Abstract
The natural history of intraneural perineurioma has been inadequately studied. The aim of this study was to characterize the clinical presentation, electrophysiologic and imaging features and outcome of intraneural perineurioma. We ask if intraneural perineurioma is a pure motor syndrome that remains confined to one nerve and should be treated by surgical resection. We examined the nerve biopsies of cases labelled perineurioma and selected those with diagnostic features. Thirty-two patients were identified; 16 children and 16 adults; 16 males and 16 females. Median age of onset of neurological symptoms was 14 years (range 0.5–55 years) and median age at evaluation was 17 years (range 2–56 years). All patients had motor deficits; however, mild sensory symptoms or signs were experienced by 27 patients; ‘prickling’ or ‘asleep numbness’ in 20, mild pain in 13 and sensory loss in 23. The sciatic nerve or its branches was most commonly affected in 15, followed by brachial plexus, radial nerve and ulnar nerve (four each). Magnetic resonance imaging demonstrated nerve enlargement (29/32), T1 isointensity (27/32), T2 hyperintensity (25/32) and contrast enhancement (20/20). Diagnoses were made based on targeted biopsy of the focal nerve enlargement identified by imaging. Neurological impairment was of a moderate severity (median Neuropathy Impairment Score was 12 points, range 2–49 points). All patients had focal involvement with 27 involving one nerve and five involving a plexus (one bilateral). Long-term follow-up was possible by telephone interview for 23 patients (median 36 months, range 2–177 months). Twelve patients also had follow-up neurologic evaluation (median 45 months, range 10–247 months). The median Neuropathy Impairment Score had changed from 12.6 to 15.4 points (P = 0.19). In all cases, the distribution of neurologic findings remained unchanged. Median Dyck Disability Score was 3 (range 2–5) indicating a mild impairment without interfering with activities of daily living. Ten patients judged their symptoms unchanged, nine slightly worse and four slightly better. We conclude intraneural perineurioma is a benign hypertrophic (non onion bulb) peripheral nerve tumour that presents insidiously in young people and is motor predominant with mild sensory involvement. It is most often a mononeuropathy, but a plexopathy can occur. Diagnosis of this condition requires clinical suspicion, imaging, targeted fascicular biopsy of the lesion and expertise of nerve pathologists. As these tumours are static or slowly progressive, remain confined to their original distribution and have low morbidity, they probably should not be resected routinely. Because intensive evaluation is needed for diagnosis, intraneural perineurioma is probably under-recognized.
Keywords: perineurioma, peripheral neuropathy, nerve sheath neoplasm, localized hypertrophic neuropathy, sciatic neuropathy
Introduction
Pathologically, there are two main forms of perineurioma: intraneural and extraneural. Intraneural (IN) perineurioma is a benign neoplasm composed exclusively of whorls of perineurial cells surrounding nerve fibres and restricted to the boundaries of a peripheral nerve (Macarenco et al., 2007). Extraneural (EN) perineurioma is also composed of perineurial cells but is found mainly in soft tissues and skin (Macarenco et al., 2007). The IN form was first described in 1964 as interstitial hypertrophic neuritis (Imaginariojda et al., 1964) and was referred to by several other names including IN neurofibroma (Lallemand and Weller, 1973), localized hypertrophic neuropathy (Hawkes et al., 1974; Snyder et al., 1977; Peckham et al., 1982; Boker et al., 1984; Iyer et al., 1988; Stanton et al., 1988; Johnson and Kline, 1989; Phillips et al., 1991; Gullotta, 1992; Suarez et al., 1994; Simmons et al., 1999) and hypertrophic neurofibrosis (Simpson and Fowler, 1966; de los Reyes et al., 1981). Early on patients with focal onion bulbs (of Schwann cell origin) and IN perineurioma (of perineurial origin) were often lumped together and their diagnosis called localized hypertrophic neuropathy, leading to confusion as to what constituted IN perineurioma. With the advent of epithelial membrane antigen (EMA) immuno-cytochemistry (Ariza et al., 1988), IN perineurioma could be differentiated from focal onion bulb formations as seen in inherited demyelinating and chronic acquired demyelinating neuropathies. IN perineurioma demonstrated EMA reactivity for the whorled formations surrounding nerve fibres whereas onion bulb formations demonstrated reactivity with a Schwann cell marker (S-100). This led to the nomenclature of ‘pseudo-onion bulbs’ when describing whorls of perineurial cells and ‘real onion bulbs’ when describing whorls of Schwann cell processes.
The pathology of IN perineurioma was nicely described in eight cases by Emory et al. (1995). In addition to the EMA reactivity of IN perineurioma, the authors noted the tumours involved only one nerve in almost all cases (one case involved the brachial plexus). One case demonstrated a clonal neoplasm associated with abnormalities of chromosome 22 (Emory et al., 1995). This was in contrast to a prior suggestion that IN perineurioma was due to trauma or reactive changes. The authors argue that this is compelling evidence for IN perineurioma being a benign peripheral nerve neoplasm. Whether this is true for all cases is unclear.
Our knowledge of the clinical features of IN perineurioma is based on individual case reports. Only one study of four patients, with a median follow-up of 8 months, exists looking at natural history and long-term outcome (Simmons et al., 1999).
Here we present a large cohort study describing the clinical features and long-term outcome of IN perineurioma. On the basis of a retrospective analysis of patients evaluated at a single institution, we addressed the following questions regarding IN perineurioma: (i) is IN perineurioma a purely motor syndrome?; (ii) if sensory involvement occurs, how severe are those symptoms and deficits and is there associated pain?; (iii) is the process always a mononeuropathy and which nerves are involved?; (iv) what are the radiographic features of IN perineurioma and are they specific? Are they focal or more widespread?; (v) what is the course of IN perineurioma and does it spread to nerves that initially were unaffected? and (vi) do patients have functional impairment?
Materials and Methods
Patient selection
With protocol approval by the Mayo Clinic Investigational Review Board (IRB), patients were identified by a search of the Mayo computerized database (January 1994 to September 2007), the Department of Pathology database (January 1985 to September 2007) and the Peripheral Nerve Laboratory database (January 1968 to September 2007) coded for perineurioma (perineuroma), pseudo-onion bulbs and hypertrophic neuropathy. Patients without pathologic specimens available at Mayo Clinic were excluded. Information was abstracted from medical records of patients who had given consent, allowing their medical records to be used for research purposes.
The pathology reports were reviewed in all patients (n = 426). In cases where there was a description potentially compatible with perineurioma (n = 48), the pathology was personally reviewed by two of the authors (M.L.M and P.J.B.D). Included cases had to be diagnostic for perineurioma: pseudo-onion bulb like formations and immunohistochemistry that demonstrated reactivity of the pseudo-onion bulbs with EMA and no reactivity of the outer leaflets for S-100. When necessary, the immunohistochemical preparations were repeated.
Scoring of neuropathy severity and disability
The clinical information was reviewed for those patients who met the pathological criteria for IN perineurioma. The Neuropathy Impairment Score (NIS) was used to score the severity of the peripheral neuropathic deficits (Dyck et al., 1980). In brief, the NIS is a summed score of a standard and representative list of motor, sensory and muscle stretch reflex impairments. Scores range from 0 (normal) to 4 (paralysed) for motor, and 0 to 2 (absent) for sensation and reflexes, for individual attributes. The NIS was calculated from the abstraction of findings from the Neurological Examination Form at Mayo Clinic visits for only those patients seen by a neurologist.
Neurophysiology
The assessment of nerve conduction studies (NCS) and needle electromyography (EMG) used methods standard for the EMG laboratory at Mayo Clinic. Quantitative sensory testing (QST) using Computerized Assisted Sensory Examination (Case IV) was performed (Dyck et al., 1984). Autonomic testing was performed using the Mayo Clinic Autonomic Reflex Screen which assesses cardiovagal, adrenergic and post-ganglionic sympathetic sudomotor function (Low, 1993).
Pattern of neuropathy
The clinical history was used to determine the presence and location of motor and sensory symptoms including pain. This information along with the clinical examination was used to categorize the neuropathy as pure motor, motor predominant or motor and sensory.
