Summary
Aim
Increases in serum cytokines have been reported after successful resuscitation from pro-longed ventricular fibrillation (VF). Pro-inflammatory cytokines can stimulate inducible nitric oxide synthase (iNOS) to produce excessive levels of nitric oxide (NO). High levels of both myocardial inflammatory cytokines and nitric oxide levels can depress myocardial contractile function. We hypothesized that myocardial pro-inflammatory cytokines and iNOS activity would increase following successful resuscitation from prolonged ventricular fibrillation cardiac arrest, and that such increases would parallel the development of post-resuscitation myocardial dysfunction.
Methods
Ventricular fibrillation cardiac arrest was induced in seven domestic swine (25 ± 5 kg). After 10 min of untreated VF, the animals were defibrillated and resuscitated. Left ventricular (LV) systolic and diastolic function measurements, serum samples (arterial and coronary sinus) for IL-8 cytokine quantification, and LV myocardial biopsies were collected before, during, and after resuscitation. Quantification of myocardial endothelial (eNOS) and inducible (iNOS) nitric oxide synthase protein levels were determined using immunoblot analyses and protein localization was examined using immunohistochemistry.
Results
Post-resuscitation LV systolic and diastolic functions were depressed while increases in both coronary sinus IL-8 levels and myocardial iNOS activity were found. Compared to prearrest baseline, levels of iNOS protein increased during VF (p ≤ 0.05) and continued to increase throughout the post-resuscitation study period of 6 h (p ≤ 0.05).
Conclusions
Myocardial inflammatory cytokines and iNOS activity increase during and after prolonged cardiac arrest and successful resuscitation. These increases correspond to the well described decrease in LV function post-resuscitation.
Keywords: Cardiopulmonary, resuscitation (CPR), Nitric oxide, Post-resuscitation, period, Stunning, Myocardial
Introduction
Initial resuscitation rates from out-of-hospital cardiac arrest can be 40% or better, particularly if rapid defibrillation is available. Unfortunately, the long-term survival of such patients remains poor, even after initial successful resuscitation, in part because of post-resuscitation myocardial dysfunction.1—3 In spite of its importance, our understanding of the etiology of such systolic and diastolic myocardial dysfunction post-resuscitation remains limited.
Increases in pro-inflammatory cytokines (including IL-1β, IL-8 and TNFα) have been found prompting Adrie et al. to suggest that successful cardiac resuscitation creates a “Sepsis-Like” syndrome.4 Myocardial dysfunction during sepsis is well described,5—7 and has been linked to the presence of a circulating ‘myocardial depressant substance’. The most likely candidates for such ‘myocardial depressant substances’ are inflammatory cytokines and/or nitric oxide (NO).8—13 Data during sepsis suggest that inflammatory cytokines and NO may work synergistically to depress left ventricular function.14,15
Nitric oxide is produced from l-arginine by a group of NO synthases (NOS). In the cardiac tissue, NO is produced by three isoforms of nitric oxide synthase, endothelial nitric oxide (eNOS), inducible nitric oxide (iNOS), and neuronal nitric oxide (nNOS). Stimulation of eNOS, a calcium-dependent enzyme, typically produces small amounts of NO, while stimulation of iNOS, a calcium-independent enzyme, can produce 10-fold higher levels of NO compared with eNOS. Pro-inflammatory cytokines have been shown to stimulate myocardial iNOS protein production.16 Increased iNOS activity and protein expression has been associated with chronic heart failure.17—19 Excessive increases in myocardial NO levels from stimulated iNOS may suppress left ventricular function.20
We hypothesized that following successful resuscitation, myocardial production and activity of the cytokine IL-8 and iNOS would increase and that such increases would parallel the development of post-resuscitation myocardial dysfunction.
Materials and methods
Animal preparation
This study was conducted with the approval of the University of Arizona Institutional Animal Care and Use Committee in accordance with the guidelines set forth in the Position of the American Heart Association on Research Animal Use. Seven healthy, young domestic swine (25—35 kg) of either sex (five females, two males) were anesthetized with 5% isoflurane inhalation anesthetic in oxygen delivered by nose cone mask. The animals were intubated and anesthesia was maintained with 1.5—3% isoflurane in room air, until the induction of ventricular fibrillation, using a rate- and volume-regulated ventilator/anesthesia machine (Narkomed 2A ventilator, North American Drager, Inc.). Initially, breaths were given at 12/min with a tidal volume (TV) of 15 ml/kg. Rate and/or TV was subsequently adjusted to maintain expired end-tidal carbon dioxide (ETCO2) at 40 ± 3 mmHg (mean ± S.D.), as monitored by an infrared capnometer (Hewlett Packard 47210A, Palo Alto, CA) and a pneumotachometer (MP45-871, Validyne Engineering Corp, Northridge, CA) placed in the airway. Electrocardiographic leads were attached to all limbs to monitor heart rate (HR) and the electrocardiogram (ECG). Vascular introducer sheaths (5 or 7F, Cordis Corp, Miami, FL) were placed in the right external and both internal jugular veins, the right carotid artery, and right femoral artery by standard cut-down procedure. Right atrial and aortic pressures were measured with solid-state micro-tipped transducers (P-500, Millar Instruments, Houston, TX). The left ventricular pressure was measured with a solid-state micro-tipped transducer (P-500, Millar Instruments, Houston, TX) for subsequent calculation of the first derivative of LV pressure overtime (dP/dt and −dP/dt), and the time course of LV isovolumic relaxation (tau). Occasionally, this solid-state micromanometer was replaced with a 7F fluid-filled pig-tail catheter (Cordis Corp, Miami, FL) to allow injection of contrast into the left ventricle (LV) for the calculation of left ventricular ejection fraction (EF). Blood was also collected periodically via this pigtail catheter for laboratory analyses of cytokine content in the arterial system. A standard myocardial bioptome (Cordis Corp, Miami, FL) was placed retrograde across the aortic valve into the LV cavity, via the carotid artery sheath, to obtain endomyocardial tissue samples for later immunohistochemical analyses. Endomyocardial samples (1 mm3) were obtained and immediately frozen in liquid nitrogen, as previously reported from our laboratory.21 A balloon-tipped pulmonary artery catheter was placed via an internal jugular vein into the right pulmonary artery for measuring cardiac output. A fluid-filled catheter was placed, via the other internal jugular vein, into the coronary sinus to periodically collect blood for analyses of cytokine content. Catheter/transducer placements were confirmed by fluoroscopy.
