Abstract
HIV encephalitis (HIVE) is accompanied by brain inflammation, leukocyte infiltration, and glial activation, and HIV patients who abuse opiates are more likely to develop HIVE. To better understand how opiates could alter HIV-related brain inflammation, the expression of astrocyte (GFAP immunoreactivity) and macrophage/microglial (F4/80 or Mac1 immunoreactivity) markers in the striatum, and the percentage of 3-nitrotyrosine (3-NT) positive macrophages/microglia, was determined following a 2 day exposure to morphine (5 mg/kg/day via time-release, subcutaneous implant) and doxycycline in GFAP-driven, doxycycline-inducible HIV-1 Tat transgenic mice. Data show that both morphine and Tat induction via doxycycline increased astrocyte activation, with significant additive increases achieved with combined morphine and doxycycline exposure. By contrast, combined Tat induction and morphine exposure, but neither manipulation alone, significantly increased the proportion of macrophages/microglia present in the striatum of transgenic mice, although morphine exposure was necessary to elevate 3-NT co-detection in Mac1-positive macrophages/microglia. Finally, Tat induction increased the percentage of neurons expressing active caspase-3, and this was even more significantly elevated by co-administration of morphine. In spite of elevations in caspase-3, neuronal TUNEL reactivity was unchanged in all groups, even after 10 days of Tat induction. Importantly, co-administration of naltrexone completely antagonized the effects of morphine. These findings indicate that morphine rapidly and significantly increases the activation of astrocytes and macrophages/microglia in the brains of inducible Tat transgenic mice, supporting the theory that early inflammatory changes in glia could underlie the development of HIVE in opiate-abusing AIDS patients.
Keywords: inflammation, neuro-acquired immunodeficiency syndrome (neuroAIDS), opioid drug abuse, oxidative stress, reactive nitrogen products, HIV transgenic mouse model, apoptosis, caspase 3
INTRODUCTION
As many as one third of HIV-1 infected individuals may develop neuroAIDS, a syndrome that includes both HIV encephalitis (HIVE) and HIV dementia (HIVD), as well as CNS infection by opportunistic agents. While the mechanism(s) by which HIV perturbs brain homeostasis is poorly understood, the development of encephalitic changes, particularly extravasation of HIV-infected peripheral immune cells, is thought to be a key early event in progression of neuroAIDS. Indeed, the invasion of the brain by HIV-infected peripheral immune cells has been postulated to be an important pathological substrate of future HIVD (reviewed in Persidsky and Poluektova, 2006; Wang et al., 2006). Despite profound changes in neuronal morphology and function in many HIV brains (Masliah et al., 1997; Kaul et al., 2001), astrocytes and microglia are the only brain-resident cells with the proper pattern of cell surface chemokine receptors to confer sensitivity to HIV-1 infection. While the mechanism(s) whereby the relatively limited viral infection in glia leads to abject changes in brain function are not understood, markers of microglial and astrocyte activation have been reported to correlate well with behavioral and cognitive changes characteristic of HIVD (Tyor et al., 1995; Glass et al., 1995; Patton et al., 1996; Gray et al., 2001; Anderson et al., 2003) leading many investigators to posit that altered or increased glial reactivity orchestrates, at least in part, the progression of neuroAIDS.
HIV-Tat is a trans-activating regulatory viral protein composed of 72 to 101 amino acids, and is actively secreted by HIV-infected cells (Tardieu et al., 1992; Ensoli et al., 1993). As anti-Tat antibodies can be detected in the serum of AIDS patients (Aldovini et al., 1986), many investigators have suggested that this extracellular pool of Tat could participate in the progression of neuroAIDS. In support of this hypothesis, Tat expression is elevated in brain tissue of patients with HIVE and HIVD (Wesselingh et al., 1993; Hofman et al., 1994; Wiley et al., 1996). Furthermore, Tat is neurotoxic in vitro and in vivo (Hayman et al., 1993; Turchan et al., 2001), and Tat has been shown to be potently pro-inflammatory both in vitro and in vivo (Philippon et al., 1994; Jones et al., 1998; Bruce-Keller et al., 2001; Bruce-Keller et al., 2003; D'Aversa et al., 2004; Minghetti et al., 2004).
The reasons why only selected AIDS patients develop neurological complications is still not understood, but there is considerable evidence that HIV-1 infected individuals who also abuse opiate-based drugs have an accelerated rate of progression to both AIDS and HIVD (Shapshak et al., 1996; Davies et al., 1997; Bell et al., 1998; Nath et al., 2002; Bell et al., 2002). Furthermore, it has been reported that opiate drug abuse in the context of HIV-1 infection increases glial reactivity in the CNS, with specific alterations in the number and phenotype of reactive astroglia and macrophages/microglia (Bell et al., 2002). As increases in glial inflammatory reactions could participate in HIVD development, experiments were designed to directly measure the effects of morphine administration on HIV-related glial reactivity in vivo. To this end, GFAP-driven, doxycycline (DOX)-inducible Tat transgenic mice were developed to evaluate the interactions of morphine and Tat within the brain. This manuscript describes the results of experiments in which control [Tat(−)] and inducible Tat transgenic [Tat(+)] mice were exposed to morphine and DOX for 2 days. Specific analyses of glial reactivity and neuronal injury were conducted in the striatum since this region is affected in human HIV (Navia et al., 1986; Wiley et al., 1998; Yiannoutsos et al., 2004) and is particularly susceptible to experimental Tat neurotoxicity in vivo (Hayman et al., 1993; Bansal et al., 2000). To determine if opiate exposure would exacerbate reactive astrogliosis and macrophage recruitment/microglial activation, acute increases in the proportion of astroglia and macrophages/microglia (identified by GFAP and F4/80 or Mac-1 immunoreactivity, respectively) were evaluated in morphine treated mice. In addition, the pattern of 3-nitrotyrosine (3-NT) immunoreactivity and the percentage of nitrotyrosine-positive microglia was evaluated following DOX and morphine administration as an estimate of the neurotoxic potential of microglia/macrophages exposed to Tat and/or opiates. The presence of 3-NT modified residues documents production of the highly neurotoxic peroxynitrite anion (ONOO-), formed by the interaction of superoxide radicals and nitric oxide (Shishehbor and Hazen, 2004; Shavali et al., 2006; Ryu and McLarnon, 2006). Lastly, the proportion of neurons possessing caspase-3 and terminal transferase-mediated UTP nick end-labeling (TUNEL) reactivity was assessed to explore whether reactive glial changes are accompanied by acute increases in neuronal injury or death.
MATERIALS AND EXPERIMENTAL PROCEDURES
Generation of DOX-inducible, brain–specific HIV-Tat transgenic mice
A tetracycline (tet) "on" system was used for generation of inducible constructs. Tat(1–86; HIV-1 IIIB) was cloned downstream of a tet responsive element (TRE) in the pTREX vector (Clonetech, Mountain View, CA). Reverse tetracycline transactivator (RTTA) was first cloned in pEGFP vector (Clonetech) at the BamHI/EcoRI site and further subcloned in GFAP promoter-driven vector pGfaLac-1 at the BamHI/BglII site. The construction of these plasmids and their validation using in vitro systems is described in a previous publication from the founder’s lab (Chauhan et al., 2003). For creation of transgenic lines, plasmid vectors were selectively digested with restriction enzymes and intact regulatory regions with respective genes and poly A sites were gel extracted. The purified fragments were used for inoculation of eggs. The founder animals (C3H × C57BL/6J) for each construct were checked for respective genes by PCR and southern blotting. Each founder line was then inbred to produce mice homozygous for the selected genes. Brain-restricted, inducible transgenic mice were created by crossing mice expressing the Tat gene under the TRE with mice engineered to express a GFAP promoter driven RTTA. Pure transgenic lines were derived by crossing homologous lines containing both the GFAP-RTTA and TRE-Tat genes. Thus, the inducible Tat transgenic mice (Tat(+)mice) described in this manuscript express both GFAP-RTTA and TRE-Tat genes, while control Tat(−)mice expressed GFAP-RTTA but not the TRE-Tat gene.
