Abstract
Alzheimer's disease is a progressive and fatal neurodegenerative disease characterized by a build up of amyloid β (Aβ) deposits, elevated oxidative stress, and deterioration of the cholinergic system. The present study investigated short-term cognitive-enhancing effects of acute intraperitoneal (i.p.) Vitamin C (ascorbate) treatment in APP/PSEN1 mice, a mouse model of Alzheimer's disease. Middle-aged (12 months) and Very old (24 months) APP/PSEN1 bigenic and wild-type mice were treated with ascorbate (125 mg/kg i.p.) or the vehicle 1 hour before testing on Y-maze spontaneous alternation and Morris water maze tasks. Very old mice performed more poorly on cognitive tasks than Middle-aged mice. Ascorbate treatment improved Y-maze alternation rates and swim accuracy in the water maze in both wild-type and APP/PSEN1 mice. Aβ deposits and oxidative stress both increased with age, and acetylcholinesterase (AChE) activity was significantly reduced in APP/PSEN1 compared to wild-type mice. However, the short course of acute ascorbate treatment did not alter Alzheimer-like neuropathological features of plaque deposition, oxidative stress, or AChE activity. These data suggest that ascorbate may have noötropic functions when administered parenterally in high doses and that the mode of action is via an acute, pharmacological-like mechanism that likely modulates neurotransmitter function.
Keywords: aging, Alzheimer's disease, APP/PSEN1, ascorbic acid, amyloid β, cognition, oxidative stress, acetylcholinesterase, vitamin C
Introduction
Alzheimer's disease is the major cause of pre-senile dementia in the United States, with a prevalence of 5 million in 2008 (2008). The main neuropathological features of Alzheimer's disease are a build up of amyloid β (Aβ) deposits, neurofibrillary tangles, elevated oxidative stress, and deterioration of the cholinergic system (Christen, 2000; Shah et al., 2008). Alzheimer's patients also have reduced plasma levels of vitamin C (ascorbic acid; ascorbate) (Charlton et al., 2004; Riviere et al., 1998) and high levels of dietary ascorbate or supplements have been suggested to lower the risk of developing the disease (Engelhart et al., 2002; Morris et al., 1998). Nevertheless, the mechanism of any protective role for ascorbate in the disease, and the relationship between ascorbate and cognition have yet to be elucidated.
Ascorbate levels in brain are generally much higher than in blood and other organs (Agus et al., 1997), and brain levels are tightly controlled. Although ascorbate is preferentially preserved in the brain relative to other organs, it is possible through dietary restriction to reduce ascorbate levels in mammals that cannot synthesize ascorbate (e.g. humans, guinea pigs, and Gulo -/- mice; (Burk et al., 2006; Harrison et al., 2008; Hodges et al., 1971; Lykkesfeldt et al., 2007)). It is more difficult to increase brain ascorbate above normal by dietary means. It has been shown that much higher levels of ascorbate can be obtained in plasma following intraperitoneal (i.p.) or intravenous (i.v.) administration than when administered by oral gavage (Chen et al., 2007). Improved cognition has been demonstrated following i.p. administration of ascorbate in mice and rats against age- and scopolamine-induced amnesia in a passive avoidance task, a memory test in the elevated plus maze, and a habituation test in light-dark activity chambers (de Angelis and Furlan, 1995; Parle and Dhingra, 2003; Shahidi et al., 2008) and in the water maze in rats treated with propionic acid which also reduced antioxidant capabilities (Pettenuzzo et al., 2002). Dietary ascorbate is vital for its antioxidant properties, which have long-term beneficial effects, whereas acutely administered ascorbate may have a greater impact on brain function in the short-term. The mechanism of action of ascorbate-induced improvement of memory is not yet known. However, it may be linked to neurotransmitter function. For example, ascorbate can attenuate the amnestic effects of the muscarinic antagonist scopolamine on memory and acetylcholinesterase (AChE) activity (de Angelis and Furlan, 1995; Lee et al., 2001; Parle and Dhingra, 2003). Much of the memory impairment in Alzheimer's disease is due to the degeneration of the basal forebrain cholinergic system, and acetylcholinesterase inhibitors are commonly used to treat the memory impairments resulting from Alzheimer's disease (Trinh et al., 2003).
The present experiment was conducted in middle-aged (12 months) and very old (24 months) APP/PSEN1 mice and wild-type controls. APP/PSEN1 mice manifest the Aβ deposits, elevated oxidative stress, and cognitive impairments found in Alzheimer's disease. APP/PSEN1 and wild-type mice were treated with ascorbate (i.p.) or the vehicle and tested in the Y-maze and Morris water maze. Following behavioral testing brain tissue was examined to investigate the effect of a short-term course of acute ascorbate treatments on ascorbate, oxidative stress, Aβ deposition and AChE activity.
Methods
Animals
APPSwe/PSEN1ΔE9 bigenic mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA; stock no. 004462) and maintained as double hemizygotes by crossing with wild-type individuals on a B6C3F1/J background strain (Jackson Laboratories stock no. 100010). These mice carry two mutations associated with early onset or familial Alzheimer's disease and exhibit amyloid aggregation and amyloid-related neuropathology, elevated oxidative stress, age-dependent cholinergic dysfunction, and cognitive decline (Bernardo et al., 2008; Lalonde et al., 2005; Machova et al., 2008; Reiserer et al., 2007). The two transgenes co-segregate in these mice such that all offspring are either wild-type or double transgenics. Genotypes were confirmed by polymerase chain reaction analysis of tail biopsies at 21 days of age and again at the end of the experiment when DNA was obtained post-mortem from a new sample of tail tissue. All mice were group-housed by gender in standard tub cages (26.5 × 17 × 12 cm) with fiber bedding under a 12/12-h light/dark cycle (lights on at 0600 h). Mice had free access to food containing a negligible amount of ascorbate (Purina lab chow 5001) and water. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Two groups of mice were used: 12-month-old (“Middle-aged”: wild-type vehicle n=4, wild-type ascorbate n=4, APP/PSEN1 vehicle n=3, APP/PSEN1 ascorbate n=3) and 24-month-old (“Very old”: wild-type vehicle n=6, wild-type ascorbate n=5, APP/PSEN1 vehicle n=4, APP/PSEN1 ascorbate n=4). All mice were behaviorally naïve at the start of testing. Groups comprised approximately equal numbers of male and female mice.