Measurements
Hemodynamic measurements
Hemodynamic data, including pressures from the aorta (AoP), right atrium (RAP), the left ventricular change in pressure overtime (dP/dt), negative dP/dt, and tau, were displayed and recorded ((D1-220-PGH, Dataq Instruments Inc, Akron, OH). In addition, ETCO2, ECG and tidal volume were continuously displayed and recorded using the same commercially available data collection software.
Left ventricular systolic function measurements
Left ventriculograms were obtained via rapid injection of 12 ml of contrast media into the LV and recorded on DVD for later determination of left ventricular ejection fraction (EF). Images taken at the peak of systole and diastole were digitized on an analyzer (Vanguard XR-55 Analyzer, Melville, NY) for computation of a single plane EF.
The rate of rise of left ventricular pressure during the isovolumic contraction period (dP/dt) was recorded as a measure of myocardial contractility. A fluid-filled pulmonary artery balloon catheter was used to measure thermodilution cardiac outputs.
Left ventricular diastolic function measurements
Diastolic left ventricular function was evaluated using negative dP/dt and the time constant of pressure fall during isovolumic relaxation (tau).
Interleukin 8 (IL-8) cytokine measurements
Concentrations of serum IL-8 were assayed from two sources (arterial blood and coronary sinus blood) using three prospectively determined time periods (pre-arrest baseline, 30 min post-resuscitation, and 5 h post-resuscitation using a standard ELISA kit from R&D Systems, (Minneapolis, MN). All tests were performed in triplicate in 96-well micro-liter plates. R&D report an assay sensitivity of <4 pg/ml, with both intra and inter-assay variabilities less than 10%.
Protein levels of eNOS/iNOS using immunoblot analysis (western blots)
Protein levels were measured and reported as arbitrary intensity units per mg (II/mg) using immunoblot techniques as described previously (22—23). Cardiac tissue was homogenized in ice-cold buffer (Hepes 5 mM, pH 7.9, glycerol 26%, v/v, MgCl2 1.5 mM, EDTA 0.2 mM, DTT 0.5 mM, phenyl-methylsulfonyl fluoride 0.5 mM), with NaCl (300 mM final), and incubated in ice for 30 min. The mixture was centrifuged at 100,000 × g at 4 °C for 20 min. The supernatant was then fractionated using 8% SDS PAGE after mixing with an equal volume of 2% SDS/1% β-mercaptoethanol. Proteins were transferred to nitrocellulose membranes. After blocking the membranes for 1 h at room temperature with 5% nonfat dry milk, 0.1% Tween-20, they were incubated with a primary monoclonal mouse anti-eNOS IgG1 antibody (1:1000) (Transduction Laboratories, BD, San Jose, CA). Endothelial nitric oxide (eNOS) was detected with horseradish peroxidase-labeled rabbit anti-mouse IgG secondary antibody (1:2000). Sections were then visualized using chemiluminescence. For iNOS, the membranes were incubated with a rabbit polyclonal IgG, NOS2 (Cat#sc-650, Santa Cruz Biotechnology, Santa Cruz, CA).
Localization of eNOS and iNOS proteins by immunohistochemistry
Protein localization was performed using standard immunohistochemical staining described previously.22—23 Serial 5-µm thick frozen sections of myocardium were dried, blocked with 5% horse serum and 0.2% bovine serum albumin, and then incubated with a primary monoclonal mouse anti-eNOS/anti-iNOS IgG1 antibody (1:50) (Transduction Laboratories, BD, San Jose, CA). After rinsing for 5 min in physiologically buffered saline (PBS), biotinylated horse anti-mouse IgG was applied for 30 min. After rinsing with PBS, avidin and biotinylated horseradish peroxidase complex was applied for 30 min. After rinsing with PBS, 0.05% diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide were applied for 5 min and washed with water. Myocardial biopsy specimens were examined for positive eNOS or iNOS protein staining (brown color) by light microscopy.
Experimental protocol
Following the collection of baseline data, all animals were electrically stimulated into ventricular fibrillation (VF) via a pacing wire temporarily inserted into the apex of the right ventricle. Ventricular fibrillation was confirmed by the characteristic ECG waveform and by the precipitous fall in aortic pressure. Assisted ventilation was discontinued and each animal remained in untreated VF for 10 min, after which cardiopulmonary resuscitation was performed following the 2005 ACLS protocol with the exceptions that each animal received immediate epinephrine (0.02 mg/kg IV) and forceful uninterrupted manual chest compressions (100/min) for 90 s prior to the first attempt at defibrillation. Previous work in our laboratory showed such modifications resulted in excellent rates (>90%) of return of spontaneous circulation.24,25 Defibrillation was performed via adhesive defibrillator pads (Quik-Combo, Medtronic PhysioControl, Redmond, WA) applied to the anterior and lateral chest wall. A single biphasic defibrillation shock of 150 J was delivered using a LifePak 12 defibrillator (Medtronic PhysioControl, Redmond, WA). Immediate chest compressions were begun after the defibrillation shock without analyzing the rhythm. If a perfusing rhythm resulted (evidenced by a pulsatile aortic pressure greater than a peak of 50 mmHg), the animals were connected to the mechanical ventilator and given 100% oxygen at an initial rate of 12 breaths per minute and a tidal volume of 15 ml/kg. Rate and/or tidal volumes were subsequently adjusted as needed to return PETCO2 to normal values. Isoflurane was added if and when the animals began to stir. No further chest compressions were administered unless the animals had recurrent VF. ROSC was defined as a peak aortic pressure of >50 mmHg and pulse pressures of >20 mmHg sustained for 1 min. If either VF or pulseless electrical activity was present after the defibrillation shock, an additional 90 s of ACLS and a second dose of epinephrine were given. This procedure was continued until a perfusing rhythm was attained or five such cycles were completed without ROSC.
