Abstract
Long-term depression (LTD) of synaptic signaling—lasting from tens of minutes to hours or longer—is a widespread form of synaptic plasticity in the brain. Neurons express diverse forms of LTD, including autaptic LTD (autLTD) observed in cultured hippocampal neurons, the mechanism of which remains unknown. We have recently reported that autaptic neurons express both endocannabinoid-mediated depolarization-induced suppression of excitation (DSE) and metabotropic suppression of excitation (MSE). We now report that activating cannabinoid CB1 receptors is necessary for the induction of autLTD. Most surprisingly, CB1 does not induce autLTD via the Gi/o proteins typically activated by this receptor nor with Gs. Rather, the requirements of presynaptic phospholipase C and filled calcium stores suggest Gq. In autLTD, a 3- to 4-min activation of the receptor by the endocannabinoid 2-arachidonoyl glycerol leads to prolonged inhibition while leaving short-term inhibition (e.g., DSE) intact. autLTD requires activation of both metabo- and ionotropic glutamate receptors. autLTD also requires MEK/ERK activation. Under certain conditions, one or more DSE stimuli will elicit autLTD. It is becoming evident that cannabinoids mediate multiple forms of plasticity at a single synapse, stretching temporally from tens of seconds (DSE/MSE) to tens of minutes (autLTD) to hours (CB1 desensitization). Our findings imply a remarkable flexibility for the cannabinoid signaling system whereby discrete mechanisms of CB1 activation within a single neuron yield temporally and mechanistically distinct forms of plasticity.
INTRODUCTION
The phenomenon of long-term depression (LTD) of neuronal synapses is ubiquitous and diverse in both its manner of induction and expression (Malenka and Bear 2004). The different forms of LTD share principally the outcome—an attenuation of synaptic transmission—but bring this about by varied mechanisms. Numerous examples of LTD have been reported in the hippocampus, with a bewildering variety of mechanisms: some are presynaptic, others postsynaptic; some require N-methyl-d-aspartate (NMDA) receptor activation, others metabotropic glutamate receptors, and still others cannabinoid receptors (see Citri and Malenka 2008 for review).
One of the first forms of LTD to be intensively studied was found in autaptic excitatory hippocampal cultures (Bekkers and Stevens 1991; Goda and Stevens 1996). While much was learned about the synaptic qualities of LTD in autaptic hippocampal neurons, the mechanisms of induction and expression of autaptic LTD (autLTD) have remained elusive. As the autaptic preparation remains useful as a simple neuronal system allowing high-resolution study of synaptic transmission in hippocampal neurons in relative isolation, an understanding of the mechanism of this form of LTD is highly desirable.
In addition to being mediators of short-term retrograde signaling (Wilson and Nicoll 2001), endocannabinoids have also been found to play a key role in several distinct forms of LTD in different brain regions (Gerdeman et al. 2002; Safo and Regehr 2005; Sjostrom et al. 2003, 2004). These distinct forms of LTD have diverse mechanisms, emphasizing the value of more simplified systems to study the signaling pathways underlying different forms of endocannabinoid-mediated LTD. Autaptic neuronal cultures are one possible system. Functional cannabinoid receptors have been reported in autaptic neurons (Straiker et al. 2002; Sullivan 1999) and we have described functional autaptic depolarization-induced suppression of excitation (DSE) and metabotropic suppression of excitation (MSE) involving production of an endocannabinoid—likely 2-arachidonyl glycerol (2-AG)—and presynaptic CB1 receptors (Straiker and Mackie 2005, 2007). In this study, we report that endocannabinoids mediate autLTD and identify several striking features of the mechanism.
METHODS
Culture preparation
All procedures used in this study were approved by the Animal Care Committee of the University of Washington or Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Animals. Mouse (CD1 strain) hippocampal neurons isolated from the CA1–CA3 region were cultured on microislands as described previously (Bekkers and Stevens 1991; Furshpan et al. 1976). Neurons were obtained from animals (age postnatal days 0–2) and plated onto a feeder layer of hippocampal astrocytes that had been laid down previously (Levison and McCarthy 1991). Cultures were grown in high-glucose (20 mM) medium containing 10% horse serum without mitotic inhibitors and used for recordings after 8 days in culture and for no more than 3 h after removal from culture medium.
Electrophysiology
When a single neuron is grown on a small island of permissive substrate, it forms synapses—or “autapses”—onto itself. All experiments were performed on isolated autaptic neurons. Whole cell voltage-clamp recordings from autaptic neurons were carried out at room temperature using Axopatch 200A amplifier (Axon Instruments, Burlingame, CA). The extracellular solution contained (in mM) 119 NaCl, 5 KCl, 2.5 CaCl2, 1.5 MgCl2, 30 glucose, and 20 HEPES and 3 μM bovine serum albumen (as a carrier for the lipophilic endocannabinoids). Continuous flow of solution through the bath chamber (∼2 ml/min) ensured rapid drug application and clearance. Drugs were typically prepared as stock, then diluted into extracellular solution at their final concentration, and used on the same day. As a rule, positive results were coupled on the same day with negative controls. Conversely, negative results for a given drug (e.g., in knockout cultures) were coupled on the same day with positive controls for that drug in control cells.
Recording pipettes of 1.8–3 MΩ were filled with (in mM) 121.5 KGluconate, 17.5 KCl, 9 NaCl, 1 MgCl2, 10 HEPES, 0.2 EGTA, 2 MgATP, and 0.5 LiGTP. Access resistance and holding current were monitored, and only cells with both stable access resistance and holding current were included for data analysis. Conventional stimulus protocol: the membrane potential was held at −70 mV and excitatory postsynaptic currents (EPSCs) were evoked every 20 s by triggering an unclamped action current with a 1.0-ms depolarizing step. The resultant evoked waveform consisted of a brief stimulus artifact and a large downward spike representing inward sodium currents followed by the slower EPSC. The size of the recorded EPSCs was calculated by integrating the evoked current to yield a charge value (in pC). Calculating the charge value in this manner yields an indirect measure of the amount of neurotransmitter released (though postsynaptic mechanisms may contribute to the magnitude of the response) while minimizing the effects of cable distortion on currents generated far from the site of the recording electrode (the soma). Data were acquired at a sampling rate of 5 kHz.
To determine the extent of LTD, the average of five data points before the LTD stimulus was compared with the average of five data points obtained 20 min after the end of the stimulus. Where possible, we interleaved drug and nondrug controls. In addition, every data set was derived from at least two cultures. It has been reported previously that autaptic neurons sometimes exhibit a washout of their EPSCs (Goda and Stevens 1998). Neurons exhibiting washout (30–50% per data set) were excluded from analysis. In the case of 4-Hz stimulus-evoked autLTD, spontaneous miniature EPSCs (mEPSCs) were obtained from the latter half of long (1 s) traces following EPSC stimulation at which point there is no appreciable contamination of mEPSCs by evoked EPSCs (Goda and Stevens 1996). All other mEPSCs were obtained in the presence of tetrodotoxin (1 μM).
LTD STIMULI.
The stimulus for induction of LTD consisted of 1 s of 4-Hz, 1-ms stimuli alternating with 1 s of rest for 180 iterations (total 6 min). After an initial exhaustion period, EPSCs recover to a new baseline that is generally below the original baseline. Recordings before and after the LTD-inducing stimulus consisted of a 1-ms depolarizing stimulus every 20 s.
DSE STIMULI.
After establishing a 10- to 20-s 0.5-Hz baseline, DSE was evoked by depolarizing to 0 mV for 1–10 s, followed by resumption of a 0.5-Hz stimulus protocol for 10–80+ s until EPSC's recovered to baseline values.
CELL IDENTIFICATION.
Hippocampal autaptic cultures consist of a variety of cell types. In this study, we focused on excitatory neurons. Inhibitory neurons could be distinguished by the distinctively slow decay time of the inhibitory postsynaptic current (IPSC). Where the identity of a neuron was in doubt, it was tested using the AMPA receptor antagonist 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX).
Drugs were purchased from Tocris Cookson (Ellisville, MO), Cayman Chemical (Ann Arbor, MI), or Sigma-Aldrich (St. Louis, MO). CB1+/− mice used to found the CB1−/− colony were generously provided by Dr. Catherine Ledent [University of Brussels, Brussels (Reibaud et al. 1999)].
