Abstract
Rationale: Nitric oxide bioactivity, mediated through the formation of S-nitrosothiols (SNOs), has a significant effect on bronchomotor tone. S-Nitrosoglutathione is an endogenous bronchodilator that is decreased in children with asthmatic respiratory failure and in adults with asthma undergoing segmental airway challenge. Recently we showed that S-nitrosoglutathione reductase (GSNOR) regulates endogenous SNOs. Mice with genetic deletion of GSNOR are protected from airway hyperresponsivity in an allergic asthma model.
Objectives: We hypothesized that GSNOR is increased in human asthma and correlates with lung SNO content and airway reactivity.
Methods: We recruited 36 subjects with mild asthma with FEV1 88.5 ± 2.3% predicted and 34 healthy control subjects with FEV1 100.7 ± 2.5% predicted. Bronchoalveolar lavage (BAL) was performed in all subjects. Cell counts, differentials, GSNOR activity, and SNO levels were determined in BAL.
Measurements and Main Results: SNO content was decreased in asthmatic BAL compared with control BAL and correlated inversely with GSNOR expression in BAL cell lysates. Furthermore, GSNOR activity measured from BAL samples was significantly increased in subjects with asthma compared with control subjects and correlated inversely with the provocative concentration of methacholine causing a 20% decrease in FEV1.
Conclusions: These findings suggest that GSNOR is an important regulator of airway SNO content and airways hyperresponsiveness in human asthma.
Keywords: asthma, S-nitrosoglutathione reductase, S-nitrosothiols, airway hyperresponsiveness
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The endogenous bronchodilator S-nitrosoglutathione (GSNO) is a nitrosothiol (SNO)—a nitric oxide (NO)–carrying molecule. The role of NO in asthma has been controversial, but increased GSNO protects against asthma-like disease in mice in the setting of robust inflammation.
What This Study Adds to the Field
Our findings in human asthma confirm that GSNOR is important in regulating airway SNOs and airway hyperresponsiveness.
S-Nitrosothiols (SNOs) are the bioactive endogenous form of nitric oxide (NO) present at substantial levels in lung lining fluid. SNOs, specifically S-nitrosoglutathione (GSNO), have been shown to exert bronchodilatory activity with potency 100 times greater than theophylline, a methylxanthine derivative used by clinicians for its bronchial smooth muscle relaxation properties (1, 2). Levels of GSNO present in lung lining fluid reflect equilibrium between GSNO production and GSNO clearance. In normal lungs, GSNO is present at concentrations of about 0.2 to 0.5 μM (3, 4). In disease states associated with inflammation and subsequent induction of inducible nitric oxide synthase (iNOS), such as lung transplantation and pneumonia, GSNO levels are typically increased several fold (3, 5). Increased concentrations of SNOs have been measured in cytokine-activated inflammatory cells and in inflamed tissues expressing iNOS (6, 7).
Interestingly, iNOS has also been found in asthma to be induced in inflamed bronchial epithelial cells and in inflammatory cells of asthmatic airways where it is hypothesized to contribute to airway reactivity (8). Indeed, an increased level of NO in exhaled breath is a signature of asthma attributed in part to increased iNOS activity in the airway (9, 10). However, in contrast to other inflammatory airway diseases in which iNOS and SNO levels are increased, SNO levels fall from 500 nM to approximately 60 nM in severe asthma (4). The drop in SNO concentration occurs despite the presence of an elevated nitrite concentration and a significantly acidic environment in asthma, which would efficiently promote the formation of GSNO by S-nitrosylation of glutathione (11, 12). This paradox of high expired NO in the presence of low concentrations of lung SNO can be explained by increased NOS activity and enhanced SNO breakdown in the airway.
The increased breakdown of GSNO in lung tissue has been demonstrated after allergen sensitization in animal models of asthma (13). Recently we showed that increased GSNO metabolism in the lung after allergen sensitization can be attributed to increased S-nitrosoglutathione reductase (GSNOR) activity. GSNOR is an enzyme that governs tissue levels of GSNO and is widely expressed in many tissues, including lung (14). GSNOR activity is elevated in a murine model of allergic asthma (15). Mice deficient in GSNOR have increased concentrations of GSNO and SNO-proteins in their lungs and developed acute lung inflammation, but not airway hyperresponsiveness, after allergen sensitization and challenge (15). These data suggest a causal role for GSNOR in the pathogenesis of airway hyperresponsiveness in allergic asthma. Moreover, new data in human studies suggest that potential host factors, which are related to GSNOR, may contribute to the airways hyperresponsiveness phenotype of asthma (16, 17).
Based on these animal studies, we hypothesize that GSNOR activity is increased in human asthma and is associated with increased airway reactivity. The objective of this study was to characterize GSNOR expression and activity in human subjects with asthma and to assess whether changes in GSNOR activity and expression predict airway reactivity and lung SNO content in asthma.