All animals were successfully resuscitated and studied for a total of 6 h post-resuscitation. Left ventricular endomyocardial biopsies are performed at the following intervals: pre-arrest; at 10 min of ventricular fibrillation; at 30 min, 2 h, and 5 or 6 h post-resuscitation. Following the final data acquisition all animals were euthanized by intravenous injection of a commercial euthanasia solution (Fatal+, Vortech Pharmaceuticals, Dearborn, Michigan).
Statistical analysis
All data analysis was performed using commercially available software (Statview 5.0; SAS Institute, Cary, NC). Baseline hemodynamic, left ventricular function, and eNOS and iNOS measurements were compared with those obtained during CPR and at serial time points post-resuscitation (30 min, 2 h, and 5 or 6 h) using repeated measures analysis of variance and a Newman—Keuls multiple comparisons procedure to identify specific differences. All results are reported as mean ± S.D. A significant difference was assumed when a p value ≤0.05 was achieved.
Results
Hemodynamics and left ventricular function parameters
Aortic pressures were significantly less than pre-arrest baseline levels at 2 and 5 h after resuscitation (Table 1). Left ventricular systolic function was depressed post-resuscitation as evidenced by a decline in left ventricular ejection fraction and LV dP/dt (Table 2). Similarly, left ventricular diastolic function declined as noted by a decrease in −dP/dt, and an increase in isovolumic relaxation time (tau). Cardiac output also declined significantly in the post-resuscitation period (Table 2). These post-resuscitation changes are consistent with our previous reports.24,25
Table 1.
Hemodynamic measurements (mmHg)
| Aortic systolic | Aortic diastolic | Aortic mean | Right atrial systolic | Right atrial diastolic | Coronary perfusion pressure | |
|---|---|---|---|---|---|---|
| Pre-arrest baseline | 82 ± 12 | 63 ± 12 | 72 ± 12 | 11 ± 5 | 8 ± 5 | — |
| CPR 5 min | 95 ± 37 | 45 ± 25 | 70 ± 30 | 121 ± 73* | 13 ± 6* | 32 ± 23 |
| Post-resuscitation 30 min | 84 ± 12 | 60 ± 18 | 72 ± 15 | 15 ± 7 | 10 ± 6 | — |
| Post-resuscitation 2 h | 65 ± 14* | 47 ± 17* | 56 ± 15* | 13 ± 7 | 9 ± 6 | — |
| Post-resuscitation 5 h | 58 ± 12* | 38 ± 8* | 48 ± 9* | 12 ± 5 | 9 ± 5 | — |
Data are mean ± S.D. N = 7
p < 0.05 vs. baseline.
Table 2.
Left ventricular function measurements
| Left ventricular ejection fraction |
Left ventricular dP/dt |
Left ventricular −dP/dt |
Tau | Cardiac output | |
|---|---|---|---|---|---|
| Pre-arrest baseline | 40 ± 7 | 1015 ± 118 | −1167 ± 302 | 42 ± 6 | 2.6 ± 0.6 |
| CPR 5 min | — | — | — | — | — |
| PR 30 min | 30 ± 11* | 1331 ± 323 | −1124 ± 378 | 47 ± 13 | 2.7 ± 1.1 |
| Post-resuscitation 2 h | 30 ± 9* | 743 ± 117 | −532 ± 223* | 72 ± 18 | 2.1 ± 0.8* |
| Post-resuscitation 5 h | 25 ± 4* | 1246 ± 1050 | −773 ± 494 | 52 ± 20 | 1.8 ± 0.8* |
Data are mean ± S.D. N = 7
p < 0.05 vs. baseline.
Inflammatory cytokines post-resuscitation
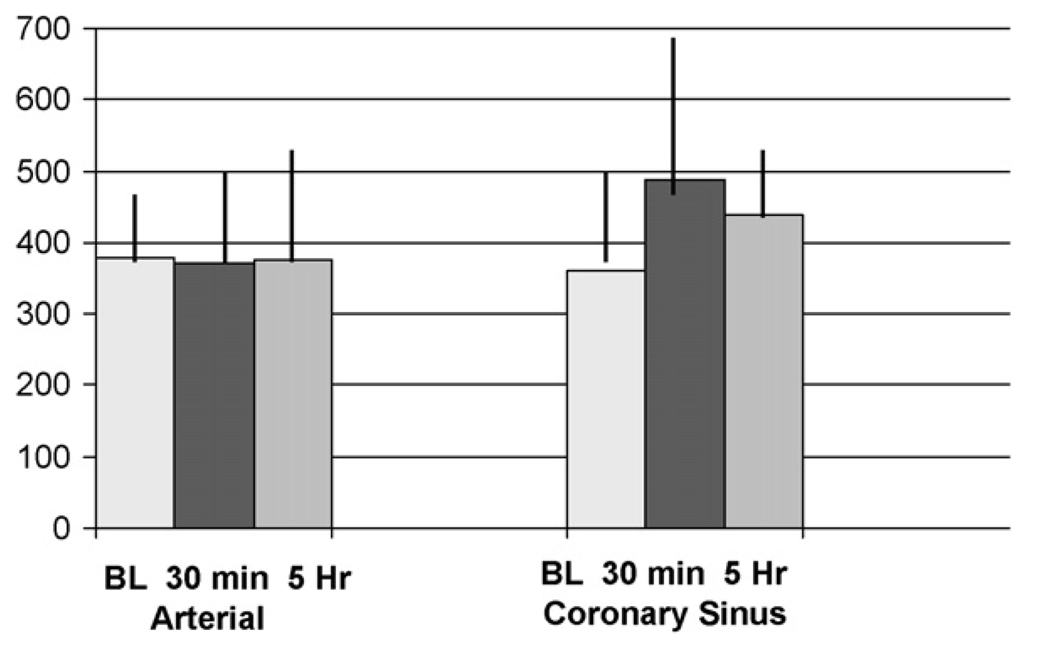
Interleukin-8 (IL-8) was measured in the arterial circulation (from the left ventricle) and in the myocardial venous circulation (from the coronary sinus). This allowed a comparison of IL-8 levels “across” the myocardium, suggesting the involvement of the myocardium or the myocardial vasculature in producing any such detectable increases in inflammatory cytokines following cardiac arrest and resuscitation. We found in the coronary sinus blood a 36% increase in IL-8 from pre-arrest baseline levels at 30 min post-resuscitation (p = 0.17) and a persistent 22% increase over baseline at 5 h post-resuscitation, while no change in IL-8 concentrations was found in the arterial blood after resuscitation (Figure 1).
