Abstract
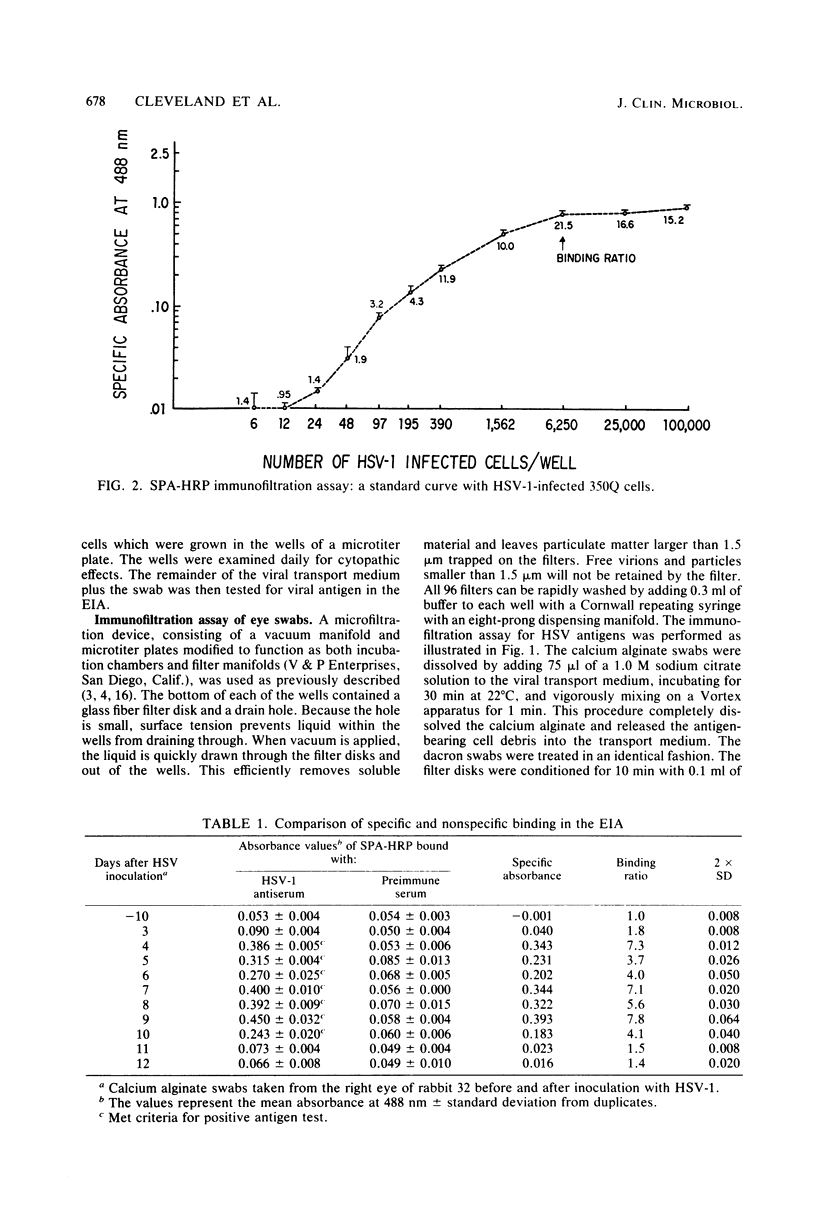
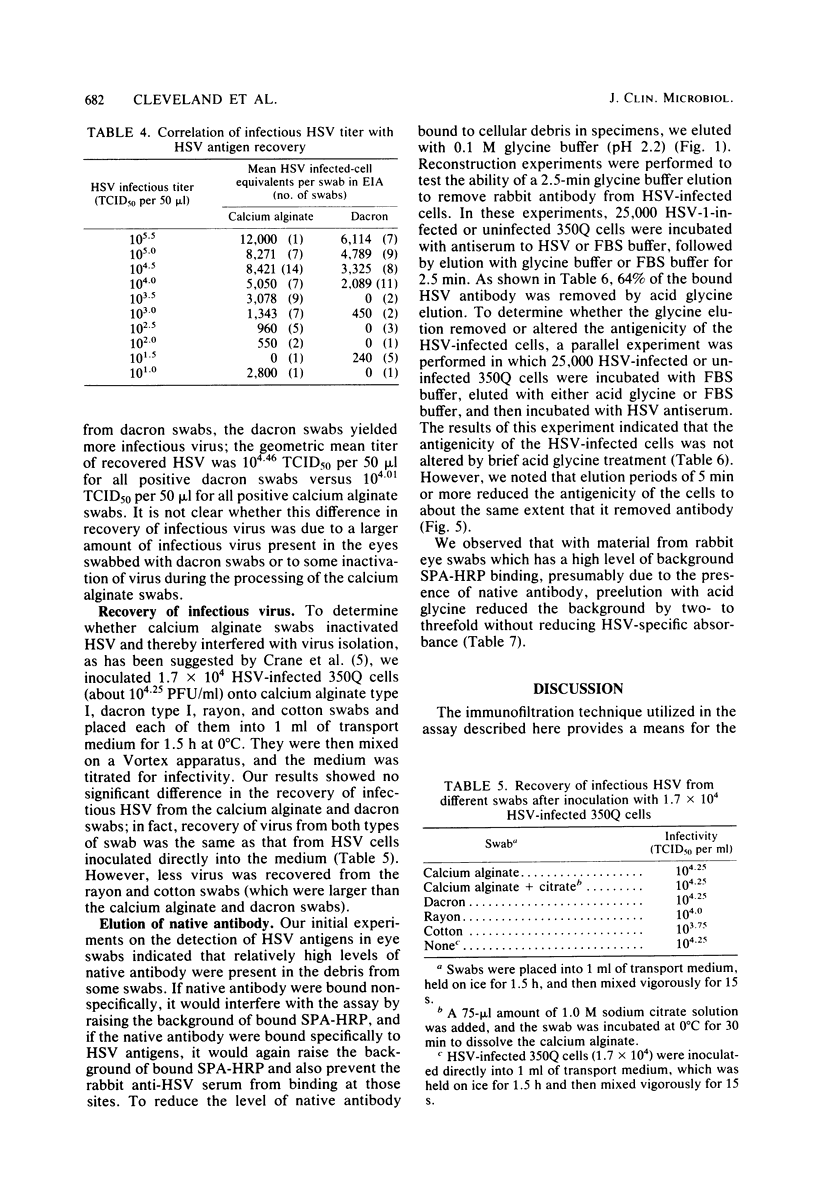
A rapid enzyme immunofiltration assay for herpes simplex virus (HSV) has been developed which is sensitive enough to detect viral antigens in eye swabs from rabbits with primary herpes keratitis. This assay employs a specially designed filter manifold to immobilize whole cells and cell debris dissociated from the swabs. Viral antigens trapped on the filters are then detected in an indirect immunoassay utilizing staphylococcal protein A conjugated with horseradish peroxidase. The assay required only 2.5 h to perform and could be read visually. Reconstruction experiments indicated that antigen from as few as 49 HSV-infected cells could be detected. Calcium alginate swabs were shown to recover more viral antigen than dacron swabs. The enzyme immunofiltration assay detected HSV antigens on 95% of the eye swabs from which infectious virus was recovered. In addition, HSV antigen was also detected in several swabs from infected eyes which did not yield infectious virus, presumably because the virus was neutralized by native antibody present in the lacrimal fluid. This enzyme immunofiltration assay technique lends itself to the elution of native antibody bound to the viral antigens, and this may be especially applicable in the diagnosis of recurrent HSV keratitis, where antiviral antibody in the lacrimal fluid may interfere with virus isolation and fluorescent-antibody or other virus detection assays.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder P. S. Herpes simplex keratitis. Surv Ophthalmol. 1977 Jan-Feb;21(4):313–331. doi: 10.1016/0039-6257(77)90113-8. [DOI] [PubMed] [Google Scholar]
