpression in VSMCs increases cell proliferation through the nuclear export of p27, without a significant reduction in total p27.
Interestingly, p27 was first discovered as a mediator of TGF-β-induced cell cycle arrest in mink lung epithelial cells (33, 34). Later studies have demonstrated that TGF-β induces p27 levels and thus decreases Cdk2 activity in cancer cells and inflammatory cells, in addition to epithelial cells (16, 22, 34, 37). These previous data highlight the novelty of our current findings, that in transformed VSMCs overexpressing Smad3, the abnormal response to TGF-β may involve the downregulation rather than the upregulation of p27 activity.
Of some relevance to our studies of vascular disease is the recent finding that cytoplasmic p27 is not inert (3). Once transported into the cytoplasm, p27 may play an essential role in cell motility and interact with proteins including RhoA and Rac (3). In light of these recent findings, not only does transportation of p27 into the cytoplasm lead to SMC proliferation, but cytoplasmic p27 may also promote SMC migration, another process that has been implicated in the formation of intimal hyperplasia.
Whereas our data indicate that the phosphorylation of p27 on S10 may be the event that leads to VSMC proliferation, other phosphorylation sites may also be important in the regulation of proliferation. For example, threonine-157 phosphorylation has been shown in breast cancer cells to promote the cytoplasmic retention of p27 (24, 45, 50). Another possible and related explanation for the effect of p27 on proliferation is the degradation of cytoplasmic p27, leading to an overall decrease in p27 levels (12, 14, 18). The ubiquitination and degradation of cytoplasmic p27 may account for the small decrease in total p27 levels that we observed after 24 h, although this decrease was not statistically significant. Lastly, while we have demonstrated an association between Smad3 overexpression and S10-pp27, the underlying mechanism for this interaction is unclear. Future research is needed to study the potential interactions between Smad3 and the PI3-kinase/Akt pathway, which has been shown to be one mechanism through which p27 is phosphorylated (2, 3, 23). Interestingly, both the Smad3 and the PI3-kinase/Akt pathways have been shown to be downstream of TGF-β.
One limitation of our study was the use of the entire arterial wall for Western blot analysis for p27. Using this approach, we observed an increase in S10 phosphorylation of p27 in arterial wall extracts 14 days after injury in AdSmad3-treated rats. We did not find a reduction in total p27 levels at this time point, and this is consistent with previous studies by Tanner et al. (48). However, it is also possible that a decrease in total p27 in VSMCs of rats treated with AdSmad3 was masked by a p27 expression in the surrounding adventitial fibroblasts or inflammatory cells. Another limitation of this study is our focus on total Smad3 rather than levels of activated or phosphorylated Smad3. In vivo, it is well accepted that TGF-β is not only present but elevated after vascular injury; therefore, we believe that Smad3 (both endogenous and exogenous) present in the tissues that we studied was in fact phosphorylated and activated by TGF-β. This was not directly tested, however, since we were unable to perform immunohistochemistry for pSmad3 due to a lack of an appropriately specific antibody reactive to rat tissue.
The results of the present study demonstrate that the TGF-β signaling protein Smad3 is upregulated after vascular injury and that this results in an increased cell proliferation secondary to p27 nuclear export and an exaggerated neointimal lesion. Thus we have begun solving the puzzle as to how TGF-β, an accepted inhibitor of cell growth, induces significant VSMC proliferation and resultant intimal hyperplasia after vascular injury. Our finding that “injured” cells have increased levels of Smad3, which results in a transformed response to TGF-β, are bolstered by the recent finding of large populations of Smad3-positive cells in atherectomy samples from restenotic lesions of the femoral arteries of humans (unpublished data, 2007). These lesions also appear to be comprised mainly of proliferating (PCNA positive) cells. A better understanding of the relationship between Smad3 and cell proliferation will provide us with further insight into the mechanism through which TGF-β produces intimal hyperplasia and also enable us to target specific aspects of this signaling mechanism as a therapy for restenosis after arterial reconstruction.
GRANTS
This work was supported by a National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL-68673 (to K. C. Kent and B. Liu); an American Heart Association Grant-in-Aid 0455859T (to B. Liu); NHLBI Grants F32-HL-086222-01-02 (to R. Kundi) and F32-HL-088818-01 (S. Tsai); a Society of University Surgeons-Ethicon Scholarship Grant Award (to S. Tsai); and National Institutes of Health Training Grants T32-CA-68971-07 (to S. T. Hollenbeck) and T32-GM-008466 (to E. J. Ryer and S. Tsai).
