Abstract
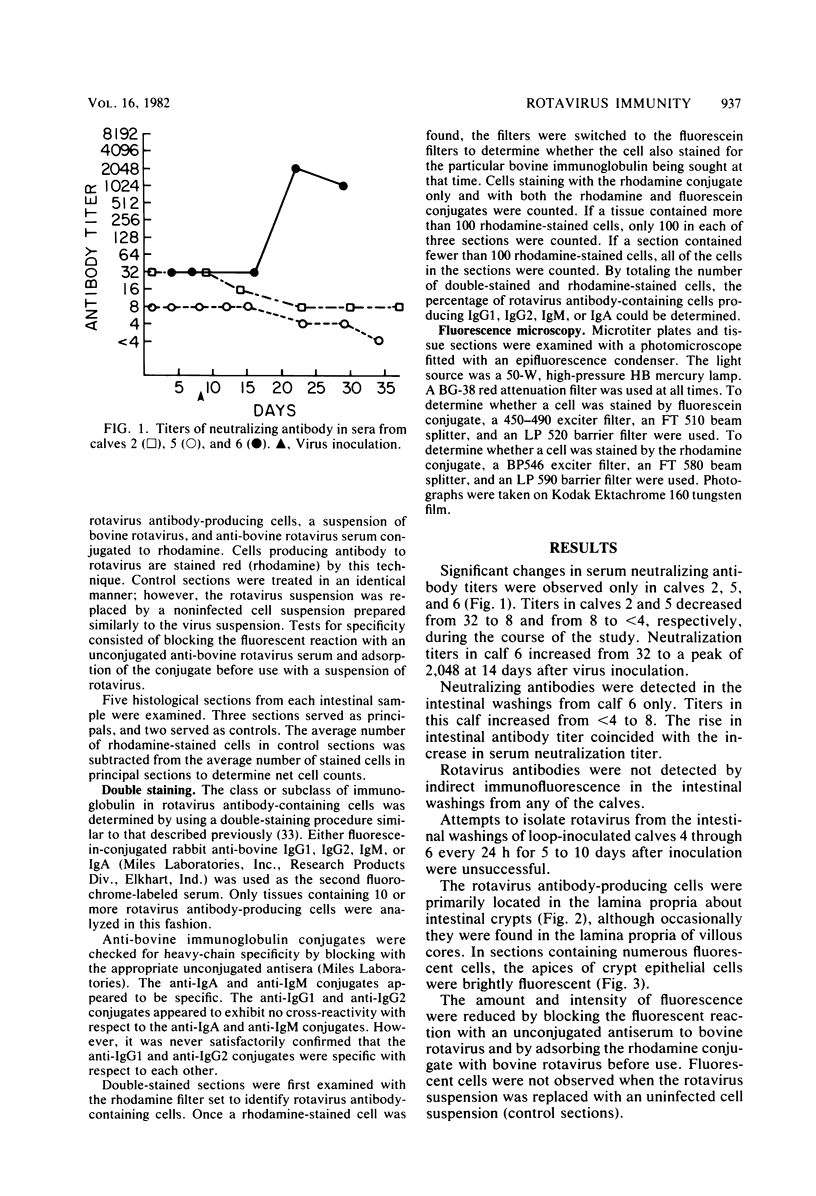
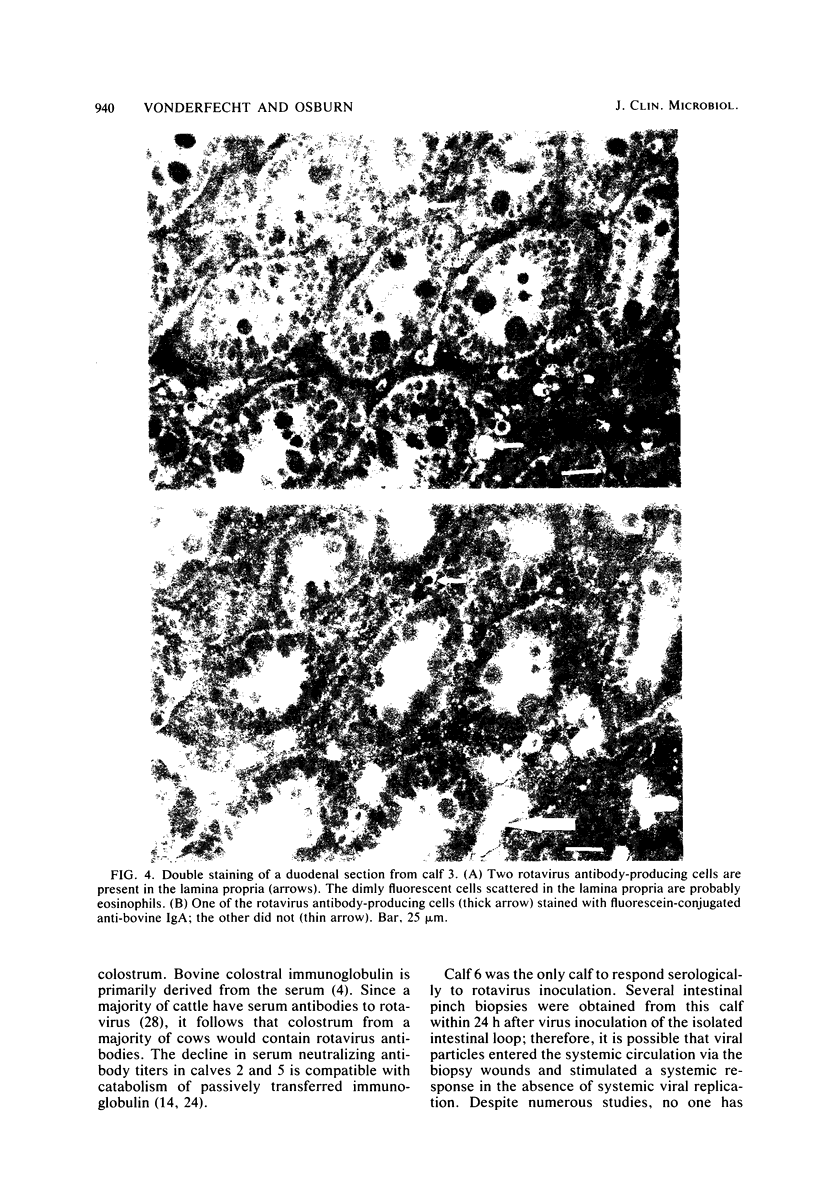
The local and systemic humoral immune responses to rotavirus were studied in six conventional neonatal calves. Attenuated bovine rotavirus was administered either orally or directly into an isolated intestinal loop. The parameters monitored were neutralizing rotavirus antibody in serum, immunofluorescent and neutralizing rotavirus antibody in intestinal loop washings, and rotavirus antibody-producing cells in intestinal mucosa. An antibody response was observed in the serum and intestinal secretions from one calf only. Viral replication was not detected in the isolated intestinal loop. Rotavirus antibody-producing cells were found in the intestinal mucosa of five calves. Double staining revealed that most of these cells produced antibody of the immunoglobulin A class. The conclusions were: (i) a previously described system to detect rotavirus antibody-producing cells can be used to study immune responses in neonatal calves, (ii) the class or subclass of antibody in rotavirus antibody-producing cells can be determined by double immunofluorescent staining, (iii) neonatal calves respond to rotavirus inoculation with a local immunoglobulin A response, and (iv) most of the rotavirus antibody-producing cells are located in the mucosa of the proximal small intestine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Immunohistochemical studies on various aspects of glandular immunoglobulin transport in man. Histochem J. 1977 Sep;9(5):553–572. doi: 10.1007/BF01002902. [DOI] [PubMed] [Google Scholar]
- Bridger J. C., Woode G. N. Neonatal calf diarrhoea: identification of a reovirus-like (rotavirus) agent in faeces by immunofluorescence and immune electron microscopy. Br Vet J. 1975 Sep-Oct;131(5):528–535. [PubMed] [Google Scholar]
- Catalano L. W., Jr, Fuccillo D. A., Sever J. L. Piggy-back microtransfer technique. Appl Microbiol. 1969 Dec;18(6):1094–1095. doi: 10.1128/am.18.6.1094-1095.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Franz J. Detection of antirotavirus immunoglobulins A, G, and M in swine colostrum, milk, and feces by enzyme-linked immunosorbent assay. Infect Immun. 1981 Feb;31(2):833–836. doi: 10.1128/iai.31.2.833-836.