Figure 1.
No change in the pro-inflammatory cytokine IL-8 (pg/ml) was seen in the arterial blood samples, but a 36% increase (p = 0.28) was seen at 30 min post-resuscitation and a 22% increase was still observed at 5 h post-resuscitation in coronary sinus blood. Mean ± S.D.
Immunoblot analysis of eNOS/iNOS
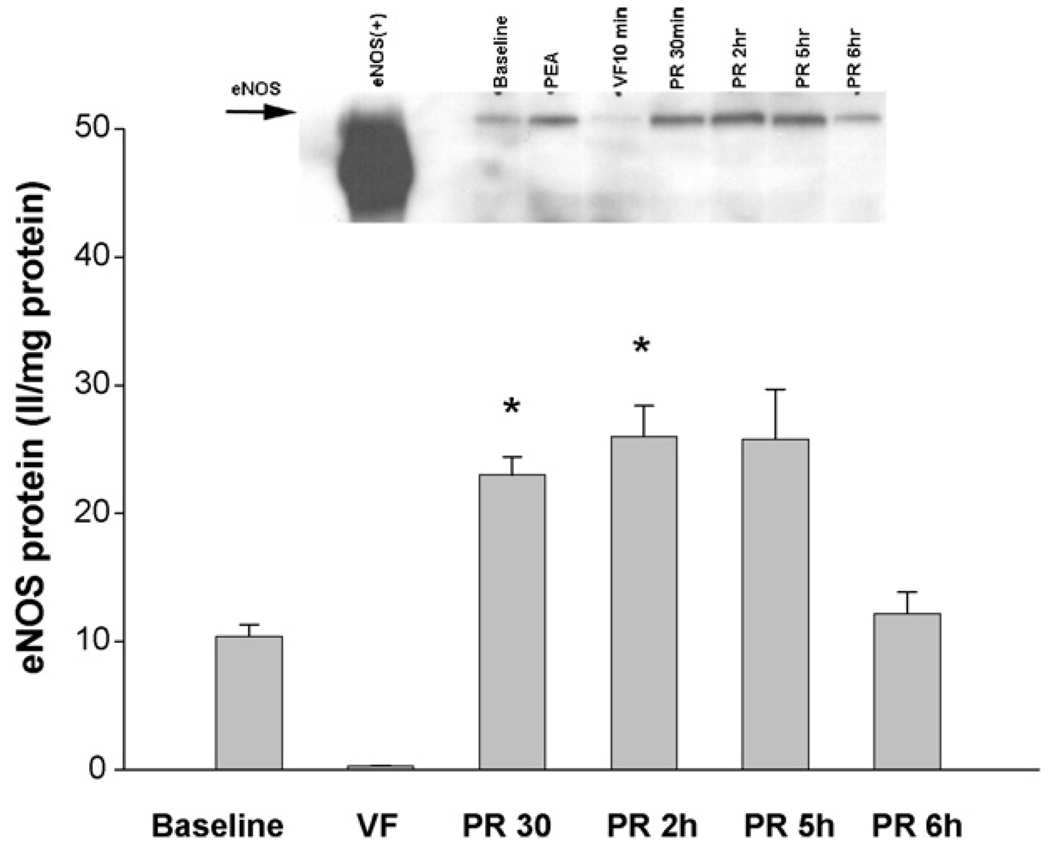
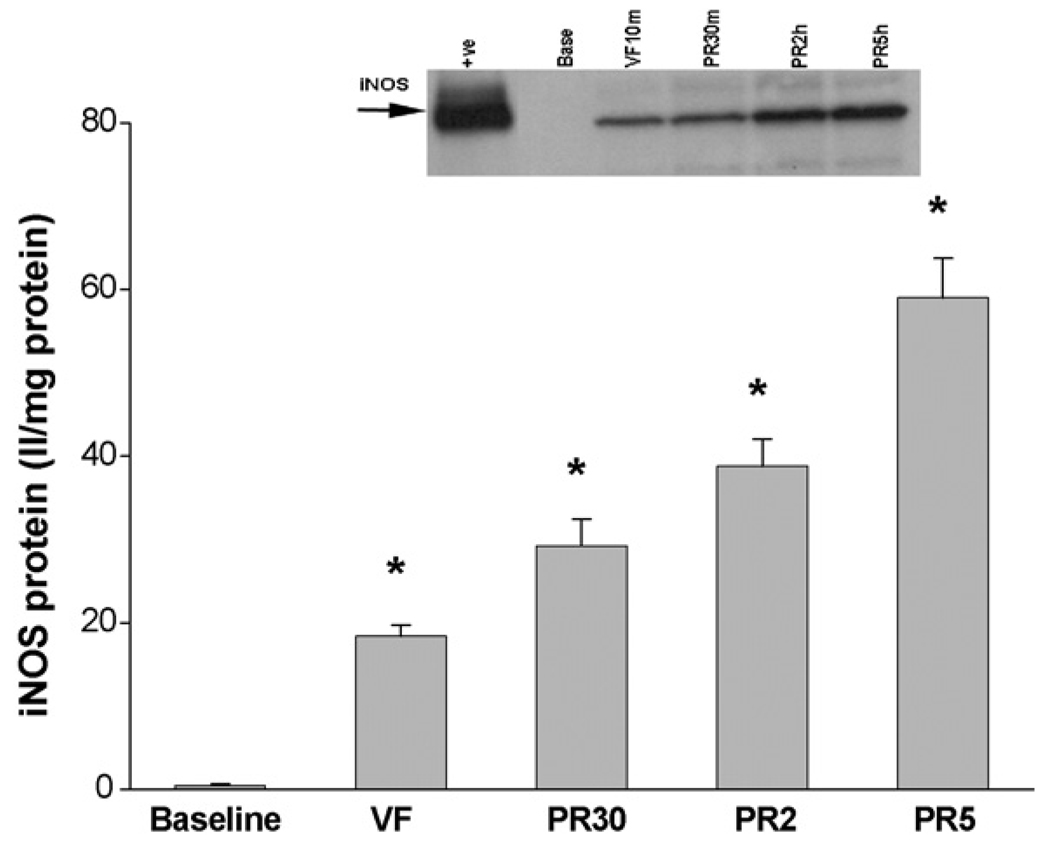
Compared to baseline, the eNOS protein levels were markedly decreased during VF (10 ± 2 II/mg at baseline vs. 0.3 ± 0.1 II/mg protein during VF, p < 0.05, Figure 2). Thirty minutes after resuscitation the eNOS protein levels increased dramatically (0.3 ± 0.1 II/mg vs. 23 ± 3 II/mg protein, p < 0.05) and were double the baseline levels. The level of eNOS remained relatively stable at 2 h (26 ± 5 II/mg protein) and at 5 h (24 ± 5 II/mg protein), returning to baseline levels by 6 h post-resuscitation (12 ± 4 II/mg protein). In contrast, levels of iNOS protein increased during VF from a baseline value of 0.5 ± 0.4 at baseline to 18 ± 3 II/mg protein (p < 0.05, Figure 3). Inducible NOS levels continued to increase with 30 min, 2 h and 5 h levels of 29 ± 7, 39 ± 7 and 59 ± 10 II/mg protein (p < 0.05). Immunohistochemical analysis confirmed the findings in immunoblots and demonstrated that both eNOS and iNOS were expressed by cardiac myocytes and vascular cells.
Figure 2.
Compared to baseline, VF decreased eNOS protein levels (10 ± 2 II/mg vs. 0.3 ± 0.1 II/mg protein, N = 5, (*p < 0.05)). Thirty minutes after resuscitation, eNOS was robustly increased compared to VF (23 ± 3 II/mg vs. 0.3 ± 0.1 II/mg protein, N = 5, (*p < 0.05)), then peaked at 2 h before returning to baseline levels by 6 h post-resuscitation (2 h: 26 ± 5; 5 h: 24 ± 5; 6 h: 12 ± 4; N = 5, respectively).
Figure 3.