RESULTS
Cannabinoid role in autLTD
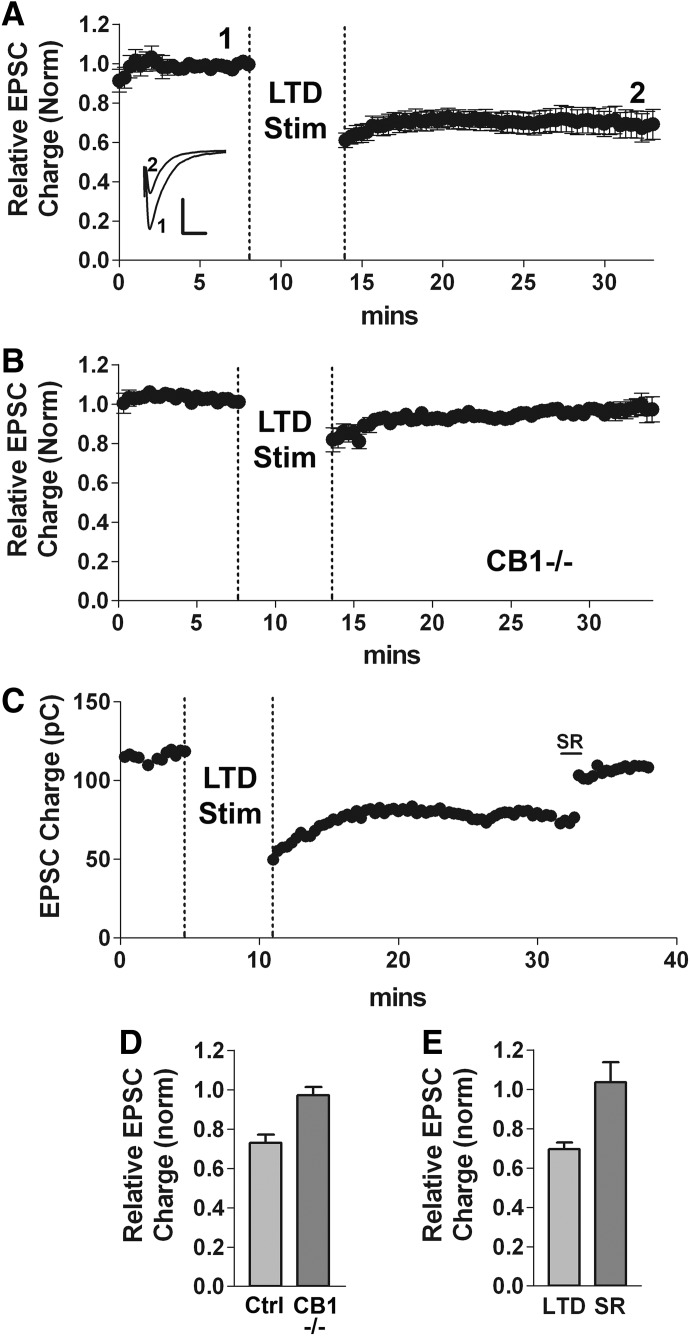
LTD is reliably elicited in autaptic neurons with an intermittent low-frequency stimulus (Fig. 1, A and D relative EPSC amplitude after LTD protocol: 0.73 ± 0.04 relative to baseline, n = 14). To test for a potential role of endocannabinoids and CB1 receptors in autLTD, we attempted to elicit LTD in cultures from mice lacking the CB1 receptor (CB1 −/−). We found LTD to be absent in CB1−/− autaptic neurons (Fig. 1, B and D, relative EPSC amplitude: 0.97 ± 0.06, n = 7; P < 0.05 1-way ANOVA with Dunnett's post hoc test vs. CB1+/+), suggesting that CB1 receptors are required for autLTD. Interestingly and surprisingly, the CB1 antagonist SR141716 (SR) reversed autLTD when applied ∼15 min after the LTD stimulus (Fig. 1, C and E, control LTD inhibition: 0.69 ± 0.03; SR reversal: 1.04 ± 0.10, n = 5, P < 0.05, paired t-test). SR has no effect on EPSC charge on its own (Straiker and Mackie 2005). These results suggest that autLTD may occur because of prolonged engagement and/or activation of CB1 receptors.
FIG. 1.
Cannabinoid CB1 receptor-dependent long-term depression (LTD). A: averaged time course of excitatory postsynaptic currents (EPSCs) (normalized). LTD was induced in autaptic neurons using a 6-min 4-Hz (1-s on-off) stimulus (see methods). Dashed vertical lines indicate the period of low-frequency stimulation. Inset: sample traces at time points indicated before (1) and after (2) induction of LTD. Scale bars: 1 nA, 10 ms. B: averaged time course of EPSCs (normalized) in response to the LTD stimulus in neurons cultured from mice lacking the CB1 receptor (CB1−/−). C: sample time course showing LTD reversal by the CB1 antagonist SR141716 (100 nM). D: bar graph summarizing data from A and B showing a comparison of relative EPSC charge under control conditions, in CB1 −/− cultures following the 4-Hz stimulus (*P < 0.001 CB1−/− vs. CB1+/+ control, 1-way ANOVA with Dunnett's post hoc test). E: bar graph summarizing reversal of established autLTD by 100 nM SR141716 (*P < 0.05, paired t-test).
Δ9-tetrahydrocannabinol antagonizes autLTD
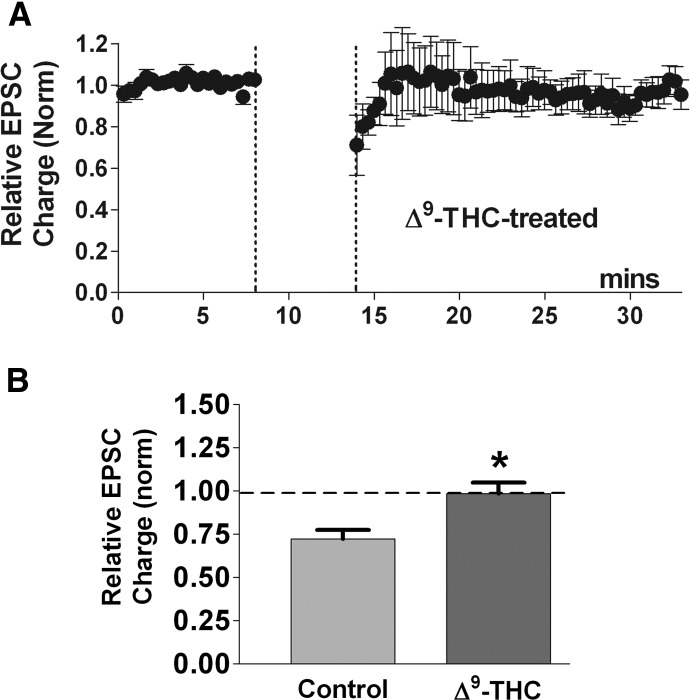
We have shown that the principal psychoactive component of marijuana, the phytocannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC), inhibits DSE and MSE in autaptic excitatory hippocampal neurons without itself inhibiting EPSCs (Straiker and Mackie 2005, 2007). The mechanism by which this occurs is curious and probably reflects the differing functional selectivities of Δ9-THC and 2-AG in inhibiting neurotransmission and eliciting desensitization (Mailman 2007) because Δ9-THC clearly both binds to and activates the receptor sufficiently to induce desensitization over the course of hours. If autLTD requires CB1 receptor activation, one would predict that Δ9-THC might also antagonize autLTD. Indeed, we found that treatment with 1 μM Δ9-THC prevented LTD [Fig. 2, LTD in Δ9-THC (∼6-min application): 0.96 ± 0.06, n = 5; P < 0.05 1-way ANOVA with Dunnett's post hoc test vs. control LTD].
FIG. 2.
Phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC) antagonizes autaptic LTD. A: Δ9-THC (1 μM) application during the LTD-inducing stimulus (•) prevents LTD (n = 5). B: bar graph summarizing the average normalized EPSC following the LTD-inducing stimulus in Δ9-THC-treated neurons—control LTD is shown for reference (*, P < 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD).
Blockade of Gi/o signaling does not prevent autLTD
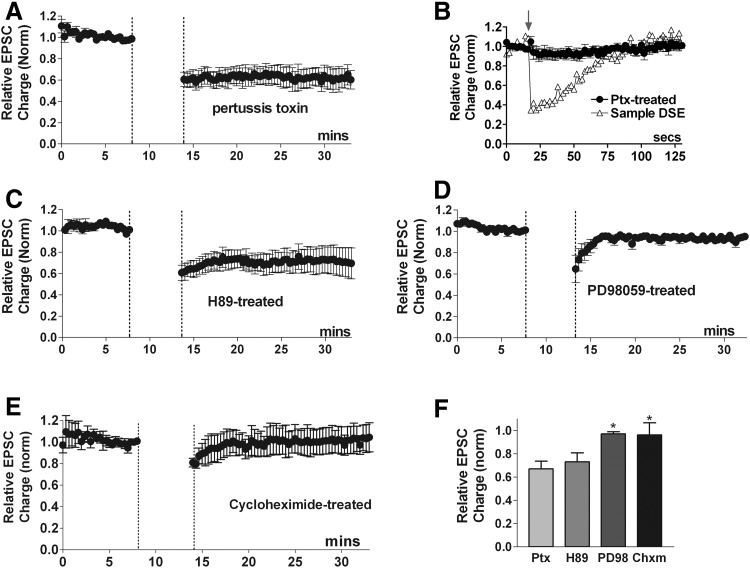
CB1 receptors primarily signal via Gi/o-type proteins. Pertussis toxin selectively and irreversibly ADP-ribosylates Gαi/o subunits, inactivating them (Hepler and Gilman 1992) and has been shown to block CB1 receptor inhibition of EPSCs, DSE, and MSE in autaptic hippocampal neurons (Straiker and Mackie 2005, 2007; Straiker et al. 2002). Thus pretreatment with pertussis toxin would be expected to prevent induction of CB1-mediated LTD. Surprisingly, overnight treatment with pertussis toxin (∼400 ng/ml) did not prevent LTD (Fig. 3 A, LTD: 0.67 ± 0.07, n = 6; P > 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD). The effectiveness of the pertussis toxin in blocking Gi/o signaling was confirmed by the absence of DSE in each neuron used in these LTD studies (Fig. 3B; DSE inhibition relative to normalized baseline: 0.95 ± 0.07, n = 7). These results clearly challenge the general expectations of CB1 receptor signaling and highlight a fundamental difference between endocannabinoid induced LTD and DSE or MSE. Yet our results, with CB1 knockouts, a CB1 antagonist and Δ9-THC, clearly indicate an involvement of CB1 cannabinoid receptors. Thus our results suggest that the LTD-inducing CB1 effect occurs via a G protein other than the Gi/o-pathway generally found to mediate CB1 actions or via a non-G-protein-mediated process.
FIG. 3.
