MicroRNAs (miRNAs) are small RNAs, ∼22 nt long, that direct proteins to repress expression of mRNAs with which they are partially complementary. miRNAs are one of several classes of small RNA guides that provide sequence specificity to RNA silencing pathways (Reviewed in Ref. 1). In many respects, miRNAs are the normal cellular counterparts to small interfering RNAs (siRNAs), which guide RNA interference (RNAi), arguably the most famous RNA silencing pathway. With the exception of some ubiquitously expressed housekeeping genes, nearly every animal mRNA may, at some time in development and in one or more cell types, be repressed by one or more miRNAs. Thus, it is not surprising that miRNAs regulate key genetic programs in cardiovascular biology and are critical for cardiac development, endothelial function, lipid metabolism, ventricular hypertrophy, and post-infarction dysrhythmias. Here, we first review the key components of the RNA silencing pathway and then examine how RNA silencing regulates cardiovascular biology. Finally, we explore the opportunities miRNAs may provide for therapeutic intervention in human cardiac disease.
History and Mechanism
RNA silencing was first observed in plants2, 3 and later in animals.4, 5 The first miRNA was identified by Ambros and colleagues in the nematode, Caenorhabditis elegans, in 1993,6 six years before the discovery of siRNAs.7 The discovery of a second nematode miRNA, let-7,8 allowed the identification of its homologs in other animals, including humans,9 and additional miRNAs were soon identified in flies, worms, and humans by direct sequencing of small RNAs.10-12 Like the original nematode miRNAs, lin-4 and let-7, these also regulate gene expression by interacting with the 3′ untranslated regions (UTRs) of their target mRNAs.13-18
MicroRNAs are but one class of “small silencing RNAs,” 21−29 nucleotide RNAs that serve as guides for proteins that repress gene expression.19 In mammals, other classes of small silencing RNAs include endogenous small interfering RNAs (endo-siRNAs) derived from double-stranded RNA formed by pairing between RNAs from complementary pseudogenes and the corresponding genes, from transposons, and from long “hairpin” RNAs, and the germ-line specific Piwi-interacting RNAs (piRNAs) that repress transposons and, perhaps, control chromosome architecture.20, 21 All small silencing RNAs guide members of the Argonaute family of proteins.22 The human genome encodes seven such Argonaute proteins: Ago1, 2, 3, and 4 bind miRNAs and, likely, siRNAs, and function ubiquitously, whereas Hiwi, Hili, and Hiwi2 bind piRNAs and function in the germ line.
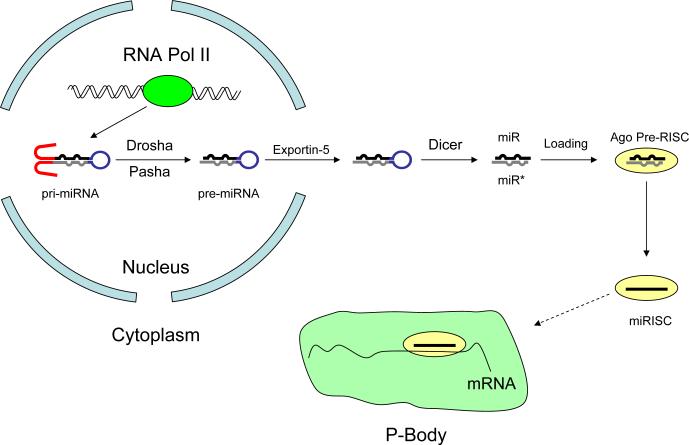
miRNAs are initially transcribed by RNA polymerase II as long primary miRNAs (pri-miRNAs), which contain a ∼65 nt stem loop; a few may be transcribed by RNA polymerase III.23 Pri-miRNAs are cleaved in the nucleus by the RNase III enzyme, Drosha, liberating the stem-loop or “pre-miRNA” (Figure 1). A few pre-miRNAs function as introns. These “mirtron” precursors are excised from the pri-miRNA by the pre-mRNA splicing machinery rather than by Drosha. The protein Exportin-5 escorts the pre-miRNA to the cytoplasm, where a second RNase III enzyme, Dicer, cleaves the pre-miRNA to produce a ∼22 nucleotide RNA duplex comprising the miRNA and its miRNA*—the ∼22-mer from the opposite arm of the stem of the pre-miRNA. The final step in miRNA maturation is the loading of the miRNA strand into an Argonaute protein and the destruction of the miRNA* strand, yielding an RNA-induced silencing complex (RISC). A few pre-miRNAs generate functional miRNAs from both arms of the pre-miRNA stem, but most generate just one abundant species.