Some of the results of these studies have been previously reported in the form of an abstract (18, 19).
METHODS
Study Population
Subjects aged 18 to 60 years were recruited by advertisement. A total of 38 subjects with asthma and 34 normal healthy control subjects were recruited from the general Durham–Chapel Hill community. Subjects with asthma fulfilled criteria for asthma as determined by the guidelines of the American Thoracic Society (20) by exhibiting a provocative concentration of methacholine resulting in a 20% decrease in FEV1 (PC20) less than 16 mg/ml, reversibility of spirometry of at least 12% or greater and 200 ml with inhaled albuterol, and post-bronchodilator FEV1 greater than 50% predicted. Subjects were maintained on as-needed short-acting inhaled β2-agonists only. No medications were discontinued. All subjects enrolled were screened by medical history, physical examination, spirometry, methacholine provocation, and chest X-ray. Exclusion criteria included use of tobacco of greater than 5 pack-years or any cigarette exposure in the last year, use of cromolyn, nedocromil, inhaled or oral corticosteroids, leukotriene modifiers, and/or theophylline within 4 weeks before the study, history of asthma exacerbation within 4 weeks, presence of significant additional pulmonary disease or nonpulmonary disease, and ongoing immunotherapy in which the maintenance dose had not been reached. The study was approved by the Duke University Medical Center Institutional Review Board, and all individuals gave informed consent.
Bronchoscopy
Subjects with mild asthma and healthy control subjects underwent bronchoscopy with bronchoalveolar lavage (BAL). BAL was performed by wedging the bronchoscope in a subsegmental bronchus of the right middle lobe or lingula and instilling 300 ml of warm, sterile 0.9% NaCl (Hospira, Inc., Lake Forest, IL) in 60-ml aliquots. Gentle suction was applied to recover infused saline. The recovered fluid volume was recorded and ethylenediaminetetraacetic acid (EDTA) (500 μM) and diethylenetriamine pentaacetate (DTPA) (100 μM) were then added to prevent metal-catalyzed degradation of SNOs. Cells derived from the BAL fluid were pelleted by centrifugation at 1,000 rpm for 10 minutes and the supernatant (BAL fluid) decanted to a fresh sterile tube and placed on ice. The cell pellet was resuspended in phosphate-buffered saline (PBS) with 500 μM EDTA and a small aliquot was used to determine cell count and differential. The remainder of cells were flash frozen and stored in a −80 C° freezer until determination of GSNOR activity and expression. The decanted supernatant was then passed through a 3-kD cutoff filter (Amicon Ultra; Millipore Corp., Bedford, MA). The fluid retained after filtration (high molecular weight [HMW] BAL) and the eluate (low molecular weight [LMW] BAL) were immediately flash frozen at −80 C°.
BAL cells were isolated by centrifugation at 1,000 × g for 10 minutes Cells were resuspended in 200 μl of PBS and counted using a hemacytometer. Cells in 200 μl of the original BAL were also centrifuged onto cytoslides (Cytospin 3; Shandon Inc., Pittsburgh, PA) and stained with hematoxylin and eosin (Hema 3, Wright-Giemsa stain; Biochemical Sciences Inc., Swedesboro, NJ). Differential counts of pulmonary inflammatory cells (alveolar macrophages, neutrophils, lymphocytes, and eosinophils) were determined via light microscopy, and numbers of eosinophils, neutrophils, and other cells per milliliter of lavage fluid were calculated.
Assessment of GSNOR Activity in BAL Fluid and BAL Cell Pellet
GSNOR activity in BAL fluid and in BAL cells was measured in PBS (20 mM, pH 8.0), containing 100 μM DTPA and 500 μM EDTA. GSNOR activity could not be detected in the LMW BAL fluid, but was measured in the HMW BAL fluid. The protein concentration of each sample (BAL and pellet) was measured using the Lowry assay (BCA assay; Pierce Biologicals, Rockford, IL). Using equal concentrations of protein, absorbance at 340 nm was measured over time in each sample in the presence of nicotinamide adenine dinucleotide reduced (NADH; final concentration, 100 μM) and after the addition of GSNO (final concentration, 100 μM). NADH-dependent GSNO reductase activity is the difference obtained in the presence and absence of GSNO. The slope of the curve generated with fluorescence intensity over time is used as relative GSNOR activity.