Neuroimaging
MRI was personally reviewed by two of the authors (M.L.M. and K.K.A.) with agreement by consensus on imaging features. The nerves that were involved radiographically, the length and diameter of the lesion and the imaging characteristics on T1, T2 and post-gadolinium imaging were determined.
Clinical follow-up
Patients were contacted and asked to return for repeat evaluation. Patients with follow-up information (more than one clinical visit) were included. The NIS score, Dyck Disability Score (DDS) (Dyck et al., 2005) and Neuropathy Symptoms and Change (NSC) questionnaire (Dyck et al., 2002) were calculated for these patients for each visit. The DDS is a scoring system used to assess the severity of the neuropathy and its restriction on acts of daily living. Scores range from 0 (no symptoms, signs or test abnormalities of neuropathy) to 8 (symptomatic neuropathy requiring constant care in an intensive care unit). When possible, NCS and EMG, QST and MRI were repeated for longitudinal follow-up.
Telephone interview
After obtaining informed consent (by letter approved by our IRB), we attempted to contact all patients who agreed to be interviewed by telephone. In the telephone interview, the patient's present symptoms and impairments, modified Rankin scale (mRS) (Bonita and Beaglehole, 1988) and present treatment were reviewed.
Results
Clinical attributes
Thirty-two patients met the pathological criteria for IN perineurioma (Figs 1 and 2) and their demographic and clinical features are summarized in Table 1. The median age of onset of neurological symptoms was 14 years (range 6 months to 55 years). The median age at time of evaluation at Mayo Clinic was 17 years (range 2–56 years). The median time from symptom onset to evaluation at Mayo Clinic was 3 years (range 6 months to 30 years). At the time of evaluation, there were 16 children and 16 adults of whom 16 were male and 16 female. The chief complaint was weakness/atrophy in 29 patients and numbness/pain in three patients. All 32 patients had symptoms of weakness develop during the course of their illness. The weakness was focal in all and caused morbidity. Twenty-four patients had sensory symptoms, 17 with ‘dead-type numbness’, six with ‘prickling’ and 13 with pain. The pain was reported as ‘stabbing’, ‘burning’ or ‘aching’ and was of mild severity. No patients had autonomic symptoms. The neuropathy was pure motor in five patients, motor predominant in 26 patients and motor and sensory in one patient. Seven of these patients (Patients 6, 9, 24, 25, 26, 27 and 28) were reported in a prior series by Emory et al. mainly reviewing the pathologic features (Emory et al., 1995). Three patients, including one from the aforementioned series, were reported in individual reports (Patients 4, 6 and 16) (Suarez et al., 1994; Hahn et al., 2007; Nguyen et al., 2007).
Figure 1.
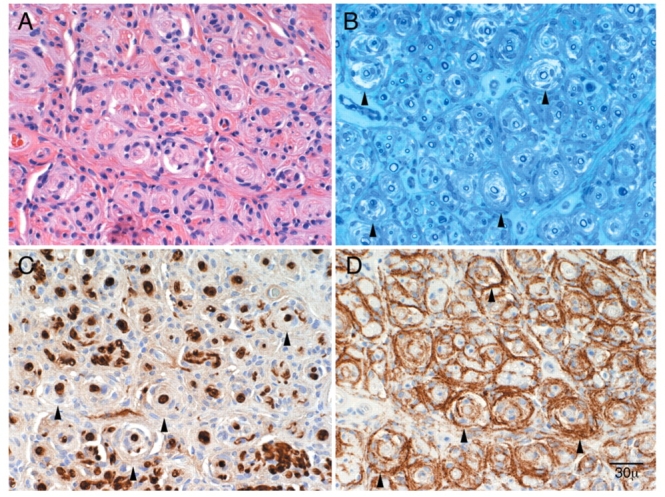
Pathological features of perineurioma. Patient 1: serial transverse sections: Arrowheads indicate pseudo-onion bulb leaflets. (A) H&E section demonstrates diffuse pseudo-onion bulb formation. (B) Epoxy section at a similar level demonstrates thinly myelinated fibres at the centre of pseudo-onion bulbs. (C) Schwann cell preparation (S-100) demonstrates reactivity of the myelinated fibres at the centre and absence of reactivity of the surrounding pseudo-onion bulbs. (D) Reactivity of pseudo-onion bulb leaflets with epithelial membrane antigen (EMA) confirming these are of perineurial origin. These findings taken together are diagnostic of perineurioma.
Figure 2.
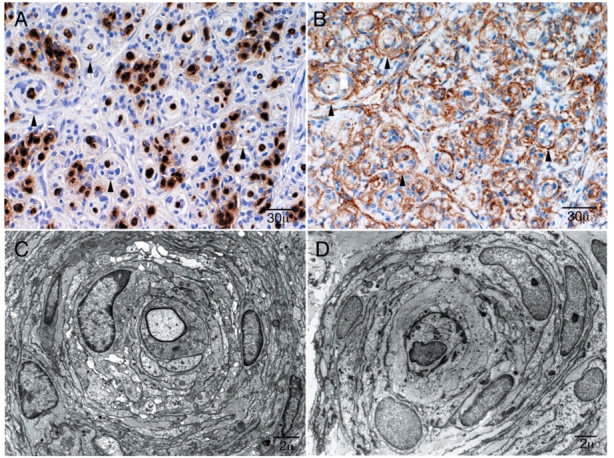
Immunohistochemistry and ultrastructural features of perineurioma. Patient 3: These pathological features are typical of intraneural perineurioma. In contrast the clinical features of this case are very unusual as this young child has bilateral disease involving multiple nerves over a long distance (see Case 2). (A) S-100 preparation demonstrates reactivity of the myelinated fibres at the centre and absence of reactivity of the surrounding pseudo-onion bulbs (arrowheads). (B) Reactivity of pseudo-onion bulb leaflets with epithelial membrane antigen (EMA) confirming these are of perineurial origin (arrowheads). (C) Electron micrograph of Patient 4 and (D) electron micrograph of Patient 8 demonstrate dense concentrically arranged cellular processes around thinly myelinated axons typical of perineurioma.
Table 1.
