Abstract
Vibrio vulnificus is a bacterial contaminant of shellfish and causes highly lethal sepsis and destructive wound infections. A definitive identification of virulence factors using the molecular version of Koch's postulates has been hindered because of difficulties in performing molecular genetic analysis of this opportunistic pathogen. For example, conjugation is required to introduce plasmid DNA, and allelic exchange suicide vectors that rely on sucrose sensitivity for counterselection are not efficient. We therefore incorporated USER friendly cloning techniques into pCVD442-based allelic exchange suicide vectors and other expression vectors to enable the rapid and efficient capture of PCR amplicons. Upstream and downstream DNA sequences flanking genes targeted for deletion were cloned together in a single step. Based on results from Vibrio cholerae, we determined that V. vulnificus becomes naturally transformable with linear DNA during growth on chitin in the form of crab shells. By combining USER friendly cloning and chitin-based transformation, we rapidly and efficiently produced targeted deletions in V. vulnificus, bypassing the need for two-step, suicide vector-mediated allelic exchange. These methods were used to examine the roles of two flagellin loci (flaCDE and flaFBA), the motAB genes, and the cheY-3 gene in motility and to create deletions of rtxC, rtxA1, and fadR. Additionally, chitin-based transformation was useful in moving antibiotic resistance-labeled mutations between V. vulnificus strains by simply coculturing the strains on crab shells. The methods and genetic tools that we developed should be of general use to those performing molecular genetic analysis and manipulation of other gram-negative bacteria.
Vibrio vulnificus is a halophilic bacterium present naturally in estuarine waters and often contaminates oysters and other shellfish (for a review, see reference 15). V. vulnificus is an opportunistic pathogen of humans, causing primary septicemia and wound infection in susceptible individuals, and is the leading cause of reported seafood-related deaths in the United States. In susceptible humans, V. vulnificus causes a rapid, fulminating disease process resulting in extensive tissue damage. Mortality rates for susceptible individuals who develop fulminating primary septicemia are greater than 50% (17). Skin infections can lead to severe cellulitis, necrotizing fasciitis, and myositis requiring surgical debridement of infected tissues or amputation of the limb (4, 29, 42). Therapeutic intervention is often difficult since death can occur in less than 24 h after contact with the bacteria. In a mouse model of infection, V. vulnificus replicates extremely rapidly in host tissues (40, 41) and kills host cells including neutrophils (41).
Over 20 years of genetic analysis, only a few virulence factors have been identified and confirmed by using the molecular version of Koch's postulates (15). Among the confirmed virulence factors are capsular polysaccharide (49), acquisition of iron (34, 52), type IV pilus (37), RTX toxins (21, 25, 30), and flagella (22, 26). Despite these advances, the full spectrum of virulence factors responsible for the rapid and destructive disease process has not been elucidated. A major hindrance to the molecular genetic analysis of V. vulnificus is the fact that the bacteria cannot be effectively electroporated, nor can the bacteria be chemically transformed. Therefore, the only effective means of introducing plasmid DNA is by conjugation. This limitation severely restricts the availability of plasmids for creating and complementing mutations for molecular genetic analysis.
A classical method to create mutants of V. vulnificus is the use of suicide vectors containing transposons, such as TnphoA (31), mini-Tn5phoA (12), mini-Tn5Km2 (12), mini-Tn5lacZ1 (12), mini-Tn10/kan (23), and Himar (1). However, transposon mutagenesis can cause polar mutations and truncated genes. The most definitive genetic analyses can be performed by deleting the genes of interest. The standard method for deleting genes is to clone the flanking upstream and downstream sequences into an allelic exchange suicide vector and introducing the plasmid into the target strain. The suicide vector integrates into the target genome via one of the flanking DNA sequences by a single-crossover event. This process is selected using antibiotic resistance encoded on the vector. The single-crossover strain is then grown without selection to allow for a second crossover that excises the allelic exchange vector. The excision event is enriched using a counterselectable marker encoded on the allelic exchange vector. A commonly used counterselectable marker is sucrose sensitivity, encoded by the sacB gene (13). If the second crossover is in the same flanking sequence as the first, the wild-type genotype is restored; however, if the second crossover is in the opposite flanking sequence, the targeted gene is deleted.