Western Blot Analyses
Cells harvested from bronchoalveolar lavage were sonicated in lysis buffer containing 20 mM Tris-HCl, pH 8.0, 0.5 mM EDTA, 100 mM DTPA, 0.1% NP-40, and 1 mM phenylmethylsulfonyl fluoride. Ten micrograms of cell lysates were separated on 4 to 20% polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with PBS with 0.1% Triton X-100 and 2% milk for 1 hour. Blots were incubated overnight with either a 1:1,000 dilution of anti-GSNOR polyclonal antibody, anti-iNOS polyclonal antibody (Upstate Biotechnology, Lake Placid, NY) or a 1:2,000 dilution of monoclonal actin antibody (Sigma-Aldrich, St. Louis, MO), washed in PBS + 0.1% Triton X-100 two times for 15 minutes each, then incubated with 1:2,500 dilution of goat anti-rabbit horseradish peroxidase labeled or goat anti-mouse horseradish peroxidase secondary antibody (Promega, Madison, WI) for 30 minutes. After washing the membranes under the same conditions stated previously, signals were detected using the Western Lightning-ECL Chemiluminescence kit (PerkinElmer Life and Analytical Sciences, Inc., Waltham, MA), and quantified by laser densitometry (LKB UltrascanXL; BioRad Laboratories, Hercules, CA).
BAL SNO Measurements
SNO content of airway lavage fluid was measured in HMW and LMW BAL fluid. Assessment of LMW SNO provided an indirect assessment of GSNO content in the eluate. SNO was measured in both samples using photolysis-chemiluminescence. The BAL samples were divided in half. One sample was treated with 1 mM HgCl2 for 20 minutes at 25°C to selectively cleave thiol-bound NO (i.e., SNO) and the other without HgCl2 treatment to measure total SNO. Samples were then subjected to photolysis-chemiluminescence detection of the released NO through reaction with ozone. SNO content was derived by subtracting the HgCl2-treated value from the untreated value. A standard curve was generated from GSNO. The assay is specific and linear to 2 pmol.
BAL Nitrate and Nitrite
To determine if the effects of GSNOR activity in asthma are independent of NO generation, nitrate (NO3−) and nitrite (NO2−) present in the LMW BALF were measured by the Griess reaction using a fluorometric nitrite/nitrate assay kit (Nitric Oxide Assay Kit; EMD Chemicals, Newark, NJ). Nitrate and nitrite standards displayed linearity between 4 pmol and 500 pmol/100 μL (r2 > 0.998 for all experiments). All samples were performed in triplicate and were detected with equal efficiency (<10% difference in detection at any concentration). Nitrate and nitrite levels in the sterile saline used for lavage were considered background and subtracted from patient sample values. Levels of nitrate and nitrite were expressed as micromolar levels in LMW BAL fluid.
BAL Cytokines
To further characterize the patient cohort studied, BAL levels of cytokines were determined using a Millipore Human Cytokine/Chemokine Panel-21Plex including interferon-inducible protein (IP)-10, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-13, IL-17, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), IFN-γ, tumor necrosis factor (TNF)–α, IL-12(P40), IL-12(P70), macrophage inflammatory protein (MIP) 1α, MIP1β, monocyte chemoattractant protein (MCP), vascular endothelial growth factor (VEGF) (Millipore, Billerica, MA) on a Luminex 1001S machine (Bio-plex System; Biorad Laboratories, Carlsbad, CA). HMW BAL fluid was analyzed as specified by the manufacturer. The analyses were performed in duplicate, and the cytokine concentrations were calculated against the standards.
Statistical Analyses
Spirometry, methacholine bronchoprovocation, GSNO reductase activity, SNO, NO2−, and NO3− levels were compared between asthma and control subjects using unpaired Student t test or Wilcoxon, depending on the distribution of the data. P values less than 0.05 were considered significant. We also used stepwise multiple regression analysis to identify significant biochemical measurements associated with methacholine PC20. The dependent variables used in the model were nitrite, nitrate, LMW SNO, HMW SNO, total SNO, GSNOR activity, IL-10, IP-10, and IL-5 in BAL fluids, BAL cell pellet GSNOR. We used a mixed forward and backward algorithm with a P in of less than 0.1 and a P out of greater than 0.1 (21). This analysis was performed on a subset of subjects of 15 subjects with asthma and 19 control subjects who had all measurements of dependent variables in the model. This analysis was performed using JMP 7.0 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Subject characteristics are shown in Table 1. Not all studies were able to be performed on all subjects. The first half of the study focused on GSNOR. Once sample processing was optimized, Western blots, nitrite, nitrate, SNO levels, and cytokine assays were performed on all study subjects.
TABLE 1.
SUBJECT CHARACTERISTICS
| Subjects with Asthma | Control Subjects | P Value | |
|---|---|---|---|
| Sex, male/female | 16/20 | 14/20 | |
| Age, years | 30 ± 1.55* | 29.7 ± 1.66* | 0.26 |
| Age, years, range | 18–57 | 18–61 | |
| BMI, kg/m2 | 26.9 ± 1.14* | 27.1 ± 0.92* | 0.91 |
| FEV1, L | 3.12 ± 0.73* | 3.44 ± 0.76* | 0.085 |
| FEV1, % pred | 88.5 ± 2.3* | 100.7 ± 2.5* | 0.0006 |
| FVC, L | 4.34 ± 0.92* | 4.33 ± 1.05* | 0.98 |
| FVC, % pred | 102.5 ± 2.4* | 106.7 ± 2.7* | 0.26 |
| FEV1/FVC, % | 82.8 ± 1.1* | 85.8 ± 0.74* | 0.051 |
| PC20 | 0.92 ± 0.16* | >16 | 0.0001 |
| Medications | β2 agonists (36)† | None |
Definition of abbreviations: BMI = body mass index; PC20 = provocative concentration of methacholine causing a 20% decrease in FEV1; % pred = percent predicted.