Patient characteristics
| Patient | Age at clinical eval (yrs) | Gender | Duration of symptoms at eval (mo) | Chief complaint | Dead numbness | Prickling | Pain | Weakness | Sensory loss | Neurologic exam findings | NIS (points) | Clinical diagnosis | EMG diagnosis | Nerve biopsied | CSF protein (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | F | 12 | Weakness | N | N | N | Y | N | MO | 12.25 | Sciatic | Sciatic (P>T) | Sciatic (P) | 22 |
| 2 | 7 | M | 15 | Weakness | N | N | N | Y | Y | MP | 12 | Sciatic | Sciatic (P>T) | Sciatic (P, T) | 15 |
| 3 | 2 | F | 24 | Weakness | N | N | N | Y | Y | MP | 30 | Lumbosacral plexus | Lumbosacral plexus | Sciatic (T) | 22 |
| 4 | 12 | F | 6 | Weakness | N | Y | Y | Y | Y | MP | 7 | Sciatic | Sciatic (P) | Sciatic (P) | 37 |
| 5 | 35 | M | 180 | Weakness | Y | N | N | Y | Y | MP | 9.5 | Tibial | Tibial | Tibial | |
| 6 | 31 | M | 84 | Atrophy | N | Y | N | Y | Y | MP | 20 | Sciatic | Tibial | Sciatic (T) | |
| 7 | 15 | F | 36 | Weakness | N | N | N | Y | N | MP | 16.25 | Sciatic | Lumbosacral plexus | Sciatic (P) | |
| 8 | 56 | M | 12 | Weakness | Y | N | N | Y | Y | MP | 7 | Radial | Radial | Radial | 45 |
| 9 | 19 | F | 14 | Weakness | N | N | Y | Y | N | MO | 5 | Femoral | Femoral | Femoral | 39 |
| 10 | 40 | M | 108 | Weakness | Y | Y | Y | Y | Y | MP | 9 | Median | Median | Median | |
| 11 | 7 | F | 7 | Weakness | N | N | N | Y | N | MO | 21 | Sciatic | Lumbosacral plexus | Sciatic (P) | 18 |
| 12 | 40 | M | 48 | Pain and numbness | Y | N | Y | Y | Y | MP | 2 | Trigeminal | ND | Auriculotemporal | |
| 13 | 34 | M | 48 | Weakness | N | N | Y | Y | Y | MP | 11.5 | Sciatic | Sciatic (P>T) | Sciatic (P) | |
| 14 | 12 | F | 48 | Weakness | N | N | N | Y | N | MO | 19 | Brachial plexus | Brachial plexus | BP (middle) | |
| 15 | 35 | M | 360 | Weakness | Y | N | N | Y | Y | MS | 49 | Brachial plexus | Brachial plexus | BP (upper) | |
| 16 | 30 | F | 48 | Pain | Y | N | Y | Y | Y | MP | 6 | Radial | Radial | Radial | |
| 17 | 14 | M | 18 | Weakness | N | N | N | Y | Y | MP | 7 | Sciatic | Sciatic (T>P) | Sciatic (T) | 42 |
| 18 | 30 | M | 78 | Weakness | N | N | Y | Y | N | MP | 22 | Sciatic | Sciatic (T) | Sciatic (T) | |
| 19 | 12 | M | 144 | Weakness | N | Y | N | Y | N | MP | 12 | Brachial plexus | Brachial plexus | BP (middle) | |
| 20 | 34 | M | 96 | Weakness | Y | N | N | Y | N | MP | 12 | Radial | Radial | Radial | |
| 21 | 8 | F | 8 | Weakness | N | N | Y | Y | Y | MP | 9 | Sciatic | Common peroneal | Common peroneal | |
| 22 | 19 | F | 60 | Weakness | Y | N | N | Y | Y | MP | NA | Ulnar | ND | Ulnar | |
| 23 | 25 | F | 8 | Pain | Y | N | Y | Y | Y | MP | 7 | Ulnar | Ulnar | Ulnar | |
| 24 | 38 | F | 9 | Weakness | N | N | N | Y | N | MO | NA | Radial (PIN) | Radial (PIN) | Radial (PIN) | |
| 25 | 15 | M | 16 | Weakness | Y | N | N | Y | Y | MP | 18 | Median | Median | Median | |
| 26 | 11 | F | 72 | Weakness | Y | N | Y | Y | Y | MP | 12 | Brachial plexus | Brachial plexus | C8/T1, BP (lower) | |
| 27 | 13 | F | 24 | Weakness | Y | N | N | Y | Y | MP | 8 | Peroneal | Common peroneal | Common peroneal | |
| 28 | 35 | F | 24 | Weakness | Y | Y | Y | Y | Y | MP | NA | Ulnar | Ulnar | Ulnar | |
| 29 | 8 | M | 90 | Weakness | Y | Y | Y | Y | Y | MP | 13 | Sciatic | Sciatic (P>T) | Sciatic (P) | 31 |
| 30 | 13 | F | 24 | Weakness | Y | N | Y | Y | Y | MP | 3.25 | Ulnar | Ulnar | Ulnar | |
| 31 | 37 | M | 77 | Weakness | Y | N | N | Y | Y | MP | 9.5 | Tibial | Tibial | Tibial | 42 |
| 32 | 12 | M | 36 | Weakness | Y | N | N | Y | Y | MP | 16 | Sciatic | Lumbosacral plexus | Sciatic (P) | 44 |
| Number | 32 | 16F, 16M | 32 | 17Y, 15N | 6Y, 26N | 13Y, 19N | 32Y, 0N | 23Y, 9N | 26MP | 29 | 11 | ||||
| Median | 17 | 36 | 12 | 37 | |||||||||||
| Range | 2–56 | 6–360 | 2–49 | 15–45 |
M = male; F = female; Y = yes; N = no; yrs = years; mo = months; MO = motor only; MP = motor predominant; MS = motor and sensory; NIS = neuropathy impairment score; NA = information not available; ND = not done; P = peroneal division; T = tibial division; BP = brachial plexus; upper = upper trunk; middle = middle trunk; lower = lower trunk; PIN = posterior interosseus nerve.
Examination findings
On examination, all 32 patients had motor weakness in the distribution of the involved nerve. Eleven patients had foot drop and three had wrist drop. Five patients required adaptive bracing, either ankle foot orthoses or wrist braces. Twenty-three patients demonstrated sensory loss on examination in the distribution of the affected nerve(s). Muscle stretch reflexes were reduced or absent in the distribution of the IN perineurioma in 16/29 patients in whom the reflexes were recorded. The median NIS was 12 points (range 2–49 points) indicating a moderate severity for a focal neuropathy. The most common nerve to be involved was the sciatic nerve or its branches in 15 patients; 12 were proximal (involving the peroneal division in two, tibial division in three, and both divisions in seven); three were distal (involving the peroneal nerve in one and tibial nerve in two). Other involved nerves included the brachial plexus (four patients), radial nerve (four), ulnar nerve (four), median nerve (two), femoral nerve (one), trigeminal nerve (one) and lumbosacral plexus (one). The pattern was most commonly a mononeuropathy (27 patients, 84%) followed by a plexopathy (five patients, 16%). Thirty-one patients had unilateral disease whereas one (Patient 3) had bilateral lumbosacral plexopathies (see Case 2 below).
Laboratory features
Twenty-two patients had extensive laboratory testing performed at Mayo Clinic, including glucose, erythrocyte sedimentation rate, antinuclear antibody, anti-neutrophil-cytoplasmic antibody, serum protein electrophoresis and immunofixation, cryoglobulins, vitamin B12, thyroid stimulating hormone, angiotensin converting enzyme, rheumatoid factor, extractable nuclear antigens human immunodeficiency virus, hepatitis profile, paraneoplastic antibodies, Lyme serology and peripheral myelin protein 22 deletion analysis for hereditary neuropathy with liability to pressure palsies all of which were unremarkable. Spinal fluid examination (CSF) was performed in 11 patients and was normal in nine patients and demonstrated a borderline elevated protein for age in two children. Median numbers of CSF white blood cells were 1 μl (range 0–4 μl), red blood cells 0 μl (range 0–36 μl), glucose 55 mg/dl (range 48–65 mg/dl) and protein 34 mg/dl (range 15–45 mg/dl).
Neurophysiology
NCS/EMG were performed in 30 patients and the focal nature of the neuropathy was generally confirmed. In one case, the abnormalities were bilateral (Patient 3). The findings suggested that axonal degeneration was the predominant pathophysiology. Fibrillation potentials were seen in 29 of 30 patients and large motor unit potentials in 29 of 30 patients (one had no voluntary motor units) in the affected nerve distribution. Paraspinal fibrillations were seen in one of 30 patients. Possible demyelination was noted in four patients: conduction block (two), dispersion (two) or slowed conduction velocities (one). In 26 of the 30 patients who had sensory NCS, there was reduction (n = 7) or absence (n = 19) of the involved sensory nerve action potential (SNAP). Overall, the EMG closely correlated with the clinical examination and demonstrated a sciatic neuropathy in seven patients, brachial plexopathy in four, radial neuropathy in four, lumbosacral plexopathy in four, tibial neuropathy in three, ulnar neuropathy in three, median neuropathy in two, common peroneal neuropathy in two and femoral neuropathy in one (Table 1).
QST was performed in 12 patients, (upper limbs = 4 and lower limbs = 8) and revealed abnormalities in 11. There was hyposensitivity to all modalities in two (Patients 7, 31), only vibration hyposensitivity in three (Patients 18, 19 and 32), only heat-pain and/or cooling hyposensitivity in four (Patients 5, 8, 10 and 14) and vibration hyposensitivity and hyperalgesia in two (Patients 11 and 29). One patient had a normal study (Patient 1).
Autonomic reflex screen showed abnormality in three of the four patients on whom it was performed and demonstrated mild distal postganglionic sympathetic sudomotor dysfunction in three (Patient 20, 31 and 32) and mild cardiovagal dysfunction in one (Patient 31).