Animal Treatments I – DOX and morphine administration
All animal procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee (IACUC) and are in keeping with AAALAC guidelines. Male and female mice aged 2–6 month-old were kept on a 14 h light/10 h dark cycle with free access to food and water. To induce Tat expression, standard mouse chow was replaced with a specially formulated chow containing 6 mg/g DOX (Harlan, Indianapolis, IN) for 2 or 10 days. Both Tat(+) and Tat(−) mice were fed the DOX chow to evaluate any non-specific actions of DOX ingestion. For analyses of combined Tat and morphine exposure, mice were given the DOX feed the night before morphine and/or naltrexone administration to allow blood levels of DOX to stabilize. Morphine administration was achieved by subscapular implantation of continuous, time-release 25 mg pellets (5 mg/day for up to 5 days; NIDA, Rockville, MD, USA) under aseptic conditions and isoflurane anesthesia as previously described (El-Hage et al., 2006a; El-Hage et al., 2006b). Although 25 mg morphine pellets are reportedly depleted at a rate of 5 mg/day, steady-state concentrations of morphine are measured in ng/ml and differ among tissues. For example, 75 mg morphine pellets typically yield steady state levels of approximately 22 µg/ml of plasma in C3HeB mice, and much of this includes conjugated morphine (see, e.g., Feng et al., 2006). Naltrexone was administered to mice via Alzet minipumps also implanted subcutaneously into the subscapular region. Specifically, 60 mg/100 µl naltrexone solubilized in 50% DMSO was used to fill 1007D model pumps (0.5 µl/h release for up to 7 days), with pumps containing 50% DMSO in sterile saline used as controls. Some mice received replacement pellets and mini-pumps after 5 days, to permit 10 total days of exposure. At 48 h or 10 days after morphine, naltrexone, or sham implantation, animals were euthanatized by perfusion with Zamboni’s fixative (2% paraformaldehyde, pH 7.4 supplemented with 0.15% picric acid). The brains were removed, cryopreserved by serial exposure to 10 and 20% sucrose, embedded in OCT compound, and stored intact at −80 °C.
Animal Treatments II – Tissue processing and Immunohistochemistry
For analyses of Tat expression, RNA was isolated from brain or ear tissue using commercially available kits (Invitrogen, Carlsbad, CA). Briefly, nucleic acids were isolated from flash-frozen brain tissue using Trizol reagent (Invitrogen) and treated with RNase-free DNase. RNA (1 µg) was reverse transcribed into cDNA using random hexamer primers and 200 units of superscript reverse transcriptase (Invitrogen) at 70 °C for 10 min and 42 °C for 1 h in 20 µl total volume. cDNA (100 ng) was used in PCR amplification with 50 pM of each primer, 0.1 mM dNTP mix, 1.25 units platinum Taq polymerase, and 1.2 mM MgCl2. The following primers were used to measure Tat expression: reverse (3’) gcg aat tct cat tgc ttt gat aga gaa act tg, and forward (5’) gcg gat cca tgg agc cag tag atc cta g. Amplified products were analyzed on 2% agarose gels with visualized with ethidium bromide.
For histology, 12 µm frozen sections were prepared from the frozen brains, and coronal sections cut at the level of the striatum and the hippocampus were collected for analysis. Specifically, 4 individual sections exactly 120 µm apart were thaw-mounted onto slides so that each slide contained representative samples from different rostral-caudal levels of the striatum. The slides were processed for immunohistochemistry using the following primary antisera: rabbit anti-mouse GFAP (1:600, Chemicon, Temecula, CA); mouse anti-Neu-N (1:100, Chemicon); rat anti-mouse F4/80 (1:500, Serotec, Raleigh, NC); rat anti-mouse CD11b (Mac-1, 1:500, Chemicon); rabbit anti-3-NT (1:500, Upstate, Charlottesville, VA); and rabbit anti-human/mouse active caspase-3 (1:2,000, R&D Systems, Minneapolis, MN). TUNEL staining was employed to detect in situ DNA framentation using the TACS2-TdT Apoptosis Detection Kit (Trevigen, Gaithersburg, MD), per manufacturer’s directions. Proportional changes of activation and reactivity in astrocytes, macrophages/microglia and neurons (see below) were quantified using indirect immunofluorescence, combined with Hoechst 33342 (15 µg/ml; Sigma St. Louis, MO) to label cell nuclei as previously described (Bruce-Keller et al., 1999; Bruce-Keller et al., 2003; El-Hage et al., 2006b). To document non-specific immunoreactivity, primary antibodies were either pre-incubated with excess antigen or omitted from the staining protocol.
To assess reactive glial changes in the striatum of each animal, the proportion of GFAP positive astrocytes, or F4/80 or Mac-1 positive macrophages/microglia were determined from the total number of Hoechst-stained cells. The total number of Hoechst-stained cells, and the total number of GFAP, Mac-1, or F4/80 positive cells, were counted within the striatum in 3–5 random fields per section, at 100× magnification (Zeiss Axioplan, Carl Zeiss Inc.), using 2 sections that were 120 µM apart per animal. Data from multiple fields and sections was then averaged to generate a final index on GFAP, F4/80, or Mac-1 immunoreactivity per animal. To evaluate microglial 3-NT immunoreactivity, sections were double-labeled with antibodies specific for Mac-1 and 3-NT, and the percentage of Mac-1 positive cells that were also 3-NT-positive was evaluated using the same approach. To assess neuronal injury and/or death, the proportion of active (cleaved) caspase-3 or TUNEL reactive, NeuN-positive neurons was determined. We examined at least 100 NeuN positive cells in adjacent fields in two different sections per animal, at 100× magnification. A Leica TCS, SP confocal microscope (Fig. 2A–C) or a Zeiss Axio Observer Z1 inverted microscope equipped with an MRm camera (Zeiss) was used to acquire and digitize the fluorescent images. In Fig. 4A–B and Fig. 5A–D, Z stacks, optimized for deconvolution microscopy, were taken through the entire section and orthogonal views assessed to demonstrate unambiguous co-localization of products in 3 dimensions (AxioVision software version 4, Zeiss). In the case of Fig. 6, deconvolution software (AutoQuant X Version 2, Media Cybernetics, Bethesda, MD) was used to better demonstrate the subcellular (nuclear) details of TUNEL, NeuN, and Hoechst dye colocalization.
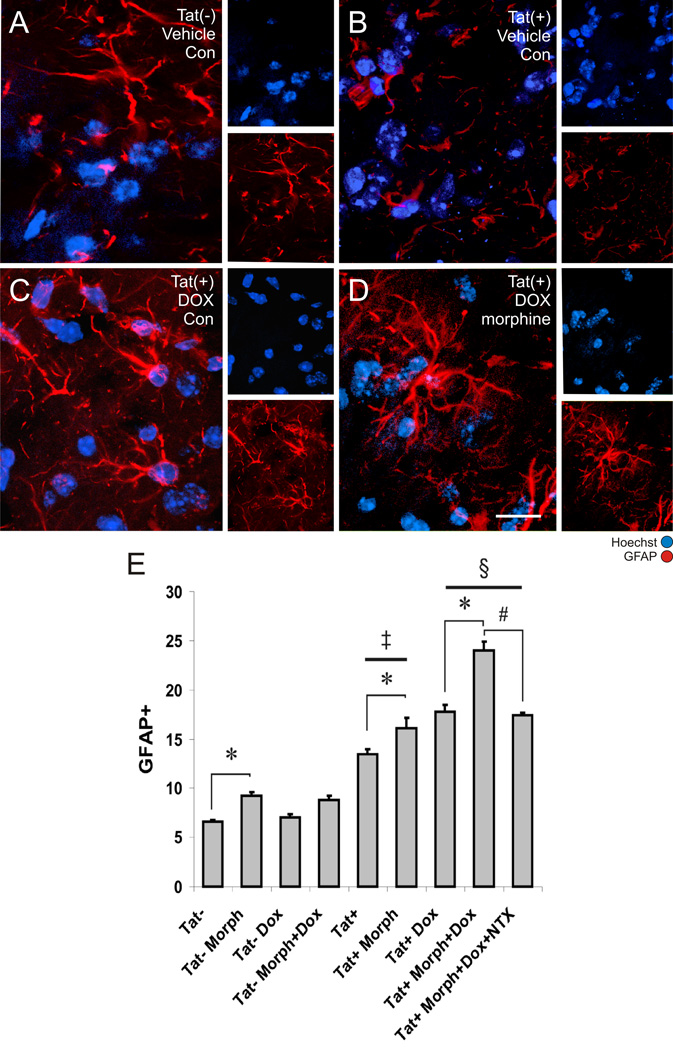
Figure 2. Astroglial responses to morphine and the inducible Tat transgene.