Data are presented as means ± SEMs.
All 36 subjects with asthma reported using as-needed β2 agonists.
BAL Cell Count and Differential
The mean percentage return volume and standard of error of the mean of BAL fluid and cellular differential counts are shown in Table 2. BAL fluid return was similar in subjects with asthma and control subjects and percent BAL eosinophils present were greater in subjects with asthma, as expected (22). There were no significant differences in total cell count observed (not shown).
TABLE 2.
BRONCHOALVEOLAR LAVAGE CELL COUNT AND DIFFERENTIAL
| Asthma* | Healthy Control* | P Value | |
|---|---|---|---|
| BALF Value % Return | 65.9 ± 1.5 | 64.1 ± 2.4 | 0.52 |
| Eosinophils, % | 1.7 ± 0.3 | 0.5 ± 0.12 | 0.008 |
| Macrophages, % | 93.1 ± 0.98 | 94.1 ± 0.9 | 0.27 |
| PMN, % | 0.69 ± 0.18 | 1.0 ± 0.2 | 0.25 |
| Lymphocytes, % | 4.8 ± 0.88 | 3.9 ± 0.76 | 0.46 |
Definition of abbreviations: BALF Value % Return = bronchioalveolar lavage fluid (BALF) recovered/total BALF instilled; PMN =polymorphonuclear cells.
Data are presented as means ± SEM.
BAL Cytokine Measurements
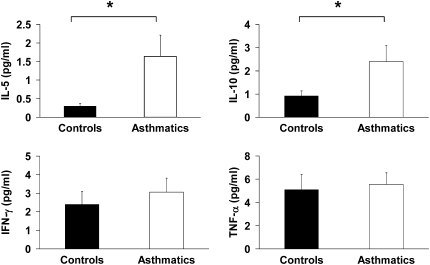
The concentrations of TH2 cytokines, including IL-5 and IL-10, are elevated in lavage fluid of subjects with asthma, consistent with the asthma phenotype (Figure 1). Although GSNOR activity and iNOS expression are increased in asthma, TH1 cytokines, IFN-γ, and TNF-α levels are similar in subjects with asthma and control subjects.
Figure 1.
Bronchoalveolar lavage (BAL) inflammatory cytokines were assessed in control subjects and subjects with asthma. TH2 cytokines IL-5 and IL-10 are shown in BAL from subjects with asthma (*P = 0.03 and **P = 0.05) compared with control subjects; subjects with asthma, n = 19; control subjects, n = 16). TH1 cytokines, IFN-γ, and TNF-α are also shown in control subjects and subjects with asthma.
GSNOR Activity and Protein Measurements
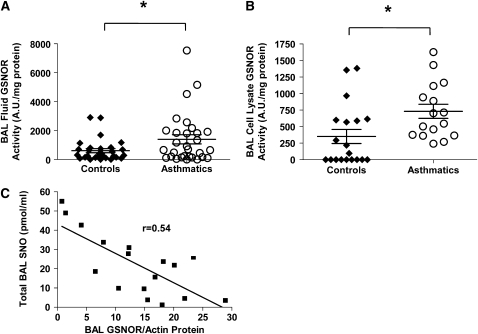
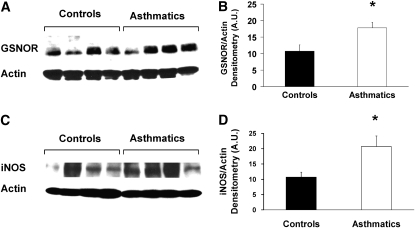
GSNOR activity was measured in BAL fluid and BAL cell pellet lysates obtained from subjects with asthma and control subjects. GSNOR activity in BAL is shown in Figure 2A, and GSNOR activity in BAL cell pellet lysates is shown in Figure 2B. BAL fluid GSNOR activity was twice as high in asthmatic lavage fluid (1223 ± 209 AU/mg protein in asthma [n = 37] vs. 537.4 ± 221 AU/mg protein in control subjects [n = 33], P = 0.03) and correlated with BAL cell pellet lysate GSNOR activity in subjects with asthma (731 ± 106 AU/mg protein in asthma [n = 16] vs. 351.5 ± 106 AU/mg protein in control subjects [n = 19], P = 0.02). The comparison analysis of GSNOR activity was performed using a Student t test after normalization of activity to protein concentration in the fluid. Protein expression of GSNOR and actin was also determined in BAL cell pellet lysates as demonstrated by a representative Western blot against GSNOR and actin shown in Figure 3A. Each sample represents a different subject. Densitometry of iNOS protein expression normalized to actin is demonstrated in Figure 3B (n = 16 samples/group; subjects with asthma 17.8 ± 1.68 vs. control subjects 10.8 ± 1.88; P = 0.01).