Ascorbate treatments
Ascorbate solutions (125 mg/kg) were prepared immediately before administration in deionised water and adjusted to pH 7. Solutions were kept in the dark in aluminum-foil covered containers and administered within 30 minutes of preparation in order to minimize the effects of oxidation. A total of 13 injections, ascorbate or the vehicle, were administered to each mouse i.p. 60 min. before each daily behavioral testing session (one Y-maze trial, 10 water maze hidden-platform acquisition sessions, one water maze probe trial, one water maze visible-platform session). Administration volume was 10 ml/kg. The dose of 125 mg/kg was selected based on results from previous published reports of changes induced in tests of cognitive function (de Angelis and Furlan, 1995; Parle and Dhingra, 2003; Shahidi et al., 2008) as well as pilot data showing that this dose blocks the amnesic effects of scopolamine (data not shown).
Learning and Memory
Y-maze spontaneous alternation
Spontaneous alternation was tested during a single trial in a standard Y-maze made of clear acrylic tubing, as described previously (Harrison et al., 2008; Reiserer et al., 2007). The maze had three identical arms 32 cm long, radiating symmetrically from the center of the maze. The number and sequence of arm entries made during a 5-min. session were recorded. Alternations were defined as an entry into each arm within three consecutive arm choices with no repetitions (e.g. ABC or BAC). Percent alternation was calculated as the number of alternations divided by the number of total arm entries minus two.
Morris water maze
Water maze acquisition trials began the day following Y-maze testing. Hidden-platform testing was conducted in a 106-cm diameter pool with a circular acrylic platform (10 cm diameter) submerged 1 cm below the surface of the water, as previously described (Bernardo et al., 2007; Harrison et al., 2008). Mice were given four acquisition trials per day for 10 days conducted in a spaced fashion, i.e., each mouse completed its first trial before the first mouse began its second trial. The water maze was located in the centre of a room with distinct, visual cues fixed to the walls that were clearly visible from the pool. Sessions were captured by an overhead camera and analyzed in real time using an NIH Image macro on a Macintosh computer written specifically for the water maze task (Miyakawa et al., 2001). Latency and path length to reach the hidden platform were the variables of interest during task acquisition. Distance from the platform, termed search error, was calculated for each acquisition trial and a daily average was calculated. This measure reflects not only search duration and path length but also proximity to the platform during the search. Twenty-four hours following target acquisition training a 60-s probe trial was conducted in which the platform was removed from the pool. During the probe trial memory for the platform location was assessed by measuring time spent swimming near the previous platform location. The time spent in the target and non-target quadrants and the average distance from the platform location (search error) were the primary dependent measures derived from the probe trial. Search error may be a more sensitive measure of selective search on the probe trial than time in quadrant (Gallagher et al., 1993). Swim speed was also assessed in the water maze to determine whether differences in performance could be attributed to non-cognitive factors. Test order was the same for all mice.
Following the hidden platform probe trial four visible-platform training trials were administered in a single session. The visible-platform version of the water maze demonstrates that mice have the sensorimotor capabilities and motivation to complete that task, and that changes in performance can be attributed to spatial learning and not to non-cognitive factors. For visible-platform training trials the platform was no longer submerged but protruded slightly (aprox. 5 mm) above the surface of the water. The platform was also marked by a conspicuous black ball on top of a pole that was fixed to the top of the platform. The platform was located in a different quadrant during each trial but was always visible throughout the trial as described above.
Neurochemistry
24 hours following the final behavioral test mice were briefly anaesthetized using isoflurane and sacrificed by decapitation. Trunk blood was collected and stored on wet ice for 1 hour, and subsequently centrifuged at 13 000 rpm for 20 minutes. Serum was collected from each sample for measurement of ascorbate levels. Liver samples were removed and weighed and kept at - 80 °C until assayed. Brains were quickly removed and cut along the midline. Cortical tissue was dissected from one hemi-brain and stored at - 80 °C until needed for ascorbate, oxidative stress and acetylcholinesterase assays. The second hemi-brain was placed in a 10% formalin solution for three days as a fixative and then stored in phosphate buffered saline for assessment of aggregated Aβ.
Ascorbate
Ascorbate levels were measured in cortex, liver and blood serum. Concentrations were measured by ion pair HPLC (Pachla and Kissinger, 1979) and electrochemical detection as previously described (May et al., 1998) except that tetrapentyl ammonium bromide was used as the ion pair reagent. Tissue samples were weighed and wet tissue was homogenized in a 1.5-ml microfuge tube with a combination of two solutions, 25% (w/v) aqueous metaphosphoric acid and 100 mM sodium phosphate buffer containing 5 mM EDTA, mixed together in a ratio of 2:7. A total of 10 μl of buffer solutions was used for each mg of tissue. The samples were then centrifuged at 13,600 g for 4 min at 4° C, and aliquots of the clear supernatant were taken for assay of ascorbate as described above following appropriate dilution with HPLC mobile phase.