Figure 1.
miRNA biosynthesis pathway. Following cleavage of the pri-miRNA by Drosha, pre-miRNA exits the nucleus and is cleaved by Dicer to a duplex. From this miRNA/miRNA* duplex, the miRNA strand is loaded into the RNA-induced silencing complex (RISC), and the miRNA* strand is destroyed. Translational repression of targeted mRNAs by RISC may occurs within the P-Body.
In mammals, miRNAs typically bind the 3′ untranslated regions (UTRs) of the mRNAs whose expression they repress. The complementarity between a miRNA and its mRNA targets is rarely complete. In fact, only a small region of the miRNA is necessary and sufficient to promote target binding: nucleotides 2−8 of the miRNA—the seed sequence—suffice to define the target repertoire of each miRNA, which can repress hundreds of different mRNAs.14, 15, 24, 25 Binding of the miRNA-programmed RISC induces translational repression or mRNA deadenylation and degradation. Consequently, inaccurate processing of a miRNA—which can change the position of the miRNA 5′ end within the stem of the pre-miRNA—completely redefines its mRNA targets.14, 15, 26
To date, 677 human miRNAs have been identified, corresponding to 545 distinct 7-nt seeds, each of which may repress tens to hundreds of different mRNAs. miRNA expression varies widely among the cell types, with some miRNAs among the most abundant RNAs in a cell (> 500,000 copies) but expressed in just a few cells, while others are present at more modest concentrations (1,000 copies per cell) nearly everywhere. The first miRNAs discovered, lin-4 and let-7 regulate developmental timing in C. elegans6, 8, but early speculation that miRNAs would generally function to regulate the timing of development proved wrong. Moreover, the dramatic phenotypes caused by loss of lin-4 or let-7 are unusual. Genetic deletion of most miRNA genes, at least in invertebrates, typically causes subtle developmental defects at best and often results in no detectable change from the wild type.27 Redundancy among miRNAs with identical seed sequences may, in part, explain these results.
Thanks to advances in expression profiling methods—especially high-throughput sequencing—and in the computational prediction and experimental validation of miRNA targets, thousands of miRNAs are now known, and they function in nearly every conceivable biological pathway, from repressing cell death to regulating glucose metabolism. miRNAs coordinate the immune response to infection through both adaptive and innate immune responses28-30 and promote stem cell fates in both the germ line and the soma. Some miRNAs can act as oncogenes; yet others are tumor suppressors, and identifying blood-borne cancers and solid tumors according to miRNA expression profiles remains a focus of ongoing investigation.31, 32
miRNAs Regulate Embryogenesis and Cell Fate Determination
Without Dicer, mice die in uter ∼7.5 days after fertilization; these defective embryos appear to lack stem cells.33 Conditional inactivation of Dicer in cultured embryonic stem cells reduces the rate of cell division, consistent with a role for miRNAs in stem cell self-renewal.34 Expression profiling studies comparing mouse stem cells with differentiated cell populations identified a subset of miRNAs restricted to embryonic stem cells, suggesting that miRNAs are important for cellular identity and for regulating pluripotency.35, 36 One of these miRNAs, miR-290, may indirectly repress expression of Oct4 and, perhaps, other pluripotency genes, by directly inhibiting expression of mRNAs encoding DNA methyltransferases.37 In adults, hematopoietic CD34+ stem cells express specific miRNAs that govern hematopoietic lineage differentiation.38
miRNAs Regulate Cardiovascular Structure and Function
Cardiomyocyte Differentiation
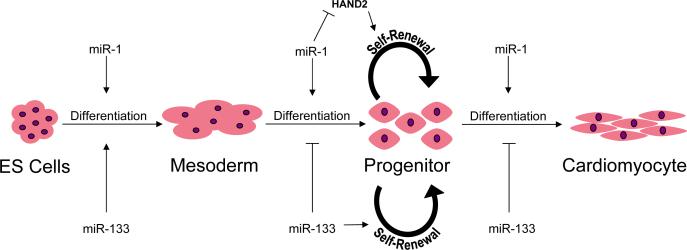
miRNAs promote the commitment of embryonic stem cells to the cardiomyocyte lineage (Figure 2). miR-1 and miR-133 are not detectable in pluripotent ES cells; their expression is markedly increased upon commitment to the precardiac mesodermal lineage. miR-1 and miR-133 are excised from a common pri-miRNA whose transcription is activated by serum response factor (SRF), MyoD, and Mef2, three proteins that promote expression of muscle-specific genes.39, 40 Artificial expression of miR-1 or miR-133 in embryonic stem cells causes expression of the early mesodermal cell marker Brachyury and suppresses commitment to the endodermal and neuroectodermal lineages. Further differentiation of mesodermal progenitor cells into cardiac and skeletal progenitor cell types is promoted by miR-1 but inhibited by miR-133, consistent with studies showing opposing effects of these two miRNAs.40, 41 The role of miRNAs in directing further differentiation into specific cardiomyocyte lineages is not yet known, although miR-1 is likely to play a part.
