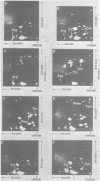
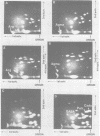
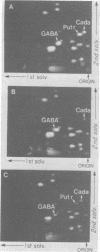
Abstract
A specific procedure has been developed for the detection of the first two enzymes involved in the arginine dihydrolase system and the detection of the decarboxylases of arginine, glutamic acid, histidine, lysine, ornithine, phenylalanine, tryptophan, and tyrosine. A loopful of growth of each organism from dihydrolase-decarboxylase induction agar medium (or broth) was washed and incubated separately with 0.2-ml samples of three test media supplemented with different amino acids. Each spent test medium was dansylated, and the dansyl derivatives were separated by two-dimensional thin-layer chromatography on polyamide sheets. The end products (citrulline, ornithine, gamma-amino-n-butyric acid, and amines) produced during incubation were estimated by comparing the fluorescent intensities of end products from the spent test media and of the corresponding parent amino acids from test medium controls after thin-layer chromatography. The method is reproducible, requiring incubation of an organism in three test media for 1 h for simultaneous detection of the first two enzymes involved in the arginine dihydrolase system and of eight amino acid decarboxylases. This method has been successfully applied to gram-positive and gram-negative microorganisms and also to Mycoplasmatales. It could simplify and improve the accuracy of the corresponding biochemical tests performed in clinical laboratories for the identification and differentiation of microorganisms, and it may prove particularly useful for the differentiation of species of Pseudomonas and Mycoplasma.
Full text
PDF

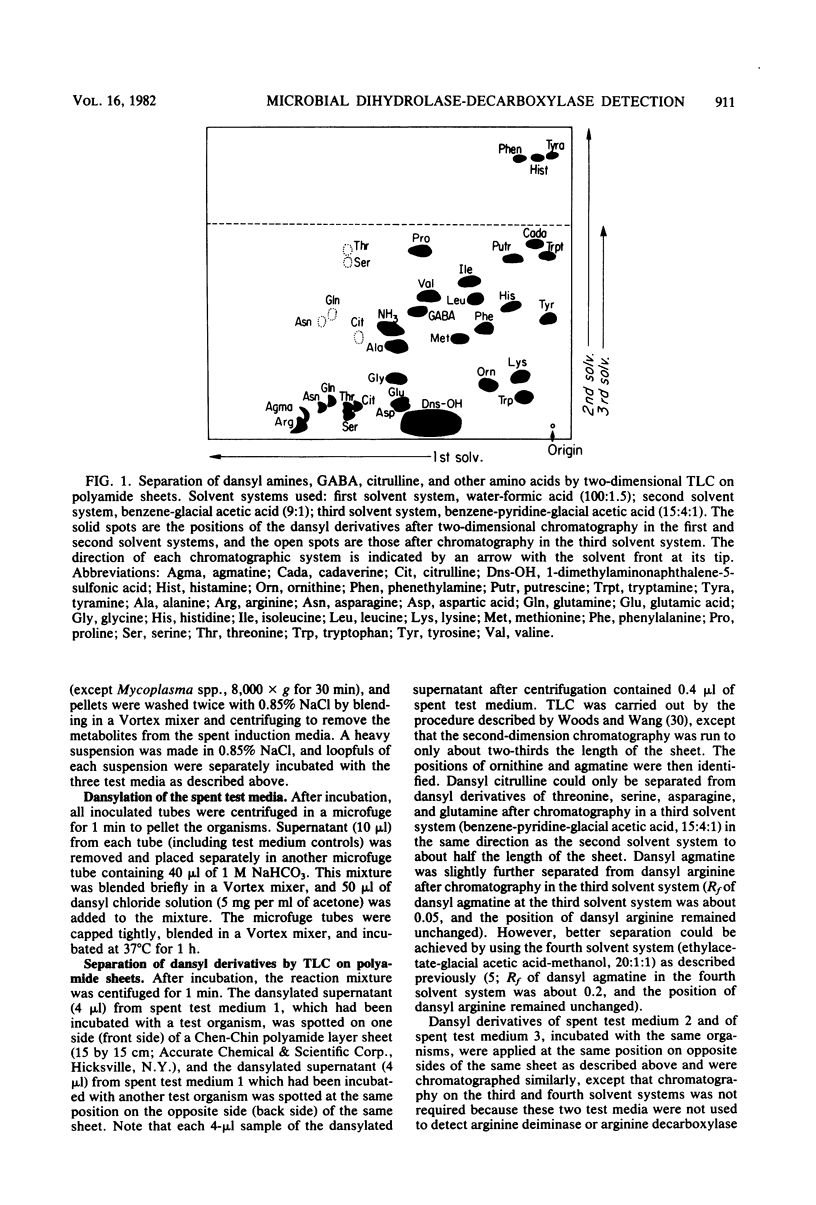
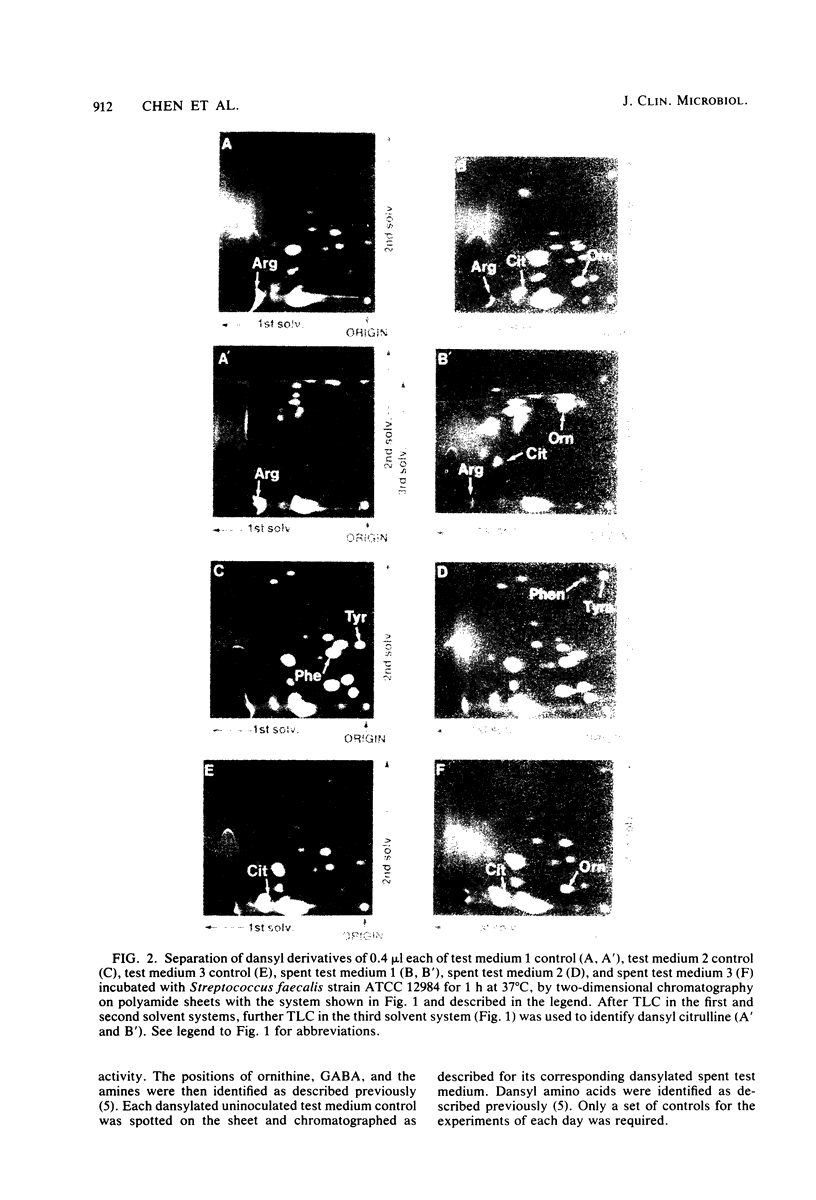
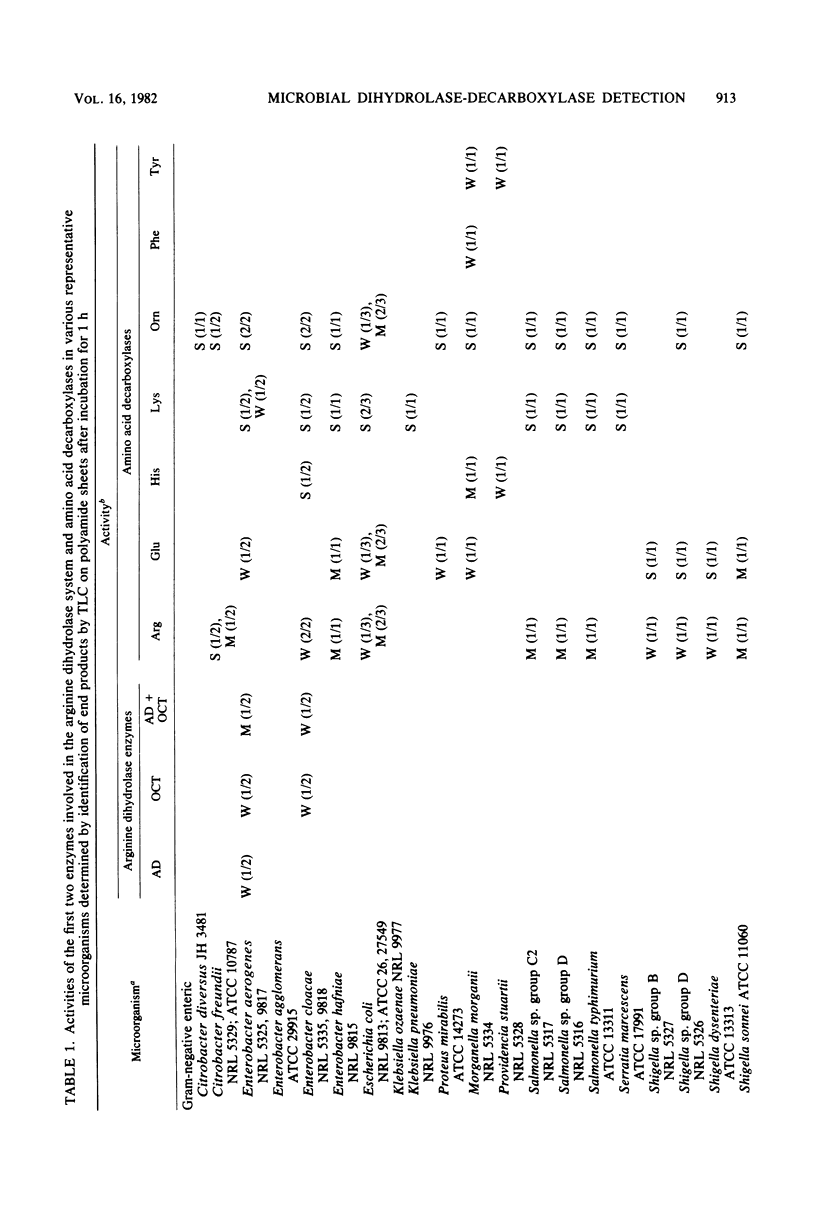
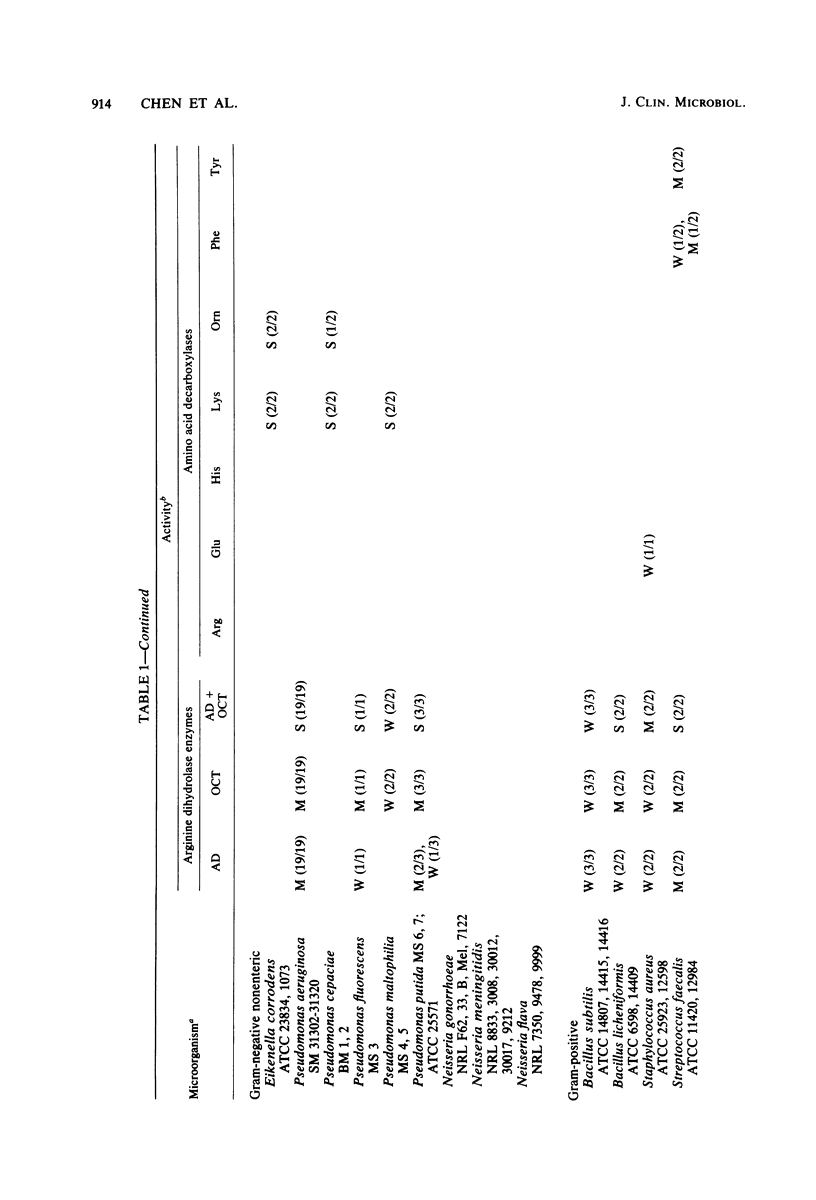

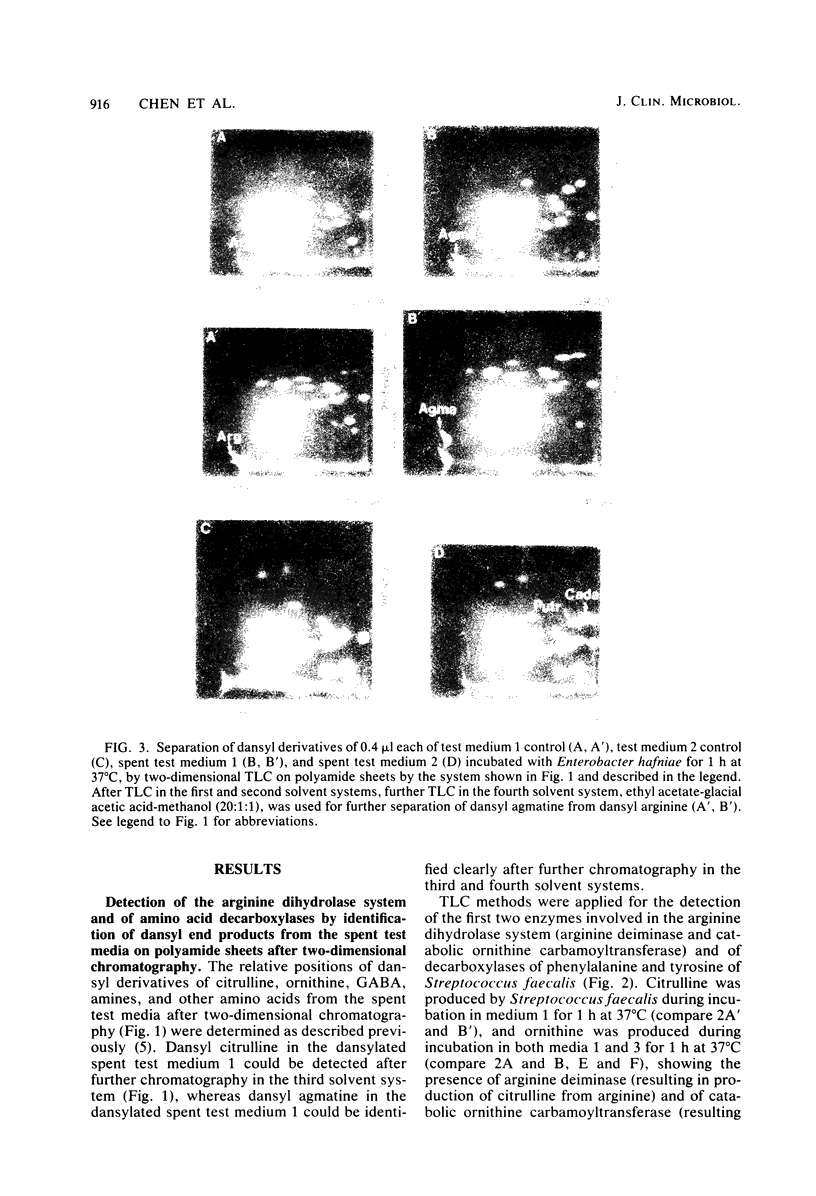
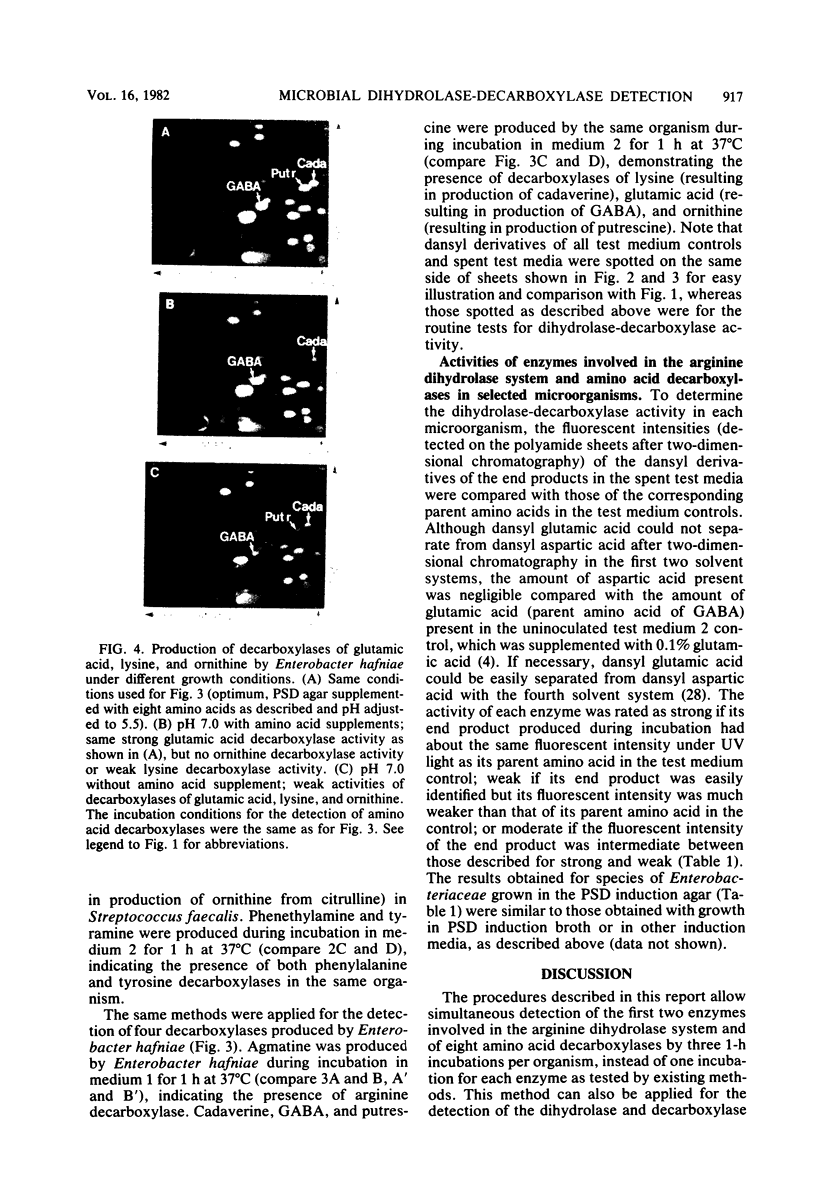


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Buchanan T. M. Hydrolases from Neisseria gonorrhoeae. The study of gonocosin, an aminopeptidase-P, a proline iminopeptidase, and an asparaginase. J Biol Chem. 1980 Feb 25;255(4):1704–1710. [PubMed] [Google Scholar]