Malondialdehyde
Malondialdehyde was measured by homogenizing small, weighed tissue samples in 1 ml 5% TCA solution. Samples were centrifuged at 13 000 rpm at room temperature for 5 mins. 250 μl of sample was reacted with the same volume of 20 mM thiobarbituric acid for 35 minutes at 95 °C, followed by 10 minutes at 4 °C. Malondialdehyde was then specifically measured using HPLC with inline fluorescence detection of the malondialdehyde-thiobarbituric acid adduct (Sabharwal and May, 2008).
Acetylcholinesterase activity
Acetylcholinesterase activity was measured using a colorimetric assay based on the methods of (Ellman et al., 1961). The protocol was modified for use with 96 well microtiterplates. Weighed tissue samples were homogenized in 0.1 M phosphate buffer (pH 8) with 0.05 % Triton X. Samples were centrifuged at 13 000 rpm at 4 °C for 30 mins and 40 μl of sample was added to separate wells of a 96-well microtiterplate that contained 260 μl 0.1 M sodium phosphate buffer (pH 8) and 10 μl of 0.01 M DTNB (5,5'-dithiobis(2-nitrobenzoic acid) Sigma Aldrich, USA). The substrate acetylthiolcholine iodide (2 μl of 0.075 M, Sigma Aldrich, USA) was added to each test well immediately before activity measurement using a kinetics program on a Spectramax M5 microplate reader (Molecular Devices, USA). The change in absorbance per minute of the sample was measured at 405 nm and the slope was calculated for the first 5 minutes of the reaction. Rates of change were calculated from the slope per mg of sample.
Amyloid β deposition
Sections (30 μm thick) were cut from the formalin-fixed hemi-brain using a freezing microtome, and mounted on glass slides. Thioflavin S (Sigma Aldrich, USA) staining was conducted and analyzed as described previously (Bernardo et al., 2008; Bernardo et al., 2007). Digital images of the hippocampus and overlying cortex were taken using a fluorescent imaging microscope with an Axiocam high-resolution camera (Carl Zeiss Microimaging Inc., Thornwood, NY, USA) at a magnification of 40X. The area of the hippocampus and overlying cortical areas occupied by amyloid plaques was determined using the freely-available Image J software (National Institute of Health, Bethesda, MD, USA). Quantification was performed by an experienced reader who was blind to the treatment condition of mice. Plaque coverage was calculated as percent of total region measured in pixels. Three sections were assayed per mouse for hippocampus and cortex, and the mean value used to calculate percent coverage for each area.
Statistics
All data were analyzed using SPSS 16.0 for Windows. There were no independent effects of sex so data were collapsed into treatment groups for further analyses. For single dependent variables in the Y-maze (arm entries and percent alternation), and neurochemical assays univariate ANOVAs were conducted with Age, Genotype and Treatment as between-groups factors. Water maze acquisition measures of escape latency, path length, swim speed, and search error were analyzed using repeated measures ANOVA (RMANOVA) with the same between-groups factors of Age, Genotype, and Treatment, and session or trial as a within-subjects factor. For water maze probe trial analysis, time spent in target and non-target quadrants was analyzed using separate RMANOVAs for each group with quadrant as the repeated measure. Probe trial search error was analyzed using Univariate ANOVAs within each age group separately with Genotype and Treatment as the between-subjects factors. In the case of violations of the assumption of sphericity analyses were corrected using Greenhouse-Geisser adjustments and we report the value for epsilon (ε) and the adjusted degrees of freedom rounded to the nearest integer (Keppel, 1991). In order to assess effect size, we also report partial Eta squared (hp 2) for significant results.
Results
Y-maze
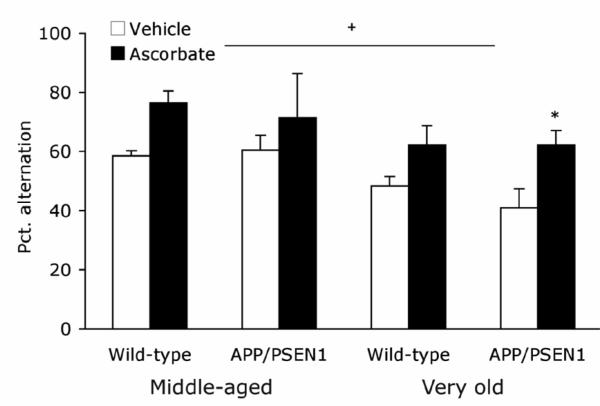
There were no effects of age, genotype, or ascorbate treatment on the number of arm entries made during the 5-min Y-maze session [Fs < 2.57, Ps >.13]. Ascorbate treatment improved alternation rates in middle aged and very old mice [F1, 25 = 11.401, P=.002, hp 2 =.31, Fig. 1]. There was no effect of genotype nor any interactions among the factors [Fs < .580, Ps >.454]. Alternation rates were approximately 10% lower in very old mice than in middle aged mice [F1, 25 = 7.945, P =.009, hp 2 =.24].
Figure 1. Ascorbate increases spontaneous alternation in middle-aged and very old APP/PSEN1 and Wild-type mice.
Spatial working memory was tested in the Y-maze spontaneous alternation task. Middle-aged and Very old ascorbate-treated mice alternated more often than vehicle-treated age-matched mice and percent alternation was lower in Very old mice relative to Middle-age mice. There was no difference between wild-type (white bars) and APP/PSEN1 mice (black bars). Symbols: Ascorbate-treated vs. vehicle-treated mice * P<.05. Very old vs. Middle-aged mice +P<.01.