Figure 2.
Assessment of S-nitrosoglutathione reductase (GSNOR) expression and activity in bronchoalveolar lavage (BAL) fluid and cell lysates. (A) Extracellular (BAL fluid) and (B) intracellular (BAL cell lysates) GSNOR activity was measured in lavage fluid of control subjects and subjects with asthma. GSNOR activity was normalized per mg protein. (A) *P = 0.03 compared with control subjects; subjects with asthma, n = 37; control subjects, n = 33; (B) *P = 0.02 compared with control subjects; subjects with asthma, n = 16; control subjects, n = 19). (C) GSNOR protein expression normalized to actin is inversely correlated with total BAL SNO content. (n = 17, r = 0.54, P = 0.008).
Figure 3.
Assessment of S-nitrosoglutathione reductase (GSNOR), inducible nitric oxide synthase (iNOS), and actin protein expression in bronchoalveolar lavage (BAL) cell lysates from control subjects and subjects with asthma. (A) Representative Western blot of GSNOR and actin protein expression in BAL cell lysates. (B) Densitometry of GSNOR protein normalized to actin (*P = 0.008 compared with control subjects; n = 16 per group). (C) Representative Western blot of iNOS and actin protein expression in BAL cell lysates. (D) Densitometry of iNOS protein normalized to actin (*P = 0.027) compared with control subjects; n = 8 per group).
No correlation between GSNOR activity and lung SNO was found; however, GSNOR protein in BAL cell lysates correlates inversely with lung SNO content and is demonstrated in Figure 2C (total n = 17, r = 0.54; P = 0.0008).
BAL Nitrite/Nitrate Measurements
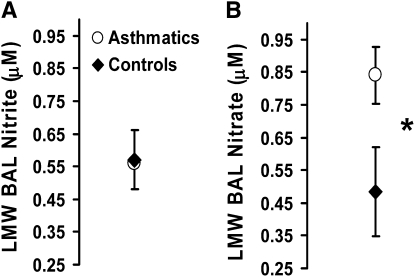
Although nitrite levels in BAL fluid were similar in subjects with asthma (n = 16, 0.57 ± 0.09 μM) and control subjects (n = 19, 0.55 ± 0.07 μM) (Figure 4A), total BAL nitrate concentration tended to be higher in subjects with asthma than in control subjects and this difference trended toward significance (0.83 ± 0.14 μM, n = 16 subjects with asthma vs. 0.53 ± 0.09 μM, n = 19 control subjects, P = 0.08) (Figure 4B). These findings are similar to previous reports by Dweik and colleagues and reflect the increase in NO· production reported in asthma (23).
Figure 4.
Evaluation of (A) nitrite and (B) nitrate in bronchoalveolar lavage fluid from control subjects and subjects with asthma. (*P = 0.8; subjects with asthma, n = 16; control subjects, n = 19).
BAL SNO Measurements
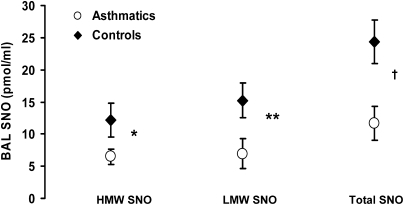
SNO concentration in BAL fluid is shown in Figure 5. We were not able to directly measure GSNO in asthmatic BAL as it was below the detection limits of our assay. This was not surprising as other investigators have reported similar difficulties (24). To measure LMW and HMW BAL SNO, we passed BAL fluid through a 3-kD Centricon filter. BAL fluid SNOs were expressed as nanomolar per ml. Analyses revealed that in contrast to healthy control subjects, baseline BAL SNO (HMW, LMW, and total) concentration is lower in mild asthma (HMW SNO, 6.5 ± 1.24 vs.12.29 ± 2.66 pmol/ml, n = 34 subjects with asthma vs. n = 32 control subjects, P = 0.05; LMW SNO, 8.3 ± 2.59 vs. 17.9 ± 3.1 pmol/ml, n = 15 subjects with asthma vs. n = 19 control subjects, P = 0.02; total SNO, 11.2 ± 2.5 vs. 23.1 ± 3.4 pmol/ml, n = 15 subjects with asthma vs. n = 19 control subjects, P = 0.01).
Figure 5.