- Chen K. C., Forsyth P. S., Buchanan T. M., Holmes K. K. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J Clin Invest. 1979 May;63(5):828–835. doi: 10.1172/JCI109382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. C., Holmes K. K. A rapid procedure for detection of bacterial amino acid decarboxylases. Anal Biochem. 1981 Feb;111(1):60–66. doi: 10.1016/0003-2697(81)90228-1. [DOI] [PubMed] [Google Scholar]
- Dunkelberg W. E., Jr, Skaggs R., Kellogg D. S., Jr Method for isolation and identification of Corynebacterium vaginale (Haemophilus vaginalis). Appl Microbiol. 1970 Jan;19(1):47–52. doi: 10.1128/am.19.1.47-52.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L. Characterization of the oxidase-negative, gram-negative coccobacilli (the Achromobacter-acinetobacter group). Antonie Van Leeuwenhoek. 1969;35(4):421–429. doi: 10.1007/BF02219161. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M. C., Lockhart B. M., Perry K. Rapid methods for determining decarboxylase activity: ornithine and lysine decarboxylases. Appl Microbiol. 1971 Sep;22(3):344–349. doi: 10.1128/am.22.3.344-349.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt M. C., Lockhart B. M. Rapid methods for determining decarboxylase activity: arginine decarboxylase. Appl Microbiol. 