Neuroimaging
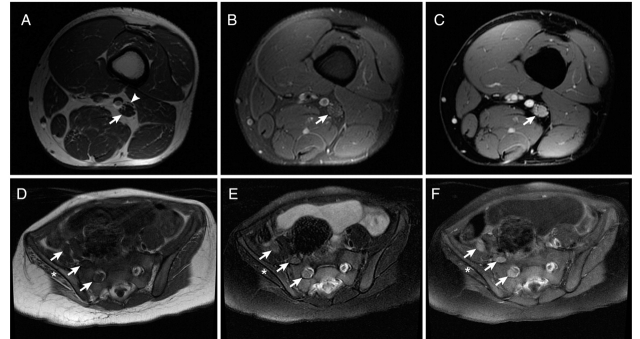
MRI of the affected nerve(s) was performed in all 32 patients. Only three were stated to be normal and these three were among the older studies and had been discarded and so were not accessible for review. Table 2 details the findings on MRI. The nerves demonstrated a characteristic MRI pattern. The abnormal nerves demonstrated an enlargement (29/32), most often a fusiform (27/32) (gradual increase of the nerve diameter over the length followed by gradual taper) enlargement. These lesions were of considerable length (median 8 cm, range 2.5–32 cm). They were consistently isointense on T1-weighted imaging (27/32), hyperintense on T2-weighted imaging (25/32) and demonstrated avid contrast enhancement (20/20) (Fig. 3) in all cases where intravenous gadolinium was administered.
Table 2.
Magnetic resonance imaging characteristics
| Patient | Nerves involved | Enlargement | Maximum diameter (cm) | Maximum length (cm) | T1 | T2 | Contrast enhancement? |
|---|---|---|---|---|---|---|---|
| 1 | L sciatic, tibial and peroneal division | Fusiform | 1.3 | 15 | Iso | Hyper | Y |
| 2 | R sciatic, peroneal division | Fusiform | 1.5 | 12 | Iso | Hyper | Y |
| 3 | R L4, L5, S1, S2, sciatic; L sacral plexus, sciatic | Fusiform** | 1.5 | 12 | Iso | Hyper | Y |
| 4 | L sciatic, peroneal division | Fusiform | 0.8 | 4 | Iso | Hyper | NA |
| 5 | L sciatic, peroneal and tibial division | Fusiform | 1.7 | 16 | Iso | Hyper | Y |
| 6* | L sciatic | Fusiform | 1.5 | 8 | Iso | Iso | NA |
| 7 | L > R sciatic, L femoral | Fusiform | 2.3 | >25 | Iso | Hyper | NA |
| 8 | L brachial plexus, medial cord | Fusiform** | 1 | 15 | Iso | Hyper | Y |
| 9* | Normal study | – | – | – | – | – | – |
| 10* | R median | Fusiform | 0.7 | 4.5 | NA | NA | Y |
| 11 | L sciatic | Fusiform | 1.5 | 6.5 | NA | NA | Y |
| 12 | R trigeminal at geniculate ganglion | Fusiform | 0.4 | 5 | Iso | Hyper | Y |
| 13 | R sciatic, peroneal division | Fusiform** | 1.8 | 23.5 | Iso | Hyper | NA |
| 14 | L C8, brachial plexus, radial | Fusiform | 1.5 | 9.7 | Iso | Hyper | Y |
| 15 | R brachial plexus, C7, C8, T1 (lower trunk) | Fusiform | 1.2 | 15 | Iso | Hyper | NA |
| 16 | R radial | Fusiform | 0.8 | 12 | Iso | Hyper | Y |
| 17 | R sciatic, tibial and peroneal division | Fusiform | 1 | 32 | Iso | Hyper | Y |
| 18 | L sciatic, tibial division | Fusiform | 1.1 | 5 | Iso | Hyper | Y |
| 19 | L brachial plexus, posterior and medial cord | Fusiform | 1.1 | 14 | Iso | Hyper | Y |
| 20 | R radial | Fusiform** | 0.7 | 3 | Iso | Hyper | Y |
| 21* | R common peroneal | ‘Mass’ | 1 | 3.5 | Iso | Hyper | Y |
| 22 | R ulnar | Fusiform | 0.9 | 6 | Iso | Hyper | Y |
| 23 | L ulnar | Fusiform | 0.9 | 2.5 | Iso | Hyper | NA |
| 24* | Normal study | – | – | – | – | – | – |
| 25* | L median | Fusiform | ‘Increased’ | Iso | Hyper | NA | |
| 26* | Normal study | – | – | – | – | – | – |
| 27 | R sciatic, peroneal division | Fusiform | 1.5 | 6 | Iso | Hyper | NA |
| 28* | L ulnar | ‘Slightly prominent’ | – | – | Iso | Iso | NA |
| 29 | L sciatic, peroneal and tibial division | Fusiform | 1.2 | 6.8 | Iso | Hyper | Y |
| 30 | L ulnar | Fusiform | 1 | 7.8 | Iso | Hyper | Y |
| 31 | L tibial | Fusiform | 1.2 | 7.2 | Iso | Hyper | Y |
| 32 | L L4, L5, S1, sciatic | Fusiform** | 1.5 | 20 | Iso | Hyper | Y |
| Number | 28 | 27 | 27Iso, 0Hyper | 2Iso, 25Hyper | 20Y | ||
| Median | 1.2 | 8.0 | |||||
| Range | 0.4–2.3 | 2.5–32 |
*Films not available for review by the authors.
**individual fascicles were enlarged.
L = left; R = right; Iso = isointense; Hyper = hyperintense; Y = yes; NA = information not available or not done.
Figure 3.
MRI characteristics of perineurioma. Patient 5: (A) 3.0-T axial T1-weighted fast spin echo (FSE) image shows marked enlargement of the individual fascicles of the tibial division of the sciatic nerve in the mid thigh (arrow). The peroneal division is unaffected (arrowhead). (B) Axial T2-weighted FSE image with fat suppression at the same level demonstrates very mild T2 hyperintensity in the tibial division of the sciatic nerve (arrow). (C) Axial T1-weighted spoiled gradient recalled (SPGR) image with fat suppression after contrast administration at the same level shows prominent enhancement of individual fascicles within the tibial division of the nerve (arrow). Patient 3: (D) 1.5-T axial T1-weighted FSE image shows marked enlargement of the femoral nerve (rightmost arrow with respect to the patient), lumbosacral plexus (middle arrow) and the S1 root (left-most arrow with respect to the patient). (E) Axial T2-weighted FSE image shows mild T2 hyperintensity of the nerves (arrows). (F) Axial T1-weighted FSE image after contrast administration shows mild hyperintensity and avid enhancement of the nerves. There is oedema within the atrophied gluteus muscles on the right consistent with a combination of subacute and chronic denervation (asterisk).
Biopsies
All cases had targeted fascicular biopsies of the affected nerve with the exception of two who had whole nerve biopsies (ulnar and trigeminal). In all but the three cases with normal imaging, the biopsies were targeted based on the MRI abnormality. The three cases with normal imaging were targeted based on the localization from the EMG examination. Only one patient reported worsening weakness after subtotal nerve resection (Patient 24).
Clinical examination follow-up
Clinical follow-up assessment was accomplished in 14 patients. Twelve had detailed neurological examination (10 were prospectively evaluated by authors M.L.M and P.J.B.D) with a median time to follow-up of 44.5 months (range 10–247 months) (Table 3). Of the 12 patients who had detailed examinations, the median initial NIS was 12.6 points (range 2–30 points) and at follow-up was 15.4 points (range 1–49 points, P = 0.19). The NSC questionnaire was used to determine if patients reported a change in symptoms over time (10 were performed at clinical follow-up, five were performed at the initial examination and patients compared their symptoms to a prior date). Patients judged their symptoms (median = 4.13, P = 0.01) and their weakness (median = –1, P = 0.04) and sensation (median = –3, P = 0.04) to be mildly worse over time. The DDS was used to determine the disability related to the neuropathy (Dyck et al., 2005). The median score was three, ranging from two to five. This score indicated the presence of neurological symptoms with the patient able to continue with usual acts of daily living. QST was performed in six patients (Table 3). Only four patients had initial and follow-up evaluations. Two patients had developed hyposensitivity to vibration at follow-up (Patients 1 and 14) and two were unchanged but abnormal (Patients 18 and 29).
Table 3.
