Abstract
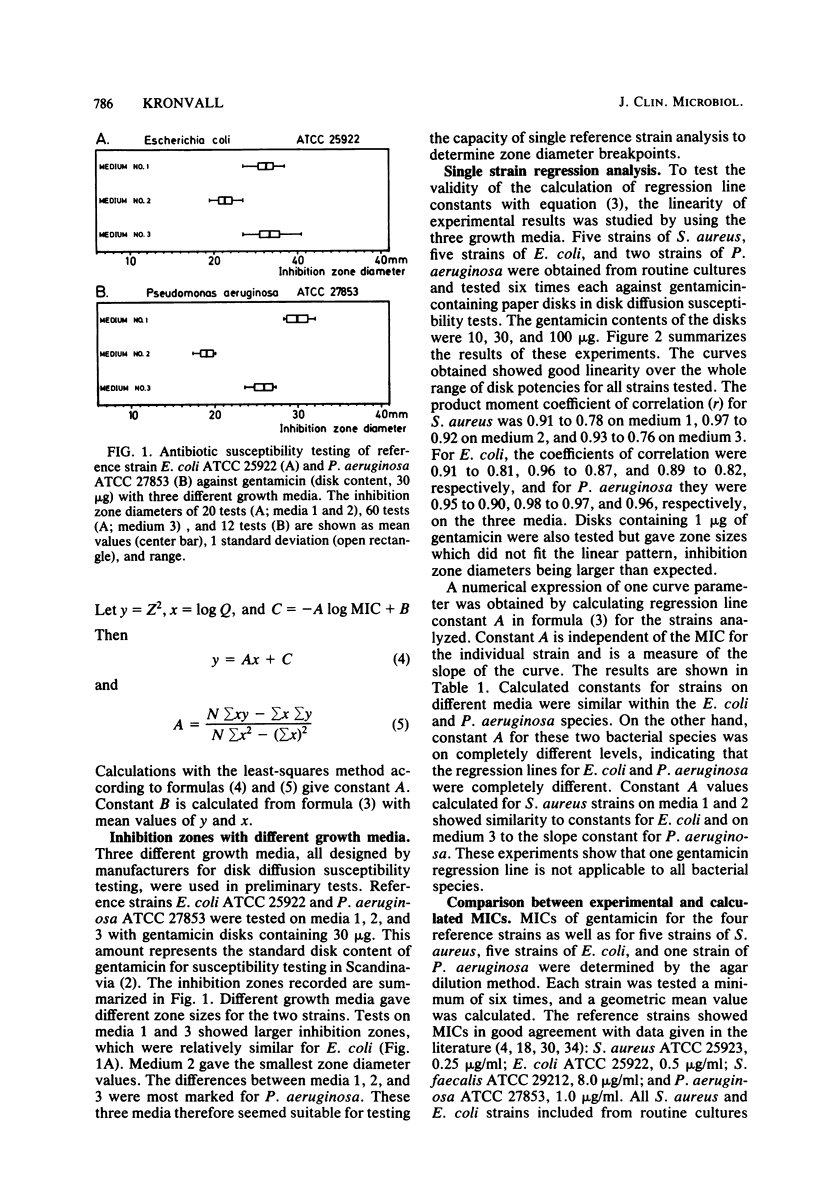
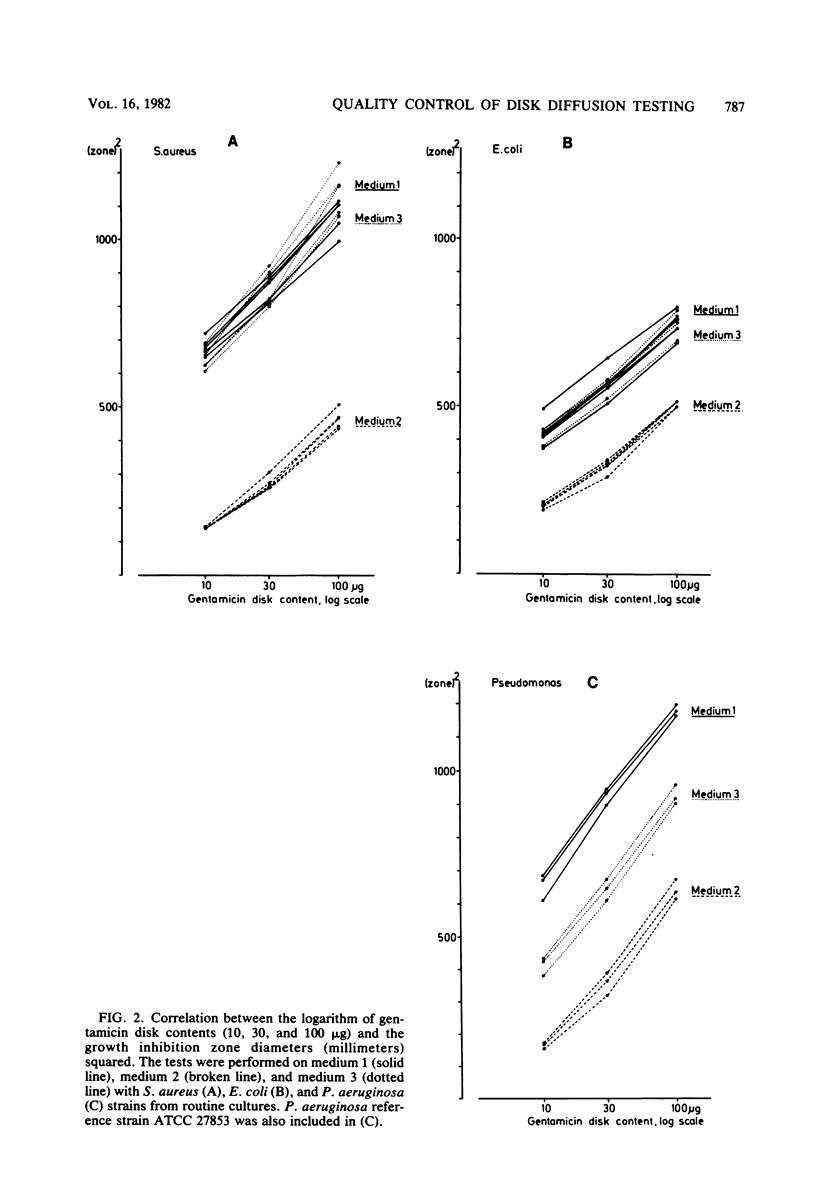
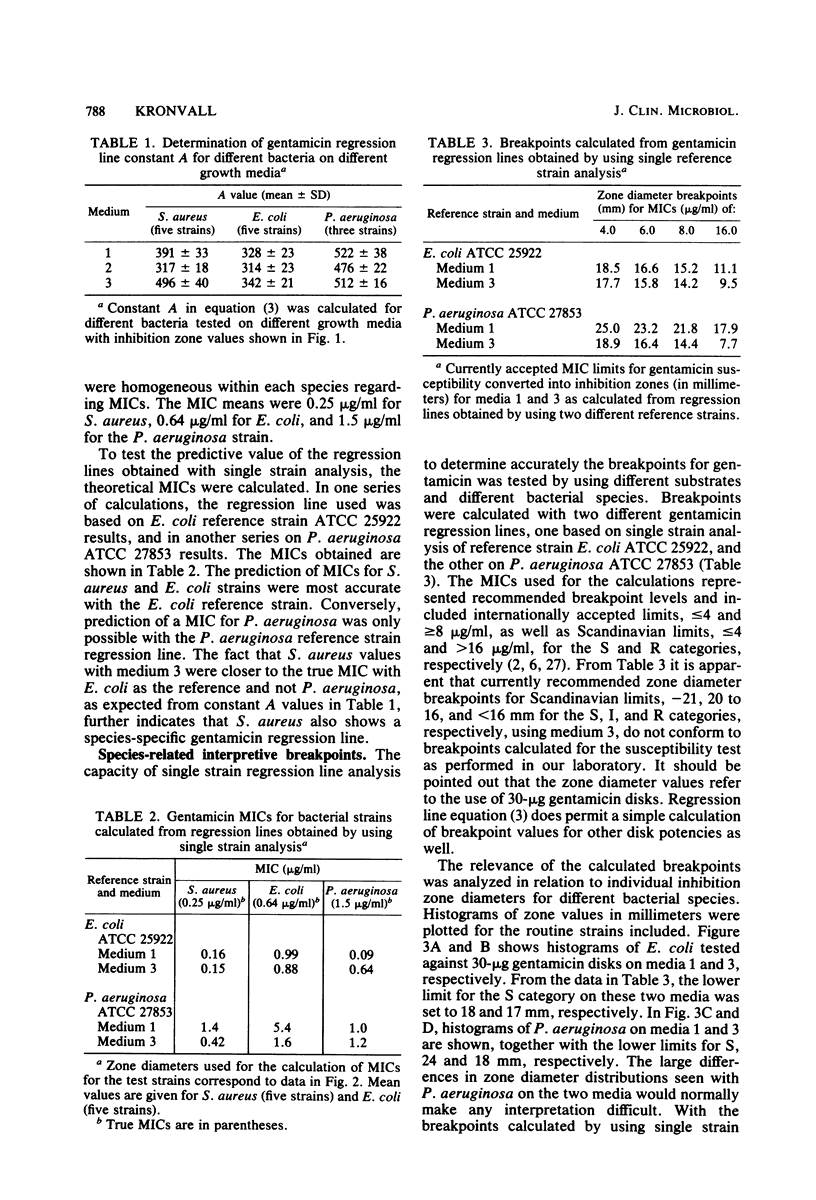
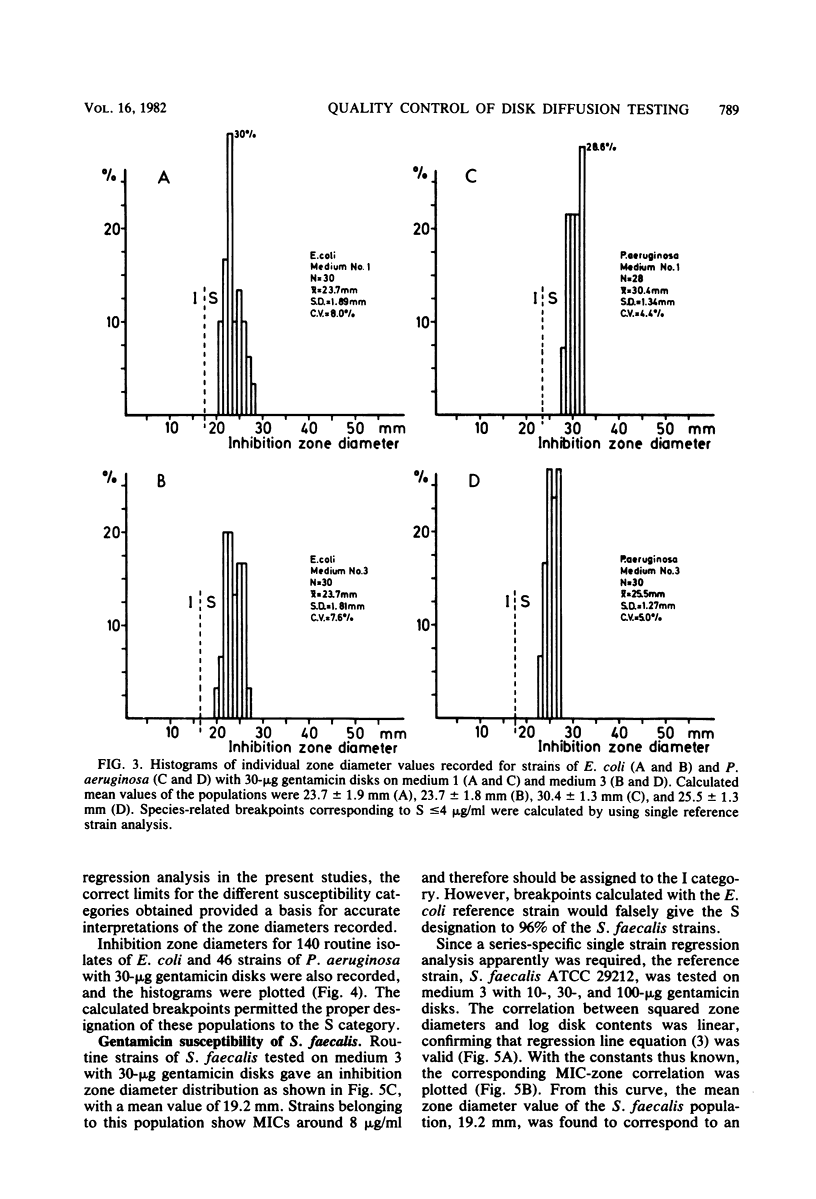
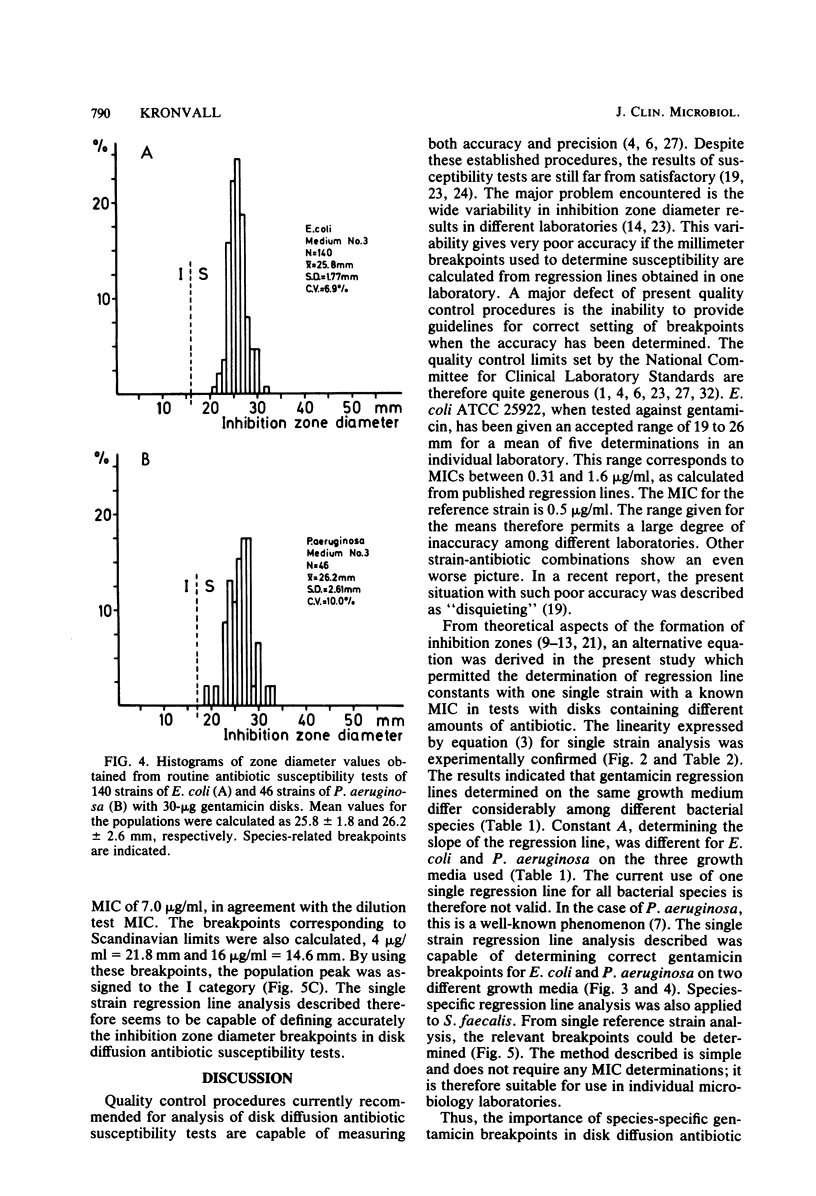
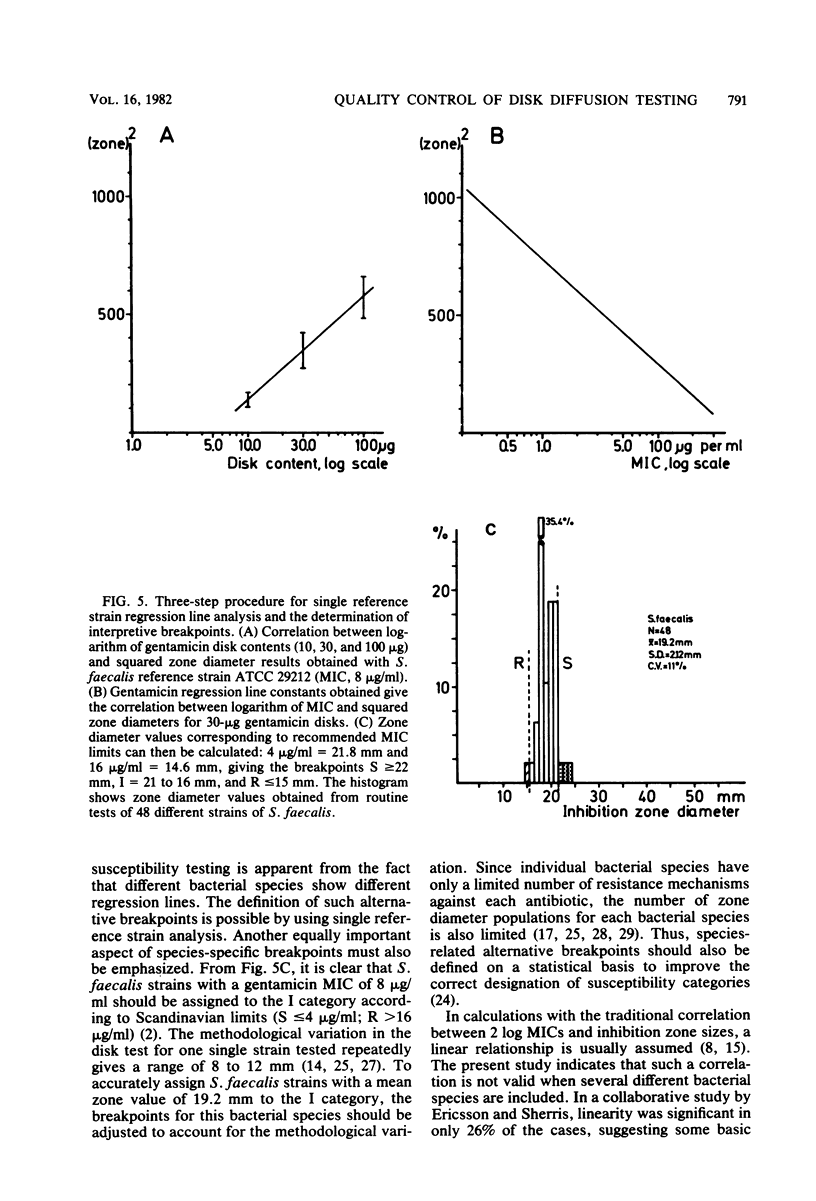
An equation was derived from known formulas to express the size of the inhibition zone diameter in the disk diffusion antibiotic susceptibility test as a function of the disk content of antibiotic. The equation permitted a calculation of regression line constants for the correlation between zone diameter and the minimum inhibitory concentration (MIC) with a single reference strain. Analysis of reference strains Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853, as well as 12 clinical isolates belonging to these species, showed a linearity between zone size squared and the logarithm of disk content in tests with 10-, 30-, and 100-micrograms gentamicin disks. All three species, however, gave regression line constants which were characteristic for the