- Blandford G., Heath R. B. Studies on the immune response and pathogenesis of Sendai virus infection of mice. I. The fate of viral antigens. Immunology. 1972 Apr;22(4):637–649. [PMC free article] [PubMed] [Google Scholar]
- Cleveland P. H., Richman D. D., Oxman M. N., Wickham M. G., Binder P. S., Worthen D. M. Immobilization of viral antigens on filter paper for a [125I]staphylococcal protein A immunoassay: a rapid and sensitive technique for detection of herpes simplex virus antigens and antiviral antibodies. J Immunol Methods. 1979;29(4):369–386. doi: 10.1016/0022-1759(79)90008-5. [DOI] [PubMed] [Google Scholar]
- Cleveland P. H., Richman D. D., Oxman M. N., Worthen D. M. Rapid serological technique for typing herpes simplex viruses. J Clin Microbiol. 1982 Mar;15(3):402–407. doi: 10.1128/jcm.15.3.402-407.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane L. R., Gutterman P. A., Chapel T., Lerner A. M. Incubation of swab materials with herpes simplex virus. J Infect Dis. 1980 Apr;141(4):531–531. doi: 10.1093/infdis/141.4.531. [DOI] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Solid phase radioimmunoassay for identification of Herpesvirus hominis types 1 and 2 from clinical materials. Appl Microbiol. 1974 Oct;28(4):661–667. doi: 10.1128/am.28.4.661-667.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. S., McQuillin J., McGuckin R. The late detection of respiratory syncytial virus in cells of respiratory tract by immunofluorescence. J Hyg (Lond) 1970 Dec;68(4):575–580. doi: 10.1017/s0022172400042509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittzus J. G., Rubin S. J. Clinical evaluation of commercial conjugates for direct immunofluorescence of herpes simplex virus. J Clin Microbiol. 1977 Dec;6(6):574–577. doi: 10.1128/jcm.6.6.574-577.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN H. E. The diagnosis of corneal herpes simplex infection by fluorescent antibody staining. Arch Ophthalmol. 1960 Sep;64:382–384. doi: 10.1001/archopht.1960.01840010384009. [DOI] [PubMed] [Google Scholar]