Water maze
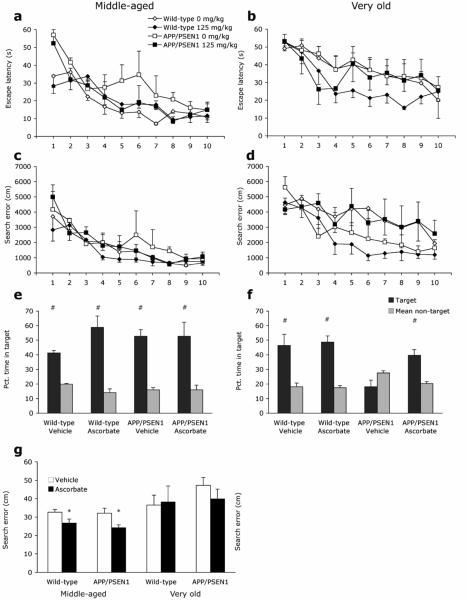
All mice learned to locate the submerged platform across the 10 days of water maze training as shown by decreasing escape latencies [F9, 216 = 26.966, P<.001, hp 2 =.53; Fig 2a-b]. Across training trials, Very old mice took longer to locate the platform than Middle-aged mice [F1, 24 = 19.485, P<.001, hp 2 =.49]. Although untreated APP/PSEN1 Middle-aged mice exhibited the poorest learning and ascorbate-treated Very old wild-type mice had the shortest escape latencies genotype differences were not significant [F1, 24 = 3.090, P=.092] and there was no difference according to treatment group during task acquisition [F1, 24 = 2.208, P=.15]. A similar improvement across training sessions was observed for path length data [F9, 216 = 30.483, P<.001, hp 2 =.56]. Overall APP/PSEN1 mice had longer path lengths than wild-type mice although this difference was not significant [F1, 24 = 4.01, P=.057] but there was no difference between ascorbate-treated and untreated mice [F1, 24 = .667, P=.422]. Significant effects of age [Age F1, 24 = 8.094, P=.009, hp 2 =.25, Age X Day F9, 216 = 1.952, P<.046, hp 2 =.08] confirmed that Very old mice performed more poorly than Middle-aged mice throughout acquisition trials. Swim speed did not vary according to genotype or treatment [Fs<.684, Ps>.416], but Very old mice swam more slowly than the Middle-aged mice [F1, 24 =5.194, P=.032, hp 2 =.18]. Search Error decreased across training sessions for all mice, indicating improved search efficiency [F9, 216 = 28.246, P<.001, ε=.533, hp 2 =.54; Fig.2 c-d]. Search error was greater in the Very old relative to the Middle-aged mice [F1, 24 = 15.281, P<.001, hp 2 =.39]. Modest improvements in search error following ascorbate treatment [F1, 24 = 3.465, P=.075] and in wild-type mice compared to APP/PSEN1 mice [F1, 24 = 3.195, P=.087] were not significant. There were no significant interaction effects [Fs<1.43, Ps>.18]. When the two age groups were considered separately a significant effect of genotype was evident in the Middle-aged [F1, 10 = 9.28, P=.012, hp 2 =.48] but not the Very old mice [F1, 14 = .93, P=.35], presumably due to the additional impairments seen in very old wild-type mice.
Figure 2. Ascorbate improves water maze performance in Middle-aged and Very old APP/PSEN1 and wild-type mice.
Spatial reference memory was tested in the Morris water maze task. (a - d) All mice improved during the 10 days of water maze acquisition as shown by decreasing escape latencies (a & b) and search error (c & d). Both escape latencies and search error were greater in Very old mice compared to Middle-aged mice during water maze acquisition. Ascorbate treatment did not affect water maze acquisition. In the water maze probe trial (e-g) memory was assessed by time spent within target and non-target quadrants (e & f) and search error (g). (e) All Middle-aged mice demonstrated memory for the platform location, spending more time swimming in the platform quadrant than non-platform quadrants. In contrast, (f) spatial memory was impaired in vehicle-treated Very old APP/PSEN1 mice, as indicated by random swimming random during the probe trial. (g) Ascorbate-treated Middle-aged mice had lower search error than vehicle-treated controls, indicating better memory for the platform location. The same pattern was not observed in Very old mice. Ascorbate treatments reversed this impairment in the Very old transgenics. Symbols: Ascorbate-treated vs. vehicle-treated mice * P<0.05. Target vs. non-target swim time # P<.05.
A 60-s probe trial was conducted 24 hours after the final acquisition trial. All Middle-aged mice demonstrated memory of the platform position by evincing a preference for the pool quadrant that had previously held the platform, compared to the other three quadrants [Wild-type vehicle F3, 9 = 25.89, P<.001, hp 2 =.90; Wild-type ascorbate F3, 9 = 12.04, P=.002, hp 2 =.80; APP/PSEN1 vehicle F3, 6 = 30.27, P<.001, hp 2 =.94; APP/PSEN1 ascorbate F3, 6 = 7.84, P=.017, hp 2 =.80; Fig. 2e]. Search error was not affected by genotype in Middle-aged mice [F1, 9 = .522, P=.49], but ascorbate-treated animals of both genotypes swam closer to the platform location than vehicle-treated mice [F1, 9 = 7.16, P=.025, hp 2 =.44; Fig. 2g]. In Very old mice, preference for swimming in the platform quadrant was observed in both groups of wild-type mice [Wildtype vehicle F3, 15 = 4.58, P=.018, hp 2 =.48; Wild-type ascorbate F3, 12 = 18.61, P<.001, hp =.82]. Vehicle-treated APP/PSEN1 mice did not show a preference for the platform location; however, ascorbate-treated transgenics were more likely to swim in the target quadrant than the non-target quadrants [APP/PSEN1 vehicle F3, 9 = 1.88, P=.203, hp 2 =.39; APP/PSEN1 ascorbate F3, 6 = 5.27, P=.041, hp 2 =.80; Fig. 2f]. The measure of search error showed a similar trend, but the treatment-induced improvements were not significant [F1, 14 = 3.54, P=.081; Fig. 2g] and there was no effect of genotype [F1, 14 = 2.76, P=.12].