The proportion of Hoechst-stained striatal cells that were glial fibrillary acidic protein (GFAP) immunoreactive at 2 days following Tat induction was assessed in untreated Tat(−) mice (A), untreated Tat(+) mice (B), Tat(+) mice treated with DOX alone (C), and Tat(+) mice treated with combined DOX and morphine (Morph) after 2 days exposure (D, scale bar = 20 µm). The proportion of GFAP immunoreactive astrocytes in the striata of untreated Tat(+) mice was significantly increased compared to Tat(−) mice irrespective of DOX and morphine treatment [‡P < 0.01 Tat(+) vs. Tat(−) mice]. Morphine administration significantly increased the proportion of astrocytes in both Tat(−) and Tat(+) mice (*P < 0.05). Tat induction by DOX further increased the proportion of GFAP astroglia compared to Tat+ mice without DOX [§P < 0.05 vs. similarly treated Tat(+) mice without DOX]. Morphine-induced increases in GFAP immunoreactivity in DOX treated Tat(+) mice were reversed by co-administering naltrexone (NTX) (#P < 0.05). Data represent the mean ± SEM of 5–6 mice/group.
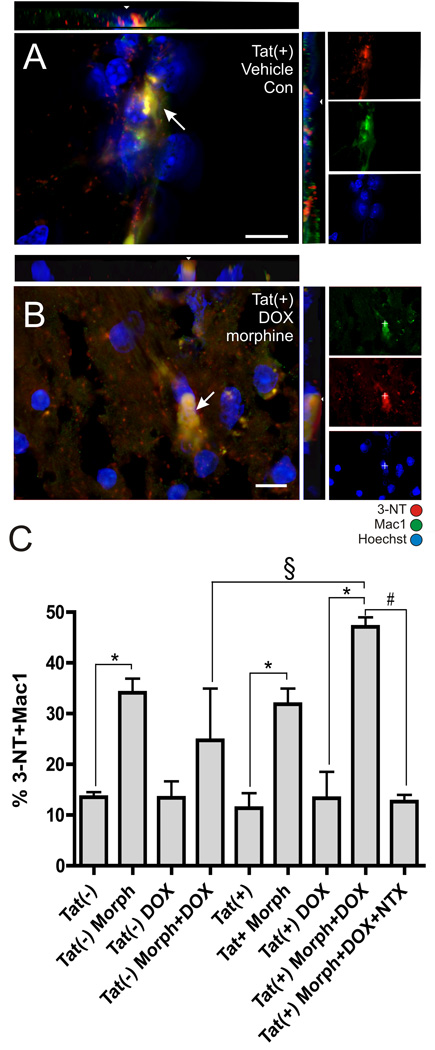
Figure 4. 3-NT-positive microglia following morphine treatment in inducible Tat transgenic mice.
Mac-1 and 3-NT immunoreactivity were co-localized (arrows) in Hoechst-stained striatum in all groups and shown here for untreated control Tat(+) mice (A) and Tat(+) mice treated with combined doxycycline (DOX) and morphine (Morph) (B) (scale bars = 10 µm; orthogonal views showing co-localization of 3-NT and Mac1 immunoreactivity in xz and yz planes are included). (C) Morphine exposure alone (*P < 0.05 vs. similar conditions without Morph) or combined morphine plus Tat induction (§P < 0.001 vs. DOX and Morph treated Tat(−) mice) significantly increased the proportion of 3-NT positive macrophages/microglia after 2 days. Morphine-induced increases in 3-NT immunoreactivity in DOX treated Tat(+) mice were reversed by co-administering naltrexone (NTX) (#P < 0.05). Data represent the mean ± SEM of 5–6 mice/group.
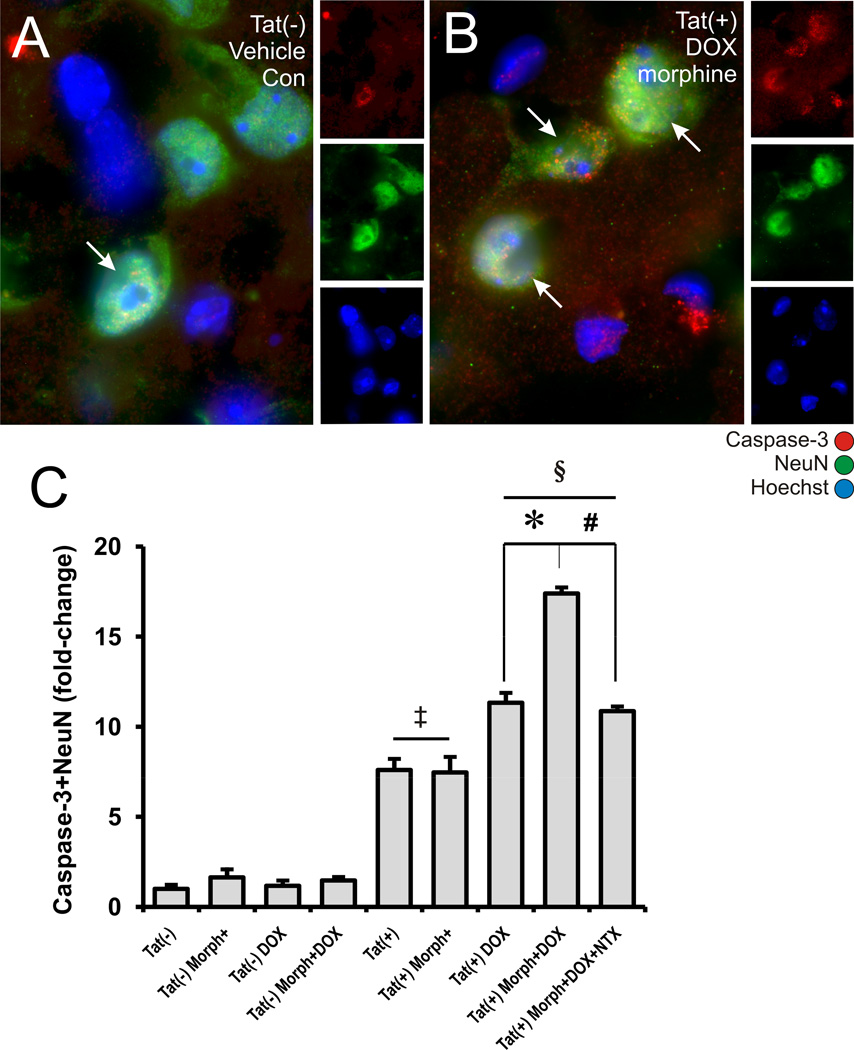
Figure 5. Effects of Tat induction and morphine exposure on active caspase-3-immunoreactivity in striatal neurons.
Although active caspase-3 and NeuN immunofluorescence could be co-localized in some neurons (arrows) in Tat(−) mice receiving placebo implants and normal feed (A), such cells appear to be increased in all Tat(+) groups (B). Significant increases in the proportion of cleaved caspase-3 were seen in (1) untreated Tat(+) mice [‡P < 0.02 vs. untreated Tat(−) mice]; (2) Tat(+) receiving doxycycline (DOX) [§P < 0.001 vs. Tat(−) mice with DOX]; or (3) with combined morphine (Morph) and DOX treatment [*P < 0.05 vs. DOX treated Tat(+) mice without morphine] (C). Morphine-induced increases in cleaved caspase-3 immunoreactivity in DOX treated Tat(+) mice were antagonized by co-administering naltrexone (NTX) (#P < 0.05) (C). Data represent the mean ± SEM of 5–6 mice/group. Scale bar in A = 10 µm (A-B are equal magnification).