Clinical follow-up
| Patient | Time to clinical follow-up (mo) | NIS at initial eval (points) | NIS at follow-up eval (points) | Dyck Score | QST | NCS/EMG | CMAP (mV) initial eval | CMAP (mV) follow-up | MRI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 12.25 | 13.75 | 3 | HS vibration | Slight progression | 11.4 | 10.7 | No change |
| 2 | 10 | 12 | 11 | 3 | HA heat pain | Slight progression | 12.8 | 11.2 | No change |
| 3 | 20 | 30 | 32.75 | 4 | No Change | 0 | 0.0 | No change | |
| 4 | 61 | 7 | 10 | 3 | No change | ||||
| 6 | 57 | 20 | 21 | No Change | 0 | 0.0 | No change | ||
| 12 | 77 | 2 | 3.25 | 3 | No change | ||||
| 14 | 41 | 19 | 20.25 | 4 | HS vibration and heat pain | Slight progression | 11 | 9.9 | No change |
| 15 | 247 | 21.5 | 49 | Progression | 8.2 | 0.0 | |||
| 16 | 48 | 6 | 1 | 2 | HS vibration | Improvement | 2.1 | 4.8 | No change |
| 18 | 24 | 22 | 24 | 4 | HS vibration | No Change | 6 | 6.4 | No change |
| 19 | 54 | 12 | 17 | 3 | Improvement | 13 | 16.6 | No change | |
| 25 | 33 | 18 | Slight progression | 0.4 | 0.0 | ||||
| 27 | 56 | 8 | No Change | 0 | 0.0 | ||||
| 29 | 14 | 13 | 12.75 | 5 | HS vibration, HA heat pain | No Change | 9.8 | 9.4 | No change |
| Number | 15 | 15 | 12 | 10 | 6 | 12 | 12 | 12 | 11 |
| Median | 44.5 | 12.6* | 15.4* | 3 | 7.1* | 5.6* | |||
| Range | 10–247 | 2–30 | 1–49 | 2–5 | 0–13 | 0–16.6 |
NIS = neuropathy impairment score; QST = quantitative sensory testing; NCS/EMG = nerve conduction studies and electromyography; CMAP = compound muscle action potential; HS = hyposensitivity; HA = hyperalgesia.
*P-values for change over time not significant using paired t-tests.
NCS/EMG were repeated in 12 patients and showed the same nerves involved as the original studies (sciatic in four, brachial plexus in three, lumbosacral plexus in one, tibial in one, radial in one, median in one and common peroneal in one). They were mildly worse in five (Patients 1, 2, 14, 15 and 25), unchanged in five (Patients 3, 6, 18, 27 and 29) and mildly improved in two (Patients 16 and 19). The median value of the compound muscle action potentials of the affected nerve or plexus demonstrated mild worsening (initial = 7.1 mV, range 0–13 mV; follow-up = 5.6 mV, range 0–16.6 mV) and this change was not significant (P = 0.58). Follow-up MRI was performed in 11 patients with no change in imaging findings compared with their initial exams.
Telephone interview
Follow-up by telephone interview was completed in 23 patients (74%) with a median time from initial evaluation of 36 months (range 2–177 months) (Table 4). The most disabling ongoing symptom was reported as weakness (19 patients), with atrophy (one patient) and pain (two patients) also reported. One patient was now asymptomatic (Patient 16). Ten patients reported no change in symptoms compared to the initial evaluation. Nine patients reported mild worsening, five in weakness, four in atrophy and two in sensation. Four patients reported mild improvement, three in sensation and two in weakness. Twenty-two patients reported focal weakness in the distribution of the affected nerve(s). Seventeen patients reported sensory symptoms, 14 with ‘dead-type numbness’, nine with ‘prickling/tingling’ and seven with pain or using pain medication. Modified Rankin Scale (mRS) was assessed; mRS = 0 (one patient); mRS = 1 (five patients); mRS = 2 (14 patients); mRS = 3 (two patients); mRS = 4 (one patient). Only five of the patients were using adaptive equipment: four wore ankle foot orthoses and one used a wrist brace). All 12 patients who had examination follow-up also had a telephone interview.
Table 4.
Telephone follow-up
| Patient | Time to telephone F/U (mo) | Most disabling symptom | Change in clinical symptoms | Sensory symptoms | Pain or pain meds | Ranking score | Assist device | Surgical outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | Weakness | No change | Tingling | N | 2 | N | |
| 2 | 6 | Weakness | No change | None | N | 2 | Y | |
| 3 | 18 | Weakness | Improvement in sensation | Numbness | N | 4 | Y | |
| 4 | 78 | Weakness | No change | Numbness and prickling | Y | 2 | N | |
| 5 | 6 | Weakness | No change | None | N | 2 | N | |
| 6 | 177 | Weakness | Improvement in stabbing sensation | Numbness, pricking | N | 1 | N | |
| 7 | 88 | Weakness | Mild increased weakness and atrophy | Numbness | N | 2 | Y | |
| 8 | 53 | Weakness | Mild increased weakness | None | N | 2 | N | TT—worsened |
| 9 | 168 | Weakness | Increased weakness and atrophy | Numbness | N | 2 | N | |
| 11 | 118 | Weakness | No change | Numbness | N | 2 | N | TT—Improved |
| 12 | 71 | Dry eyes > facial pain | No change | Burning, prickling, tingling | Y | 1 | N | |
| 13 | 45 | Weakness | Increased numbness | Numbness, tingling | Y | 2 | N | |
| 14 | 37 | Weakness | Increased weakness | None | Y | 2 | Y | |
| 15 | 36 | Weakness | Worsened temperature discrimination | Numbness and prickling | N | 2 | N | TT—unchanged |
| 16 | 27 | No symptoms | Improvement in weakness and numbness | None | N | 0 | N | TT—improved |
| 17 | 24 | Atrophy | Increased atrophy | Numbness | N | 2 | N | |
| 18 | 18 | Weakness | No change | None | N | 1 | N | |
| 19 | 34 | Weakness | Increased atrophy | Tingling | N | 3 | N | |
| 21 | 135 | Weakness | Improvement in weakness | Numbness and hyperalgesia | N | 1 | N | NG, TT—improved |
| 22 | 127 | Weakness | No change | Numbness and prickling | N | 1 | N | NG, TT—improved |
| 29 | 2 | Pain | No change | Numbness | Y | 3 | Y | TT—unchanged |
| 30 | 8 | Weakness | Mild increased weakness | Numbness and tingling | Y | 2 | N | |
| 31 | 6 | Weakness | No change | Numbness | Y | 2 | N | |
| Number | 23 | 19 weak | 17Y, 6N | 7Y, 16N | 23 | 5Y, 18N | 7 | |
| Median | 36 | 2 | ||||||
| Range | 2–177 | 0–4 |
F/U = follow-up; N = no; Y = yes; TT = tendon transfer; NG = nerve graft.
Surgical treatment
Thirteen patients had additional surgical interventions to treat their neurological deficits. Eight patients had various tendon transfers; three had tendon transfers in addition to resection of the lesion and nerve grafting; and two had lesion resection with nerve grafting only. Seven of these patients were contacted in telephone follow-up (five with tendon transfers alone and two with tendon transfers and lesion resection with nerve grafting) (Table 4). Of these seven, four patients felt the surgery had improved their strength and function (Patients 11, 16, 21 and 22). Two had initial improvement followed by progression of weakness such that there was no perceived improvement after several months (Patients 15 and 29). One patient demonstrated arthrofibrosis after the tendon transfers and despite additional surgery and aggressive physical therapy reported increased stiffness and joint contractures and was dissatisfied with the functional outcome (Patient 8). Of the other six patients in whom there was no telephone interview, postoperative follow-up was variable and often relatively short. One of the patients who underwent resection of a radial nerve lesion and nerve grafting (Patient 24) had worsening of her wrist and finger extension immediately postoperatively; gradual improvement (though not to baseline) was noted but clinical follow-up was limited to 6 months.
Representative cases
Case 1—Patient 2 (Fig. 4A and B)
Figure 4.