All mice were able to locate the platform when marked by an obvious visible cue, despite its being located in a different quadrant on each trial. Performance improved for all groups across the 4-trial session [Middle-aged F3, 30 = 15.02, P<.001, hp 2 =.60; Very old F3, 42 = 5.44, P=.003, hp 2 =.28], indicating that mice were capable of using visual cues to locate the target. There were no effects of treatment or genotype and no interactions for either age group [Fs<3.75, Ps>.073].
Neurochemistry
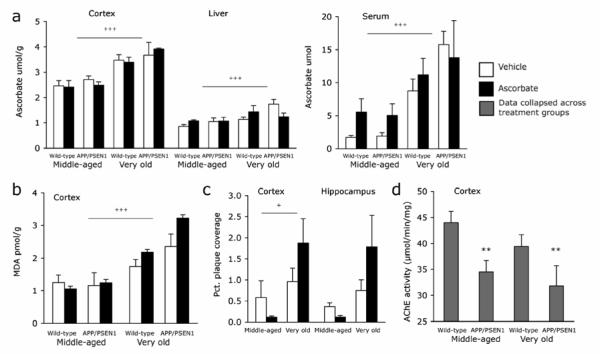
Mice that had been tested in the Y-maze and water maze were sacrificed 24 hours after the water maze probe session. Ascorbate was measured in the cortex, liver and serum to determine whether acute treatments had altered long-term storage or circulating levels of ascorbate. Ascorbate levels were higher in cortex, liver, and serum of Very old compared to Middle-aged mice [Fs>13.794, Ps <.001], but no effects of genotype or treatment were observed in either age group 24 hours following the final administration [Fs <2.218, Ps>.149; Fig. 3a]. Malondialdehyde was quantified as a marker of lipid peroxidation. No main effects of genotype or treatment were observed in the cortex in Middle-aged or Very old mice [Fs<1.718, Ps>.202, Fig. 3b]. Malondialdehyde levels were significantly higher in Very old compared to Middle-aged mice [F1, 24 = 44.307, P<.001, hp 2 =.65]. There were no significant effects of genotype or treatment on malondialdehyde levels [Fs< 1.718, Ps>.202]. Deposited Aβ plaques were quantified in the hippocampus and cortex of Middle-aged and Very old mice. Very old APP/PSEN1 mice had greater plaque deposition in the cortex than Middle-aged mice [F1, 8 = 5.27, P<.05, hp 2 =.40; Fig. 3c and Fig. 4], but not in the hippocampus [F1, 8 = 3.33, P=.11]. Treatment with ascorbate did not affect plaque deposition in either the cortex or hippocampus [Fs<48, Ps>.51]. Acetylcholinesterase activity was significantly lower in APP/PSEN1 mice than wild-types at both ages [F1, 24 = 8.811, P=.007, hp 2 =.25; Fig. 3d]. There was a small decrease in enzyme activity with age but this difference was not significant and there was no main effect of treatment and no interactions between the factors [Fs<1.583, P>.220].
Figure 3. Ascorbate, oxidative stress and amyloid deposits increase with age.
Relative to Middle-aged mice, Very old mice had (a) higher ascorbate levels in cortex, liver and serum, and (b) increased malondialdehyde levels in the cortex. Neither genotype nor treatment affected ascorbate or malondialdehyde levels. (c) Aβ plaque deposits increased in the cortex with age but were unaffected by treatment. (d) AChE activity was significantly lower in APP/PSEN1 mice than wild-type mice but did not vary significantly according to treatment or age. Data shown are collapsed across treatment group. Symbols: Very old vs. Middle-aged mice +++ P<0.001; APP/PSEN1 vs. wild-type mice ** P<0.01.
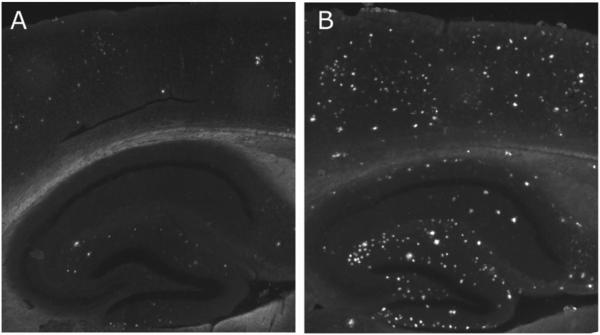
Figure 4. Very old APP/PS1 mice exhibit increased levels of Aβ deposits compared with Middle-aged APP/PS1 mice.
Representative images of Thioflavin-S staining in hippocampus and overlying cortex in (a) Middle-aged and (b) Very old APP/PS1 mice. Images shown reflect the median percent plaque coverage for each age group.
Discussion
In the present study, acute parenteral treatment with ascorbate reversed some of the spatial learning and memory deficits found in APP/PSEN1 transgenic and aged wild-type mice. The ascorbate treatments had acute effects that were independent of long-term changes in lipid peroxidation, acetylcholinesterase activity or Alzheimer-type neuropathology in transgenic mice.