In contrast to eNOS, iNOS activity was undetected at pre-arrest baseline and then began to appear during cardiac arrest after just 10 min of VF. [iNOS protein levels = 0.5 ± 0.4 II/mg vs. 18 ± 3 II/mg protein, N = 5, (p < 0.05*)]. iNOS activity incrementally increased post-resuscitation at 30 min, 2 h, and 5 h (29 ± 7 II/mg vs. 39±7 II/mg vs. 59 ± 10 II/mg vs. II/mg, N = 5, respectively (p < 0.05*)).
Immunohistochemical analysis of eNOS/iNOS
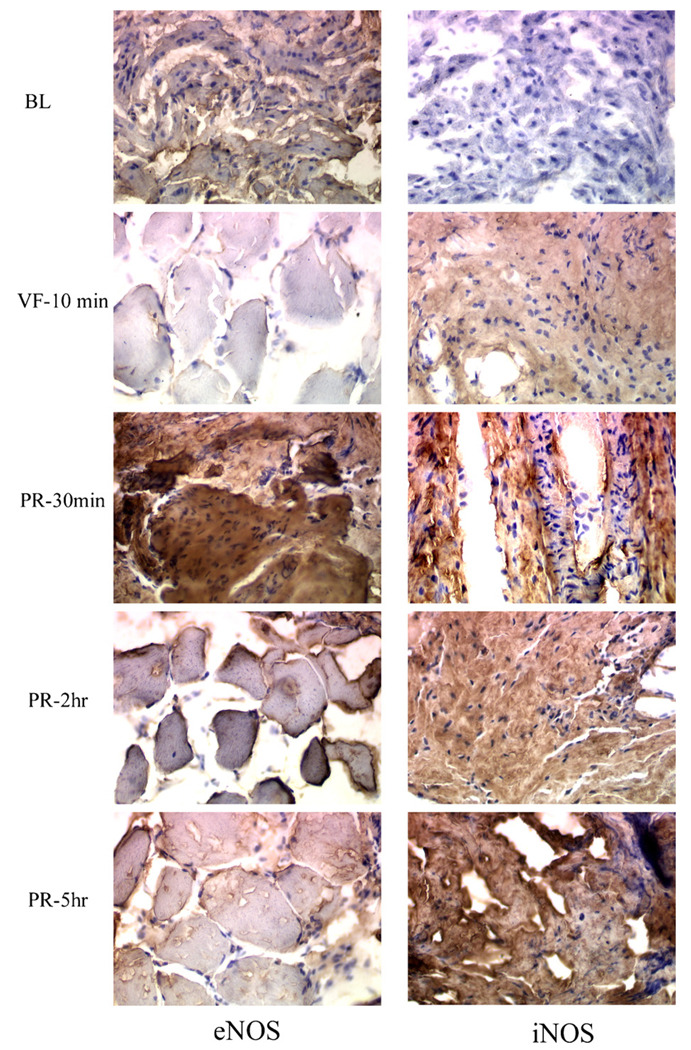
To determine the localization of eNOS and iNOS protein within the myocardium, immunohistochemical analysis was performed on heart biopsies collected and snap frozen in 2-methylbutane-suspended liquid nitrogen (Figure 4). The left panel shows eNOS protein expression and the right panel shows iNOS protein expression measured at baseline (BL), 10 min of ventricular fibrillation (VF-10 min), thirty minutes after resuscitation (PR-30 min), 2 h after resuscitation (PR-2 h), and 5 h after resuscitation (PR-5 h). Data indicate that eNOS is expressed at baseline and diminished after 10 min of cardiac arrest. Resuscitation with restoration of flow appears to induce a rapid and peak eNOS protein expression early, as shown in the third row, left panel (PR-30 min). In contrast, no iNOS is detected at baseline (first row, right panel). iNOS protein expression is not seen at baseline, but becomes evident after 10 min of VF cardiac arrest (second row, right panel). Similar to eNOS, iNOS protein expression appears to be induced by resuscitation, but in contrast to eNOS, iNOS expression appears to continue to increase throughout the 5 h post-resuscitation period. These data suggest that eNOS and iNOS respond differently to cardiac arrest and resuscitation. Resuscitation and return of spontaneous circulation induces both nitric oxide synthase isoenzymes with an early robust upregulation of eNOS, which returned to baseline level by 6 h, as opposed to a slower, more sustained upregulation of iNOS.