Supplementary Material
Acknowledgments
We thank Sophia Chu for preparation of adenoviruses.
REFERENCES
- 1.Baldassarre G, Belletti B, Bruni P, Boccia A, Trapasso F, Pentimalli F, Barone MV, Chiappetta G, Vento MT, Spiezia S, Fusco A, Viglietto G. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J Clin Invest 104: 865–874, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J 21: 3390–3401, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borriello A, Cucciolla V, Oliva A, Zappia V, Della Ragione F. p27Kip1 metabolism: a fascinating labyrinth. Cell Cycle 6: 1053–1061, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Krasinski K, Sylvester A, Chen J, Nisen PD, Andres V. Downregulation of cyclin-dependent kinase 2 activity and cyclin A promoter activity in vascular smooth muscle cells by p27(KIP1), an inhibitor of neointima formation in the rat carotid artery. J Clin Invest 99: 2334–2341, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clowes AW, Clowes MM, Fingerle J, Reidy MA. Regulation of smooth muscle cell growth in injured artery. J Cardiovasc Pharmacol 14, Suppl 6: S12–S15, 1989. [PubMed] [Google Scholar]
- 6.Clowes MM, Lynch CM, Miller AD, Miller DG, Osborne WR, Clowes AW. Long-term biological response of injured rat carotid artery seeded with smooth muscle cells expressing retrovirally introduced human genes. J Clin Invest 93: 644–651, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Farb A, Kolodgie FD, Hwang JY, Burke AP, Tefera K, Weber DK, Wight TN, Virmani R. Extracellular matrix changes in stented human coronary arteries. Circulation 110: 940–947, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg MW, Shimizu K, Lebedeva M, Haspel R, Takayama K, Chen Z, Frederick JP, Wang XF, Simon DI, Libby P, Mitchell RN, Jain MK. Essential role for Smad3 in regulating MCP-1 expression and vascular inflammation. Circ Res 94: 601–608, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem 277: 28706–28713, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Halloran BG, Prorok GD, So BJ, Baxter BT. Transforming growth factor-beta 1 inhibits human arterial smooth-muscle cell proliferation in a growth-rate-dependent manner. Am J Surg 170: 193–197, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S, Nakayama K. Degradation of p27(Kip1) at the G(0)-G(1) transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem 276: 48937–48943, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res 120: 288–294, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama KI. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J Biol Chem 277: 14355–14358, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Yamamura S, Nelson PR, Mureebe L, Kent KC. Differential effects of platelet-derived growth factor isotypes on human smooth muscle cell proliferation and migration are mediated by distinct signaling pathways. Surgery 120: 427–431, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Kamesaki H, Nishizawa K, Michaud GY, Cossman J, Kiyono T. TGF-beta 1 induces the cyclin-dependent kinase inhibitor p27Kip1 mRNA and protein in murine B cells. J Immunol 160: 770–777, 1998. [PubMed] [Google Scholar]
- 17.Kamiya K, Sakakibara K, Ryer EJ, Hom RP, Leof EB, Kent KC, Liu B. Phosphorylation of the cyclic AMP response element binding protein mediates transforming growth factor beta-induced downregulation of cyclin A in vascular smooth muscle cells. Mol Cell Biol 27: 3489–3498, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, Yoshida M, Nakayama K, Nakayama KI. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol 6: 1229–1235, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kanzaki T, Tamura K, Takahashi K, Saito Y, Akikusa B, Oohashi H, Kasayuki N, Ueda M, Morisaki N. In vivo effect of TGF- beta 1. Enhanced intimal thickening by administration of TGF- beta 1 in rabbit arteries injured with a balloon catheter. Arterioscler Thromb Vasc Biol 15: 1951–1957, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Yokote K, Fujimoto M, Yamashita K, Sakamoto A, Kitahara M, Kawamura H, Maezawa Y, Asaumi S, Tokuhisa T, Mori S, Saito Y. Targeted disruption of TGF-beta-Smad3 signaling leads to enhanced neointimal hyperplasia with diminished matrix deposition in response to vascular injury. Circ Res 96: 904–912, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kubota K, Okazaki J, Louie O, Kent KC, Liu B. TGF-beta stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J Surg Res 109: 43–50, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lecanda J, Parekh TV, Gama P, Lin K, Liarski V, Uretsky S, Mittal K, Gold LI. Transforming growth factor-beta, estrogen, and progesterone converge on the regulation of p27Kip1 in the normal and malignant endometrium. Cancer Res 67: 1007–1018, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Lee JG, Kay EP. Two populations of p27 use differential kinetics to phosphorylate Ser-10 and Thr-187 via phosphatidylinositol 3-kinase in response to fibroblast growth factor-2 stimulation. J Biol Chem 282: 6444–6454, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Itoh H, Louie O, Kubota K, Kent KC. The signaling protein Rho is necessary for vascular smooth muscle migration and survival but not for proliferation. Surgery 132: 317–325, 2002. [DOI] [PubMed] [Google Scholar]