The administration of noötropic compounds has been shown to improve Y-maze spontaneous alternation performance following administration of scopolamine (Kim et al., 2004), intracerebroventricular administration of Aβ(25-35) (Tsunekawa et al., 2008), or in APP + PS1 transgenic mice (Joseph et al., 2003). In the present study APP/PSEN1 mice were unimpaired in the Y-maze compared to wild-type controls, a finding that has been reported previously in these mice (Reiserer et al., 2007). Untreated, Middle-aged APP/PSEN1 and wild-type mice had normal levels of alternation (aprox. 65%), and untreated Very old mice had lower alternation levels (aprox. 40-50%) than Middle-aged mice regardless of genotype. Ascorbate-treated Middle-aged mice alternated at significantly higher rates (~80%) than vehicle-treated controls. This rate is also higher than that reported in younger mice of this mutant line (~62%) tested in the same environment at 7 months of age (Reiserer et al., 2007), and indicates that ascorbate may improve cognition in less-compromised systems.
We observed modest, non-significant group differences during water maze acquisition. As shown in Figure 2c-d, the most superior performance was seen in the ascorbate-treated wild-type mice, although treatment effects were not significant. In Very old mice the data were less clear than in Middle-aged mice with the absence of a significant impairment in the transgenic animals. Group heterogeneity tends to increase with age in several aspects of animal and human cognition (Gallagher and Rapp, 1997; Morse, 1993), and overall, poorer performance was observed in Very old mice of both genotypes compared to Middle-aged mice. Nevertheless, significant genotype and treatment effects in spatial memory were evident on the probe trial. All Middle-aged mice exhibited memory for the platform location, showing a preference for swimming in the platform quadrant over non-platform quadrants. Very old, untreated transgenic mice did not show preferential swim patterns during the probe trial, whereas the ascorbate-treated transgenic mice and both wild-type groups demonstrated a clear preference for swimming in the quadrant that had previously contained the platform. Search error is a more precise measure of search accuracy and revealed improved efficiency of search in Middle-aged ascorbate-treated mice compared to untreated mice. In Very old mice, the visible difference in group performance in the quadrant time measure following ascorbate treatment was not significant in the search error data. This result suggests that the improvement was not as great as that seen in the Middle-aged group, especially considering the greater search error observed in all of the very old mice compared to the younger animals in this experiment. Thus, spatial memory was improved by ascorbate treatments, albeit in a heterogeneous manner, in both Middle-aged mice (search error) and Very old mice (target quadrants).
Good performance on the visible platform version of the water maze supports the idea that the deficits, and partial rescue by ascorbate, were due to spatial learning and not to impaired sensorimotor abilities. The greatest benefit of ascorbate treatment was observed in Very old mice that had the lowest untreated Y-maze alternation rates and poorest water-maze search accuracy, indicating that ascorbate treatment may exert its greatest benefit in systems that are heavily compromised. These results are consistent with those reported by Arzi and colleagues (Arzi et al., 2004), in which orally-administered treatments of vitamin C combined with vitamin E improved performance in a passive avoidance task in aged (15 months) but not young (3 months) mice. Consistent with Wilder's law of initial value (Wilder, 1967), cases in which there are no improvements in cognition, such as in young mice, may reflect baseline cognitive performance that is too good to be able to detect an effect of treatment. Alternatively, the mechanism of action of ascorbate may only be effective in systems that have undergone some thus-far unidentified change due to age or disease. Furthermore, although only one dose of ascorbate (125 mg/kg) was used in this study, it may be that administration of higher doses would lead to even greater effects in aged mice and also improve performance in less-impaired animals. Either way, the data presented here suggest that cognitive rescue can be achieved to some degree even in animals suffering from severe neuropathology.
Ascorbate-induced improvements were observed in mice following a single treatment of ascorbate. In the water maze the largest changes were seen in the probe trial on the 12th day of ascorbate administration. Although no cumulative effects of ascorbate injections were detectable in ascorbate or MDA levels 24 hours following the final injection, this does not preclude the possibility that some sensitization or other mechanism caused a cumulative effect on cognitive ability, making later injections more potent. Thus it is possible that if Y maze testing had been conducted following water maze testing even greater effects would have been observed.
Thus far the mechanism of action for ascorbate on cognition is not clear. Ascorbate (i.p.) has previously been shown to have anti-cholinesterase properties (Dhingra et al., 2006), however, this result has not been widely investigated. Acetylcholinesterase inhibitors (AChEIs) are the largest class of drugs available with which to treat Alzheimer's disease. We present data to show decreased AChE activity in the APP/PSEN1 mice and posit that this change reflects an overall diminishing of the cholinergic activity in these mice as previously reported (Machova et al., 2008; Savonenko et al., 2005). Nevertheless, the data presented here are insufficient to ascribe a mechanistic relationship and other potential mechanisms are also likely. For example, ascorbate is an essential co-factor for the synthesis of norepinephrine from dopamine and thus additional exogenous ascorbate may impact catecholaminergic signaling. Furthermore, increases in glutamate in the striatum, hippocampus and other forebrain areas and cortical projections lead to the release of ascorbate from neurons (Cammack et al., 1991; O'Neill et al., 1984; Pierce and Rebec, 1993). This relationship persists whether the glutamate increase results from exogenous administration of glutamate or its release from within neurons (O'Neill et al., 1984). Specifically, ascorbate release is associated with reuptake of glutamate. Excess glutamate can be neurotoxic and thus the functional relationship with ascorbate may be to provide a protective role against glutamatergic neurotoxicity. Furthermore, many neurotransmitter receptors show a decreased affinity to oxidized neurotransmitter forms. It is possible the release of intracellular ascorbate may provide antioxidant protection to neurotransmitters at the synaptic cleft and thus play a vital functional role in maintaining optimal neurotransmitter signaling. Thus ascorbate release, rather than overall brain tissue levels may help to paint a clearer picture of how ascorbate treatments interact with cognition.