Evaluation of S-nitrosothiol (SNO) content in bronchoalveolar lavage fluid from control subjects and subjects with asthma. High molecular weight (HMW) SNO (≥3 kD) and low molecular weight (LMW) SNO (<3 kD) were measured in control subjects and subjects with asthma. *P = 0.05 compared with control subjects; subjects with asthma, n = 34, control subjects, n = 32 for HMW SNO. **P = 0.02 compared with control subjects; subjects with asthma, n = 15, control subjects, n = 19 for LMW SNO. †P = 0.01 compared with control subjects; subjects with asthma, n = 15, control subjects, n = 19 for total SNO (HMW SNO + LMW SNO).
Association between GSNOR Activity and Airway Hyperresponsiveness
Using the stepwise multiple linear regression model, we identified IL-5 and intracellular GSNOR activity in BAL as two independent variables associated with PC20 (Table 3). The correlations were both negative, indicating that an increase in IL-5 and GSNOR activity would be associated with a lower PC20 or greater airway hyperresponsiveness to methacholine. None of the other variables were significant in our model (21).
TABLE 3.
SIGNIFICANT PARAMETERS ASSOCIATED WITH PROVOCATIVE CONCENTRATION OF METHACHOLINE CAUSING A 20% DECREASE IN FEV1 BASED ON STEPWISE MULTIPLE LINEAR REGRESSION ANALYSIS
| Parameter | Estimate | P Value |
|---|---|---|
| BAL IL-5 | ∼6.61 | 0.0061 |
| BAL cell lysate GSNOR activity | ∼0.0088 | 0.008 |
Definition of abbreviations: BAL = bronchoalveolar lavage; GSNOR = S-nitrosoglutathione reductase.
r2 = 0.53.
DISCUSSION
Our data demonstrate for the first time that GSNOR activity is elevated in the BAL fluid of subjects with mild asthma and show that resident lung BAL cells have increased GSNOR activity and expression in asthma compared with healthy control subjects. GSNOR, in the presence of NADH, metabolizes GSNO to oxidized glutathione (GSSG) and ammonium (NH4+). Theoretically, the metabolism of GSNO by GSNOR may increase GSSG. However, the concentrations of LMW SNOs measured in this study were quite low (∼10 nM) compared with reduced glutathione (GSH) and would not be expected to significantly contribute to the increased GSSG levels (∼300 nM) previously reported to be present in the lavage fluid in asthma (25).
BAL SNO levels have been shown to be diminished in subjects with mild and severe asthma at baseline (4, 22). Previously two soluble protein fractions from guinea pig lung homogenate have been identified, which break down SNO and prevent airway smooth muscle relaxation in vitro (26). Moreover, studies in mice containing deletions of GSNOR (15) support these findings by demonstrating a threefold increase in total lung SNO content and decreased airway hyperresponsiveness to allergen challenge. We have shown that in human asthma GSNOR is upregulated, providing a plausible mechanism to explain decreased lung SNO levels. The increased GSNOR activity may explain the paradox seen in asthma—decreased SNO levels despite increased exhaled NO.
The subjects included in this study have mild asthma with increased airway hyperresponsiveness documented by decreased FEV1% and PC20 values in the population with asthma compared with healthy control subjects. The distribution of aged subjects was parallel in both control subjects and subjects with asthma and therefore age was not believed to be a contributor to airway reactivity (27). Despite the mild nature of the disease, BAL SNO content is decreased in the cohort with asthma studied. This decrease in SNO is occurring in the presence of increased airway inflammation as demonstrated by higher BAL eosinophilia, nitrite production, and iNOS expression in subjects with asthma than in control subjects. In our study GSNOR activity was found to be increased in asthmatic BAL fluid and BAL fluid cell lysates. Because GSNO is in equilibrium with protein-SNOs (14), a decrease in lung GSNO would likely be reflected by a decrease in protein-SNO content. In fact, we found that SNO (HMW, LMW, and total SNO) was decreased in BAL fluid of subjects with asthma compared with healthy control subjects and correlated inversely with BAL cell lysate GSNOR protein expression. Our finding of increased GSNOR activity in BAL cell lysates, in part, explains the decreased SNO bioavailability in the lung in asthma.
The origin of the GSNOR activity in BAL is unknown; one possibility is that it represents increased cellular breakdown. Another possibility is that, in asthma, there is increased transport of GSNOR out of the cell into the lavage fluid. Because macrophages constituted the majority of cells in BAL fluid and epithelial cells are in direct contact with the lavage fluid, we hypothesized that the increased GSNOR activity represented macrophage or epithelial cell GSNOR activity. Both lung macrophages and epithelial cells have been shown to be primary locations for NOS activity and expression in asthmatic airways (15, 23) and are involved in regulating bronchial hyperresponsiveness (28, 29) and airway tone (30). Furthermore, these cells have also been shown to be primary locations for GSNOR expression in allergic asthma (15) and demonstrated high levels of GSNOR activity (14), which is specific for GSNO. Indeed, in this asthma cohort, GSNOR activity was two times higher than it was in control subjects in BAL cell lysates. The contribution of macrophages and epithelial cells as a source of GSNOR in human asthma will be examined in future studies.