- 26.McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, Bush HL Jr. Decreased type II/type I TGF-beta receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-beta1. J Clin Invest 96: 2667–2675, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaffrey TA, Du B, Fu C, Bray PJ, Sanborn TA, Deutsch E, Tarazona N, Shaknovitch A, Newman G, Patterson C, Bush HL Jr. The expression of TGF-beta receptors in human atherosclerosis: evidence for acquired resistance to apoptosis due to receptor imbalance. J Mol Cell Cardiol 31: 1627–1642, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Mii S, Ware JA, Kent KC. Transforming growth factor-beta inhibits human vascular smooth muscle cell growth and migration. Surgery 114: 464–470, 1993. [PubMed] [Google Scholar]
- 29.Morgan DO Principles of CDK regulation. Nature 374: 131–134, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 190: 300–309, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Nikol S, Isner JM, Pickering JG, Kearney M, Leclerc G, Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest 90: 1582–1592, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372: 570–573, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev 8: 9–22, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78: 59–66, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen LM, Wolf YG, Ruoslahti E. Vascular smooth muscle cells from injured rat aortas display elevated matrix production associated with transforming growth factor-beta activity. Am J Pathol 147: 1041–1048, 1995. [PMC free article] [PubMed] [Google Scholar]
- 36.Reidy MA, Fingerle J, Lindner V. Factors controlling the development of arterial lesions after injury. Circulation 86: III43–III46, 1992. [PubMed] [Google Scholar]
- 37.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 9: 1831–1845, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Rodier G, Montagnoli A, Di Marcotullio L, Coulombe P, Draetta GF, Pagano M, Meloche S. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J 20: 6672–6682, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roque M, Kim WJ, Gazdoin M, Malik A, Reis ED, Fallon JT, Badimon JJ, Charo IF, Taubman MB. CCR2 deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol 22: 554–559, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Ryan ST, Koteliansky VE, Gotwals PJ, Lindner V. Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J Vasc Res 40: 37–46, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Ryer EJ, Hom RP, Sakakibara K, Nakayama KI, Nakayama K, Faries PL, Liu B, Kent KC. PKCdelta is necessary for Smad3 expression and transforming growth factor beta-induced fibronectin synthesis in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 26: 780–786, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, Kent KC, Liu B. Protein kinase C delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem 280: 35310–35317, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther 315: 1005–1012, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Sgambato A, Ratto C, Faraglia B, Merico M, Ardito R, Schinzari G, Romano G, Cittadini AR. Reduced expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27(Kip1) in human colon cancer. Mol Carcinog 26: 172–179, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8: 1145–1152, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Singh SP, Lipman J, Goldman H, Ellis FH Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res 58: 1730–1735, 1998. [PubMed] [Google Scholar]
- 47.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res 93: 783–790, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Tanner FC, Yang ZY, Duckers E, Gordon D, Nabel GJ, Nabel EG. Expression of cyclin-dependent kinase inhibitors in vascular disease. Circ Res 82: 396–403, 1998. [DOI] [PubMed] [Google Scholar]
- 49.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci 29: 265–273, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, Fusco A, Santoro M. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 8: 1136–1144, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Viglietto G, Motti ML, Fusco A. Understanding p27(kip1) deregulation in cancer: down-regulation or mislocalization. Cell Cycle 1: 394–400, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Wolf YG, Rasmussen LM, Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J Clin Invest 93: 1172–1178, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.