Figure 2.
miR-1 and miR-133 regulate progenitor cell self-renewal and cardiomyocyte differentiation from embryonic stem (ES) cells.
Cardiomyocyte differentiation and proliferation require temporal and transcriptional regulation of specific genetic programs. As with skeletal and smooth muscle differentiation, SRF and basic helix-loop-helix (bHLH) transcription factors turn on genes necessary for these programs.42, 43 The bHLH transcription factor HAND2 controls temporal regulation of cardiomyocyte growth by enhancing cellular proliferation within the linear heart tube and, later, preferentially within the right ventricle. HAND1 likely serves a similar role within the developing left ventricle, but is expressed over a different time course and is regulated independently from HAND2. SRF enhances miR-1−2 expression, which in turn represses HAND2 in cardiomyocytes and the developing heart. (Mice and humans have two miR-1 genes: miR-1−1 and miR-1−2.)
Cardiac Development
miR-1 repression of HAND2 mRNA is important for normal right ventricular development and septation.44 Normal cardiac development begins when mesodermally derived precursor cells within the cardiac crescent proliferate to form the linear heart tube, which then undergoes rightward looping and further maturation to form the right and left ventricles. Two similar but discrete cardiomyocyte mesodermal precursor cell types from the primary and secondary heart fields contribute to the formation of the left and right ventricles, respectively, and differences between the genetic programs of these precursor cells may explain in part the origin of septal and right ventricular developmental abnormalities.45, 46 About half of mice lacking miR-1−2 die near the end of gestation or soon after birth, displaying ventricular septal defects consistent with inappropriate HAND2 expression.44 Conversely, HAND2 protein expression is depressed in transgenic mice over expressing miR-1. These animals have decreased cardiomyocyte expansion, leading to thin, dilated ventricles.40
Ventricular development and septation are also regulated by miR-133a, a bicistronic gene that clusters with miR-1 on chromosomes 2 and 18. Combined deletion of miR-133a-1 & miR-133a-2 is lethal in > 50% of mice just one day after birth and is associated with ventricular septal defects that form primarily near the apex.47 Mice surviving to adulthood (25%) have markedly impaired systolic function due to extensive fibrosis and sarcomere structural abnormalities. The developmental defects in these double-deficient mice are associated with increased cardiomyocyte proliferation and apoptosis. Transgenic mice over expressing miR-133a die late in gestation and exhibit either VSDs or thin walled ventricles attributed to a decrease in cardiomyocyte proliferation. These gain and loss of function studies demonstrate that miR-133a regulates ventricular development by decreasing cardiomyocyte proliferation in contrast to its growth promoting function seen in satellite cell-derived myoblasts.39
Ventricular Hypertrophy and Congestive Heart Failure
miRNAs regulate genes important for ventricular hypertrophy and fibrosis. Congestive heart failure is a growing problem that reduces the life span and lowers the quality of life of those it afflicts. Congestive heart failure is characterized by neurohumoral activation and a decline in cardiac output. Several miRNAs are regulated uniformly across all causes of heart failure. In addition, ventricular pressure overload accompanying aortic stenosis and systemic hypertension may elicit expression of a set of miRNAs not induced by other causes of heart failure, such as ischemic and dilated cardiomyopathies. Ikeda and coworkers determined the miRNA expression profiles of biopsy specimens or explanted hearts from sixty-seven patients diagnosed with aortic stenosis, ischemic cardiomyopathy, or idiopathic cardiomyopathy.48 Of 87 miRNAs evaluated, the expression of 43 was significantly different from that in normal hearts in at least one group; seven showed responses common to all (let-7c, miR-23a, miR-100, miR-103, miR-140*, miR-214 increased, while miR-126* decreased), four of which previously had been described in earlier studies. These data suggest that while levels of only a few miRNAs are increased or reduced in response to heart failure, many others are regulated in direct response to the pathologic cause of the heart failure syndrome.