Second messengers in autaptic LTD. A: neurons treated overnight with pertussis toxin (400 ng/ml), an irreversible inactivator of Gαi/o proteins, still exhibited LTD (•, n = 5). B: averaged depolarization-induced suppression of excitation (DSE) time course (•) in the same pertussis toxin-treated neurons as in A showing DSE is absent. An example of DSE time course (▵) from an untreated neuron is shown for reference. C: treatment with the PKA inhibitor H89 (500 nM) does not prevent LTD (n = 6). D: treatment with the mitogen activated protein kinase kinase (MEK) inhibitor PD98059 (20 μM) prevents LTD, indicating that this pathway is involved in autaptic LTD (n = 5). E: pretreatment with the protein synthesis inhibitor cycloheximide (30 μM) prevents the induction of LTD (n = 7). F: bar graph summarizes results from above time courses. *, P < 0.05 1-way ANOVA with Dunnett's post hoc test vs. control LTD.
CB1 cannabinoid receptors have been reported to activate the Gs cAMP/PKA pathway (Glass and Felder 1997) under limited conditions. To test for a possible role of this pathway, we blocked protein kinase A (PKA) signaling using the inhibitor H89. H89 (500 nM) did not affect EPSC amplitude and did not inhibit LTD, indicating that cannabinoids do not induce autLTD via activation of cAMP/PKA [Fig. 3, C and F: LTD in H89 (500 nM): 0.73 ± 0.08, n = 6; P > 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD].
CB1 activation via the Gq pathway has been observed (Lauckner et al. 2005), although 2-AG was explicitly found not to activate signaling via this pathway in HEK293 cells. If autLTD occurs via Gq, one would expect activation of phospholipase C (PLC), which in turn activates protein kinase C (PKC) by producing diacylglycerol (DAG) and increasing intracellular calcium. Unfortunately, PLC activation is necessary for the production of 2-AG in autaptic cultures, and selective pre- or postsynaptic pharmacological blockade of PLC is impractical in autaptic neurons.
Role for MEK/ERK and protein synthesis in autLTD
Extracellular signal-regulated receptor kinase (ERK) may have a role in the induction of some forms of LTD (Thiels et al. 2002). Cannabinoid receptors are known to activate ERK in a variety of cells (Davis et al. 2003; Galve-Roper et al. 2002; Karanian et al. 2005) including excitatory hippocampal neurons (Derkinderen et al. 2003). Although ERK signaling via CB1 receptors is generally mediated by pertussis-toxin (PTX)-sensitive G proteins (Howlett et al. 2002), exceptions have been reported (Daigle et al. 2008a). Thus we examined the effect of PD98059 (20 μM), an inhibitor of mitogen activated protein kinase kinase (MEK), a kinase that phosphorylates and activates ERK. We found that the MEK inhibitor also prevented autLTD, indicating that the signaling pathway that gives rise to autLTD also requires MEK (Fig. 3, D and F: LTD in PD98059: 0.97 ± 0.02, n = 5; P < 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD). The same PD98059 treatment did not prevent DSE (data not shown).
Protein synthesis has been implicated in several forms of LTD including endocannabinoid-mediated LTD (e.g., Yin et al. 2006). To examine a potential role for protein synthesis in autLTD, we pretreated neurons with the protein synthesis inhibitor cycloheximide. We found that LTD was prevented by pretreatment with cycloheximide [Fig. 3, E and F: LTD in cycloheximide (70 μM, 2–4 h pretreatment): 0.96 ± 0.10, n = 7; P < 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD]. Cycloheximide does not prevent DSE (data not shown). Thus induction of autLTD requires ongoing protein synthesis.
Either ionotropic or group I metabotropic glutamate receptor activation is required for induction of autLTD
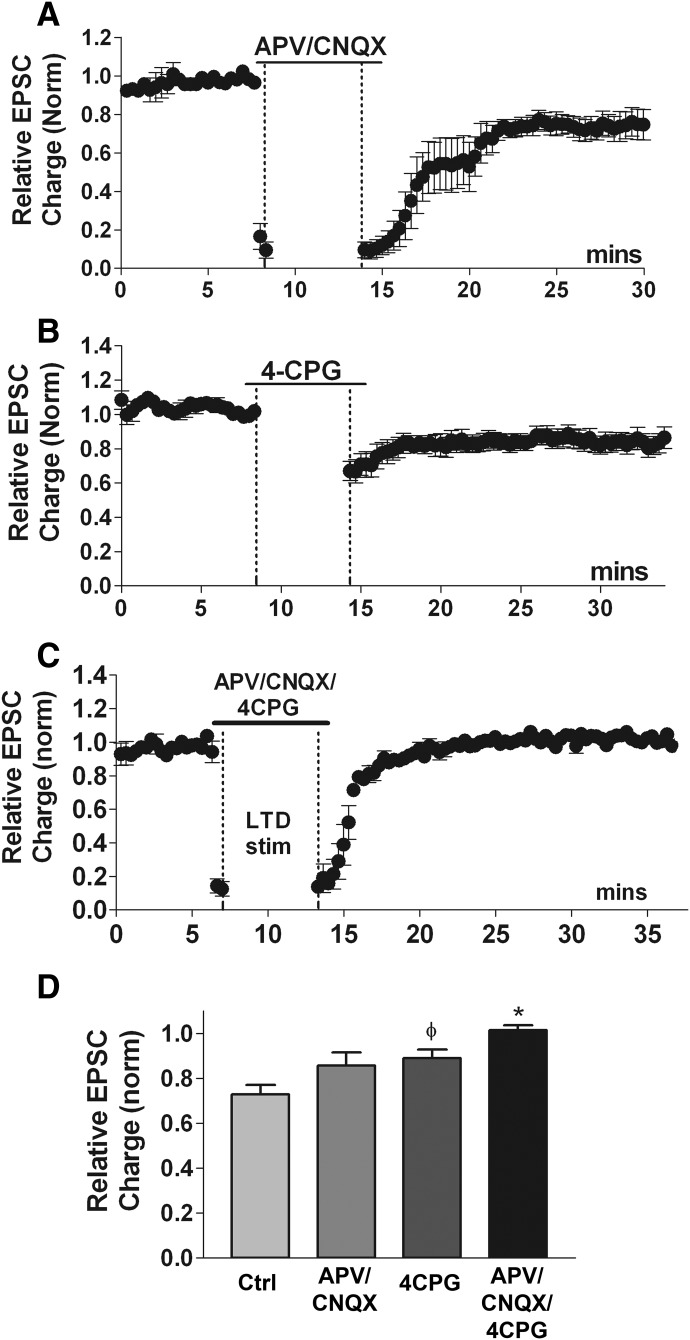
The involvement of ionotropic glutamate receptors in LTD is variable. Some studies have demonstrated a central role for AMPA or NMDA glutamate receptors either in the induction or expression of endocannabinoid-mediated LTD (Nosyreva and Huber 2006; Safo and Regehr 2005; Sjostrom et al. 2003, 2004), but other forms of endocannabinoid-mediated LTD do not require these receptors (Gerdeman et al. 2002; Robbe et al. 2002). Autaptic EPSCs under our experimental conditions are carried largely by AMPA receptors, as shown by nearly complete EPSC inhibition by CNQX (Straiker and Mackie 2005). However, it is possible that the persistent low-frequency stimulation used to induce LTD might activate NMDA receptors. To examine potential roles for these receptors in the induction of LTD, we treated neurons with antagonists for AMPA and NMDA receptors, CNQX and 2-amino-5-phosphonopentanoic acid (APV), respectively, during the LTD stimulus protocol. We found that treatment with CNQX (10 μM) and APV (25 μM) did not prevent LTD (Fig. 4, A and C, CNQX + APV: 0.86 ± 0.06, n = 7; P > 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD). Thus neither NMDA nor AMPA receptors are required for autLTD.
FIG. 4.
Either ionotropic or group I metabotropic glutamate receptor activation is required for induction of autaptic LTD. A: time course of EPSC charge (normalized) shows that the AMPA receptor antagonist 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX, 10 μM) in combination with the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (APV, 25 μM) does not block LTD. B: summary LTD time course for neurons treated with the group I mGluR antagonist (S)4-carboxyphenylglycine (4-CPG, 50 μM) indicates that blocking this class of metabotropic receptor does not prevent autaptic LTD, though there is a trend toward statistical significance (see following text). C: averaged time course of autLTD in presence of CNQX/APV/4-CPG showing that combined antagonism of group I mGluRs and ionotropic glutamate receptors prevents LTD D, Bar graph summarizes results from A–C for LTD inhibition in presence of CNQX/APV (n = 7) or 4-CPG (n = 6) or CNQX/APV/4-CPG (n = 4). φ, P = 0.07, *, P < 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD.
Several reports have implicated Group I metabotropic receptors in LTD (Bolshakov and Siegelbaum 1994; Chevaleyre and Castillo 2003; Gerdeman et al. 2002; Oliet et al. 1997; Robbe et al. 2002). We have recently reported that these receptors mediate one form of MSE (Straiker and Mackie 2006) in autaptic cultures and others have established their role in MSE in other preparations (Hirasawa et al. 2004; Kushmerick et al. 2004). Thus in autaptic cultures Group I mGluR's are present and in a position to participate in the production of the endocannabinoids required to activate CB1 receptors and induce autLTD. If autLTD occurs via postsynaptic group I mGluR-induced production of endocannabinoids, one would expect a blockade of these receptors to prevent autLTD. However, the selective group I mGluR antagonist (S)4-carboxyphenylglycine (4-CPG, 50 μM) did not prevent LTD when applied during the LTD stimulus protocol (Fig. 4, B and C; LTD inhibition in 4-CPG: 0.86 ± 0.03, n = 6; P = 0.07 t-test vs. control LTD). This concentration of 4-CPG fully blocks DHPG (50 μM)-induced MSE [(Straiker and Mackie 2007); data not shown; 1.02 ± 0.03, n = 4]. Metabotropic (group I) glutamate receptors are therefore not required for induction of autLTD. This leaves two likely possibilities: either activation of metabotropic or ionotropic glutamate receptors is required, or the 4-Hz stimulus protocol sufficiently depolarizes the postsynaptic terminals to induce production of endocannabinoids (eCBs), and either of these is sufficient to produce autLTD. To test the former possibility, we elicited LTD in the presence of CNQX, APV, and 4-CPG. This completely abolished LTD (Fig. 4, C and D; LTD inhibition in CNQX/APV/4-CPG: 1.02 ± 0.02, n = 4; P < 0.05 vs. control LTD), suggesting a permissive arrangement where activation of either group I mGluR's or ionotropic glutamate receptors is sufficient for induction of autLTD.