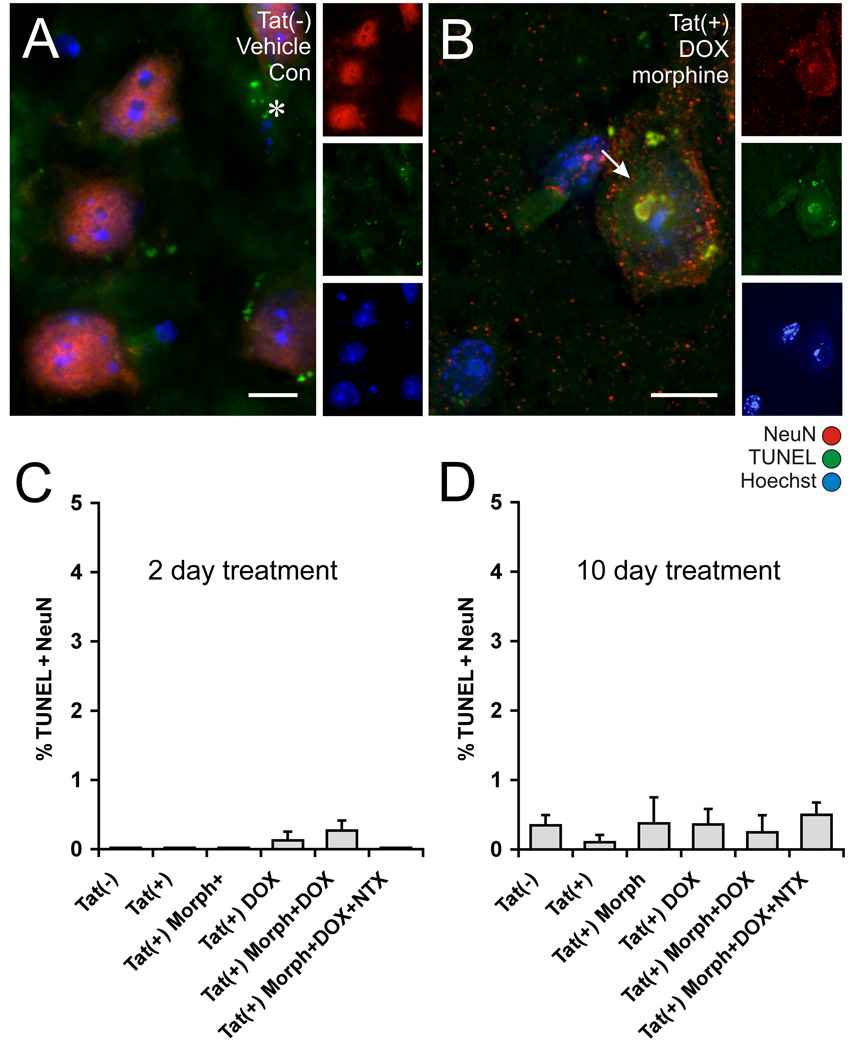
Figure 6. Effects of Tat induction and morphine exposure on TUNEL reactivity in striatal neurons.
Tissues from the same mice analyzed for caspase-3 immunostaining after 2 days of treatment (Fig. 5) were analyzed for TUNEL labeling (A). Additional mice were analyzed after 10 days of treatment (B). TUNEL reactivity was rarely detected in NeuN immunoreactive neurons in the striatum of Tat transgenic mice, irrespective of DOX or Morph exposure (A), although an occasional TUNEL positive neuron was seen in Tat(+) mice receiving DOX, or combined DOX and Morph (B). Some TUNEL-positive cells that were not NeuN+ were present in all tissues, and likely represent dying glia (*). The proportion of TUNEL-reactive neurons was unaffected by the Tat transgene or by treatments with DOX and/or Morph following either 2 days (C), or 10 days (D) of exposure. Data represent the mean ± SEM of 5–6 mice/group. Scale bars in A and B = 10 µm.
An important goal of these studies was to assess the acute response of neurons and glia to HIV ± opiates. We were particularly interested in assessing relative changes in acute inflammation and injury within specific neuron and glial populations, in part to determine the extent to which a rapid response would be coordinated among diverse neural cell types. Our current model for opiate-HIV interactions predicts that while opiates per se can affect the response of astrocytes, microglia and neurons individually to HIV, synergistic neuronal injury is likely a consequence of inflammatory signaling between astroglia, microglia, and neurons that spirals out of control. In addition, assessing the relative changes in the proportion of GFAP-positive astrocytes and F4/80-positive macrophages/microglia also permitted the direct comparison of current results in Tat transgenic mice with our earlier studies in which Tat was injected intrastriatally (El-Hage et al., 2006a; El-Hage et al., 2006b).
Statistical analyses
All data were analyzed using one-way ANOVA, followed by Tukey’s post-hoc analyses to determine statistical significance. In the case of the TUNEL data, the variances among treatment groups were non-homogeneous. For this reason, Kruskal Wallis nonparametric ANOVA was used to assess significant differences among treatment groups. P values < 0.05 were designated as statistically significant.
RESULTS
1. Effect of DOX administration on HIV-Tat expression in inducible Tat transgenic mice
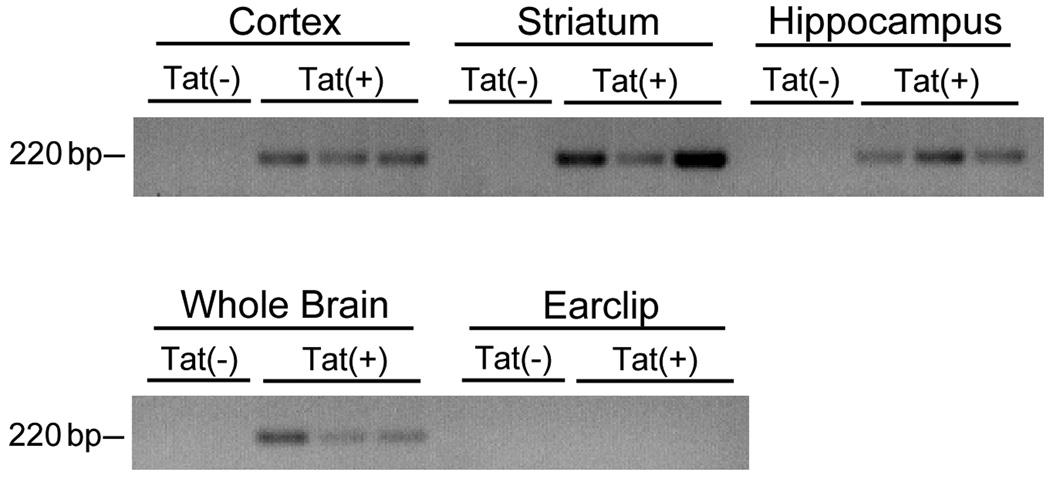
Although published reports have shown feed-based DOX administration can control the expression of target Tet-responsive genes in brain cells initial experiments were designed to confirm the induction of Tat expression in the brains of the DOX-treated transgenic mice. Adult Tat(−) and Tat(+) mice were administered DOX for 2 days, after which the brains were removed, and specific brain regions micro-dissected to determine the regional patterns of Tat expression. Additionally, RNA was extracted from peripheral tissue to confirm that Tat expression was restricted to the brain. Tat mRNA expression was determined by RT-PCR analyses as described in Methods. Evaluation of PCR band intensities confirmed that DOX administration caused robust inductions of Tat mRNA expression in the cortex, striatum, and hippocampus of Tat(+) mice, without noticeable regional variation (Fig. 1). Tat mRNA was not detected in tissue taken by earclip from DOX-treated Tat(+) mice or in any tissue examined from the Tat(−) mice (Fig. 1).
Figure 1. Regional specificity of inducible Tat expression.
RT-PCR was used to examine Tat transgene expression in adult Tat(+) and Tat(−) transgenic mice after 2 days of DOX administration. Images depict the presence or absence of Tat mRNA (220 bp) in samples derived from specific brain regions (cortex, striatum, hippocampus) as well as peripheral tissue (earclip) from three Tat(+) and two Tat(−) mice.
2. Effects of morphine administration on markers of astrocyte reactivity in inducible Tat transgenic mice
To assess the early physiological effects of brain-restricted Tat expression, Tat(−) and Tat(+) mice were administered DOX for 2 days, and selected mice were treated also with morphine and/or naltrexone, as described in Methods. These mice were generated from a C3H × C57Bl/6 background and are, like humans, sensitive to morphine toxicity. Thus a small amount of lethality (< 5%) occurred due to the initial effects of 25 mg/kg/day morphine, prior to development of tolerance and dependence at 12–36 h. There was no relationship between morphine-induced toxicity and either genotype, irrespective of DOX administration. While sensitivity to morphine differs among mouse strains and particular outcome measures (Brase et al., 1977), 75 mg/kg/day morphine is reported to be well-tolerated by both C3HeB and C57Bl/6 mice (Rahim et al., 2003; Peart and Gross, 2004).