individual bacterial species. Calculations of zone diameter breakpoints corresponding to recommended MIC limits with E. coli and P. aeruginosa reference strains gave an accurate prediction of gentamicin susceptibility. Histogram analysis of 48 strains of Streptococcus faecalis from clinical specimens showed a distribution of zone diameter values which would result in false classification of susceptibility with breakpoints calculated for the other bacterial species studied. Single reference strain analysis of S. faecalis ATCC 29212 (gentamicin MIC, 8 micrograms/ml) permitted the calculation of breakpoints which accurately assigned the strains tested to the intermediate category of susceptibility. Single reference strain analysis offers a quality control method for individual laboratories that allows the determination of inhibition zone diameter breakpoints corresponding to recommended MIC limits with no MIC determinations required.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A revised system for antibiotic sensitivity testing. The Swedish Reference Group for Antibiotics. Scand J Infect Dis. 1981;13(2):148–152. doi: 10.3109/inf.1981.13.issue-2.13. [DOI] [PubMed] [Google Scholar]
- Arvidson S., Dornbusch K., Ericsson H. Interpretation of the agar diffusion method for bacterial susceptibility testing. J Antimicrob Chemother. 1981 Jan;7(1):5–14. doi: 10.1093/jac/7.1.5. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. Gentamicin and amikacin disk susceptibility tests with Pseudomonas aeruginosa: definition of minimal inhibitory concentration correlates for susceptible and resistant categories. J Clin Microbiol. 1981 May;13(5):1000–1003. doi: 10.1128/jcm.13.5.1000-1003.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- COOPER K. E., LINTON A. H., SEHGAL S. N. The effect of inoculum size on inhibition zones in agar media using staphylococci and streptomycin. J Gen Microbiol. 1958 Jun;18(3):670–687. doi: 10.1099/00221287-18-3-670. [DOI] [PubMed] [Google Scholar]