Evidence that the eCB 2-AG is required for autLTD
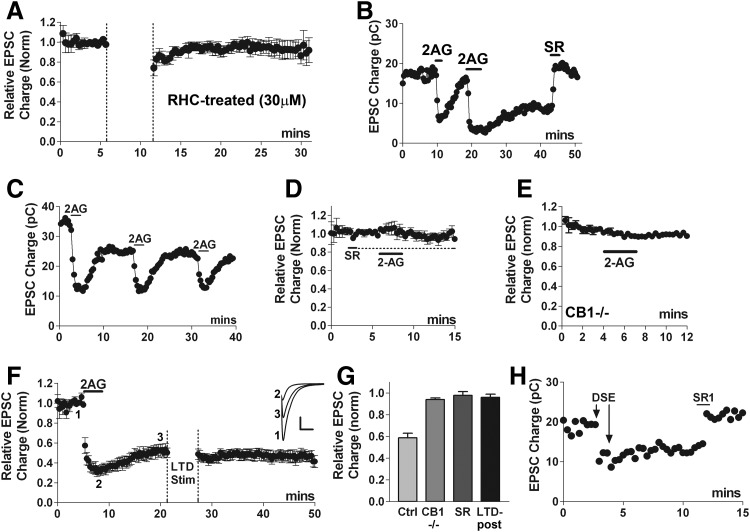
Strong evidence supports 2-AG as the eCB mediating DSE and MSE in autaptic hippocampal neurons (Straiker and Mackie 2005, 2006). A favored mechanism of 2-AG production involves cleavage of DAG by DAG lipase (Piomelli 2003) and inhibition of DAG lipase attenuates the extent of autaptic DSE (Straiker and Mackie 2005). We found that LTD was strongly diminished when neurons were treated with the DAG lipase inhibitor RHC-80267 (30 μM; Fig. 5 A, RHC-treated: 0.96 ± 0.03, n = 7; P < 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD). This suggests that 2-AG is the messenger mediating autLTD.
FIG. 5.
2-arachidonyl glycerol (2-AG) is necessary and sufficient to induce autaptic LTD. A: time course plotting normalized EPSC values shows that pretreatment with the diacylglycerol (DAG) lipase inhibitor RHC-80267 (30 μM, 20+ min) prevents LTD (n = 7). P < 0.05 1-way ANOVA, Dunnett's post hoc test vs. control LTD. B: representative time course shows that while a brief application of 2-AG (1 μM) strongly inhibits EPSCs with full recovery, on prolonged application (>3 min), the inhibition does not fully recover on washout of 2-AG. Subsequent treatment with CB1 antagonist SR141716 (100 nM) induces full recovery, indicating that the inhibition requires continued activation of CB1 receptors. C: 5 μM 2-AG still induces acute inhibition with full recovery after induction of aut-LTD. D: averaged time course indicates that pretreatment with a CB1 antagonist (100 nM SR141716) blocks the 2-AG-induced inhibition (n = 5). E: 2-AG is also without effect in CB1−/− cultures (n = 5). F: averaged time course shows partial recovery with prolonged 2-AG treatment and occlusion of subsequent LTD (n = 5). Inset: sample traces at time points indicated before 2-AG treatment (1) after acute inhibition by 2-AG (2) and after onset of LTD (3). Scale bars: 1 nA, 10 ms. G: summary of 2-AG data indicating that 2-AG induces a ∼40% long-term inhibition of EPSCs but not in CB1 knockouts or after SR treatment. Nor is it possible to elicit autLTD after a long-term 2-AG inhibition. H: time course showing pair of DSE stimuli eliciting a long-term inhibition, reversed by SR1 (200 nM).
Prolonged activation of CB1 receptors is sufficient to induce autLTD
It has been suggested that hippocampal eCB LTD may simply be the result of prolonged activation of CB1 receptors (Chevaleyre et al. 2006; Kano et al. 2009). While we have previously tested brief applications of 2-AG and tested long-term 2-AG treatment with respect to receptor desensitization (Straiker and Mackie 2005), we have not systematically examined the consequences of prolonged 2-AG treatment by examining the degree of recovery after 2-AG washout. To our surprise, we found that in contrast to brief applications (∼1 min), application of 5 μM 2-AG for 3–4 min induced an acute inhibition of EPSCs with recovery to a new lower baseline (Fig. 5, B, F, and G; 0.58 ± 0.05, n = 7), mimicking autLTD. The CB1 receptor antagonist SR141716 when applied 20–30 min after 2-AG had been washed from the bath resulted in a complete recovery to the original baseline value (Fig. 5B, 0.98 ± 0.05, n = 7). This sustained, LTD-like inhibition by 2-AG did not prevent subsequent applications of 2-AG from acutely inhibiting EPSCs with full recovery (Fig. 5C), indicating that the acute and long-term actions are separable. No acute or long-term EPSC inhibition was observed after prior application of SR141716 (Fig. 5, D and G, 0.98 ± 0.03, n = 5) or in CB1−/− cultures (E and G, 0.94 ± 0.01, n = 5) both experiments confirming the involvement of CB1 receptors. Furthermore, the 4-Hz LTD stimulus failed to elicit LTD when applied after this 2-AG LTD-like inhibition, demonstrating that autLTD is occluded by prolonged 2-AG-induced inhibition. Taken together these results suggest that although brief application of 2-AG does not induce an LTD-like response, several minutes of 2-AG application are sufficient to induce autLTD.
If 3–4 min of 2-AG application are sufficient to induce autLTD, then it seems likely that under some circumstances it should be possible for DSE, which is highly likely to occur via 2-AG in this preparation, to induce autLTD. For instance, pairs of DSE stimuli within a few minutes might be sufficient to elicit autLTD. We have found that a second DSE stimulus elicited before full recovery from the first can bring about a lasting inhibition, and this inhibition is fully reversed by SR1 [e.g., Fig. 5H; DSE-LTD: 0.60 ± 0.6, n = 6; reversal by SR (200 nM): 1.15 ± 0.07, n = 4, P < 0.05 by unpaired t-test], mimicking the properties of autLTD. In a few cases where DSE recovery from a single stimulus is particularly slow, a prolonged inhibition may also occur (data not shown).
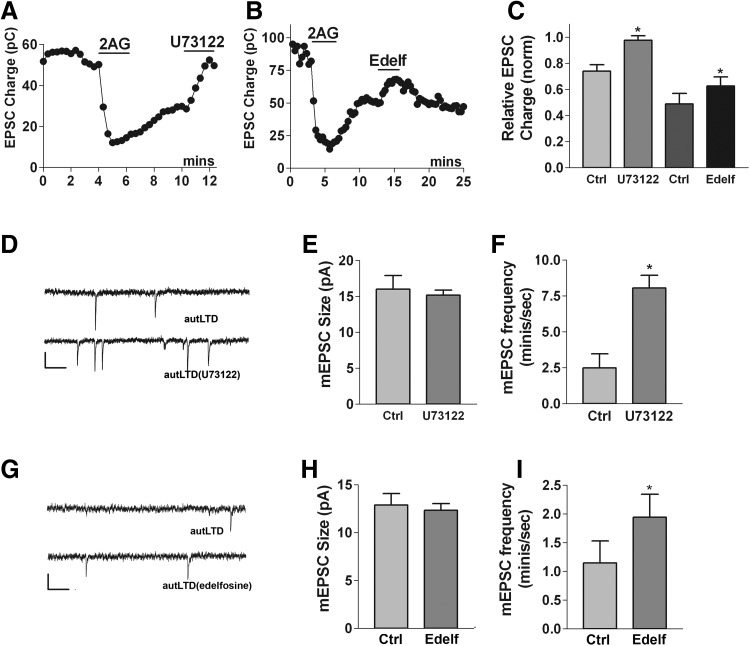
As mentioned earlier, there is evidence for CB1 signaling via Gq-coupled receptors, but the role for PLC, the chief effector of these receptors, is difficult to assess in 4 Hz-induced autLTD insofar as PLC may be involved in eCB production and/or that PLC inhibitors may act directly at CB1 (Hashimotodani et al. 2008). However, because 2-AG is sufficient to induce autLTD, and autLTD appears to involve prolonged activation of CB1-dependent signaling, then if autLTD is PLC-dependent, it should be possible to reverse autLTD with a PLC inhibitor. Indeed, we found that in cells with autLTD, a brief treatment with U73122 (2 μM) readily and completely reversed autLTD [Fig. 6, A and C, same-cell experiments: autLTD: 0.74 ± 0.05; after U73122 treatment (3 min): 0.98 ± 0.03, n = 6, P < 0.05 paired t-test]. As a control, we also tested the PLC inhibitor edelfosine (10 μM), which resulted in a partial reversal of autLTD [Fig. 6, B and C, same cell experiments: autLTD: 0.49 ± 0.08; after edelfosine (3 min): 0.63 ± 0.07, n = 5]. To test whether this action was presynaptic, we measured spontaneous miniature EPSCs (“minis”) under the preceding conditions (but in 1 μM of the Na channel blocker tetrodotoxin). In same-cell experiments, the amplitude of minis did not change as U73122 reversed autLTD, but their frequency increased (Fig. 6, D–F; amplitude under autLTD conditions: 16 ± 1.9 pA, amplitude after reversal by U73122: 15 ± 10.7 pA; frequency under autLTD: 2.5 ± 1.0/s, after U73122 reversal: 8.1 ± 0.9/s, n = 4, P < 0.05 by paired t-test). The results for edelfosine were similar—mini amplitude remained the same while mini frequency rose (Fig. 6, G–I; amplitude under autLTD conditions: 13 ± 1.2, amplitude after partial reversal by edelfosine: 12 ± 0.8; frequency under autLTD: 1.2 ± 0.4, after partial edelfosine reversal: 2.0 ± 0.4). Because CB1 receptors in this preparation are highly enriched in presynaptic terminals, it is likely that the site of action of these drugs is presynaptic. It is highly unlikely that two structurally dissimilar PLC blockers would both inhibit CB1. Instead the most plausible explanation for our findings is that autLTD occurs via activation of presynaptic PLC, downstream of CB1.