- Olding-Stenkvist E., Grandien M. Rapid diagnosis of influenza A infection by immunofluorescence. Methodological problems and clinical material. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):296–302. doi: 10.1111/j.1699-0463.1977.tb01978.x. [DOI] [PubMed] [Google Scholar]
- PETTIT T. H., KIMURA S. J., PETERS H. THE FLUORESCENT ANTIBODY TECHNIQUE IN DIAGNOSIS OF HERPES SIMPLEX KERATITIS. Arch Ophthalmol. 1964 Jul;72:86–98. doi: 10.1001/archopht.1964.00970020088020. [DOI] [PubMed] [Google Scholar]
- Redfield D. C., Richman D. D., Oxman M. N., Kronenberg L. H. Psoralen inactivation of influenza and herpes simplex viruses and of virus-infected cells. Infect Immun. 1981 Jun;32(3):1216–1226. doi: 10.1128/iai.32.3.1216-1226.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Cleveland P. H., Oxman M. N., Zaia J. A. A rapid radioimmunoassay using 125I-labeled staphylococcal protein A for antibody to varicella-zoster virus. J Infect Dis. 1981 May;143(5):693–699. doi: 10.1093/infdis/143.5.693. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Gallo D., Devlin V., Woodie J. D., Emmons R. W. Direct immunofluorescence staining for detection of herpes simplex and varicella-zoster virus antigens in vesicular lesions and certain tissue specimens. J Clin Microbiol. 1980 Nov;12(5):651–655. doi: 10.1128/jcm.12.5.651-655.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber L. H., Brasier F., Couch R. B., Greenberg S. B., Jones D., Knight V. Diagnosis of herpes simplex virus infection by immunofluorescence. J Clin Microbiol. 1976 Mar;3(3):309–312. doi: 10.1128/jcm.3.3.309-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