As astroglial reactivity is a prominent pathological feature in the brains of opiate-abusing HIV patients (Bell et al., 2002; Anderson et al., 2003), the effects of Tat expression, with or without morphine exposure, were determined on astrocyte reactivity. The proportion of Hoechst-stained cells that were GFAP-positive were assessed in wild type and Tat(+) mice ± opiates and DOX. Evaluation of fluorescent images of the striatum confirmed that while significant GFAP immunoreactivity could be detected in Tat(−) mice, increases in GFAP immunoreactivity were apparent in Tat(+) mice treated with DOX or combined DOX and morphine (Fig. 3A). No visible immunoreactivity could be detected when non-specific staining was examined (data not shown). Blinded cell counts revealed that in Tat(−) mice, morphine alone caused significant increases in the proportion of total cells that were GFAP-immunoreactive astrocytes compared to mice not treated with morphine (Fig. 3B). Administration of DOX had no effect on GFAP-positive cells in Tat(−) mice (Fig. 3B). In Tat(+) mice, administration of either morphine or DOX alone significantly increased astrocyte activation compared to untreated mice (Fig. 3B). Furthermore, combined morphine and DOX exposure caused significant additive increases in the proportion of GFAP-immunoreactive astrocytes in the striatum of Tat(+) mice compared to mice receiving either morphine or DOX alone (Fig. 3B). To confirm that the effects of morphine were mediated through opioid receptors, the selective opioid receptor antagonist naltrexone was administered concurrently with DOX and morphine. Data show that the effects of morphine were completely antagonized by naltrexone (Fig. 3B), supporting a critical role for opioid receptors in these biological effects of morphine.
Figure 3. Microglial responses to morphine and the inducible Tat transgene.
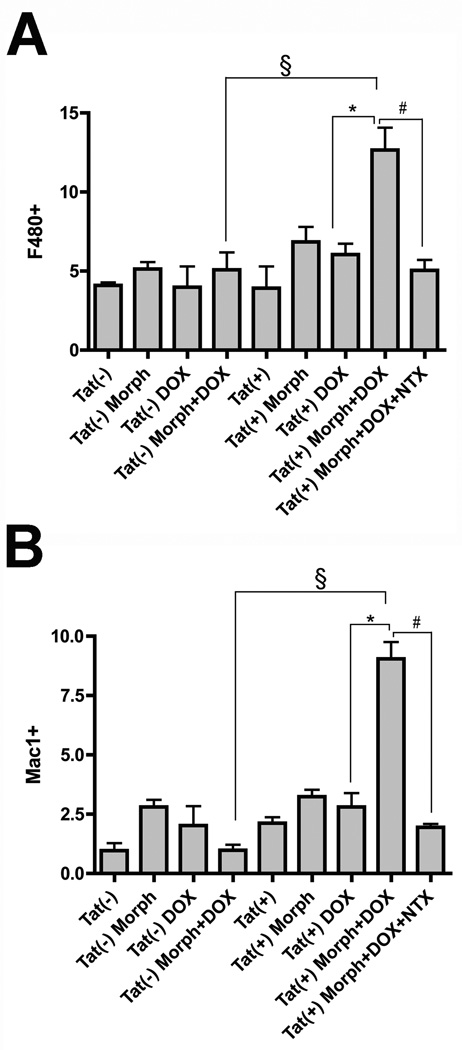
In combination, morphine (Morph) exposure and Tat induction significantly increased the proportion of F4/80 (A) or Mac-1 (B) immunoreactive macrophages/microglia after 2 days [§P < 0.001 vs. DOX and Morph treated Tat(−) mice; *P < 0.001 vs. DOX treated Tat(+) mice without Morph], while neither Tat induction nor Morph alone had any effect. Morphine-induced increases in F4/80 and Mac-1 immunoreactivity in DOX treated Tat(+) mice were reversed by co-administering naltrexone (NTX) (#P < 0.05). Data represent the mean ± SEM of 5–6 mice/group.
3. Effects of morphine administration and Tat induction on the proportion of macrophages/microglia
As microglia are also responsive to opiates (Rogers and Peterson, 2003; El-Hage et al., 2006b), and the number of reactive macrophages/microglia has been shown to correlate well with the degree of HIVD (Tyor et al., 1995; Glass et al., 1995; Patton et al., 1996; Gray et al., 2001; Anderson et al., 2003), the effects of DOX and/or morphine treatment on macrophage/microglial activation was determined in Tat(−) and Tat(+) mice. The proportion of reactive microglia/macrophages was quantified in the striatum after a 2 day exposure to DOX, morphine, or combined DOX/morphine using both F4/80 (Carson et al., 1998) and Mac-1 (Wirenfeldt et al., 2003) as described in the Methods. Blinded cell counts revealed that DOX or morphine exposure alone did not alter the percentage of Hoechst-stained cells in either the Tat(−) or Tat(+) striatum that expressed either F4/80 (Fig. 3A) or Mac-1 (Fig. 3B). However, combined Tat induction and morphine exposure caused significant increases in the proportion of both F4/80 (Fig. 3A) and Mac-1 (Fig. 3B) positive cells in Tat(+) mice as compared to either untreated mice or mice treated with DOX alone. Co-administration of naltrexone completely reversed morphine-induced alterations in F4/80 and Mac-1 immunoreactivity in Tat(+) mice.
4. Effects of morphine administration and Tat induction on 3-NT immunoreactivity in macrophages/microglia
In inflammatory cells such as microglia or macrophages, 3-NT immunoreactivity has been used to identify cells with an inflammatory phenotype capable of inducing significant oxidative stress (Shavali et al., 2006; Shishehbor and Hazen, 2004; Ryu and McLarnon, 2006). To determine if Tat and/or morphine exposure could induce a neurotoxic phenotype in microglia, 3-NT immunoreactivity in macrophages/microglia was evaluated in Tat(−) and Tat(+) mice with or without DOX and/or morphine treatment. Tissue sections from mice were processed for specific 3-NT and Mac-1 immunoreactivity as described in Methods, and sections were inspected to verify the specificity of the antibodies and to determine the pattern of co-localization.
Evaluation of images indicated that 3-NT immunoreactivity was readily detected in Mac1-positive cells (Fig. 4A & B). Two days of morphine treatment alone significantly increased the proportion of 3-NT-immunopositive Mac1-labeled macrophages/microglia, irrespective of Tat genotype (Fig. 4C). DOX administration alone did not alter the percentage of Mac-1 cells that expressed 3-NT, suggesting that Tat alone did not elevate 3-NT (Fig. 4C). There was a synergistic interaction between Tat and morphine since DOX-induced Tat(+) mice showed significantly more 3-NT staining when morphine was co-administered, as compared to Tat(−) mice that also received DOX and morphine (§ in Fig. 4C). The synergy was entirely opiate-dependent since co-administration of naltrexone completely reversed morphine-induced alterations in 3-NT immunoreactivity (Fig. 4C).
5. Effects of morphine exposure and Tat induction on caspase-3 activation and TUNEL reactivity in neurons
The presence of activated caspase-3 in neurons was highly sensitive to the Tat transgene. In fact, cleaved caspase-3 was markedly elevated in the neurons of Tat(+) mice even in the absence of doxycycline, suggesting “leakiness” in the transgene (Fig. 5C). Despite increased background levels of caspase-3 expression in the absence of DOX, Tat-induction significantly increased the proportion of active caspase-3 immunoreactive neurons, suggesting that levels of caspase-3 activity were directly proportional to levels of Tat expression.
Morphine treatment alone failed to increase the proportion of neurons staining for cleaved caspase-3 from control values in both Tat(−) and Tat(+) mice (Fig. 5). Importantly, however, when morphine was co-administered with DOX in Tat(+) mice, there were synergistic increases in the percentage of neurons expressing cleaved caspase-3 (Fig. 5C). Thus, morphine appeared to have a differential effect on cleaved caspase-3 levels that was dependent upon whether or not Tat expression was induced by DOX. This suggested that there may be different threshold titers for different actions of Tat, and that DOX-induction was necessary to reach this threshold before interactive effects with opiates upregulating caspase-3 cleavage became apparent. Alternatively, morphine interactions with very low levels of Tat that upregulate caspase may require more than 2 days of co-exposure to become apparent.
In contrast to caspase-3, increased Tat expression did not increase DNA fragmentation as assessed by TUNEL reactivity in neurons after either 2 days or 10 days of continuous DOX administration (Fig. 6). DNA fragmentation is a characteristic feature of apoptotic cell death. Despite morphine co-administration, no increases in TUNEL reactivity were apparent in striatal neurons, even in DOX-treated Tat(+) mice. In rare instances, dying neurons were observed with TUNEL positive nuclei (Fig. 6B), but this represented ≤0.5 % of all NeuN+ cells at both timepoints, and the numbers did not approach significance (Fig. 6C & D). TUNEL reactive neurons also showed other signs of degeneration including chromatin condensation, and nuclear shrinkage and fragmentation that were evident with Hoechst staining. Our findings indicate that caspase-3 activation is quite sensitive to levels of Tat expression and dramatically elevated with concurrent morphine exposure, but this was not accompanied by increased chromatin condensation and DNA fragmentation.