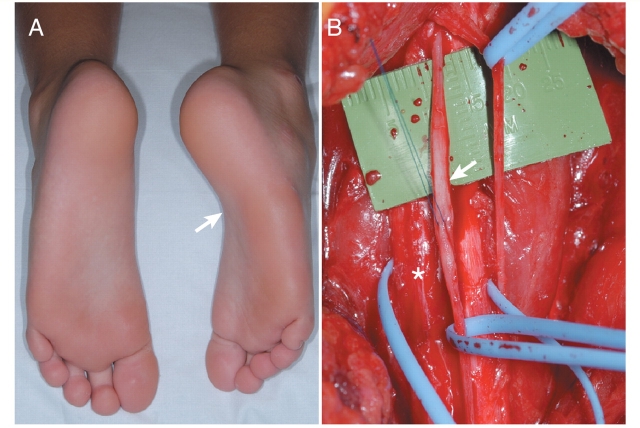
Patient 2. (A) Photograph of the feet demonstrating atrophy and inversion of the affected foot due to chronic sciatic neuropathy (arrow). (B) Intra-operative photograph demonstrating focal fusiform enlargement of the tibial division (arrow) of the sciatic nerve (asterisk).
A 7-year-old boy presented with inward deviation of his right foot at age 6. He developed gradually progressive painless weakness of the right foot with associated atrophy requiring bracing. He had no complaints of sensory loss but developed painless skin breakdown on the right foot. There was occasional tingling on the lateral aspect of the right lower leg and the sole of his foot. Examination demonstrated right calf and foot (Fig. 4) atrophy with profound weakness in peroneal and tibial innervated muscles. There was subtle sensory loss to light touch, pinprick and temperature on the dorsum of the right foot and a reduced right ankle reflex. NCS/EMG demonstrated a long-standing, severe right sciatic neuropathy in the thigh, with greater involvement of the peroneal division. The right sural sensory response was reduced. MRI demonstrated fusiform enlargement of the right sciatic nerve beginning at the ischial tuberosity and extending 12 cm with T2 hyperintensity and enhancement of individual fascicles with contrast. Laboratory and CSF examination were normal. MRI targeted fascicular nerve biopsy showed focal fusiform enlargement of involved fascicles (Fig. 4). Fascicles from both the peroneal and tibial divisions of the sciatic nerve were taken and demonstrated typical pathological findings of IN perineurioma (pseudo-onion bulbs reactive to EMA). At telephone interview, 6 months after the initial evaluation, there was no change in symptoms. Modified Rankin scale was 2 and he continued to use an ankle foot orthosis. Clinical follow-up 10 months after evaluation demonstrated a change in NIS from 12 to 11 points. DDS was 3. QST demonstrated hyperalgesia to heat pain on the right foot. NCS/EMG demonstrated slight progression and MRI was unchanged.
Case 2—Patient 3 (Figs 2A and B, 3D, E and F)
A 2-year-old girl was the product of a full-term pregnancy without complications. Her mother noted she never crawled well and tended to drag her right leg. She began walking at 12 months and limped on the right. At 18 months of age, her parents noted asymmetry in the size of her lower extremities. At 23 months, she was unable to lift her right knee and developed a right foot drop. Laboratory and CSF examinations were normal. Examination demonstrated proximal and distal right lower limb weakness, an absent right ankle reflex, and decreased sensation of the right lower limb. NCS/EMG demonstrated bilateral lumbosacral plexopathies, right greater than left. MRI examination demonstrated markedly enlarged right L5–S4 nerve roots, femoral and sciatic nerves (Fig. 3). Abnormalities were present in the left sided nerves but were much milder. There was marked denervation atrophy of right gluteal muscles. MRI targeted fascicular nerve biopsy of the tibial division of the right sciatic nerve disclosed the typical pathological features of IN perineurioma (Fig. 2). At telephone interview 18 months later, her mother reported that she continued to have profound lower limb weakness but had improvement in sensation. Modified Rankin scale was 4 and she required a right lower limb brace. Clinical follow-up 20 months later demonstrated a mild worsening of NIS from 30 to 32.75 points. DDS was 4 as her mother had to assist her with motor activities that she should normally be able to do by age 4. There was no change in NCS/EMG or MRI findings. At age 5 years (37-month follow-up), she began to have occasional (approximately once per week) episodes of urinary incontinence at school.
Discussion
This study describing the typical clinical presentation, distribution, electrophysiological profile, MRI findings and long-term outcome of patients with IN perineurioma was possible because we were able to identify a large series. Two-thirds (n = 21, 66%) of these patients were personally evaluated by the authors both initially and in follow-up. There have been individual case reports and very small series (four or less patients) describing the clinical features of IN perineurioma (Imaginariojda et al., 1964; Lallemand and Weller, 1973; Snyder et al., 1977; Mitsumoto et al., 1980; de los Reyes et al., 1981; Peckham et al., 1982; Bilbao et al., 1984; Boker et al., 1984; Tranmer et al., 1986; Iyer et al., 1988; Phillips et al., 1991; Gullotta, 1992; Mitsumoto et al., 1992; Simmons et al., 1999; Jazayeri et al., 2000; Heilbrun et al., 2001; Hamazaki et al., 2004; Huguet et al., 2004; Rankine et al., 2004; Boyanton et al., 2007; Hahn et al., 2007; Nguyen et al., 2007), but there is little information on long-term follow-up data. The large series reported here is important because despite these reports IN perineurioma remains under-recognized by neurologists. This is probably due to the rarity of the condition, physicians’ unfamiliarity with it and its requirement of specialized techniques for diagnosis. Furthermore, until this series, the typical electrophysiologic and radiographic features of this condition have been unknown.
Our study confirms that IN perineurioma is a disease of childhood and young adulthood as has been found in other smaller series (Emory et al., 1995; Boyanton et al., 2007), but also shows that middle aged and older patients can occasionally present with IN perineurioma. Our series also verified the lack of gender predominance in this disorder. Despite other series reporting a predominance of upper limb nerve involvement (Boyanton et al., 2007), especially the posterior interosseus or radial nerve (Imaginariojda et al., 1964; Hawkes et al., 1974; Boker et al., 1984; Isaac et al., 2004; Cortes et al., 2005; Nguyen et al., 2007), our series demonstrates that IN perineurioma presents equally in upper and lower limb nerves, but most commonly in the sciatic nerve or its branches. The location of the IN perineurioma was always focal but it is not always a mononeuropathy. In approximately one-sixth of cases, more than one nerve was involved and they were plexus neuropathies. In one severe case presenting at a very young age multiple lower limb nerves were involved bilaterally (Case 2, Patient 3).
Prior series have emphasized the motor predominance of the presentation (Emory et al., 1995; Cortes et al., 2005; Boyanton et al., 2007). While our series supports this finding, we stress that sensory symptoms, impairments and test abnormalities are almost always present and are important to recognize as they can aid in the characterization and recognition of this disorder. Other studies have not quantified the sensory involvement or the types of sensory fibres involved. We found that there are symptoms and signs of both small and large sensory fibre dysfunction that are confined to the focal area of nerve involvement on nerve conduction and QST. Pain has been previously reported to be uncommon by others (Alfonso et al., 2001), but in our series 13 of 32 (41%) patients described painful symptoms. Overall the sensory symptoms are common but are mild in severity.
Prior to the identification of the reactivity to EMA, IN perineurioma was initially thought to be a reactive process, possibly related to trauma (Johnson and Kline, 1989; Mitsumoto et al., 1992; Tsang et al., 1992). Our study confirms that the development of IN perineurioma rarely follows a traumatic event. Only four patients in our series recalled any prior trauma, and these were mild and may not have even involved the affected limb (Patients 9, 13, 15 and 18). None of the tumours occurred at typical sites of compression. Perhaps the strongest evidence against a reaction to trauma as the cause of IN perineurioma, are the young children (such as Patient 3) without a history of birth injury or childhood trauma who develop IN perineuriomas. Our series also helps to confirm that IN perineurioma is a benign peripheral nerve tumour and is not part of a more systemic disorder. The family history in all of our patients was unremarkable. Three patients had one café au lait spot without other signs of neurofibromatosis.