Figure 4.
The left panel shows eNOS protein expression and the right panel shows iNOS protein expression measured at baseline (BL), at 10 min of ventricular fibrillation (VF = 10 min), and serially after resuscitation at 30 min (PR = 30 min), at 2 h (PR = 2 h), and at 5 h (PR = 5 h). Data indicate that eNOS is expressed at baseline and diminished after just 10 min of cardiac arrest. Resuscitation appears to induce eNOS protein expression as shown in the third row, left panel (PR = 30 min). Then, eNOS expression appears to stabilize, then decrease by 5 h compared to 30 min after resuscitation. In contrast, no iNOS is detected at baseline (first row, right panel). Likewise, in contrast to eNOS, significant iNOS protein expression is evident after 10 min of VF cardiac arrest (second row, right panel). Similar to eNOS, iNOS protein expression appears to be induced by resuscitation, but iNOS expression appears to increase incrementally throughout the 5 h post-resuscitation period. Thus, it appears that eNOS and iNOS respond differently to cardiac arrest, while resuscitation seems to induce both nitric oxide synthase isoenzymes with an early, robust upregulation of eNOS and a slower, more sustained upregulation of iNOS.
Discussion
Left ventricular dysfunction is common in the immediate post-resuscitation period.24,25 We demonstrated significant left ventricular systolic and diastolic dysfunction as early as 30 min post-resuscitation, and persisting throughout the post-resuscitation study period of 5—6 h. During the same period, the inflammatory cytokine IL-8 was increased in the myocardial venous effluent (coronary sinus). These data confirm that pro-inflammatory cytokines increase after resuscitation from prolonged cardiac arrest.26—28 In our study, this increase in IL-8 was maximal at 30 min post-resuscitation and began to decline, though still present, at 5 h post-resuscitation (Figure 1). The likely origin of this increased IL-8 cytokine in the coronary sinus blood is either the myocardium itself or its inherent vasculature.
Quantitative measurements of myocardial eNOS protein expression during and after resuscitation efforts show that this constitutive isoform, found in the vascular bed within the myocardium interstitium, had a bimodal response to cardiac arrest and resuscitation (Figure 2). eNOS activity during ventricular fibrillation initially declined from its pre-arrest baseline level, then temporarily increased during the first few hours post-resuscitation, before returning to near pre-arrest baseline level by 6 h post-resuscitation.
Protein expression from the inducible isoform iNOS showed a very different pattern (Figure 3). While eNOS was expressed at pre-arrest baseline conditions, iNOS was not. Following 10 min of untreated ventricular fibrillation, eNOS protein expression diminished while the protein expression of iNOS was increased. Thirty minutes after resuscitation, eNOS expression was dramatically enhanced, while iNOS levels were only slightly elevated. Five hours after resuscitation, eNOS expression began to decrease and returned to near pre-arrest baseline levels by 6 h, while at 5 h post-resuscitation, iNOS expression had continued to steadily increase (Figure 3).
Previous investigators have shown that tumor necrosis factor-ά and other pro-inflammatory cytokines can decrease myocardial function .29,12 Some have demonstrated that excessive nitric oxide can depress myocardial contractility,9,30—33 while others have found that inhibition of nitric oxide synthase can improve myocardial function following ischemia and reperfusion.34,35 Myocardial dysfunction often accompanies sepsis and septic shock.5,6 Both systolic (decrease in left ventricular ejection fraction) and diastolic (loss of left ventricular compliance) dysfunction occur with sepsis.7 The myocardial dysfunction of sepsis seems to result from circulating depressant factors rather than hypoperfusion. These myocardial depressant factors include inflammatory cytokines and tumor necrosis factor-alpha8,10,29,12 and selective inhibition of such substances has ameliorated myocardial dysfunction in sepsis.36,37 Excessive nitric oxide production is one proposed cellular pathway for septic depression of myocardial inotropic and lusitropic functions.9,14,15 Our data suggest the same mechanism may also be functioning post-resuscitation.
We found both localized increases in inflammatory cytokines in the myocardial venous effluent and substantially activated iNOS protein production in the myocardium during the first 6 h post-resuscitation. Though ‘cause and effect’ cannot be determined from these data, a possible association between increased iNOS activity and concurrently depressed left ventricular ejection fraction was noted. Our findings suggest that excessive nitric oxide produced by upregulation of iNOS may be contributing to resuscitation-induced myocardial stunning. Whether manipulation of selective isoforms of NOS will improve post-resuscitation myocardial dysfunction is an important but yet unresolved issue.