Serum ascorbate increases substantially following i.p. administration of ascorbate and can remain high for up to 2 hours following treatment (Chen et al., 2007). In the present study 12 days of daily ascorbate injections did not increase stored ascorbate levels in the brain or circulating levels in serum when measured 24-hours following the final treatment. This reflects the tight regulation of ascorbate levels in mammals. These data also support the idea that the effects of treatment in the present study were acute and via pharmacological-type mechanisms rather than via the long-term antioxidant role of ascorbate. Although there was no difference between genotypes, ascorbate levels were elevated in the Very old mice in the present study. Antioxidant levels have been shown to decline with age concomitant with age-related increases in oxidative stress (Harman, 1981). However, many studies have reported unchanged or increased ascorbate levels in brain and other tissues in older animals (Lykkesfeldt and Moos, 2005; Rikans and Moore, 1988). In the present study malondialdehyde levels increased in the cortex with age. It is possible that the age-dependent rise in ascorbate seen in the present study reflects increased ascorbate synthesis in very old mice in response to the presence of increased free radicals or to oxidative injury. Because mice can synthesize their own ascorbate, this may be an additional defense mechanism available to them to defend against oxidative stress. Nevertheless, it is possible that there are conditions when the oxidative damage is too great and overwhelms such mechanisms. Another possible explanation for our findings is that the increased oxidant stress associated with normal and pathological aging may have upregulated vitamin C transport into the cells via the SVCT2 transporter (Sotiriou et al., 2002; Tsukaguchi et al., 1999). Increased transport would allow increased storage of ascorbate in the brain; however, this mechanism of upregulation has not been investigated.
The short, 12-day series of acute ascorbate treatments did not affect deposited Aβ. This result is not surprising since plaques are first detectable at 4-5 months in these mice (Garcia-Alloza et al., 2006) and had thus been developing for 7-8 months in the Middle-aged group and 16-17 months in the Very old mice. Aβ plaques are considered end points in the disease process, detectable following a series of changes in normal cellular function and reflectingting a lifetime of accumulation. It is possible that ascorbate had an effect on soluble Aβ, which has been shown to be amnestic (McDonald et al., 1994). Soluble Aβ was not measured in this experiment and might have been susceptible to the drug-like actions of ascorbate, although the mechanism through which this might be achieved is not known. Although ascorbate treatments did not lower oxidative stress or affect Aβ aggregation in the brain, they improved memory in wild-type as well as APP/PSEN1 mice. This suggests that ascorbate acted independently of aggregated Aβ and related neuropathology to reverse memory impairments in the transgenics and even provide some measure of cognitive enhancement in wild-type mice. In fact many different facets of neuropathology, such as oxidative stress and changes in Aβ secretion, occur early in the disease process before cognitive deficits are detectable. We therefore suggest that earlier interventions with ascorbate might mediate some of these changes and thus play a preventative role against disease development as well as ameliorating deficits in older, more compromised mice as we have demonstrated here. In the present study, cognitive ability was measured an hour following ascorbate administration and neurochemical assays were performed on tissue from mice sacrificed 24 hours following treatment in order to assess long-term changes in ascorbate levels Aβ deposition, and oxidative stress. There may, therefore, have been acute changes in brain ascorbate or oxidative stress that were not detected.
A discussion of the data presented here must consider that peripheral effects of ascorbate may have contributed to improved cognitive ability. For example, if ascorbate had conferred a cardiovascular or visual (retinal) advantage, or reduced anxiety, it is possible that performance may have been modestly affected independent of cognition. Control measures such as swim speed, peripheral swimming, floating, or tail shakes, and proficient performance on the cued platform task make it unlikely that the improvements are due to the peripheral actions of ascorbate. Nevertheless, until the underlying mechanism of improvement has been identified, such actions cannot be entirely ruled out and must be carefully controlled for in any future experiments.
The present experiments show clearly that ascorbate can improve learning and memory in aged wild-type and APP/PSEN1 transgenic mice and that the beneficial effects of systemic ascorbate are likely via alternative pathways to the long-term antioxidant effects that are activated by continuous dietary intake of ascorbate. Although the mechanism of the ascorbate-induced improvements in cognition remains to be elucidated, exploration of ascorbate as a potential pharmacological avenue for the treatment of Alzheimer's disease, mild cognitive impairment (MCI), and age-related cognitive decline is warranted.