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Monié H. J., Gowans J. L. The natural history of the cells producing IgA in the gut. Ciba Found Symp. 1977 Apr 26;(46):29–54. doi: 10.1002/9780470720288.ch3. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Brandt C. D., Kapikian A. Z., Wyatt R. G., Arrobio J. O., Rodriguez W. J., Chanock R. M., Parrott R. H. Human reovirus-like agent infection. Occurrence in adult contacts of pediatric patients with gastroenteritis. JAMA. 1977 Aug 1;238(5):404–407. doi: 10.1001/jama.238.5.404. [DOI] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Mebus C. A., Torres-Medina A., Twiehaus M. J., Bass E. P. Immune response to orally administered calf reovirus-like agent and coronavirus vaccine. Dev Biol Stand. 1976;33:396–403. [PubMed] [Google Scholar]
- Orstavik I., Haug K. W., Sovde A. Rotavirus-associated gastroenteritis in two adults probably caused by virus reinfection. Scand J Infect Dis. 1976;8(4):277–278. doi: 10.3109/inf.1976.8.issue-4.12. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sircar B. K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978 Jul;21(1):185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P. Immunoglobulins in bovine mammary secretions. Quantitative changes in early lactation and absorption by the neonatal calf. Immunology. 1972 Aug;23(2):225–238. [PMC free article] [PubMed] [Google Scholar]
- Porter P., Noakes D. E., Allen W. D. Intestinal secretion of immunoglobulins in the preruminant calf. Immunology. 1972 Sep;23(3):299–312. [PMC free article] [PubMed] [Google Scholar]
- Porter P., Parry S. H., Allen W. D. Significance of immune mechanisms in relation to enteric infections of the gastrointestinal tract in animals. Ciba Found Symp. 1977 Apr 26;(46):55–75. doi: 10.1002/9780470720288.ch4. [DOI] [PubMed] [Google Scholar]
- Rodriguez W. J., Kim H. W., Brandt C. D., Yolken R. H., Richard M., Arrobio J. O., Schwartz R. H., Kapikian A. Z., Chanock R. M., Parrott R. H. Common exposure outbreak of gastroenteritis due to type 2 rotavirus with high secondary attack rate within families. J Infect Dis. 1979 Sep;140(3):353–357. doi: 10.1093/infdis/140.3.353. [DOI] [PubMed] [Google Scholar]