We showed that cardiac arrest induced by VF has a differential effect on eNOS and iNOS protein expression. The current study is the first to show that resuscitation from ventricular fibrillation up-regulates iNOS protein expression. Our data is consistent with a report that demonstrates differential expression of eNOS and iNOS mRNA in the rat heart after endotoxin administration.38 Though no data previously existed concerning the potential role of iNOS in the etiology of myocardial injury and subsequent dysfunction following ventricular fibrillation cardiac arrest and resuscitation, there is evidence suggesting that following hemorrhagic shock and resuscitation, injury to non-myocardial organs such as hepatic and lung injuries, result from enhanced tissue iNOS activity. Experimental studies have shown that inhibition of iNOS, both by pharmacological inhibition and by gene manipulation, results in a marked reduction in such hepatic and pulmonary injuries.39—41 Recently, Collins and co-workers have shown that the upregulation of iNOS during and after hemorrhagic shock can occur within 1 h.42 They demonstrated that iNOS mRNA is elevated by 60 min of shock, suggesting that hepatic iNOS expression is an early molecular response to hemorrhagic shock and resuscitation. A few recent studies have also examined the effect of NOS inhibition on cardiac arrest and resuscitation dynamics.43—46 Krismer et al. showed an increased coronary perfusion pressure and return of spontaneous circulation after administration of l-NAME, a non-selective inhibitor of nitric oxide synthase.43 Zhang et al. found no difference in return of spontaneous circulation after non-selective NOS inhibition administered prior to the induction of VF cardiac arrest.44 Wu et al. showed a decrease in left ventricular ejection fraction at 2 h post-resuscitation in subjects receiving non-selective NOS inhibitors compared to placebo.45 Mean systemic blood pressure was significantly higher in such animals and the resultant increased systemic vascular resistance further compromised the post-resuscitation stunned myocardial function. This same group found that selective iNOS inhibition with aminoguanidine improved post-resuscitation left ventricular function measured as LVEF, fractional shortening, and wall motion index.46
Hence, it appears that nitric oxide synthase activity may have a role in post-resuscitation myocardial dysfunction, and that manipulation of the nitric oxide synthase isoforms could become a potential area of interest in post-resuscitation care.
Limitations
The major limitation to these preliminary observations of NOS activity during and after cardiac arrest is the lack of a non-arrest control group. The pre-arrest baseline data provides a glimpse of non-cardiac arrest data, but changes overtime potentially due to other factors than VFCA and resuscitation cannot be identified. It is possible that certain factors such as supplemental oxygen provided post-resuscitation could have some effect on NOS isoform activity. These observations of increasing IL-8 and iNOS activity in the post-resuscitation period parallel the well-documented development of post-resuscitation myocardial dysfunction. However, a cause and effect relationship is not yet established and will require further study.
Conclusions
In summary, our study shows that the inflammatory cytokine IL-8 increases in the myocardium after ventricular fibrillation cardiac arrest and resuscitation, and that myocardial isoforms of nitric oxide synthase also react to the stimulus of cardiac arrest and resuscitation. Their respective responses are quite different, with an early increase then decline in eNOS activity compared to a steady and sustained increase in activity of iNOS for at least 6 h post-resuscitation. At the same time as these biochemical changes, post-resuscitation left ventricular systolic and diastolic function are impaired. Further study is needed to define any cause and effect relationship.
Acknowledgment
This research was funded by a grant from the Arizona Disease Control Research Commission.
Footnotes
Conflict of interest
No author has any conflict of interest in regards to this work.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics: 2006 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. doi: 10.1161/CIRCULATIONAHA.105.171600. http://circ.ahajournals.org/cgi/reprint/113/6/e85. [DOI] [PubMed]
- 2.Schoenenberger RA, von Planta M, von Planta I. Survival after failed out-of-hospital resuscitation. Are further therapeutic efforts in the emergency room futile? Arch Intern Med. 1994;154:2433–2437. [PubMed] [Google Scholar]
- 3.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopul-monary resuscitation after cardiac arrest as “Sepsis-Like” Syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 5.Weisel RD, Vito L, Dennis RC, Hechtman HB. Myocardial depression during sepsis. Am J Surg. 1977;133:512–521. doi: 10.1016/0002-9610(77)90141-6. [DOI] [PubMed] [Google Scholar]
- 6.Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 7.Poelaert J, Declert C, Vogelaers D, Colardyn F, Visser CA. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997;23:553–560. doi: 10.1007/s001340050372. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor-alpha and interleukin 1-beta are responsible for depression of in vitro myocardial cell contractility induced by serum from humans with septic shock. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkel MS, Oddis CV, Jacobs TD, Watkins DC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 10.Vincent JL, Bakker J, Marecaux G, Schandene L, Kahn RJ, Dupont E. Administration of anti-TNF antibody improves left ventricular function in septic shock patients: results of a pilot study. Chest. 1992;101:810–815. doi: 10.1378/chest.101.3.810. [DOI] [PubMed] [Google Scholar]
- 11.Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. IL-1 and TNF inhibit cardiac myocyte adrenergic responsiveness. Proc Natl Acad Sci. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walley KR, Hebert PC, Wakai Y, Wilcox PG, Road JD, Cooper DJ. Decrease in left ventricular contractility after tumor necrosis factor-α infusion in dogs. J Appl Physiol. 1994;76:1060–1067. doi: 10.1152/jappl.1994.76.3.1060. [DOI] [PubMed] [Google Scholar]
- 13.Kinugawa K, Takahashi T, Kohmoto O, et al. Nitric oxide-mediated effects of IL-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994;75:285–295. doi: 10.1161/01.res.75.2.285. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Kreiger A, Symeoneides S, Kumar A, Parillo JE. Myocardial dysfunction in septic shock: Part II. Role of cytokines and nitric oxide. J Cardiothorac Vasc Anesth. 2001;15:485–511. doi: 10.1053/jcan.2001.25003. [DOI] [PubMed] [Google Scholar]