The Ikeda study also helps identify how cardiac-specific miRNAs (miR-1, mir-133, and miR-208) are mis-expressed in cardiomyopathic as compared with normal human hearts. Like previous studies of patients with hypertrophic cardiomyopathy and of mice subjected to thoracic aortic constriction, miR-1 was reduced in the group of patients with aortic stenosis.49, 50 miR-1 was not increased in patients with chronic ischemic cardiomyopathy, contrasting with an acute myocardial ischemia model in which miR-1 expression increased.51 In mice, miR-133 expression was unaltered up to 14 days after aortic constriction49, consistent with the finding that miR-133 expression was unchanged across all patient groups.48 However, these findings contrast with a previous study showing that thoracic banding and hypertrophic cardiomyopathy significantly decreases miR-133 expression.50 The discrepancies may reflect differences in the extent of disease, tissue sampling, experimental sample size, or methodology. Collectively, the studies suggest that cardiac-specific miRNAs participate in the development of ventricular hypertrophy and heart failure, but how miR-1 participates in heart failure following myocardial infarction requires further clarification.
Over-expression and inhibition of specific miRNAs have provided further insight into their roles in hypertrophy, fibrosis, and heart failure. Microarray analysis of myocardium from mice following ligation of the left anterior descending coronary artery revealed significantly reduced miR-29 expression. Systemic administration of a miR-29 inhibitor increased collagen expression, suggesting that miR-29 regulates cardiac fibrosis and perhaps ventricular remodeling by targeting collagen genes expressed after myocardial infarction.52 Over-expression of miR-133 blocks cardiac hypertrophy in Akt transgenic mice predisposed to left ventricular hypertrophy (LVH), whereas wild-type mice developed LVH in response to administration of a miR-133 antisense oligonucleotide (ASO) inhibitor.50 However, LVH was not observed in miR-133 deficient mice, and whether these contrasting findings are as a result of chronicity or the complete absence of miR-133 is unclear.47 Although reports vary as to whether and to what extent miR-195 expression changes during hypertrophy or cardiac dysfunction48, 53, 54, miR-195 over expression resulted in LVH in wild-type mice, accompanied by increased expression of brain natriuretic peptide and β-MHC, markers of progressive heart failure and LVH.55
Key molecular events leading to heart failure include the induction of fetal gene expression and a reduction of the more rapidly contractile but less energy efficient α-MHC instead of its β-MHC counterpart. This change in the ratio of α to β isoform expression may be partly restored—and contractility improved—by β-blocker therapy. Remarkably, the cardiac-specific miR-208 is encoded by intron 27 of the α-MHC gene. Expression of miR-208 indirectly enhances β-MHC expression in response to hypertrophic stimuli such as pressure overload conditioning, but not under normal conditions.54 As α-MHC expression decreases in response to stress, miR-208 expression also decreases, thereby moderating the resulting increase in β-MHC expression. Such fine-tuning of gene expression is hypothesized to be the primary function of miRNAs.56 Expression profiling and target gene analysis of cardiomyocytes suggest that miRNAs expressed preferentially in fetal and failing hearts (miR-21, mir-129, mir-212) also help regulate cellular hypertrophy.57
Thus, in response to environmental perturbations, cardiomyocytes alter the expression of miRNAs that participate in programs governing ventricular hypertrophy, fibrosis, and contractile protein expression. These changes in miRNA expression profiles can either promote or avert progressive ventricular dysfunction and congestive heart failure.