1971 Sep;22(3):350–357. doi: 10.1128/am.22.3.350-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman F. W., Framer J. J., 3rd, Steigerwalt A. G., Brenner D. J. Unusual groups of Morganella ("Proteus") morganii isolated from clinical specimens: lysine-positive and ornithine-negative biogroups. J Clin Microbiol. 1980 Jul;12(1):88–94. doi: 10.1128/jcm.12.1.88-94.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Effect of urea concentration on growth of Ureaplasma urealyticum (T-strain mycoplasma). J Bacteriol. 1977 Oct;132(1):144–150. doi: 10.1128/jb.132.1.144-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E. Heat-lability and organic solvent-solubility of mycoplasma antigens. Ann N Y Acad Sci. 1967 Jul 28;143(1):676–681. doi: 10.1111/j.1749-6632.1967.tb27713.x. [DOI] [PubMed] [Google Scholar]
- Lambert M. A., Moss C. W. Use of gas chromatography for detecting ornithine and lysine decarboxylase activity in bacteria. Appl Microbiol. 1973 Oct;26(4):517–520. doi: 10.1128/am.26.4.517-520.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccani J. E. Aerobically incubated medium for decarboxylase testing of Enterobacteriaceae by replica-plating methods. J Clin Microbiol. 1979 Dec;10(6):940–942. doi: 10.1128/jcm.10.6.940-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Cherry W. B. Use of gas chromatography for determining catabolic products of arginine by bacteria. Appl Microbiol. 1972 May;23(5):889–893. doi: 10.1128/am.23.5.889-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MØLLER V. Simplified tests for some amino acid decarboxylases and for the arginine dihydrolase system. Acta Pathol Microbiol Scand. 1955;36(2):158–172. doi: 10.1111/j.1699-0463.1955.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Nord C-E, Linberg A. A., Dahlbäck A. Four hour-test for the identification of Enterobacteriaceae. Med Microbiol Immunol. 1975 Sep 19;161(4):231–238. doi: 10.1007/BF02122710. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Arginine dihydrolase activity in species of the genus Bacillus revealed by thin-layer chromatography. J Gen Microbiol. 1974 Sep;84(1):209–213. doi: 10.1099/00221287-84-1-209. [DOI] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M., Stanier R. Y., Solánes R. E., Mandel M. Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. J Gen Microbiol. 1970 Feb;60(2):215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- SMITH P. F. Amino acid metabolism of PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:543–550. doi: 10.1111/j.1749-6632.1960.tb42721.x. [DOI] [PubMed] [Google Scholar]
- Smith H. L., Jr, Bhat-Fernandes P. Modified decarboxylase-dihydrolase medium. Appl Microbiol. 1973 Oct;26(4):620–621. doi: 10.1128/am.26.4.620-621.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. J., WHITBY J. L. PSEUDOMONAS PYOCYANEA AND THE ARGININE DIHYDROLASE SYSTEM. J Clin Pathol. 1964 Mar;17:122–125. doi: 10.1136/jcp.17.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Williams G. A., Blazevic D. J., Ederer G. M. Detection of arginine dihydrolase in nonfermentative gram-negative bacteria by use of thin-layer chromatography. Appl Microbiol. 1971 Dec;22(6):1135–1137. doi: 10.1128/am.22.6.1135-1137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zolg W., Ottow J. C. Improved thin-layer technique for detection of arginine dihydrolase among the Pseudomonas species. Appl Microbiol. 1973 Dec;26(6):1001–1003. doi: 10.1128/am.26.6.1001-1003.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolg W., Ottow J. C. Thin-layer chromatography of arginine, lysine and ornithine decarboxylase activity among Pseudomonas spp. and Enterobacteriaceae. Microbios. 1974 May;10(40):225–231. [PubMed] [Google Scholar]