- 15.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz Rm Nava E, Moncada S. Induction and potential biological relevance of a Ca2+-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992;105:575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Q, Fortin AJ, Lu X, et al. Effects of l-arginine on endothelial and cardiac function in rats with heart failure. Eur J Pharmacol. 1999;376:37–44. doi: 10.1016/s0014-2999(99)00360-x. [DOI] [PubMed] [Google Scholar]
- 18.Haywood GA, Tsao PS, von der Leyen HE, et al. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1194. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 19.Drexler H, Kastner S, Strobel A, et al. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol. 1998;32:955–963. doi: 10.1016/s0735-1097(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferdinandy P, Danial H, Ambrus I, et al. Peroxynitrite is a major contributor cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 21.Kern KB, Garewal HS, Sanders AB, et al. Depletion of myocardial adenosine triphosphate during prolonged untreated ventricular fibrillation: effect on defibrillation success. Resuscitation. 1990;20:221–229. doi: 10.1016/0300-9572(90)90005-y. [DOI] [PubMed] [Google Scholar]
- 22.Gaballa MA, Goldman S. Overexpression of endothelial nitric oxide synthase reverses the diminished vasorelaxation in the hindlimb vasculature in ischemic heart failure in vivo. J Cell Mol Cardiology. 1999;31:1243–1252. doi: 10.1006/jmcc.1999.0956. [DOI] [PubMed] [Google Scholar]
- 23.Gaballa MA, Goldman S. Gene transfer of endothelial nitric oxide isoform decreases rat hindlimb vascular resistance in vivo. Hum Gene Ther. 2000;11:1637–1646. doi: 10.1089/10430340050111296. [DOI] [PubMed] [Google Scholar]
- 24.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28(1):232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 25.Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction: treatment with dobutamine. Circulation. 1997;95:2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Saitoh D, Fukuzuka K, et al. Significance of elevated serum interleukin-8 in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2001;51:47–53. doi: 10.1016/s0300-9572(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 27.Shyu K-G, Chang H, Lin C-C, Huang F-Y, Hung C-R. Concentrations of serum interleukin-8 after successful cardiopulmonary resuscitation in patients with cardiopulmonary arrest. Am Heart J. 1997;134:551–556. doi: 10.1016/s0002-8703(97)70094-2. [DOI] [PubMed] [Google Scholar]
- 28.Niemann JT, Garner D, Lewis RJ. Tumor necrosis factor-ά is associated with early post-resuscitation myocardial dysfunction. Crit Care Med. 2004;32:1753–1758. doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 29.Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-α and interleukin-1β synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–1318. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Kelly RA, Balligand J-L, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- 31.Ungureanu-Longrois D, Balligand J-L, Keely RA, Smith TW. Myocardial contractile dysfunction in the systemic inflammatory response syndrome: role of a cytokine-inducible nitric oxide synthetase in cardiac myocytes. J Mol Cell Cardiol. 1995;27:155–167. doi: 10.1016/s0022-2828(08)80015-6. [DOI] [PubMed] [Google Scholar]
- 32.Wildhirt SM, Weismueller S, Schulze C, Conrad N, Kornberg A, Reichart B. Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc Res. 1999;43:698–711. doi: 10.1016/s0008-6363(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Davies LR, Martin SM, et al. The nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP) increases free radical generation and degrades left ventricular function after myocardial ischemia-reperfusion. Resuscitation. 2003;59:345–352. doi: 10.1016/s0300-9572(03)00240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wildhirt SM, Schulze C, Conrad N, Kornberg A, Horstman D, Reichart B. Aminoguanidine inhibits inducible NOS and reverses cardiac dysfunction late after ischemia and reperfusion-implications for iNOS-mediated myocardial stunning. Thorac Cardiovasc Surg. 1999;47:137–143. doi: 10.1055/s-2007-1013128. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Bissing JW, Xu L, et al. Nitric oxide synthase inhibitors decrease coronary sinus-free radical concentration and ameliorate myocardial stunning in an ischemia-reperfusion model. J Am Coll Cardiol. 2001;38:546–554. doi: 10.1016/s0735-1097(01)01400-0. [DOI] [PubMed] [Google Scholar]
- 36.Fisher CJ, Jr, Dhaiaut JF, Opal SM, et al. Recombinant human IL-1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double blind placebo controlled trial. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 37.Fisher CJ, Jr, Slotner GJ, Opal SM, et al. Initial evaluation of human recombinant IL-1 receptor antagonist in the treatment of the sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Ishiwata T, Guo F, Naito Z, Asano G, Nishigaki R. Differential distribution of eNOS and iNOS mRNA in rat heart after endotoxin administration. Jpn Heart J. 1997;38:445–455. doi: 10.1536/ihj.38.445. [DOI] [PubMed] [Google Scholar]
- 39.Hierholzer C, Harbrecht B, Menezes JM, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menezes J, Hierholzer C, Watkins SC, et al. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol. 1999;277:G144–G151. doi: 10.1152/ajpgi.1999.277.1.G144. [DOI] [PubMed] [Google Scholar]
- 41.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock. 2002;17:13–18. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Collins JL, Vodovotz Y, Hierholzer C, et al. Characterization of the expression of inducible nitric oxide synthase in rat and human liver during hemorrhagic shock. Shock. 2003;19(2):117–122. doi: 10.1097/00024382-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Krismer AC, Lindner KH, Wenzel V, Rainer B, Mueller G, Lingnau W. Inhibition of nitric oxide improves coronary perfusion pressure and return of spontaneous circulation in a porcine cardiopulmonary resuscitation model. Crit Care Med. 2001;29:482–486. doi: 10.1097/00003246-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Boddicker KA, Rhee BJ, Davies LR, Kerber RE. Effect of nitric oxide synthase modulation on resuscitation success in a swine ventricular fibrillation cardiac arrest model. Resuscitation. 2005;67:127–134. doi: 10.1016/j.resuscitation.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Wu D, Bassuk J, Arias J, et al. Different roles of nitric oxide synthase isoforms in cardiopulmonary resuscitation in pigs. Resuscitation. 2007;73:144–153. doi: 10.1016/j.resuscitation.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 46.Adams JA, Wu D, Bassuk J, et al. Nitric oxide synthase isoform inhibition before whole body ischemia reperfusion in pigs: vidal or protective? Resuscitation. 2007;74:516–525. doi: 10.1016/j.resuscitation.2007.02.009. [DOI] [PubMed] [Google Scholar]