The location of the perineurioma was suspected by clinical examination and further defined by NCS/EMG. However because many of the patients’ tumours were in proximal locations inaccessible by NCS/EMG, MRI is a necessary tool to further delineate the site of abnormality. Many of the patients with reportedly normal outside imaging were found to have MRI abnormalities on our examination. We suspect that the three normal studies, with modern MRI techniques, would have shown abnormalities. Our findings and the findings of others (Simmons et al., 1999; Takao et al., 1999; Heilbrun et al., 2001) suggest that IN perineurioma has a very stereotypical finding on MRI—it has a fusiform enlargement, is isointense on T1, hyperintense on T2-weighted images and shows avid enhancement with gadolinium. Our study confirms the need for a high-field (3.0 T) MRI with specific coils designed to image specific nerves and a skilled peripheral nerve radiologist. One patient (Patient 4) with very subtle imaging findings had a normal 1.5-T MRI but an abnormal 3.0 T MRI that was detected by a peripheral nerve radiologist (K.K.A.) (Hahn et al., 2007).
The differential diagnosis of IN perineurioma is broad and includes other benign nerve tumours such as neurofibroma, schwannoma, angiomyofibromas, perhaps inherited hypertrophic neuropathy, as well as acquired processes such as injury neuroma, focal inflammatory demyelination [focal chronic inflammatory demyelinating polyneuropathy (CIDP)], sarcoidosis, leukaemia and lymphoma. Often the most important differentiation is between IN perineurioma and chronic demyelinating mononeuropathy or focal CIDP. The CSF protein is almost always normal in IN perineurioma (only 2 of 11 patients with CSF examination had a borderline elevated protein) and is helpful in differentiating it from CIDP in which CSF protein is usually elevated (Dyck et al., 1975). The MRI findings may also be helpful in differentiating these entities, as CIDP does not usually demonstrate enhancement with gadolinium administration. At this time, fascicular nerve biopsy is the only definitive method for differentiating these focal mononeuropathies.
Prior to our study, the knowledge about the long-term course of patients with IN perineurioma was limited. The series of four patients by Simmons et al. (1999) had a median follow-up time of 8 months. In a literature review of 51 previously published cases of definitive IN perineurioma, the follow-up of 35 cases ranged from 1 to 72 months, with a median of 12 months (Boyanton et al., 2007). No patients demonstrated tumour recurrence or metastatic disease. Although our series is mostly a retrospective analysis, a standard neurologic examination scoring method allows for calculation of an NIS. Furthermore prospective clinical follow-up examination in 12 patients over 3.5 years demonstrated very mild slow progression of motor and sensory deficits. Also none of these patients had the tumour spread to nerves not initially involved. This slow progression was confirmed by telephone interview, examination (NIS), NCS/EMG and MRI follow-up (Tables 3 and 4). We have found no cases where there was transformation to a malignant tumour.
Although IN perineurioma is a benign peripheral nerve tumour, it does cause problematic morbidity. The median NIS score in our series at the time of presentation was 12.0 points which correlates to a moderate neurologic deficit given the focal nature of these tumours. At telephone follow-up, most of our patients had a mRS of at least 2 which corresponds to slight disability meaning patients are unable to carry out all previous activities, but able to look after own affairs without assistance. This was supported by the DDS at clinical follow-up where 4 of 10 patients had a score of 4 or more (unequivocal limitation of usual work, acts of daily living, recreational activity or family and social obligations).
In our current practice, targeted fascicular nerve biopsy at the site of MRI lesion is being performed as the mainstay of diagnosis. This technique allows for a much more focused nerve biopsy with the ability to minimize scarring, reduce surgical deficit and remove a portion of the nerve most likely to demonstrate pathology. When a definitive diagnosis of IN perineurioma is established, we then consider performing other surgical interventions as a second stage procedure. Some surgeons have suggested resection of the lesion with interpositional nerve grafting when the intra-operative nerve action potential recording is absent or low amplitude (Gruen et al., 1998). We believe that this approach may be considered with focal lesions in more distal locations that are not associated with long-term muscle atrophy. In our experience the majority of our cases would not meet these criteria and so nerve resection should usually not be performed. In addition, a surgeon would risk further iatrogenic deficit with nerve grafting, as was seen in one of our patients. A theoretical advantage of resecting the lesion in its entirety would be for prevention of longitudinal spread. In our series, we found no such cases in which tumour spread to initially uninvolved nerves on surveillance MRIs, including one patient who was followed for 20 years and the distribution remained confined. Still, we recognize the need for longer follow-up examination to definitively answer this question. Other surgical options [i.e. tendon transfers or distal nerve transfers (distal to the site of the IN perineurioma)] could be considered when donors are available. In these cases, there should be evidence of static disease, although that time frame needs to be defined with longer clinical follow-up.
In conclusion, IN perineurioma is probably an under-diagnosed focal neuropathy because biopsy from proximal mixed nerves is required to make the diagnosis. Many of these cases are probably labelled as idiopathic and no further evaluation is done. Through the use of a multi-disciplinary practice with experts in peripheral nerve care, electrophysiology, peripheral nerve imaging, peripheral nerve surgery and peripheral nerve pathology, many more cases can now be diagnosed. Consequently through use of MRI targeted fascicular nerve biopsy, IN perineurioma is now a relatively common diagnosis in our clinical practice. We have been able to do these procedures with relatively little surgical morbidity and a high diagnostic yield. Our study demonstrates that IN perineurioma presents with a slowly progressive, motor predominant focal neuropathy or plexopathy with mild sensory symptoms and signs. MRI of the focal nerve lesions demonstrates T2 hyperintensity and avid contrast enhancement. At this point, we believe that most of these patients should receive a targeted fascicular nerve biopsy from the MRI lesion at a centre that has expertise in peripheral nerve care. However, in cases with nerve lesions that are difficult to access without major invasive surgery, it may be reasonable to follow clinically with imaging to verify clinical stability.
Funding
This research was published with support from NS36979 and Mayo Funds.
Glossary
Abbreviations
- CIDP
Chronic inflammatory demyelinating polyneuropathy
- CSF
cerebrospinal fluid
- DDS
Dyck disability score
- EMA
epithelial membrane antigen
- EMG
electromyography
- IN
intraneural
- IRB
institutional review board
- MRI
magnetic resonance imaging
- mRS
modified Rankin scale
- NCS
nerve conduction studies
- NIS
Neuropathy Impairment Score
- NSC
Neuropathy Symptoms and Change
- QST
quantitative sensory testing
- SNAP
sensory nerve action potential
References
- Alfonso DT, Sotrel A, Grossman JA. Carpal tunnel syndrome due to an intraneural perineurioma in a 2-year-old child. J Hand Surg [Br] 2001;26:168–70. doi: 10.1054/jhsb.2000.0528. [DOI] [PubMed] [Google Scholar]
- Ariza A, Bilbao JM, Rosai J. Immunohistochemical detection of epithelial membrane antigen in normal perineurial cells and perineurioma. Am J Surg Pathol. 1988;12:678–83. doi: 10.1097/00000478-198809000-00004. [DOI] [PubMed] [Google Scholar]