FIG. 6.
Phospholipase C (PLC) inhibition reverses autLTD presynaptically. A: 2-AG-induced autLTD is reversed by treatment with the PLC blocker U73122 (2 μM). B: similarly, 2-AG-induced autLTD is partially reversed by treatment with the PLC inhibitor edelfosine (10 μM). C: bar graph summarizes same-cell data for cells with 2-AG-induced autLTD (Ctrl) and either subsequent treatment with U73122 (n = 6) or edelfosine (n = 5). D: sample spontaneous miniature PSCs from a neuron with 2-AG-induced LTD (autLTD) and after treatment with U73122 (autLTD/U73122). Scale bars: 15 pA, 100 ms. E and F: U73122 increases frequency but not amplitude of mEPSCs. Bar graph summarizes same-cell miniature EPSC (mEPSC) amplitudes and frequencies for conditions described in D (n = 4). G: sample spontaneous miniature PSCs from neuron with 2-AG-induced LTD (autLTD) and after treatment with edelfosine (autLTD/edelfosine). Scale bars: 15 pA, 100 ms. H and I: edelfosine increases frequency but not amplitude of mEPSCs. Bar graph summarizes same-cell miniature EPSC amplitudes and frequencies for conditions described in G (n = 5). *, P < 0.05 by paired t-test.
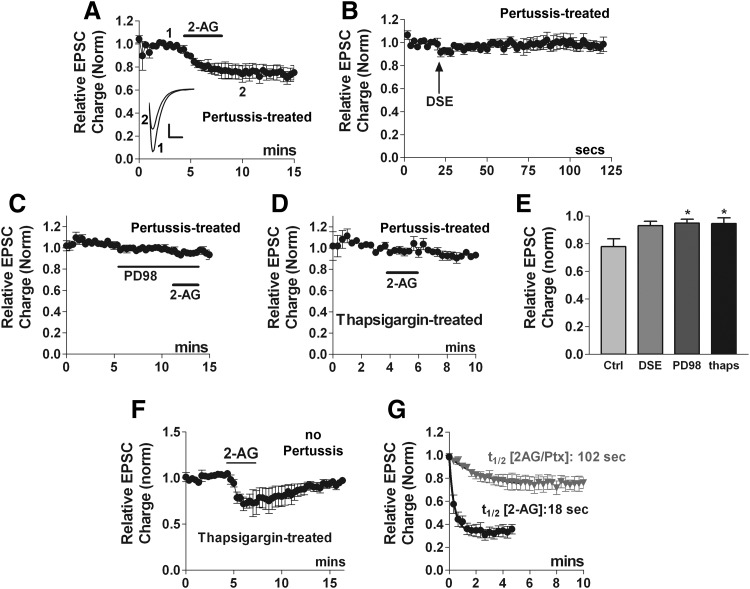
As Fig. 3A shows, we found that pertussis toxin did not prevent autLTD. Yet, we have previously shown that acute 2-AG inhibition of EPSCs is pertussis toxin sensitive (Straiker and Mackie 2005). If autLTD is mediated by a prolonged activation of CB1 receptors by 2-AG, then one would predict that prolonged 2-AG inhibition of EPSCs is also insensitive to pertussis toxin. We found that pertussis toxin treatment reliably blocked DSE (Fig. 7 B, 0.93 ± 0.03, n = 6) but, in what is perhaps our most surprising finding, 5 μM 2-AG (3–4 min), induced no acute inhibition in the same PTX-treated neurons but did induce a slow-onset inhibition (Fig. 7, A and E, EPSC at 5 min post 2-AG: 0.76 ± 0.07, n = 5). The time required to reach 50% inhibition (t1/2) was 102 s, much longer than the 18 s for 2-AG in absence of pertussis toxin (Fig. 7G, 95% confidence intervals are nonoverlapping).
FIG. 7.
2-AG induces autLTD in a pertussis toxin-independent manner. A: 2-AG (5 μM) inhibits EPSCs even after pertussis toxin treatment (overnight, 400 ng/ml). Inset: sample EPSC traces at time points indicated, before (1) and after 2-AG-induced LTD (2). Scale bars: 1 nA, 10 ms. B: in the same cells as A, DSE is abolished by pertussis toxin treatment. C: MEK inhibitor PD98059 (PD98, 20 μM) blocks pertussis-toxin independent 2-AG inhibition. D: Ca-ATPase inhibitor thapsigargin (1 μM, >20mins), also blocks pertussis-toxin independent 2-AG inhibition. E: bar graph summarizes results from A, B, and D. F: averaged time course indicating that in cells treated with thapsigargin alone, 2-AG inhibition reverses fully. G: t1/2 values are calculated from the time courses from Figs. 7A (2AG/pertussis) and 6E (2AG) emphasizing the difference in kinetics between EPSC inhibition by 2-AG signaling via pertussis-toxin (PTX)-independent and -dependent pathways. *, P < 0.05 by 1-way ANOVA with Dunnett's post hoc test vs. control inhibition (2-AG after PTX treatment).
Our previous results with autLTD suggest that 2-AG-induced, pertussis-toxin-insensitive EPSC inhibition should be attenuated by MEK blockers. Indeed we found that in PD98059 and pertussis toxin-treated neurons 2-AG did not induce a prolonged inhibition (20 μM for 5 min: 2-AG, EPSC at 5 min: 0.95 ± 0.03, n = 5, P < 0.05 vs. control autLTD).
We separately revisited the question of Gs-dependent signaling using an adenylyl cyclase inhibitor. Our earlier experiments involving the PKA blocker H89 do not rule out PKA-independent cAMP signaling pathways activated by a hypothetical CB1-Gs-pathway. We therefore examined whether an adenylyl cyclase inhibitor, SQ22536 (80 μM, 5 min) (Harris et al. 1979; Hourani et al. 2001) would block the ability of 2-AG to induce autLTD. We found that SQ22536 had no effect on EPSCs or on 2-AG induction of autLTD (SQ22536 inhibition of EPSCs: 1.04 ± 0.07, n = 5; 10 min after 2-AG plus SQ22536: 0.45 ± 0.08, n = 5).
If autLTD occurs via activating presynaptic PLC, then autLTD may require presynaptic release of calcium from stores. It was not possible to assess such a presynaptic role for autLTD elicited via 4-Hz stimulation because Ca release is also implicated in production of eCBs in this preparation (Straiker and Mackie 2005). However, because 2-AG can induce autLTD directly, we were able to examine the role of presynaptic calcium stores by pretreating cells with the Ca-ATPase inhibitor thapsigargin (1 μM, 20 min). We found that in cells pretreated with pertussis toxin and thapsigargin, 2-AG did not inhibit EPSCs, indicating that slow inhibition of EPSCs by 2-AG requires filled calcium stores (Fig. 7, D and E, 2-AG inhibition after pertussis/thapsigargin pretreatment: 0.95 ± 0.04, n = 5). In contrast, in cells treated only with thapsigargin brief 2-AG inhibition occurred (Fig. 7F, 2-AG acute inhibition thapsigargin pretreatment: 0.63 ± 0.04, recovery: 0.93 ± 0.02, n = 5), indicating that these modes of 2-AG inhibition are pharmacologically separable by their requirements for filled calcium stores.
It has recently been reported that presynaptic activity is necessary for establishment of eCB-dependent LTD in hippocampal slices (Heifets et al. 2008) and in striatal slices (Adermark et al. 2009). To test whether the 20-s interval test stimulus may play an enabling role in 2-AG-induced LTD, we examined EPSCs before and after 2-AG (5 μM, 3 min) treatment. We found that 2-AG still induced LTD even without the test stimulus (data not shown, EPSC inhibition 10 min after 2-AG treatment: 0.44 ± 0.14 n = 4).
Expression of autLTD: pre- vs. postsynaptic
CB1 receptors in excitatory autaptic neurons are known to be present and to act presynaptically (Straiker and Mackie 2005; Sullivan 1999). If presynaptic CB1 receptors are required for the induction of LTD, it seems likely that the expression of LTD is also presynaptic.