Vascular Biology
miRNAs also participate in angiogenesis and endothelial cell function, inflammatory cell recruitment, and the response to arterial injury. The global importance of miRNAs to endothelial cell physiology was evaluated following siRNA-mediated depletion of Dicer in human endothelial cells, the result of which was to increase the expression of endothelial nitric oxide synthase, cell adhesion molecules, and growth factor receptors and to decrease the expression of cytokines and chemokines. No morphologic differences were apparent, but endothelial cell proliferation decreased.58 Deletion of the endothelial cell-specific miR-126 impairs maintenance of vascular integrity during embryogenesis and reduces angiogenesis following myocardial infarction in mice at least in part by increasing expression of the angiogenesis inhibitor, Sprouty-related protein-1.59
In miRNA profiling studies performed following carotid balloon injury in rats, expression of the anti-apoptotic miRNA, miR-21, increased rapidly, suggesting a role for miR-21 in neointimal growth following arterial injury.60-62 Administration of an antisense inhibitor of miR-21 increased apoptosis of cultured vascular smooth muscle cells; administration of the antisense inhibitor at the time of arterial injury similarly increased apoptosis and decreased VSMC proliferation in the developing neointimal lesion in rats.63 Moreover, intravenous administration of an antisense inhibitor of miR-21 three weeks after initiating pressure overload-induced hypertrophy reduced cardiac ERK-MAPkinase activity, inhibited interstitial fibrosis and attenuated cardiac dysfunction.64
Therapy
Results like these in disease models suggest that inhibiting inappropriately expressed miRNAs using ASOs and, perhaps, replacing missing miRNAs using siRNAs can be used to treat human cardiac diseases. Treating human disease using siRNAs is being explored for a wide range of diseases, including neurodegenerative disorders, familial hypercholesterolemia, diabetes, pulmonary and systemic viral infections, cancer, and retinopathy. Increasing protein expression by inhibiting miRNAs or reducing protein expression with siRNAs would make proteins previously considered ‘non-druggable’ into new targets for therapy.
Gene over expression and antisense therapies for treating cardiovascular diseases have been explored extensively, and many of the limitations previously encountered apply to RNA silencing strategies as well. These include the obstacles inherent in bypassing the reticuloendothelial system and endogenous nuclease degradation; delivery of the therapeutic to the target tissue and cell type; transmembrane uptake; escape from endosomes and release into the cytoplasm; and target message specificity. Modifications to RNA structure and conjugation that prevent urinary excretion and hepatic clearance have proven useful in improving bioavailability. Among the more common approaches employed for siRNA delivery are the use of cationic liposomes and nanoparticles that resist reticuloendothelial system clearance and facilitate cell uptake by promoting fusion with the cell membrane. In cynomolgus monkeys, intravenous administration of an apoB-targeting siRNA encapsulated in modified cationic liposomes accumulated in the liver and resulted in >90% silencing of the target mRNA, with accompanying reductions in serum total and LDL cholesterol detectable at least 11 days later.65
Further enhancements in siRNA delivery to tissues or regions of interest may be accomplished by cell surface marker recognition. When complexed with a positively charged protamine-monoclonal antibody fusion protein, systemically administered siRNAs were specifically delivered to HIV-infected cells in vitro66 and in a humanized mouse model.67 Similarly, an anti-integrin antibody was used to direct multilamellar vesicles containing a Cyclin-D1 siRNA to mononuclear leukocytes. Delivery of the siRNA by this route reduced leukocyte proliferation, inflammatory cytokine expression, and colitis in a mouse disease model.68
Similar delivery strategies may enable specific administration of siRNAs to the heart. Cationic microbubbles tagged with VCAM-1 antibodies have been used to image arteriosclerotic plaque, and microbubble-assisted delivery of siRNAs to the myocardium has been demonstrated in mice. We can envision using microbubbles and ultrasound to deliver siRNAs directly to the cardiovascular system.69-71 Alternatively, aptamer-siRNA chimeras have shown promise in targeting prostate cancer in a xenograft model. These chimeras are considerably smaller and easier to synthesize compared to antibody- or lipid-based delivery schemes and can provide high affinity binding to specific cell-surface proteins, imparting cell-type specificity.72
Cardiovascular miRNA Targeting Therapies
Individual miRNA species can be blocked in cultured cells and in whole animals using modified ASOs. The first studies to demonstrate effective ASO-mediated inhibition of miRNAs in cultured human cells and in vivo in nematodes were important not only for proof of concept but also for demonstrating that ASOs block small RNAs after they are assembled into silencing complexes.73, 74 Subsequent improvements in ASO chemistry have extended this approach first to mice75 and later to non-human primates.76 For both, ASOs were delivered intravenously to block the function of miR-122, whose function in the liver is required for cholesterol biosynthesis. One study employed cholesterolconjugated, 21 nt long 2′-O-methyl oligonucleotides (‘antagomirs’), whereas the other used locked nucleic acid (LNA) modifications of a 15 nt DNA oligonucleotide containing phosphorothioate linkages in place of the standard phosphodiester bonds (‘LNA-anti-miR’). Both types of anti-miR-122 ASOs reduced plasma cholesterol. In the African green monkey study, three doses of LNA-anti-miR, administered intravenously on three successive days, detectably reduced plasma cholesterol for as long as 3 months.76 Studies in mouse and rat similarly suggest that inhibition of miR-21 using intravenously administered ASOs may provide therapy for failing hearts.63, 64
Local inhibition of miRNAs by ASOs also holds promise. Wang and coworkers used transepicardial injection of a 2’-O-methyl ASO targeting miR-1 prior to ligation of the left anterior descending coronary artery to suppress post-infarction ventricular arrhythmia, suggesting that such ASOs could be used therapeutically.51 Conversely, administration of a miRNA-mimic to increase miR-1 expression increased post-infarction ventricular arrhythmias. Computational analysis suggests that miR-1 represses the GJA1 and KCNJ2 mRNAs, which encode the predominant protein expressed at gap junctions (Connexin 43) and the inward rectifying K+ channel (Kir2.1), respectively. The increase in arrhythmogenesis caused by miR-1 appears to be at least in part mediated by a reduction in expression of these genes, as administration of siRNAs targeting either also increased ventricular arrhythmias, even when co-administered with the miR-1 antisense inhibitor.