DISCUSSION
This manuscript describes data obtained from the brains of novel, brain-specific, inducible Tat transgenic mice. Our goal in engineering HIV-1 Tat transgenic mice was to explore the intrinsic effects of HIV-1 Tat in the CNS. Despite considerable evidence from cell culture studies and in vivo findings using Tat injection, the role of HIV-1 Tat per se in the pathogenesis of HIVE and neuroAIDS is incompletely understood. Our findings suggest that the Tat transgenic mouse is a useful model for mechanistic studies concerning the intrinsic effects of Tat in HIVE and neuroAIDS pathogenesis, in the absence of alternative toxic viral proteins such as gp120 (reviewed by Kaul et al., 2001), Rev, Vpr, and Nef, which may also contribute to neural inflammation and injury (Avison et al., 2003)..
In addition, the experiments were designed to evaluate if opiate exposure could combine with Tat expression to alter glial inflammation in vivo. This was prompted by prior experiments suggesting specific, synergistic interactions between opioid abuse and the Tat viral protein in the CNS. Data show that astrocyte reactivity was sensitive to both morphine exposure and Tat expression, with significant additive increases achieved when morphine was administered in the context of Tat expression. Conversely, the expression of microglial/macrophage cell markers was only increased under conditions of combined morphine exposure and Tat expression, although chronic morphine by itself was able to increase 3-NT immunoreactivity within the microglia/macrophage population even in control Tat(−) mice. This agrees with a number of reports indicating that morphine may act through a variety of interrelated mechanisms to modulate the production of (or cellular response to) reactive nitrogen products. Acute morphine exposure can protect astrocytes or SH-SY5Y neuroblastoma cells against peroxynitrite-induced insults (Kanesaki et al., 1999; Kim et al., 2001), while chronic morphine exposure increases the production of tyrosine-nitrated proteins in antinociceptive circuits associated with the spinal cord (Muscoli et al., 2007). Although morphine preferentially activates µ opioid receptors, it can also activate δ and κ receptors at high concentrations. Similarly, because naltrexone is a broad-acting opioid receptor antagonist, its use does not discern whether µ opioid receptors are specifically involved. While it is likely that morphine is acting preferentially via μ opioid receptors at the dosages used (5 mg/day), this assumption needs to be confirmed using highly selective μ; agonists and antagonists. Nevertheless, the effects of morphine were completely antagonized by naltrexone, supporting a critical role for opioid receptors in the biological effects of morphine. Collectively, these data suggest that opiate abuse could further the progression of neuroAIDS by inducing early and significant inflammatory changes in brain-resident glial cells. Furthermore, these data also lend support to prior studies indicating that HIV-1 Tat has an intrinsic ability to elicit an inflammatory reaction and contribute to the pathology of HIVE (Bruce-Keller et al., 2003; Eugenin et al., 2005; King et al., 2006; El-Hage et al., 2006b), further indicating that Tat per se could contribute to the pathogenesis of neuroAIDS.
Synergistic increases in the proportion of NT-3 positive macrophages/microglia and cleaved caspase-3 positive neurons suggest that the response of the HIV infected CNS to opioids is rapid and highly coordinated among glia and neurons. Acute increases in the proportion of reactive astroglia with morphine exposure alone and in combination with Tat induction support the hypothesis that astrocytes contribute meaningfully to opioid-induced exacerbation of HIVE (El-Hage et al., 2005; El-Hage et al., 2006b). Recent evidence suggests that GFAP expression may be limited to a subset of “reactive” astrocytes (Cahoy et al., 2008), which may increase with trauma or inflammatory diseases such as HIV. Interestingly, previous studies showed that morphine by itself causes reactive hypertrophy and GFAP immunoreactivity in μ opioid receptor expressing astrocytes isolated in vitro (Stiene-Martin et al., 1993; Hauser et al., 1996), without affecting survival (Gurwell and Hauser, 1993). Especially when considering the rapidity of the response, the proportional increase in the number of astrocytes noted in the present study likely reflects increased GFAP expression and cellular hypertrophy within existing populations of astrocytes.
A careful analysis of these data suggests that the astroglial response may be a more sensitive index of Tat expression than the response of macrophages/microglia. While HIV-1 infection of astrocytes is not thought to contribute heavily to CNS viral titers, astroglia are highly sensitive to the effects of HIV (Avgeropoulos et al., 1998; Kramer-Hammerle et al., 2005), and alterations in their phenotype are frequent early findings in patients with HIV infection (Navia et al., 1986; Petito and Roberts, 1995). Likewise, astrogliosis is evident in rodents injected with Tat or in SCID mice inoculated with HIV-1-infected monocytes intracranially (Tyor et al., 1993; Persidsky et al., 1996; Bansal et al., 2000). Thus, it was not surprising that the number of GFAP immunoreactive astroglia in Tat(+) mice was increased after only 48 hours of Tat induction. Furthermore, unlike the response of macrophages/microglia, moderate but significant increases in GFAP immunoreactivity were evident in untreated Tat(+) mice as compared to untreated Tat(−) mice, likely in response to “leaky” activation of the TRE promoter (Baron and Bujard, 2000; Zhu et al., 2001).
Morphine administration in the absence of Tat expression increased the proportion of GFAP-immunoreactive astrocytes to a lesser, although statistically significant, extent. These data suggest that Tat and morphine can act independently, as well as synergistically, to induce reactive increases in GFAP expression in striatal astrocytes. In support of these independent effects, reactive astrocytic changes have been noted in the brains of heroin addicts in the absence of HIV infection (Gosztonyi et al., 1993; Anderson et al., 2003; Hauser et al., 2005), and intrastriatal Tat infusion increased the reactivity of both μ opioid and non-μ-opioid receptor immunoreactive subpopulations of astrocytes in vivo (El-Hage et al., 2006b). Reports from our labs have as well demonstrated other incidences of synergy, in that opiates and HIV-1 Tat cause synergistic increases in intracellular calcium that are accompanied by increased chemokine release in cultured astroglia (Hauser et al., 1996; Haughey et al., 1999; El-Hage et al., 2005).
The results also demonstrate that combined Tat and morphine exposure evoke significant increases in the proportion of F4/80 and Mac-1-immunoreactive macrophages/microglia within the brain after only 48 hrs. Such increased levels of microglial/macrophage activation could set the stage for significant clinical declines if exposure conditions were to become chronic, as is the case with HIV-infected drug abusers. Brain macrophages/microglia are thought to contribute to neuroAIDS through a variety of mechanisms, including by serving as viral reservoirs and sites of HIV-1 replication, as well as sources of neurotoxic cytokines and pro-oxidants (reviewed in (Kramer-Hammerle et al., 2005). Emerging evidence suggests that opiates can affect many key aspects of the macrophage/microglia response to HIV-1. For example, µ-opioid receptor agonists increase HIV replication in monocytes and microglial cells (Peterson et al., 1990; Carr and Serou, 1995; Peterson et al., 1999; Li et al., 2002). Narcotics may also affect the intrinsic cytokine and reactive/oxidative response of macrophage/microglia to innate microbial insults (Wetzel et al., 2000; Rahim et al., 2003; Wang et al., 2005; Qin et al., 2005). Indeed, data in this manuscript indicate that the proportion of 3-NT-positive Mac-1 positive cells was increased by morphine alone, with a trend toward further increases in 3-NT when Tat induction and morphine are combined. Together, proportional increases in macrophage/microglial numbers combined with marked increases in 3-NT activity in individual cells suggest that morphine potentiates macrophage/microglial reactivity and neurotoxic oxidative stress, especially in the context of elevated Tat viral protein. These findings in inducible Tat transgenic mice are in agreement with an earlier study in which morphine was found to exacerbate the effects of intrastriatal Tat injection on F4/80+, macrophage/microglial numbers, seemingly through morphine-induced increases in CCL2 (El-Hage et al., 2006a; El-Hage et al., 2006b). CCL2, a major chemokine signaling macrophage recruitment and microglial activation, is an extremely reliable biomarker for cognitive decline with HIV or simian immunodeficiency viral models of neuroAIDS (Sevigny et al., 2004; Mankowski et al., 2004; Chang et al., 2004). The present study also agrees with clinical findings of elevated macrophages in patients with a history of combined drug abuse and HIV (Bell et al., 2002), which may involve increased turnover of perivascular macrophages (Anthony et al., 2005).