We also showed that GSNOR activity in BAL cells inversely correlates with PC20, an indicator of the severity of airway hyperresponsiveness. We had previously demonstrated decreased sensitivity to nonspecific airway challenge in GSNOR-deficient mice; these mice did not develop bronchoconstriction after ovalbumin sensitization and challenge. We have now confirmed these associations in human asthma. We also showed that GSNOR-deficient mice are protected from desensitization to β-receptor agonists (15) and that SNOs increase β-adrenergic receptor expression (31). Our cohort with asthma only required intermittent albuterol therapy and should not have desensitization to β-agonist. The low GSNO content in these patients would be consistent with the absence of desensitization.
There were significant individual variations in GSNOR activity and expression in our cohort with asthma. This variability in GSNOR may be a reflection of genetic differences in the host (16, 17). GSNOR polymorphism is present in 31 to 45% of the population with asthma (17). Some of these single-nucleotide polymorphisms may affect the activity of GSNOR. Currently little is known about the regulation of GSNOR in asthma. Although IL-13 and steroids have been previously reported to affect epithelial expression of GSNOR (32), the mechanism by which this occurs is still under investigation.
In summary, our novel findings suggest that GSNOR activity regulates airway hyperresponsiveness and SNO content in individuals with asthma. Inhibition of GSNOR activity may represent a new therapeutic target in the treatment of asthma.
Supported by NIH RO1 HL086887 (L.G.Q.), NIH RO1 HL064619 and P50 HL084917 (M.K.), and by NIEHS 5 U19-ES012496 (J.S.S.).
Originally Published in Press as DOI: 10.1164/rccm.200901-0158OC on April 24, 2009
Conflict of Interest Statement: L.G.Q. shares a patent with J.S.S.: Duke Reference # 2809—Imipramine for the Treatment of Asthma and Atherosclerosis. Z.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S.S. has equity and thus financial interest in N30 Pharma, which is developing NO-based therapies for respiratory disorders, and has issued patents and/or patents pending on the related subject matter; J.S.S.'s institution, Duke University, has equity and thus financial interest in N30 Pharma, which is developing NO-based therapies for respiratory disorders. N.L.L. received $50,001 to $100,000 from Merck in industry-sponsored grants for an investigator-initiated study on obesity and asthma. M.K. received research funding from Genentech and Novartis from 2007 to 2009, GE Healthcare in 2008 to 2009, Bronchus in 2007 to 2009, Asthmatx from 2007 to 2009, GlaxoSmithKline from 2008 to 2009, and Biomarck in 2008 to 2009. M.K. received consulting fees from advisory board participation from Astra-Zeneca in 2007, Merck in 2007, GlaxoSmithKline in 2008, Novartis in 2007 to 2008, and Sepracor in 2008. M.K. also received lecture fees from Merck in 2007 to 2008, Astra-Zeneca in 2007 to 2008, Schering in 2007, and GlaxoSmithKline in 2008 for participation in their speakers' bureau.
References
- 1.Bannenberg G, Xue J, Engman L, Cotgreave I, Moldeus P, Ryrfeldt A. Characterization of bronchodilator effects and fate of s-nitrosothiols in the isolated perfused and ventilated guinea pig lung. J Pharmacol Exp Ther 1995;272:1238–1245. [PubMed] [Google Scholar]
- 2.Gaston B, Drazen JM, Jansen A, Sugarbaker DA, Loscalzo J, Richards W, Stamler JS. Relaxation of human bronchial smooth muscle by s-nitrosothiols in vitro. J Pharmacol Exp Ther 1994;268:978–984. [PubMed] [Google Scholar]
- 3.Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ. Endogenous nitrogen oxides and bronchodilator s-nitrosothiols in human airways. Proc Natl Acad Sci USA 1993;90:10957–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaston B, Sears S, Woods J, Hunt J, Ponaman M, McMahon T, Stamler JS. Bronchodilator s-nitrosothiol deficiency in asthmatic respiratory failure. Lancet 1998;351:1317–1319. [see comment]. [DOI] [PubMed] [Google Scholar]
- 5.Mayer B, Pfeiffer S, Schrammel A, Koesling D, Schmidt K, Brunner F. A new pathway of nitric oxide/cyclic gmp signaling involving s-nitrosoglutathione. J Biol Chem 1998;273:3264–3270. [DOI] [PubMed] [Google Scholar]
- 6.Eu JP, Liu L, Zeng M, Stamler JS. An apoptotic model for nitrosative stress. Biochemistry 2000;39:1040–1047. [DOI] [PubMed] [Google Scholar]
- 7.Hilliquin P, Borderie D, Hernvann A, Menkes CJ, Ekindjian OG. Nitric oxide as s-nitrosoproteins in rheumatoid arthritis. Arthritis Rheum 1997;40:1512–1517. [DOI] [PubMed] [Google Scholar]
- 8.Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet 1993;342:1510–1513. [DOI] [PubMed] [Google Scholar]
- 9.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J 1993;6:1368–1370. [PubMed] [Google Scholar]
- 10.Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994;343:133–135. [DOI] [PubMed] [Google Scholar]
- 11.Hunt J, Gaston B. Airway nitrogen oxide measurements in asthma and other pediatric respiratory diseases. J Pediatr 2000;137:14–20. [DOI] [PubMed] [Google Scholar]
- 12.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 2000;161:694–699. (see comment). [DOI] [PubMed] [Google Scholar]
- 13.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 1997;99:2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for s-nitrosothiol conserved from bacteria to humans. Nature 2001;410:490–494. [DOI] [PubMed] [Google Scholar]
- 15.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 2005;308:1618–1621. [see comment]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhry S, Que LG, Liu L, Eng C, Nazario S, Casas J, Torres A, Gomez I, Salas J, Chapela R, et al. S-nitrosoglutathione reductase and beta 2-adrenergic receptor gene-gene interaction is associated with asthma in latinos. Proc Am Thorac Soc 2007;175.