Conclusions
Clearly, miRNAs play a significant role in cardiovascular development and disease. miRNAs are important for regulating cardiomyocyte self-renewal and differentiation, as well as for normal cardiac structural integrity. Identification of those miRNAs and target genes that contribute to adult cardiac pathology is likely to suggest new targets for therapy. Obstacles encountered previously with gene therapy and antisense drugs remain an impediment to delivering miRNAs, but pre-clinical studies for antisense miRNA inhibitors have been promising. Similarly, both preclinical studies and phase II human trials evaluating siRNA therapies have been encouraging, suggesting that miRNA replacement strategies and miRNA inhibitors will find their way into clinical use in the near future.
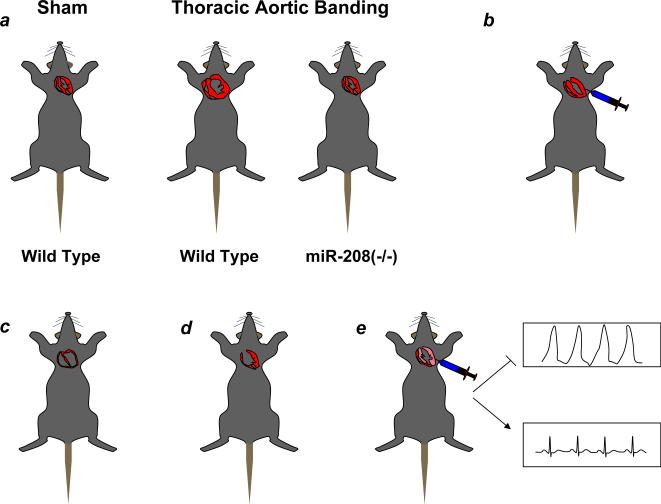
Figure 3.
miRNAs regulate cardiac development and the stress response in mice. A, miR-208 deletion abrogates left ventricular hypertrophy induced by thoracic aortic banding51. B, Transepicardial injection of a miR-133 inhibitor induces LVH in mice. C, miR-1 and miR-195 over expression produces a phenotype exhibiting thinned and dilated ventricles. D, miR-1−2 deficient mice have ventricular septal defects. E, Transepicardial injection of a 2′-O-methyl antisense oligonucleotide targeting miR-1 prior to coronary artery ligation reduces post-infarction dysrhythmias.
Acknowledgments
The authors acknowledge their colleagues in the Aronin and Zamore laboratories for helpful discussions.
Footnotes
Conflict of Interest Disclosures
P.D.Z. is a cofounder and member of the scientific advisory board of Alnylam Pharmaceuticals, Inc., and a member of the scientific advisory board of Regulus Therapeutics, L.L.C.