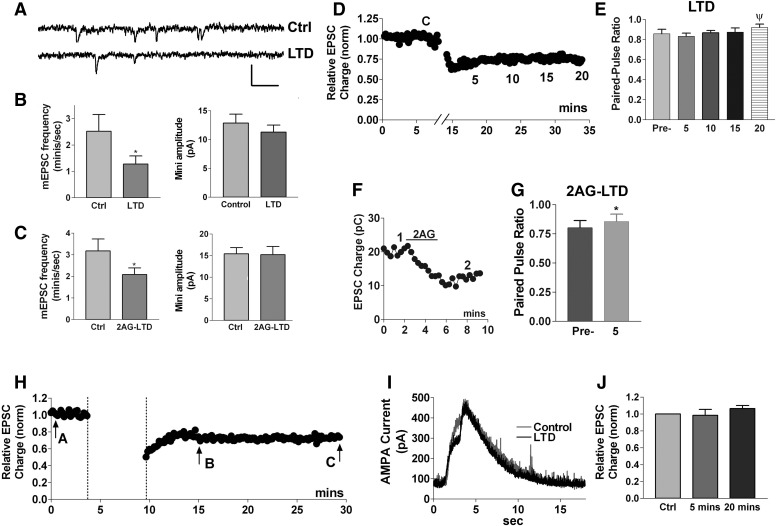
To explore this question further, we examined spontaneous miniature synaptic currents (minis, mEPSCs). These currents are due to release of individual neurotransmitter-containing vesicles. A change in the frequency of minis is consistent with a presynaptic site of action, whereas a change in mini amplitude indicates a postsynaptic action. Examples of postsynaptic action might be: a change in glutamate receptor number, or change in responsiveness or coupling to downstream effectors. Consistent with previous findings in rat (Goda and Stevens 1996), we found that the frequency of minis in mouse neurons decreased following LTD (Fig. 8, A and B; mini frequency before LTD protocol: 2.5 ± 0.6/s, frequency after LTD protocol: 1.3 ± 0.3/s, n = 6; P < 0.005 by paired t-test). The amplitude of minis did not change significantly (Fig. 8, A and B; mini amplitude control: 12.9 ± 1.6 pA; after LTD: 11.3 ± 1.2, n = 5). We obtained similar results for minis obtained after 2-AG-induced LTD in pertussis-toxin-treated cells (Fig. 8C; mini frequency before 2-AG: 3.2 ± 0.6/s; after 2-AG: 2.1 ± 0.3/s; mini amplitude before 2-AG: 15.4 ± 1.5 pA, after 2-AG: 15.2 ± 1.0 pA, n = 7, P < 0.05 by paired t-test for frequency but not amplitude). Taken together these findings point to a presynaptic mode of action for autLTD.
FIG. 8.
Synaptic properties of autaptic LTD are consistent with presynaptic site of action. A: sample traces showing miniature spontaneous EPSCs before (Ctrl) and after (LTD) LTD induction. Scale bars: 25 pA/100 ms. B, left: an analysis of frequency of spontaneous miniature PSCs before and after induction of LTD, (n = 6, *, P < 0.05 paired t-test). Right: an analysis of spontaneous miniature postsynaptic current amplitude before and after induction of LTD (n = 5). C, left: an analysis of frequency of spontaneous miniature PSCs before and after induction of 2-AG-induced LTD (pretreated with PTX; n = 7, *, P < 0.05 paired t-test). Right: an analysis of spontaneous miniature postsynaptic current amplitude before and after 2-AG induction of LTD (n = 7). D: sample LTD timecourse. E: bar graph shows paired-pulse ratio (60-ms interpulse duration) before initiation of LTD (control, C) and at 5-min intervals (noted in representative LTD time course in C) after the end of LTD-inducing stimulus (n = 9, times indicated below LTD time course in top panel, ψ, P = 0.09 paired t-test vs. control). F: sample 2-AG-induced LTD (pretreated with pertussis toxin). G: bar graph shows paired-pulse ratio for 2-AG-induced LTD (pretreated with pertussis toxin) at time points 1 and 2 shown in sample F (n = 7, P < 0.05 by paired t-test). H: sample LTD time course with arrows indicating time points of 0.5- to 2-s puff application of AMPA-Br (4 μM) before (arrow A), 5 min after (arrow B), and 20 min after (arrow C) the LTD-inducing stimulus. I: sample time course of a current response to an AMPA-Br puff. J: bar graph summarizing peak current responses to AMPA-Br puffs.
As a separate test of the site of action for autLTD, we examined paired pulse ratios. The ratios of responses to two sets of closely spaced action potentials (interstimulus interval ∼60 ms, see methods) offers a way to estimate synaptic release probability. The size of the response to the second action potential reflects a balance of two opposing trends following from the first action potential—the effect of residual calcium in the presynaptic terminal on the one hand and the exhaustion of synaptic vesicles due to release of neurotransmitter on the other (Debanne et al. 1996; Mennerick and Zorumski 1995). Release probability will be enhanced by the former and attenuated by the latter. Our initial results for paired pulse ratios in autaptic neurons were surprising: the ratios do not change significantly with electrically induced LTD at 5, 10, or even 20 min after the end of the LTD stimulus protocol (Fig. 8, D and E, control paired-pulse ratio: 0.86 ± 0.04; 5 min: 0.83 ± 0.03; 10 min: 0.87 ± 0.02; 15 min: 0.87 ± 0.04; 20 min: 0.92 ± 0.03, n = 9; ψ, P = 0.09 paired t-test control vs. 20 min), although the ratio trends toward statistical significance (P = 0.09) by 20 min. However, because electrically induced autLTD is often nonsaturating, we tested the paired-pulse ratios of 2-AG-induced LTD, which does saturate at 5 μM (see Fig. 5C for example). We found that the increase in paired pulse ratios was statistically significant for 2-AG-induced LTD (Fig. 8, F and G, control paired-pulse ratio: 0.80 ± 0.06; ratio after 2-AG-LTD: 0.86 ± 0.06, n = 7; *, P < 0.05 by paired t-test). Thus the evidence using paired-pulse ratios also favors a presynaptic expression of autaptic eCB-mediated LTD.
In principle, this combination of reduced mini frequency and a modest rise in paired-pulse ratio could indicate that autLTD occurs via a silencing of AMPA synapses. Such a silencing would reduce mini frequency while leaving amplitude unchanged (because entire synapses are being taken off-line) yet would yield no changes in paired pulse ratios. If the silencing involves a sufficiently large number of synaptic AMPA receptors (as a percentage of total AMPA responses), then it might also be accompanied by a measurable reduction of current responses to puffed AMPA receptor agonists. To test this, we briefly puffed AMPA-Br (4 μM, 0.5–2 s) onto neurons ∼5 min before initiation of LTD stimulus as well as 5 and 20 min after the end of the LTD stimulus. No significant change was seen in response to the puffed AMPA [Fig. 8, H–J; Peak AMPA response 5 min after end of LTD stimulus protocol: 0.98 ± 0.07, n = 6 of control (normalized to 1); after 20 min: 1.06 ± 0.03 of control, n = 5, P > 0.05 by 1-way ANOVA.] Thus LTD did not affect the response to puffed AMPA at either time point. Taken together, these experiments best fit with autLTD that occurs via presynaptic modulation of neurotransmitter release.
DISCUSSION
LTD is a decrease of synaptic strength between neurons that lasts tens of minutes to hours or even longer. It is likely to be a fundamental and essential underpinning of neuronal plasticity (Chevaleyre et al. 2006; Citri and Malenka 2008). Numerous examples of LTD have been encountered in the CNS. The CA1 region of the hippocampus alone is home to at least four major forms of LTD (Malenka and Bear 2004): NMDA-receptor-dependent LTD, a postsynaptically expressed LTD, the manifestation of which appears to be fundamentally dependent on NMDA receptor subunit expression; two forms of mGluR LTD, one pre- and one postsynaptic (Nosyreva and Huber 2006); and eCB LTD (Chevaleyre and Castillo 2003). The last of these is probably the least well understood, apparently occurring chiefly at the synapses of specific inhibitory neurons (CCK-positive basket cells) onto CA1 pyramidal neurons or via heterosynaptic LTD in excitatory neurons in young (P2-10) hippocampus (Yasuda et al. 2008). Autaptic hippocampal LTD was first reported >10 yr ago (Goda and Stevens 1996), but the underlying mechanism has remained elusive. Our studies with autaptic hippocampal neurons suggest that autLTD in fact falls into the last LTD category, that of eCB LTD, but with important distinctions that make it very different from the forms of cannabinoid-mediated hippocampal LTD described thus far.
Two forms of synaptic modulation via temporal modulation of a single stimulus
It is highly likely that autaptic DSE and MSE occur in a retrograde manner with autaptic neurons in possession of postsynaptic machinery to produce eCBs (Straiker and Mackie 2005, 2007). We therefore propose that the low-frequency stimulus results in sustained release of glutamate, which acting postsynaptically on either or both metabotropic and ionotropic glutamate receptors, produces eCBs (likely 2-AG) via activation of DAG lipase (see Fig. 9). 2-AG then retrogradely crosses the synapse, activating presynaptic CB1 receptors. Approximately 3 min of CB1 receptor activation are sufficient to induce autLTD via a nontraditional pathway independent of Gs or Gi/o G proteins. After this, ongoing eCB production is not required; CB1 receptors remain activated either because cannabinoids loiter presynaptically or because CB1 has been switched to a continuously active mode, perhaps independent of agonist. For this reason, SR treatment reverses autLTD even after establishment. Downstream of CB1, activation of Gq proteins is most consistent given the requirements for PLC and filled calcium stores and represents a favored mode of action. Protein synthesis is required for autLTD while MEK (and presumably ERK) activation is also needed, likely via PLC activation. The ultimate mode of action involves a reduction in release probability. Thus CB1 is necessary both for autLTD induction and maintenance.