- Bilbao JM, Khoury NJ, Hudson AR, Briggs SJ. Perineurioma (localized hypertrophic neuropathy) Arch Pathol Lab Med. 1984;108:557–60. [PubMed] [Google Scholar]
- Boker DK, Schonberg F, Gullotta F. Localized hypertrophic neuropathy—a rare, clinically almost unknown syndrome. Clin Neuropathol. 1984;3:228–30. [PubMed] [Google Scholar]
- Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- Boyanton BL, Jr, Jones JK, Shenaq SM, Hicks MJ, Bhattacharjee MB. Intraneural perineurioma: a systematic review with illustrative cases. Arch Pathol Lab Med. 2007;131:1382–92. doi: 10.5858/2007-131-1382-IPASRW. [DOI] [PubMed] [Google Scholar]
- Cortes W, Cheng J, Matloub HS. Intraneural perineurioma of the radial nerve in a child. J Hand Surg [Am] 2005;30:820–5. doi: 10.1016/j.jhsa.2005.02.018. [DOI] [PubMed] [Google Scholar]
- de los Reyes RA, Chason JL, Rogers JS, Ausman JI. Hypertrophic neurofibrosis with onion bulb formation in an isolated element of the brachial plexus. Neurosurgery. 1981;8:397–9. doi: 10.1227/00006123-198103000-00013. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Hughes RAC, O'Brien PC. Quantitating overall neuropathic symptoms, impairments and outcomes. Philadelphia: Elsevier; 2005. [Google Scholar]
- Dyck PJ, Karnes J, O’Brien PC, Zimmerman IR. Detection thresholds of cutaneous sensation in humans. Philadelphia: W.B. Saunders; 1984. [Google Scholar]
- Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic inflammatory polyradiculoneuropathy. Mayo Clin Proc. 1975;50:621–37. [PubMed] [Google Scholar]
- Dyck PJ, Sherman WR, Hallcher LM, Service FJ, O’Brien PC, Grina LA, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8:590–6. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Turner DW, Davies JL, O’Brien PC, Dyck PJB, Rask CA. Electronic case-report forms of symptoms and impairments of peripheral neuropathy. Can J Neurol Sci. 2002;29:258–66. doi: 10.1017/s0317167100002043. [DOI] [PubMed] [Google Scholar]
- Emory TS, Scheithauer BW, Hirose T, Wood M, Onofrio BM, Jenkins RB. Intraneural perineurioma. A clonal neoplasm associated with abnormalities of chromosome. Am J Clin Pathol. 1995;103:696–704. doi: 10.1093/ajcp/103.6.696. [DOI] [PubMed] [Google Scholar]
- Gruen JP, Mitchell W, Kline DG. Resection and graft repair for localized hypertrophic neuropathy. Neurosurgery. 1998;43:78–83. doi: 10.1097/00006123-199807000-00051. [DOI] [PubMed] [Google Scholar]
- Gullotta F. Localized hypertrophic neuropathy (LHN) Pathol Res Pract. 1992;188:815–6. doi: 10.1016/s0344-0338(11)80189-5. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Mauermann ML, Dyck PJB, Keegan BM. A 16-year-old girl with progressive weakness of the left leg. Neurology. 2007;69:84–90. doi: 10.1212/01.wnl.0000267406.82078.8f. [DOI] [PubMed] [Google Scholar]
- Hamazaki S, Fujiwara K, Okada S. Intraneural perineurioma involving the ulnar nerve. Pathol Int. 2004;54:371–5. doi: 10.1111/j.1440-1827.2004.01634.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Jefferson JM, Jones EL, Thomas Smith W. Hypertrophic mononeuropathy. J Neurol Neurosurg Psychiatry. 1974;37:76–81. doi: 10.1136/jnnp.37.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbrun ME, Tsuruda JS, Townsend JJ, Heilbrun MP. Intraneural perineurioma of the common peroneal nerve. Case report and review of the literature. J Neurosurg. 2001;94:811–5. doi: 10.3171/jns.2001.94.5.0811. [DOI] [PubMed] [Google Scholar]
- Huguet P, de la Torre J, Pallares J, Carrera M, Soler F, Espinet B, et al. Intraosseous intraneural perineurioma: report of a case with morphological, immunohistochemical and FISH study. Med Oral. 2004;9:64–8. [PubMed] [Google Scholar]
- Imaginariojda G, Coelho B, Tome F, Luis ML. [Monosymptomatic interstitial hypertrophic neuritis] J Neurol Sci. 1964;64:340–7. [PubMed] [Google Scholar]
- Isaac S, Athanasou NA, Pike M, Burge PD. Radial nerve palsy owing to localized hypertrophic neuropathy (intraneural perineurioma) in early childhood. J Child Neurol. 2004;19:71–5. doi: 10.1177/08830738040190010711. [DOI] [PubMed] [Google Scholar]
- Iyer VG, Garretson HD, Byrd RP, Reiss SJ. Localized hypertrophic mononeuropathy involving the tibial nerve. Neurosurgery. 1988;23:218–21. doi: 10.1227/00006123-198808000-00015. [DOI] [PubMed] [Google Scholar]
- Jazayeri MA, Robinson JH, Legolvan DP. Intraneural perineurioma involving the median nerve. Plast Reconstr Surg. 2000;105:2089–91. doi: 10.1097/00006534-200005000-00026. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Kline DG. Localized hypertrophic neuropathy: possible focal perineurial barrier defect. Acta Neuropathol (Berl) 1989;77:514–8. doi: 10.1007/BF00687253. [DOI] [PubMed] [Google Scholar]
- Lallemand RC, Weller RO. Intraneural neurofibromas involving the posterior interosseous nerve. J Neurol Neurosurg Psychiatry. 1973;36:991–6. doi: 10.1136/jnnp.36.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA. Autonomic nervous system function. J Clin Neurophysiol. 1993;10:14–27. doi: 10.1097/00004691-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Macarenco RS, Ellinger F, Oliveira AM. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625–36. doi: 10.5858/2007-131-625-PADAUP. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Estes ML, Wilbourn AJ, Culver JE., Jr Perineurial cell hypertrophic mononeuropathy manifesting as carpal tunnel syndrome. Muscle Nerve. 1992;15:1364–8. doi: 10.1002/mus.880151212. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Wilbourn AJ, Goren H. Perineurioma as the cause of localized hypertrophic neuropathy. Muscle Nerve. 1980;3:403–12. doi: 10.1002/mus.880030504. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Dyck PJB, Daube JR. Intraneural perineurioma of the radial nerve visualized by 3.0 Tesla MRI. Muscle Nerve. 2007;36:715–20. doi: 10.1002/mus.20795. [DOI] [PubMed] [Google Scholar]
- Peckham NH, O'Boynick PL, Meneses A, Kepes JJ. Hypertrophic mononeuropathy. A report of two cases and review of the literature. Arch Pathol Lab Med. 1982;106:534–7. [PubMed] [Google Scholar]
- Phillips LH, 2nd, Persing JA, Vandenberg SR. Electrophysiological findings in localized hypertrophic mononeuropathy. Muscle Nerve. 1991;14:335–41. doi: 10.1002/mus.880140408. [DOI] [PubMed] [Google Scholar]
- Rankine AJ, Filion PR, Platten MA, Spagnolo DV. Perineurioma: a clinicopathological study of eight cases. Pathology. 2004;36:309–15. doi: 10.1080/00313020410001721663. [DOI] [PubMed] [Google Scholar]
- Simmons Z, Mahadeen ZI, Kothari MJ, Powers S, Wise S, Towfighi J. Localized hypertrophic neuropathy: magnetic resonance imaging findings and long-term follow-up. Muscle Nerve. 1999;22:28–36. doi: 10.1002/(sici)1097-4598(199901)22:1<28::aid-mus6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Simpson DA, Fowler M. Two cases of localized hypertrophic neurofibrosis. J Neurol Neurosurg Psychiatry. 1966;29:80–4. doi: 10.1136/jnnp.29.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Cancilla PA, Batzdorf U. Hypertrophic neuropathy simulating a neoplasm of the brachial plexus. Surg Neurol. 1977;7:131–4. [PubMed] [Google Scholar]
- Stanton C, Perentes E, Phillips L, VandenBerg SR. The immunohistochemical demonstration of early perineurial change in the development of localized hypertrophic neuropathy. Hum Pathol. 1988;19:1455–7. doi: 10.1016/s0046-8177(88)80239-9. [DOI] [PubMed] [Google Scholar]
- Suarez GA, Giannini C, Smith BE, Windebank AJ, Litchy WJ, Dyck PJ. Localized hypertrophic neuropathy. Mayo Clin Proc. 1994;69:747–8. doi: 10.1016/s0025-6196(12)61093-3. [DOI] [PubMed] [Google Scholar]
- Takao M, Fukuuchi Y, Koto A, Tanaka K, Momoshima S, Kuramochi S, et al. Localized hypertrophic mononeuropathy involving the femoral nerve. Neurology. 1999;52:389–92. doi: 10.1212/wnl.52.2.389. [DOI] [PubMed] [Google Scholar]
- Tranmer BI, Bilbao JM, Hudson AR. Perineurioma: a benign peripheral nerve tumor. Neurosurgery. 1986;19:134–8. doi: 10.1227/00006123-198607000-00024. [DOI] [PubMed] [Google Scholar]
- Tsang WY, Chan JK, Chow LT, Tse CC. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756–63. doi: 10.1097/00000478-199208000-00003. [DOI] [PubMed] [Google Scholar]