References
- 1.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napoli C, Lemieux C, Jorgensen RA. Introduction of a chimeric chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Krol AR, Mur LA, Beld M, Mol JNM, Stuitji AR. Flavonoid genes in petunia: Addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990;2:291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo S, Kemphues KJ. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- 5.Bahramian MB, Zarbl H. Transcriptional and posttranscriptional silencing of rodent alpha1(I) collagen by a homologous transcriptionally self-silenced transgene. Mol Cell Biol. 1999;19:274–283. doi: 10.1128/mcb.19.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 8.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 9.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 10.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of Novel Genes Coding for Small Expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 11.Lee RC, Ambros V. An Extensive Class of Small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 12.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 13.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 14.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 16.Grun D, Wang YL, Langenberger D, Gunsalus KC, Rajewsky N. microRNA target predictions across seven Drosophila species and comparison to mammalian targets. PLoS Comput Biol. 2005;1:e13. doi: 10.1371/journal.pcbi.0010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 18.Rajewsky N, Socci ND. Computational identification of microRNA targets. Dev Biol. 2004;267:529–535. doi: 10.1016/j.ydbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Matranga C, Zamore PD. Small silencing RNAs. Curr Biol. 2007;17:R789–93. doi: 10.1016/j.cub.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Hartig JV, Tomari Y, Forstemann K. piRNAs--the ancient hunters of genome invaders. Genes Dev. 2007;21:1707–1713. doi: 10.1101/gad.1567007. [DOI] [PubMed] [Google Scholar]
- 21.Nilsen TW. Endo-siRNAs: yet another layer of complexity in RNA silencing. Nat Struct Mol Biol. 2008;15:546–548. doi: 10.1038/nsmb0608-546. [DOI] [PubMed] [Google Scholar]
- 22.Tolia NH, Joshua-Tor L. Slicer and the Argonautes. Nat Chem Biol. 2007;3:36–43. doi: 10.1038/nchembio848. [DOI] [PubMed] [Google Scholar]
- 23.Du T, Zamore PD. Beginning to understand microRNA function. Cell Res. 2007;17:661–663. doi: 10.1038/cr.2007.67. [DOI] [PubMed] [Google Scholar]
- 24.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 26.Seitz H, Ghildiyal M, Zamore PD. Argonaute Loading Improves the 5' Precision of Both MicroRNAs and Their miRNA(*) Strands in Flies. Curr Biol. 2008;18:147–151. doi: 10.1016/j.cub.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miska EA, Alvarez-Saavedra E, Abbott AL, Lau NC, Hellman AB, McGonagle SM, Bartel DP, Ambros VR, Horvitz HR. Most Caenorhabditis elegans microRNAs Are Individually Not Essential for Development or Viability. PLoS Genet. 2007;3:e215. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 30.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 32.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 34.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houbaviy HB, Dennis L, Jaenisch R, Sharp PA. Characterization of a highly variable eutherian microRNA gene. RNA. 2005;11:1245–1257. doi: 10.1261/rna.2890305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 37.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, Zavolan M, Svoboda P, Filipowicz W. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–267. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 38.Georgantas RW, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM, Civin CI. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2007;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 41.Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, Srivastava D. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1−2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 45.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31:367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 49.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 50.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 51.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 52.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bashirullah A, Pasquinelli AE, Kiger AA, Perrimon N, Ruvkun G, Thummel CS. Coordinate regulation of small temporal RNAs at the onset of Drosophila metamorphosis. Dev Biol. 2003;259:1–8. doi: 10.1016/s0012-1606(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 54.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 55.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 57.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116:258–267. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 58.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 59.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 61.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 63.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 64.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JTR, Muckenthaler M, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. Role of miR-21 in MAPK signalling and myocardial disease. Nature. 2008 doi: 10.1038/nature07511. in press. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, Maclachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 66.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 67.Kumar P, Ban H-S, Kim S-S, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang Y-G, Jeong J-H, Lee K-Y, Kim Y-H, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee S-K, Shankar P. T Cell-Specific siRNA Delivery Suppresses HIV-1 Infection in Humanized Mice. Cell. 2008 doi: 10.1016/j.cell.2008.06.034. 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 70.Bekeredjian R, Grayburn PA, Shohet RV. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol. 2005;45:329–335. doi: 10.1016/j.jacc.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 71.Tsunoda S, Mazda O, Oda Y, Iida Y, Akabame S, Kishida T, Shin-Ya M, Asada H, Gojo S, Imanishi J, Matsubara H, Yoshikawa T. Sonoporation using microbubble BR14 promotes pDNA/siRNA transduction to murine heart. Biochem Biophys Res Commun. 2005;336:118–127. doi: 10.1016/j.bbrc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 72.McNamara JO, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 73.Hutvagner G, Simard MJ, Mello CC, Zamore PD. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2:E98. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 76.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]