The demonstration that opiates can increase macrophage/microglia reactivity is significant because the presence of activated macrophages is highly correlated with the incidence and severity of HIV dementia (Tyor et al., 1995; Glass et al., 1995; Patton et al., 1996; Gray et al., 2001; Anderson et al., 2003). While mechanisms leading to widespread glial activation in HIV-1 brains are not understood, there is ample evidence supporting an important role for the Tat viral protein in neuroAIDS (reviewed in King et al., 2006). However, existing animal models available to assess the role of Tat in specific brain responses have relied mainly on direct stereotaxic Tat administration (Bansal et al., 2000; Bruce-Keller et al., 2003; Pu et al., 2003; El-Hage et al., 2006a; El-Hage et al., 2006b). Injection creates an artificial Tat gradient and causes significant damage to the blood-brain-barrier and brain parenchyma, and therefore may not be optimal for measures of glial and macrophage responses. Nonetheless, the data in this manuscript are generally supportive of those stereotaxic injection studies. The phenotype of the mice described in the present manuscript is much less severe than that described in a previous report using a similar, but not identical, system of Tat induction in transgenic mice (Kim et al., 2003). In that study, a murine GFAP promoter was used to drive DOX-inducible, brain-specific Tat expression, reportedly resulting in highly significant behavioral and histopathological consequences, including motor disturbances, cognitive deficits, seizures, and premature death (Kim et al., 2003). Additional reported histological consequences of Tat expression included brain edema, astrocytosis, neuronal apoptosis and neurite degeneration, and infiltration of peripheral immune cells (Kim et al., 2003). The mice described in our manuscript have a much less severe phenotype, and there are several key differences in the lines that might account for this dissimilarity. These include the use of a human GFAP promoter in our line, the relatively early time point (2 days) chosen for study, and the relatively high gene copy number (up to 10 copies) used in the previous study.
Increased expression of active caspase-3 has been previously shown in neurons exposed to Tat (Kruman et al., 1998; Kruman and Mattson, 1999; James et al., 1999; Bonavia et al., 2001; Singh et al., 2004), and we observed a similar increase in striatal neurons in the Tat transgenic mice after 2 days of DOX and/or morphine. However, corresponding increases in TUNEL labeling were not observed in the same mice. Since we considered that cell death, and hence TUNEL labeling, might require a longer exposure to Tat and/or morphine we treated another group of mice for a ten day period, with identical results. We do not believe that the lack of significance in neuronal TUNEL staining reflects inadequate sampling because we have detected slight, but significant, differences in TUNEL staining within the oligodendrocyte population in these same animals at only 2 days of exposure (Hauser et al, in review). We cannot exclude the possibility that prolonged periods of Tat and/or morphine exposure would eventually lead to increased TUNEL in neurons. However, our present data lead us to conclude that 10 days of Tat induction and/or morphine treatment did not result in neuron death, at least as indicated by TUNEL labeling, in this model. The active caspase-3 observed within neurons may reflect one of several non-lethal functions for caspase-3 that have been proposed, including synaptic culling or dendritic pruning (e.g., synaptic apoptosis) associated with remodeling during development, or after cellular injury (Oomman et al., 2004; Yang et al., 2004; Garrido and Kroemer, 2004; Launay et al., 2005). We noted caspase-3 increases in Tat(+) mice that had never received DOX, and took this as an indication of low, constitutive levels of promoter activity. Assuming that the promoter is “leaky” throughout life, we might expect chronic Tat production and the resulting caspase-3 activity to cause dramatic neuronal losses and overt behavioral changes. These were not apparent in our mice. Based on these considerations, we speculate that Tat-induced caspase-3 activity may be causing non-lethal neuron injury. Although additional studies are needed to confirm or deny this suggestion, elevations in caspase and reductions in dendritic complexity and synapses have been reported in HIV associated dementia, while neuronal apoptosis is not always apparent (Sa et al., 2004; Bellizzi et al., 2005) or may only accompany severe or end-stage dementia (Everall et al., 1999; Bellizzi et al., 2006). If cell death in response to caspase-3 depends on total enzyme activity within a cell, the caspase-3 activity that we detected may be below the threshold required to initiate neuron death. This is impossible to determine based on immunocytochemistry.
It is noteworthy that opiates potentiated increases in cleaved caspase-3 in Tat(+) mice in the presence of DOX, but not in its absence, despite evidence that the Tat transgene was “leaky” and elevated caspase-3 was present in untreated Tat(+) mice. This suggests that a threshold for Tat action (perhaps dependent on concentration or duration of Tat exposure) must be attained before combined opiate and Tat-interactive effects are apparent. As noted above, Tat can affect multiple targets in the CNS, and Tat may independently trigger each in a dose-dependent or time-dependent manner. Opiates may interact with some of the events and not with others.
In conclusion, data in this manuscript support the theory that Tat, as a significant component of HIV-1, could contribute per se to the pathological response of astroglia and microglia to HIV infection in the brain. Data show that morphine acts synergistically with Tat to increase the pathological responses of astroglia and microglia, reinforcing the notion that chronic opiate exposure is a major co-morbid factor in neuroAIDS. The glial response to combined morphine/Tat induction occurs rapidly, within 2 days, suggesting that even moderate or short-term doses of opiates could sensitize the brain to the future development of neuroAIDS.
ACKNOWLEDGEMENTS
The authors thank Dr. Valeria Adjan and Dr. Guanghan Wu for expert technical and scientific assistance. This work was supported by grants from the NIH (DA19398, DA18633, NS046267, and P20 RR15592).
References
- Aldovini A, Debouck C, Feinberg MB, Rosenberg M, Arya SK, Wong-Staal F. Synthesis of the complete trans-activation gene product of human T-lymphotropic virus type III in Escherichia coli: demonstration of immunogenicity in vivo and expression in vitro. Proc Natl Acad Sci U S A. 1986;83:6672–6676. doi: 10.1073/pnas.83.18.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, Simmonds P, Bell JE. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol Appl Neurobiol. 2003;29:378–388. doi: 10.1046/j.1365-2990.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31:325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Avgeropoulos N, Kelley B, Middaugh L, Arrigo S, Persidsky Y, Gendelman HE, Tyor WR. SCID mice with HIV encephalitis develop behavioral abnormalities. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:13–20. doi: 10.1097/00042560-199805010-00003. [DOI] [PubMed] [Google Scholar]
- Avison M, Berger JR, McArthur JC, Nath A. HIV meningitis and dementia. In: Nath A, Berger JR, editors. Clinical Neurovirology. New York: Marcel Dekker, Inc.; 2003. pp. 251–276. [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Baron U, Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: Principles and advances. Methods in Enzymology. 2000;327:401–421. doi: 10.1016/s0076-6879(00)27292-3. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and Drug Misuse in the Edinburgh Cohort. J Acquir Immune Defic Syndr. 2002;31 Suppl 2:S35–S42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Lu SM, Masliah E, Gelbard HA. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005;115:3185–3192. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellizzi MJ, Lu S-M, Gelbard HA. Protecting the synapse: evidence for a rational strategy to treat HIV-1 associated neurologic disease. J Neuroimmune Pharmacol. 2006;1:20–31. doi: 10.1007/s11481-005-9006-y. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Brase DA, Loh HH, Way EL. Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther. 1977;201:368–374. [PubMed] [Google Scholar]
- Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol. J Neurochem. 2001;78:1315–1324. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Serou M. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology. 1995;31:59–71. doi: 10.1016/0162-3109(95)00033-6. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, St HC, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278:13512–13519. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- D'Aversa TG, Yu KO, Berman JW. Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein Tat. J Neurovirol. 2004;10:86–97. doi: 10.1080/13550280490279807. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–1150. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Ambati J, Bruce-Keller AJ, Knapp PE, Hauser KF. CCR2 mediates increases in glial activation caused by exposure to HIV-1 Tat and opiates. J Neuroimmunol. 2006a;178:9–16. doi: 10.1016/j.jneuroim.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006b;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan R, Wingfield P, Gallo R. Release, uptake, and effects of extracellular human immunodeficiency virus type-1Tat protein on cell growth and viral replication. Journal of Virology. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Dyer G, Calderon TM, Berman JW. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: Possible role in NeuroAIDS. Glia. 2005;49:501–510. doi: 10.1002/glia.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E HNRC Group. HIV Neurobehavioral Research Center. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Rahim RT, Cowan A, Liu-Chen LY, Peng X, Gaughan J, Meissler JJ, Jr., Adler MW, Eisenstein TK. Effects of mu, kappa or delta opioids administered by pellet or pump on oral Salmonella infection and gastrointestinal transit. Eur J Pharmacol. 2006;534:250–257. doi: 10.1016/j.ejphar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Garrido C, Kroemer G. Life's smile, death's grin: vital functions of apoptosis-executing proteins. Curr Opin Cell Biol. 2004;16:639–646. doi: 10.1016/j.ceb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Gosztonyi G, Schmidt V, Nickel R, Rothschild MA, Camacho S, Siegel G, Zill E, Pauli G, Schneider V. Neuropathologic analysis of postmortal brain samples of HIV-seropositive and -seronegative i.v. drug addicts. Forensic Sci Int. 1993;62:101–105. doi: 10.1016/0379-0738(93)90052-c. [DOI] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- Gurwell JA, Hauser KF. Morphine does not affect astrocyte survival in developing primary mixed-glial cultures. Dev Brain Res. 1993;76:293–298. doi: 10.1016/0165-3806(93)90222-v. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 Protein Tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, Bruce-Keller AJ, Knapp PE. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M, Arbuthnott G, Harkiss G, Brace H, Filippi P, Philippon V, Thomson D, Vigne R, Wright A. Neurotoxicity of peptide analogues of the transactivating protein tat from Maedi-Visna virus and human immunodeficiency virus. Neuroscience. 1993;53:1–6. doi: 10.1016/0306-4522(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Hofman FM, Dohadwala MM, Wright AD, Hinton DR, Walker SM. Exogenous tat protein activates central nervous system-derived endothelial cells. J Neuroimmunol. 1994;54:19–28. doi: 10.1016/0165-5728(94)90226-7. [DOI] [PubMed] [Google Scholar]
- James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, Gelbard HA. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–386. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Jones M, Olafson K, Del Bigio MR, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- Kanesaki T, Saeki M, Ooi Y, Suematsu M, Matsumoto K, Sakuda M, Saito K, Maeda S. Morphine prevents peroxynitrite-induced death of human neuroblastoma SH-SY5Y cells through a direct scavenging action. Eur J Pharmacol. 1999;372:319–324. doi: 10.1016/s0014-2999(99)00206-x. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Cheong YP, So HS, Lee KM, Kim TY, Oh J, Chung YT, Son Y, Kim BR, Park R. Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase) Biochem Pharmacol. 2001;61:779–786. doi: 10.1016/s0006-2952(01)00541-x. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene. 2005;24:5137–5148. doi: 10.1038/sj.onc.1208524. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Tian S, Guo CJ, Douglas SD, Ho WZ. Methadone enhances human immunodeficiency virus infection of human immune cells. J Infect Dis. 2002;185:118–122. doi: 10.1086/338011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I HNRC Group. The HIV Neurobehavioral Research Center. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Visentin S, Patrizio M, Franchini L, jmone-Cat MA, Levi G. Multiple actions of the human immunodeficiency virus type-1 Tat protein on microglial cell functions. Neurochem Res. 2004;29:965–978. doi: 10.1023/b:nere.0000021241.90133.89. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31 Suppl 2:S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Oomman S, Finckbone V, Dertien J, Attridge J, Henne W, Medina M, Mansouri B, Singh H, Strahlendorf H, Strahlendorf J. Active caspase-3 expression during postnatal development of rat cerebellum is not systematically or consistently associated with apoptosis. J Comp Neurol. 2004;476:154–173. doi: 10.1002/cne.20223. [DOI] [PubMed] [Google Scholar]
- Patton HK, Benveniste EN, Benos DJ. Astrocytes and the AIDS dementia complex. J NeuroAIDS. 1996;1:111–131. doi: 10.1300/j128v01n01_06. [DOI] [PubMed] [Google Scholar]
- Peart JN, Gross GJ. Morphine-tolerant mice exhibit a profound and persistent cardioprotective phenotype. Circulation. 2004;109:1219–1222. doi: 10.1161/01.CIR.0000121422.85989.BD. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice [see comments] Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Poluektova L. Immune privilege and HIV-1 persistence in the CNS. Immunol Rev. 2006;213:180–194. doi: 10.1111/j.1600-065X.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr. Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis [see comments] Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Philippon V, Vellutini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, Filippi P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J. 2005;19:550–557. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Zhang L, Adler MW, Rogers TJ, Eisenstein TK. Withdrawal from morphine in mice suppresses splenic macrophage function, cytokine production, and costimulatory molecules. J Neuroimmunol. 2003;144:16–27. doi: 10.1016/s0165-5728(03)00273-x. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Ryu JK, McLarnon JG. Minocycline or iNOS inhibition block 3-nitrotyrosine increases and blood-brain barrier leakiness in amyloid beta-peptide-injected rat hippocampus. Exp Neurol. 2006;198:552–557. doi: 10.1016/j.expneurol.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol (Berl) 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- Shapshak P, Crandall KA, Xin KQ, Goodkin K, Fujimura RK, Bradley W, McCoy CB, Nagano I, Yoshioka M, Petito C, Sun NC, Srivastava AK, Weatherby N, Stewart R, Delgado S, Matthews A, Douyon R, Okuda K, Yang J, Zhangl BT, Cao XR, Shatkovsky S, Fernandez JB, Shah SM, Perper J. HIV-1 neuropathogenesis and abused drugs: current reviews, problems, and solutions. Adv Exp Med Biol. 1996;402:171–186. doi: 10.1007/978-1-4613-0407-4_23. 171–186. [DOI] [PubMed] [Google Scholar]
- Shavali S, Combs CK, Ebadi M. Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson's disease. Neurochem Res. 2006;31:85–94. doi: 10.1007/s11064-005-9233-x. [DOI] [PubMed] [Google Scholar]
- Shishehbor MH, Hazen SL. Inflammatory and oxidative markers in atherosclerosis: relationship to outcome. Curr Atheroscler Rep. 2004;6:243–250. doi: 10.1007/s11883-004-0038-1. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Mattson MP, Hauser KF. Opiates selectively increase intracellular calcium in developing type 1 astrocytes: Role of calcium in morphine-induced morphologic differentiation. Dev Brain Res. 1993;76:189–196. doi: 10.1016/0165-3806(93)90207-q. [DOI] [PubMed] [Google Scholar]
- Tardieu M, Hery C, Peudenier S, Boespflug O, Montagnier L. Human immunodeficiency virus type 1-infected monocytic cells can destroy human neural cells after cell-to-cell adhesion. Ann Neurol. 1992;32:11–17. doi: 10.1002/ana.410320104. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci U S A. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Wesselingh SL, Griffin JW, McArthur JC, Griffin DE. Unifying hypothesis for the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. Journal of AIDS and Human Retroviruses. 1995;9:379–388. [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- Wang T, Rumbaugh JA, Nath A. Viruses and the brain: from inflammation to dementia. Clin Sci (Lond) 2006;110:393–407. doi: 10.1042/CS20050278. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Eisenstein TK, Adler MW, Henderson EE, Rogers TJ. Mu-opioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-gamma-inducible protein-10 expression in human peripheral blood mononuclear cells. J Immunol. 2000;165:6519–6524. doi: 10.4049/jimmunol.165.11.6519. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL. Expression of regulatory and structural mRNA in the central nervous system. AIDS. 1996;10:943–947. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirenfeldt M, Dalmau I, Finsen B. Estimation of absolute microglial cell numbers in mouse fascia dentate using unbiased and efficient stereological cell counting principles. Glia. 2003;44:129–139. doi: 10.1002/glia.10277. [DOI] [PubMed] [Google Scholar]
- Yang JY, Michod D, Walicki J, Widmann C. Surviving the kiss of death. Biochem Pharmacol. 2004;68:1027–1031. doi: 10.1016/j.bcp.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–935. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Lee CG, Zheng T, Chupp G, Wang J, Homer RJ, Noble PW, Hamid Q, Elias JA. Airway inflammation and remodeling in asthma. Lessons from interleukin 11 and interleukin 13 transgenic mice. Am J Respir Crit Care Med. 2001;164:S67–S70. doi: 10.1164/ajrccm.164.supplement_2.2106070. [DOI] [PubMed] [Google Scholar]