Acknowledgements
This work was supported by grants from the National Institute of Health (AG023138 to James M May) and (AG022439 to Michael P McDonald).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alzheimer's Association 2008 Alzheimer's disease facts and figures. Alzheimers Dement. 2008;4:110–33. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Agus DB, et al. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest. 1997;100:2842–8. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, et al. Effect of vitamins C and E on cognitive function in mouse. Pharmacol Res. 2004;49:249–52. doi: 10.1016/j.phrs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Bernardo A, et al. Elimination of GD3 synthase improves memory and reduces amyloid-beta plaque load in transgenic mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Bernardo A, et al. Impaired spatial memory in APP-overexpressing mice on a homocysteinemiainducing diet. Neurobiol Aging. 2007;28:1195–205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Burk RF, et al. A combined deficiency of vitamins E and C causes severe central nervous system damage in guinea pigs. J Nutr. 2006;136:1576–81. doi: 10.1093/jn/136.6.1576. [DOI] [PubMed] [Google Scholar]
- Cammack J, et al. The pharmacological profile of glutamate-evoked ascorbic acid efflux measured by in vivo electrochemistry. Brain Res. 1991;565:17–22. doi: 10.1016/0006-8993(91)91731-f. [DOI] [PubMed] [Google Scholar]
- Charlton KE, et al. Lowered plasma vitamin C, but not vitamin E, concentrations in dementia patients. J Nutr Health Aging. 2004;8:99–107. [PubMed] [Google Scholar]
- Chen Q, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104:8749–54. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- de Angelis L, Furlan C. The effects of ascorbic acid and oxiracetam on scopolamine-induced amnesia in a habituation test in aged mice. Neurobiol Learn Mem. 1995;64:119–24. doi: 10.1006/nlme.1995.1050. [DOI] [PubMed] [Google Scholar]
- Dhingra D, et al. Comparative brain cholinesterase-inhibiting activity of Glycyrrhiza glabra, Myristica fragrans, ascorbic acid, and metrifonate in mice. J Med Food. 2006;9:281–3. doi: 10.1089/jmf.2006.9.281. [DOI] [PubMed] [Google Scholar]
- Ellman GL, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, et al. Dietary intake of antioxidants and risk of Alzheimer disease. Jama. 2002;287:3223–9. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- Gallagher M, et al. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–70. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, et al. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–24. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981;78:7124–8. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, et al. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. J Neurochem. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges RE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432–43. doi: 10.1093/ajcn/24.4.432. [DOI] [PubMed] [Google Scholar]
- Joseph JA, et al. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–62. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis : a researcher's handbook, vol. Prentice Hall; Englewood Cliffs, N.J.: 1991. [Google Scholar]
- Kim HK, et al. Effects of green tea polyphenol on cognitive and acetylcholinesterase activities. Biosci Biotechnol Biochem. 2004;68:1977–9. doi: 10.1271/bbb.68.1977. [DOI] [PubMed] [Google Scholar]
- Lalonde R, et al. Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+PS1/DeltaE9 mice with amyloid plaques. Neurosci Lett. 2005;390:87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Lee L, et al. Effect of supplementation of vitamin E and vitamin C on brain acetylcholinesterase activity and neurotransmitter levels in rats treated with scopolamine, an inducer of dementia. J Nutr Sci Vitaminol (Tokyo) 2001;47:323–8. doi: 10.3177/jnsv.47.323. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Moos T. Age-dependent change in Vitamin C status: a phenomenon of maturation rather than of ageing. Mech Ageing Dev. 2005;126:892–8. doi: 10.1016/j.mad.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, et al. Vitamin C deficiency in weanling guinea pigs: differential expression of oxidative stress and DNA repair in liver and brain. Br J Nutr. 2007;98:1116–9. doi: 10.1017/s0007114507787457. [DOI] [PubMed] [Google Scholar]
- Machova E, et al. Impairment of muscarinic transmission in transgenic APPswe/PS1dE9 mice. Neurobiol Aging. 2008;29:368–78. doi: 10.1016/j.neurobiolaging.2006.10.029. [DOI] [PubMed] [Google Scholar]
- May JM, et al. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–9. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- McDonald MP, et al. Effects of an exogenous beta-amyloid peptide on retention for spatial learning. Behav Neural Biol. 1994;62:60–7. doi: 10.1016/s0163-1047(05)80059-7. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, et al. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–75. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- Morris MC, et al. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:121–6. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- Morse CK. Does variability increase with age? An archival study of cognitive measures. Psychol Aging. 1993;8:156–64. doi: 10.1037//0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- O'Neill RD, et al. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci Lett. 1984;52:227–33. doi: 10.1016/0304-3940(84)90166-6. [DOI] [PubMed] [Google Scholar]
- Pachla LA, Kissinger PT. Analysis of ascorbic acid by liquid chromatography with amperometric detection. Methods Enzymol. 1979;62:15–24. doi: 10.1016/0076-6879(79)62183-3. [DOI] [PubMed] [Google Scholar]
- Parle M, Dhingra D. Ascorbic Acid: a promising memory-enhancer in mice. J Pharmacol Sci. 2003;93:129–35. doi: 10.1254/jphs.93.129. [DOI] [PubMed] [Google Scholar]
- Pettenuzzo LF, et al. Ascorbic acid prevents cognitive deficits caused by chronic administration of propionic acid to rats in the water maze. Pharmacol Biochem Behav. 2002;73:623–9. doi: 10.1016/s0091-3057(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Rebec GV. Intraneostriatal administration of glutamate antagonists increases behavioral activation and decreases neostriatal ascorbate via nondopaminergic mechanisms. J Neurosci. 1993;13:4272–80. doi: 10.1523/JNEUROSCI.13-10-04272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiserer RS, et al. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Moore DR. Effect of aging on aqueous-phase antioxidants in tissues of male Fischer rats. Biochim Biophys Acta. 1988;966:269–75. doi: 10.1016/0304-4165(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Riviere S, et al. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int J Geriatr Psychiatry. 1998;13:749–54. doi: 10.1002/(sici)1099-1166(1998110)13:11<749::aid-gps860>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Sabharwal AK, May JM. alpha-Lipoic acid and ascorbate prevent LDL oxidation and oxidant stress in endothelial cells. Mol Cell Biochem. 2008;309:125–32. doi: 10.1007/s11010-007-9650-z. [DOI] [PubMed] [Google Scholar]
- Savonenko A, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–17. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Shah RS, et al. Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother. 2008;62:199–207. doi: 10.1016/j.biopha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Shahidi S, et al. Ascorbic acid supplementation could affect passive avoidance learning and memory in rat. Brain Res Bull. 2008;76:109–13. doi: 10.1016/j.brainresbull.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Sotiriou S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med. 2002;8:514–7. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- Trinh NH, et al. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. Jama. 2003;289:210–6. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–5. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Tsunekawa H, et al. Synergistic effects of selegiline and donepezil on cognitive impairment induced by amyloid beta (25-35) Behav Brain Res. 2008;190:224–32. doi: 10.1016/j.bbr.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wilder J. Stimulus and Response: The Law Of Initial Value. Wright; Bristol, England: 1967. [Google Scholar]