- COOPER K. E. Theory of antibiotic inhibition zones in agar media. Nature. 1955 Sep 10;176(4480):510–511. doi: 10.1038/176510b0. [DOI] [PubMed] [Google Scholar]
- ERICSSON H., TUNEVALL G., WICKMAN K. The paper disc method for determination of bacterial sensitivity to antibiotics. Relationship between the diameter of the zone of inhibition and the minimum inhibitory concentration. Scand J Clin Lab Invest. 1960;12(4):414–422. doi: 10.3109/00365516009065406. [DOI] [PubMed] [Google Scholar]
- ERLANSON P. Determination of the sensitivity in vitro of bacteria to chemotherapeutic agents. Acta Pathol Microbiol Scand Suppl. 1951;85:1–162. [PubMed] [Google Scholar]
- Furtado G. L., Medeiros A. A. Single-disk diffusion testing (Kirby-Bauer) of susceptibility of Proteus mirabilis to chloramphenicol: significance of the intermediate category. J Clin Microbiol. 1980 Oct;12(4):550–553. doi: 10.1128/jcm.12.4.550-553.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L., Fuchs P. C., Gerlach E. H., Matsen J. M., Reller L. B., Thornsberry C., Thrupp L. D. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981 Jul;14(1):67–72. doi: 10.1128/jcm.14.1.67-72.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. In vitro veritas? Antimicrobial susceptibility tests and their clinical relevance. J Infect Dis. 1981 Oct;144(4):380–385. doi: 10.1093/infdis/144.4.380. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., LIGHTBOWN J. W. A general theory for plate assay of antibiotics with some practical applications. J Gen Microbiol. 1952 Aug;7(1-2):129–143. doi: 10.1099/00221287-7-1-2-129. [DOI] [PubMed] [Google Scholar]
- Kahlmeter G. Some causes for variation in aminoglycoside plate assays. J Antimicrob Chemother. 1980 Jan;6(1):43–52. doi: 10.1093/jac/6.1.43. [DOI] [PubMed] [Google Scholar]
- Knowles R. C., Moore T. D. Quality control of agar diffusion susceptibility tests. Am J Clin Pathol. 1979 Aug;72(2 Suppl):365–370. [PubMed] [Google Scholar]
- Krasemann C., Hildenbrand G. Interpretation of agar diffusion tests. J Antimicrob Chemother. 1980 Mar;6(2):181–187. doi: 10.1093/jac/6.2.181. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Runehagen A. Förbättrad Känslighetsbedömning av bakterier medelst artrelaterat SIR-system. Lakartidningen. 1981 Sep 30;78(40):3483–3485. [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Kent R. L., Medeiros A. A. Computer-generated plots of results of antimicrobial-susceptibility tests. JAMA. 1969 Oct 6;210(1):84–92. [PubMed] [Google Scholar]
- Reimer L. G., Stratton C. W., Reller L. B. Minimum inhibitory and bactericidal concentrations of 44 antimicrobial agents against three standard control strains in broth with and without human serum. Antimicrob Agents Chemother. 1981 Jun;19(6):1050–1055. doi: 10.1128/aac.19.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R., Hedges A. J., Edwards R. J. Distribution of levels of penicillin resistance among freshly isolated strains of N. gonorrhoeae. Application of a novel sensitivity assay. Br J Vener Dis. 1975 Aug;51(4):246–250. doi: 10.1136/sti.51.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]