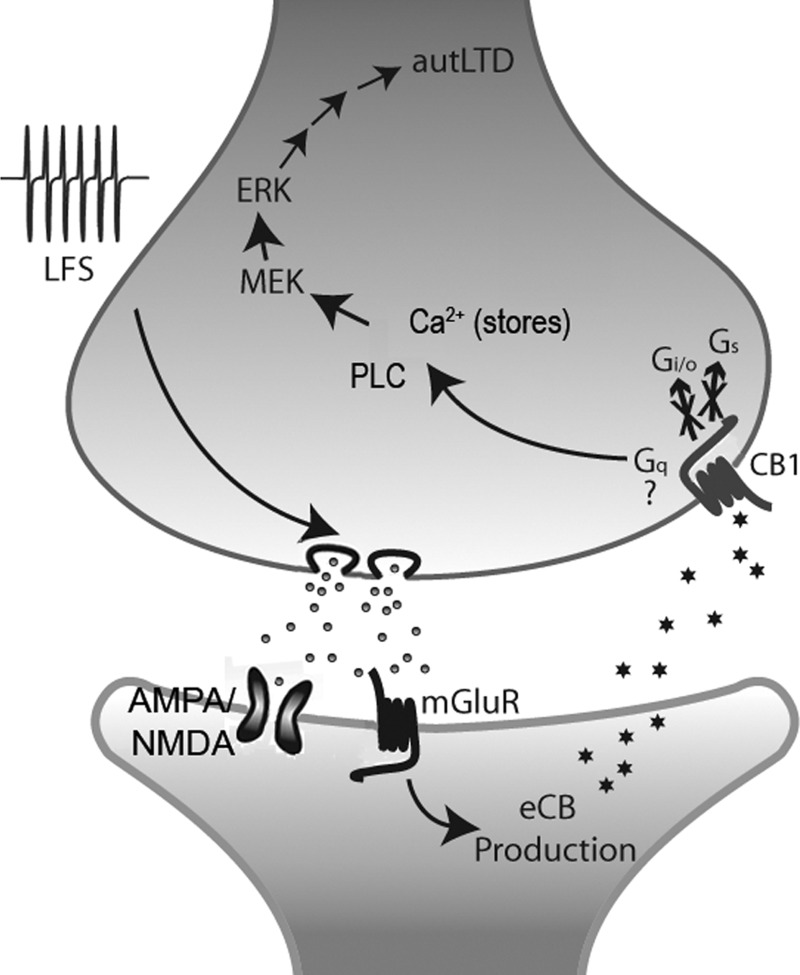
FIG. 9.
Schematic representation of autLTD. Schematic representation of pre- (upper) and postsynaptic (lower) terminals of an autaptic hippocampal neuron with a hypothesized sequence of events leading to autLTD. Low-frequency stimulus (LFS) results in sustained release of glutamate, which acting postsynaptically on either or both metabo- and ionotropic glutamate receptors, produces endocannabinoids (likely 2-AG) via activation of DAG lipase. 2AG retrogradely crosses the synapse, activating presynaptic CB1 receptors. Approximately 3 min of CB1 receptor activation is then sufficient to induce autLTD via a nontraditional pathway independent of Gs or Gi/o G proteins. After this, CB1 receptors remain activated either because cannabinoids loiter presynaptically or because CB1 has been switched to a continuously active mode. Downstream of CB1, activation of Gq-proteins is most consistent given the requirements for PLC and filled calcium stores and represents a favored mode of action. Protein synthesis is required for autLTD while MEK (and presumably extracellular signal-regulated receptor kinase) activation are also needed, likely via PLC activation. The ultimate mode of action involves a reduction in presynaptic release probability.
In addition to the reversal of autLTD by SR, this arrangement has several striking aspects. Our most unexpected finding is that while CB1 receptors are required for induction of LTD, Gi/o proteins are not. Even before cloning of the first cannabinoid receptor, it was established that CB1 cannabinoid receptors primarily act via Gi/o G proteins (Howlett et al. 1986). The notion that this is the dominant arrangement has persisted despite intermittent evidence that other pathways may be activated by CB1 (Glass and Felder 1997; Lauckner et al. 2005; Rhee et al. 1998). The role of Gi/o G proteins in eCB-mediated synaptic plasticity has been difficult to address directly because pertussis toxin treatment is challenging in neuronal slice preparations, where most synaptic transmission work is done. Our results are suggestive of Gq coupling for autLTD. Indeed, using HEK cells (Lauckner et al. 2005), Gq G protein activation has been observed for the synthetic CB1 agonist WIN55212. That a similar activation was not seen for 2-AG may be due to differential cell-dependent activation profiles. For instance, Daigle et al. (2008b) reported very different signaling for identical CB1 mutants in HEK versus AtT20 cells. Separately, Daigle et al. (2008a) reported that under some conditions, ERK activation in HEK cells was PTX insensitive and thus not mediated by Gi/o proteins. Therefore the current observation of a role for ERK1/2 in a CB1-mediated, yet PTX-insensitive, process is not entirely surprising. The original view that most CB1 signaling is Gi/o mediated is proving to be too simple; G-protein pathways activated by CB1 receptors are quite variable and depend both on ligand and cell type.
Our results also point to a role for presynaptic calcium stores because presynaptic PLC and filled calcium stores are required for 2-AG-induced autLTD. The role of calcium in cannabinoid signaling has generated great interest, but the focus has been almost exclusively postsynaptic where calcium plays a key role in the generation of eCBs (Hashimotodani et al. 2005; Isokawa and Alger 2006; Stella et al. 1997). In autaptic neurons, calcium from stores may play an additional part by mediating an alternative CB1 signal. Our results may also explain the findings of Hashimotodani et al. (2008), who reported a direct inhibition of CB1 receptor signaling by the PLC inhibitor U73122. Our observation that the structurally distinct PLC inhibitors U73122 and edelfosine each at least partially block the prolonged form of CB1 inhibition suggests that U73122 may be blocking a component of cannabinoid signaling downstream of the CB1 receptor rather than at the receptor itself.
Last, our results indicate that a simple change in the duration of CB1 receptor activation by an agonist induces a profound switch from acute inhibition lasting tens of seconds to one lasting tens of minutes or more. The switch occurs as a summation of postsynaptic stimulation and does so via differential downstream signaling by a single receptor population. The pertussis-toxin-insensitive component activates more slowly and is coupled to an inhibition that lasts far longer (we did not see reversal, even in recordings >1 h). Importantly, it leaves the acute inhibition intact—we found that subsequently stimulated CB1 receptors clearly retained their short-term inhibition of neurotransmission, in the form of DSE or 2-AG inhibition. It is worth noting that a series of overlapping DSE stimuli can be sufficient to induce autLTD; in some cases where DSE decay times are especially slow, autLTD can be brought on by even a single DSE stimulus.
Although autaptic hippocampal neurons are derived from neonatal hippocampal neurons, autLTD is very different from the forms of persistent cannabinoid-dependent plasticity that have been described thus far in the hippocampus. For instance, autLTD differs substantially from the hippocampal eCB-mediated LTD described by Chevaleyre and Castillo (2003): in addition to its reversibility and mediation by an alternative signaling pathway, autLTD is homo- rather than heterosynaptic and involves only excitatory circuitry rather than interactions between excitatory and inhibitory neurons. The heterosynaptic LTD described by Yasuda et al. (2008) is of particular interest here given that the expression is early postnatal (P2-10) and may therefore be expected to share some qualities of neuronal cultures derived from the same early postnatal starting material. However, this form of LTD shares little else aside from provenance: even the time course (tens of minutes) of onset differs greatly from autLTD. The lack of a role for PKA in autLTD is also somewhat surprising given compelling recent evidence for a PKA role in eCB-mediated LTD (Heifets et al. 2008). Looking at other forms of eCB-mediated hippocampal plasticity, Edwards et al. (2008) have reported a persistent upregulation of DSI; however, this plasticity involves eCB mobilization, whereas after an activation period of several minutes, autLTD appears to be independent of eCB release. AutLTD also differs fundamentally from the DSI potentiation reported by Chen et al. (2007) in the wake of tetanic stimulation at Schaffer collateral synapses: rather than a potentiation of DSI (or DSE), autLTD represents a shift in the “tone” of the cannabinoid signaling. Interestingly, Zhu and Lovinger (2007) recently described a low-frequency stimulus-induced depression of evoked IPSCs in the hippocampus that in some respects resembles autLTD. This depression was prevented by SR, but the authors did not test whether it was reversed with a post hoc application of SR. They found that SR reversed LTP at glutamatergic synapses that appeared to be an outgrowth of the persistent synaptic depression at CA1 GABAergic synapses, finding that SR did not reverse the LTP. This does not entirely rule out the possibility that the LTD was in fact reversible by SR.
In summary, given the fact that the presynaptically expressed autLTD can be induced by ∼3-min application of 2-AG and thereafter reversed by CB1 antagonists or even a PLC inhibitor 10–15 min after induction and the lack of involvement of Gi/o in this form of LTD, autLTD appears to be very different from other forms of eCB-dependent LTD seen in the hippocampus thus far.
Δ9-THC as an antagonist of eCB-mediated plasticity
We have previously shown that Δ9-THC, the chief psychoactive ingredient of cannabis and hashish (Gaoni and Mechoulam 1964), acts at CB1 receptors to antagonize eCB-mediated DSE and MSE (Straiker and Mackie 2005, 2006). Yet Δ9-THC clearly activates the receptor sufficiently to induce desensitization as completely as the synthetic CB1 agonist WIN 55212–2 (Straiker and Mackie 2005). A recent publication (Roloff and Thayer 2008) found that the modulation of excitatory transmission in conventional hippocampal culture switched from agonistic to antagonistic in nature depending on the prevailing firing rate. Here we find that Δ9-THC again antagonizes endogenous cannabinoid signaling, now in the form of CB1-dependent LTD. Interestingly, however, this antagonistic action occurs with an interstimulus interval (20 s) that would be expected to confer agonist properties to Δ9-THC based on the Roloff study. It is possible that the relatively restricted growth conditions of autaptic neurons shifts the balance of sensitivity to firing rate toward antagonistic Δ9-THC action. That Δ9-THC antagonizes three forms of eCB-mediated plasticity suggests that a major mechanism of action may in fact be to antagonize endogenous cannabinoid (i.e., 2-AG) signaling at some synapses, a role that stands in marked contrast to what is commonly assumed.