- 17.Wu H, Romieu I, Sienra-Monge JJ, Estela Del Rio-Navarro B, Anderson DM, Jenchura CA, Li H, Ramirez-Aguilar M, Del Carmen Lara-Sanchez I, London SJ. Genetic variation in s-nitrosoglutathione reductase (GSNOR) and childhood asthma. J Allergy Clin Immunol 2007;120:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Que L, Yang, Z, Church, TD, McMahon, TJ, Liu, L, and Kraft, M. Dysregulation of GSNO metabolism in patients with mild asthma. Proc Am Thorac Soc 2007;3:A834. [Google Scholar]
- 19.Que L, Yang, Z, Chien, T, Church, TD, Slade, D, Beavers, D, Jinwright, DM, and Kraft, M. Increased s-nitrosoglutathione reductase activity and expression in human asthma. Am J Respir Crit Care Med 2008;177:A764. [Google Scholar]
- 20.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS board of directors, November 1986. Am Rev Respir Dis 1987;136:225–244. [DOI] [PubMed] [Google Scholar]
- 21.Kleinbaum DG, Kupper LL, Muller KE, and Nizam A, editors. Applied regression analysis and other multivariable methods. 3rd ed. Pacific Grove, CA: Duxbury Press; 1998.
- 22.Kraft M, Bettinger CM, Wenzel SE, Irvin CG, Ackerman SJ, Martin RJ. Methacholine challenge does not affect bronchoalveolar fluid cell number and many indices of cell function in asthma. Eur Respir J 1995;8:1966–1971. [DOI] [PubMed] [Google Scholar]
- 23.Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, Calhoun W, Erzurum SC. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol 2000;164:5970–5980. [DOI] [PubMed] [Google Scholar]
- 24.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 2001;98:2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999;354:482–483. [DOI] [PubMed] [Google Scholar]
- 26.Fang K, Johns R, Macdonald T, Kinter M, Gaston B. S-nitrosoglutathione breakdown prevents airway smooth muscle relaxation in the guinea pig. Am J Physiol 2000;279:L716–L721. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz J, Schindler C, Zemp E, Perruchoud AP, Zellweger JP, Wuthrich B, Leuenberger P, Ackermann-Liebrich U. Predictors of methacholine responsiveness in a general population. Chest 2002;122:812–820. [DOI] [PubMed] [Google Scholar]
- 28.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol 2004;31:22–27. [DOI] [PubMed] [Google Scholar]
- 29.Careau E, Proulx LI, Pouliot P, Spahr A, Turmel V, Bissonnette EY. Antigen sensitization modulates alveolar macrophage functions in an asthma model. Am J Physiol 2006;290:L871–L879. [DOI] [PubMed] [Google Scholar]
- 30.Hjoberg J, Shore S, Kobzik L, Okinaga S, Hallock A, Vallone J, Subramaniam V, De Sanctis GT, Elias JA, Drazen JM, et al. Expression of nitric oxide synthase-2 in the lungs decreases airway resistance and responsiveness. J Appl Physiol 2004;97:249–259. [DOI] [PubMed] [Google Scholar]
- 31.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, et al. Regulation of beta-adrenergic receptor signaling by s-nitrosylation of g-protein-coupled receptor kinase 2. Cell 2007;129:511–522. [DOI] [PubMed] [Google Scholar]
- 32.Carraro S, Palmer L, DeRonde K, Brown-Steinke K, Zaman K, Que L, Stamler JS, Gaston B. Upregulation of the asthma enzyme, S-nitrosoglutathione reductase, by interleukin 13 [abstract]. Proc Am Thorac Soc 2006:A586.