- Schlafer D. H., Scott F. W. Prevalence of neutralizing antibody to the calf rotavirus in New York cattle. Cornell Vet. 1979 Jul;69(3):262–271. [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. Rotavirus infection in lambs: studies on passive protection. Arch Virol. 1976;52(3):201–205. doi: 10.1007/BF01348017. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. The immunoprophylaxis of of rotavirus infections in lambs. Vet Rec. 1978 Feb 18;102(7):146–148. doi: 10.1136/vr.102.7.146. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Wells P. W. The influence of colostrum on neonatal rotaviral infections. Ann Rech Vet. 1978;9(2):335–338. [PubMed] [Google Scholar]
- Totterdell B. M., Chrystie I. L., Banatvala J. E. Cord blood and breast-milk antibodies in neonatal rotavirus infection. Br Med J. 1980 Mar 22;280(6217):828–830. doi: 10.1136/bmj.280.6217.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S. L., Osburn B. I. Detection of anti-rotavirus antibody-producing cells in paraffin-embedded tissue sections. Am J Vet Res. 1982 Feb;43(2):356–359. [PubMed] [Google Scholar]
- Wells P. W., Snodgrass D. R. The effect of vaccination on titres of antibody to rotavirus in colostrum and milk. Ann Rech Vet. 1978;9(2):265–267. [PubMed] [Google Scholar]
- Woode G. N., Bew M. E., Dennis M. J. Studies on cross protection induced in calves by rotaviruses of calves, children and foals. Vet Rec. 1978 Jul 8;103(2):32–34. doi: 10.1136/vr.103.2.32. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Crouch C. F. Naturally occurring and experimentally induced rotaviral infections of domestic and laboratory animals. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):522–526. [PubMed] [Google Scholar]
- Woode G. N. Epizootiology of bovine rotavirus infection. Vet Rec. 1978 Jul 15;103(3):44–46. doi: 10.1136/vr.103.3.44. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Jones J., Bridger J. Levels of colostral antibodies against neonatal calf diaahoea virus. Vet Rec. 1975 Aug 23;97(8):148–149. doi: 10.1136/vr.97.8.148. [DOI] [PubMed] [Google Scholar]
- Woode G. N. Pathogenic rotaviruses isolated from pigs and calves. Ciba Found Symp. 1976;(42):251–271. doi: 10.1002/9780470720240.ch15. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Mebus C. A., Yolken R. H., Kalica A. R., James H. D., Jr, Kapikian A. Z., Chanock R. M. Rotaviral immunity in gnotobiotic calves: heterologous resistance to human virus induced by bovine virus. Science. 1979 Feb 9;203(4380):548–550. doi: 10.1126/science.216077. [DOI] [PubMed] [Google Scholar]