Summary
In summary, we have found that autLTD in cultured hippocampal neurons is mediated by eCBs (likely 2-AG) acting at cannabinoid CB1 receptors. Indeed prolonged (∼3 min) activation by 2-AG is sufficient to induce autLTD. The mechanism for this involves activation of PLC and MEK and requires filled calcium stores but not PKA. Ongoing protein synthesis is needed for autLTD expression. Importantly, autLTD does not occur via the expected CB1 activation of pertussis-toxin-sensitive Gi/o G proteins. We propose that CB1 receptors in the presynaptic terminals of autaptic hippocampal neurons integrate their eCB inputs; once a threshold of postsynaptic activation is crossed, cannabinoid signaling transitions from short term (tens of seconds) to long term (tens of minutes or longer) signaling. The switch may occur via differential coupling to distinct G proteins—perhaps their relatively inefficient coupling to Gq proteins nonetheless triggers a pronounced LTD of glutamatergic transmission that is well-suited to protect neurons from excitotoxicity. Like other forms of eCB-mediated plasticity in autaptic hippocampal neurons, Δ9-THC antagonizes autLTD.
Thus a single autaptic hippocampal neuron comes equipped with the signaling machinery to generate DSE (Straiker and Mackie 2005), MSE (Straiker and Mackie 2007), and autLTD. These three forms of plasticity, together with CB1 desensitization, implicate CB1 receptors in modulation of neuronal activity spanning a temporal spectrum from seconds to hours. This represents a considerable flexibility on the part of CB1 receptors and the eCB system, and underscores their involvement in diverse forms of synaptic plasticity.
GRANTS
This work was supported by National Drug Abuse Institute Grants DA-11322 and DA-021696.
Acknowledgments
We thank J. Sullivan for a critical reading of the manuscript.
REFERENCES
- Adermark et al. 2009.Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci 29: 32–41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers and Stevens 1991.Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA 88: 7834–7838, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov and Siegelbaum 1994.Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264: 1148–1152, 1994. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2007.Chen K, Neu A, Howard AL, Foldy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci 27: 46–58, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre and Castillo 2003.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38: 461–472, 2003. [DOI] [PubMed] [Google Scholar]
- Chevaleyre et al. 2006.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29: 37–76, 2006. [DOI] [PubMed] [Google Scholar]
- Citri and Malenka 2008.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33: 18–41, 2008. [DOI] [PubMed] [Google Scholar]
- Daigle et al. 2008a.Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 54: 36–44, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle et al. 2008b.Daigle TL, Kwok ML, Mackie K. Regulation of CB(1) cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J Neurochem 106: 70–82, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis et al. 2003.Davis MI, Ronesi J, Lovinger DM. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J Biol Chem 278: 48973–48980, 2003. [DOI] [PubMed] [Google Scholar]
- Debanne et al. 1996.Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol 491: 163–176, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkinderen et al. 2003.Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci 23: 2371–2382, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards et al. 2008.Edwards DA, Zhang L, Alger BE. Metaplastic control of the endocannabinoid system at inhibitory synapses in hippocampus. Proc Natl Acad Sci USA 105: 8142–8147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan et al. 1976.Furshpan EJ, MacLeish PR, O'Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA 73: 4225–4229, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galve-Roperh et al. 2002.Galve-Roperh I, Rueda D, Gomez del Pulgar T, Velasco G, Guzman M. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol 62: 1385–1392, 2002. [DOI] [PubMed] [Google Scholar]
- Gaoni and Mechoulam 1964.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: 1646–1647, 1964. [Google Scholar]
- Gerdeman et al. 2002.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5: 446–451, 2002. [DOI] [PubMed] [Google Scholar]
- Glass and Felder 1997.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 17: 5327–5333, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda and Stevens 1996.Goda Y, Stevens CF. Long-term depression properties in a simple system. Neuron 16: 103–111, 1996. [DOI] [PubMed] [Google Scholar]
- Goda and Stevens 1998.Goda Y, Stevens CF. Readily releasable pool size changes associated with long term depression. Proc Natl Acad Sci USA 95: 1283–1288, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris et al. 1979.Harris DN, Asaad MM, Phillips MB, Goldenberg HJ, Antonaccio MJ. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res 5: 125–134, 1979. [PubMed] [Google Scholar]
- Hashimotodani et al. 2008.Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology 54: 58–67, 2008. [DOI] [PubMed] [Google Scholar]
- Hashimotodani et al. 2005.Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cbeta serves as a coincidence detector through its Ca(2+) dependency for triggering retrograde endocannabinoid signal. Neuron 45: 257–268, 2005. [DOI] [PubMed] [Google Scholar]
- Heifets et al. 2008.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci USA 105: 10250–10255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler and Gilman 1992.Hepler JR, Gilman AG. G proteins. Trends Biochem Sci 17: 383–387, 1992. [DOI] [PubMed] [Google Scholar]
- Hirasawa et al. 2004.Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol 559: 611–624, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourani et al. 2001.Hourani SM, Boon K, Fooks HM, Prentice DJ. Role of cyclic nucleotides in vasodilations of the rat thoracic aorta induced by adenosine analogues. Br J Pharmacol 133: 833–840, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett et al. 2002.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161–202, 2002. [DOI] [PubMed] [Google Scholar]
- Howlett et al. 1986.Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 29: 307–313, 1986. [PubMed] [Google Scholar]
- Isokawa and Alger 2006.Isokawa M, Alger BE. The ryanodine receptor regulates endogenous cannabinoid mobilization in the hippocampus. J Neurophysiol 95: 3001–3011, 2006. [DOI] [PubMed] [Google Scholar]
- Kano et al. 2009.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380, 2009. [DOI] [PubMed] [Google Scholar]
- Karanian et al. 2005.Karanian DA, Brown QB, Makriyannis A, Bahr BA. Blocking cannabinoid activation of FAK and ERK1/2 compromises synaptic integrity in hippocampus. Eur J Pharmacol 508: 47–56, 2005. [DOI] [PubMed] [Google Scholar]
- Kushmerick et al. 2004.Kushmerick C, Price GD, Taschenberger H, Puente N, Renden R, Wadiche JI, Duvoisin RM, Grandes P, von Gersdorff H. Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J Neurosci 24: 5955–5965, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner et al. 2005.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55,212–2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci USA 102: 19144–19149, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison and McCarthy 1991.Levison SW, McCarthy KD. Characterization and partial purification of AIM: a plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J Neurochem 57: 782–794, 1991. [DOI] [PubMed] [Google Scholar]
- Mailman 2007.Mailman RB GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci 28: 390–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka and Bear 2004.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004. [DOI] [PubMed] [Google Scholar]
- Mennerick and Zorumski 1995.Mennerick S, Zorumski CF. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol 488: 85–101, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva and Huber 2006.Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol 95: 3291–3295, 2006. [DOI] [PubMed] [Google Scholar]
- Oliet et al. 1997.Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron 18: 969–982, 1997. [DOI] [PubMed] [Google Scholar]
- Piomelli 2003.Piomelli D The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–884, 2003. [DOI] [PubMed] [Google Scholar]
- Reibaud et al. 1999.Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharmacol 379: R1–2, 1999. [DOI] [PubMed] [Google Scholar]
- Rhee et al. 1998.Rhee MH, Bayewitch M, Avidor-Reiss T, Levy R, Vogel Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neurochem 71: 1525–1534, 1998. [DOI] [PubMed] [Google Scholar]
- Robbe et al. 2002.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA 99: 8384–8388, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff and Thayer 2009.Roloff AM, Thayer SA. Modulation of excitatory synaptic transmission by {Delta}9-tetrahydrocannabinol switches from agonist to antagonist depending on firing rate. Mol Pharmacol 75: 892–900, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo and Regehr 2005.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron 48: 647–659, 2005. [DOI] [PubMed] [Google Scholar]
- Sjostrom et al. 2004.Sjostrom PJ, Turrigiano GG, Nelson SB. Endocannabinoid-dependent neocortical layer-5 LTD in the absence of postsynaptic spiking. J Neurophysiol 92: 3338–3343, 2004. [DOI] [PubMed] [Google Scholar]
- Sjostrom et al. 2003.Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39: 641–654, 2003. [DOI] [PubMed] [Google Scholar]
- Stella et al. 1997.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388: 773–778, 1997. [DOI] [PubMed] [Google Scholar]
- Straiker and Mackie 2005.Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569: 501–517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker and Mackie 2006.Straiker A, Mackie K. Cannabinoids, electrophysiology, and retrograde messengers: challenges for the next 5 years. Aaps J 8: E272–276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker and Mackie 2007.Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol 578: 773–785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker et al. 2002.Straiker AJ, Borden CR, Sullivan JM. G-Protein alpha subunit isoforms couple differentially to receptors that mediate presynaptic inhibition at rat hippocampal synapses. J Neurosci 22: 2460–2468, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan 1999.Sullivan JM Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol 82: 1286–1294, 1999. [DOI] [PubMed] [Google Scholar]
- Thiels et al. 2002.Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci 22: 2054–2062, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson and Nicoll 2001.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592, 2001. [DOI] [PubMed] [Google Scholar]
- Yasuda et al. 2008.Yasuda H, Huang Y, Tsumoto T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc Natl Acad Sci USA 105: 3106–3111, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. 2006.Yin HH, Davis MI, Ronesi JA, Lovinger DM. The role of protein synthesis in striatal long-term depression. J Neurosci 26: 11811–11820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu and Lovinger 2007.Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol 97: 4386–4389, 2007. [DOI] [PubMed] [Google Scholar]