This method of mutagenesis is problematic for V. vulnificus. First, one of the easiest ways to clone PCR amplicons is by TA topoisomerase-mediated ligation (TOPO TA cloning) with commercially available vectors such as pCR2.1 TOPO (Invitrogen, Carlsbad, CA). Unfortunately, these vectors are not mobilizable; hence, they cannot be used directly with V. vulnificus. Furthermore, there are no available TA topoisomerase allelic exchange vectors. Site-specific recombination vectors such as Gateway (Invitrogen) have facilitated PCR-mediated cloning and gene expression; however, none of the relevant vectors is mobilizable. Therefore, essentially all cloning for expression in or mutagenesis of V. vulnificus requires multiple subcloning steps. Second, sucrose sensitivity counterselection is not very efficient with V. vulnificus, as it is time-consuming and involves the screening of large numbers of colonies to find truly sucrose-resistant colonies. In some cases, sacB counterselection has completely failed. Another method for introducing mutations into target strains is the lambda phage red recombinase system (11). However, this procedure involves introducing additional helper plasmids into the target strain, and mobilizable helper plasmids have not been made. Finally, no generalized transducing phages and no methods for transformation have been described for V. vulnificus. Therefore, if one desires to move mutations between strains, the mutations must be cloned into suicide vectors, and the inefficient two-step allelic exchange process must be repeated for each strain.
We describe here the adaption of USER (uracil-specific excision reagent) friendly cloning (3, 14) into allelic exchange and expression vectors that are commonly used with V. vulnificus. USER friendly cloning enables the creation of 3′ overhangs in PCR amplicons by use of deoxyuridine in PCR primers and treatment with USER enzyme mix (uracil DNA glycosylase and DNA glycosylase-lyase endonuclease VIII). The overhangs are complementary to overhangs created in vectors. The method is very adaptable, with immense leeway in choosing the DNA sequences of the overhangs independent of restriction enzyme cleavage sites. USER friendly cloning methods and vectors enable the rapid creation of upstream-downstream clones to delete genes or loci of interest. To alleviate inefficiencies of subcloning into V. vulnificus vectors, we created an array of USER friendly cloning allelic exchange and expression vectors that should be useful to many investigators.
Meibom et al. (32) recently described the ability of Vibrio cholerae to become naturally transformable during growth in the presence of chitin, as either chitohexose or crab shell fragments. We adapted their crab shell system and determined that V. vulnificus can also become naturally transformable during growth on chitin. The bacteria take up linear DNA; hence, as long as selectable markers are used, allelic exchange mutagenesis can be accomplished without the need for inefficient counterselection involving sucrose. We used these methods to create antibiotic resistance-marked deletions in V. vulnificus of the two loci encoding flagellins, flaCDE and flaFBA, and the genes encoding the following functions: the flagellar motor motAB; a critical element of signal transduction for chemotaxis, cheY-3; an RTX toxin, rtxA1; a putative RtxA-modifying enzyme, rtxC; and a regulator of fatty acid metabolism, fadR. These genetic tools and methods will facilitate the molecular genetic analysis of V. vulnificus as well as those of other gram-negative bacteria.
MATERIALS AND METHODS
Bacteria, plasmids, and oligonucleotide primers.
The Escherichia coli and V. vulnificus strains used are listed in Table 1, plasmid constructs are listed in Table 2, and oligonucleotides are listed in Table 3.
TABLE 1.
Bacterial strains used in this work
| Strain | Relevant genotype or descriptiona | Source or reference |
|---|---|---|
| E. coli | ||
| S17-1λpir | RP4 2-Tc::Mu-Km::Tn7 pro thi recA HsdR−M+ λpir | 39 |
| Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG | Invitrogen |
| EC100Dpir+ | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG pir+(DHFR) | Epicentre |
| V. vulnificus | ||
| CMCP6 | Wild type, biotype 1 clinical isolate | 20 |
| M06-24/O | Wild type, biotype 1 clinical isolate | 49 |
| CVD752 | M06-24/O wza::TnphoA | 48 |
| FLA591 | CMCP6 ΔrtxC; constructed using pGTR265 | This work |
| FLA614 | CMCP6 ΔfadR::aph; constructed using pGTR2010 | 6 |
| FLA674 | CMCP6 ΔmotAB::aph; constructed using pGTR1141 | This work |
| FLA677 | CMCP6 ΔflaCDE::aph; constructed using pGTR1136 | This work |
| FLA680 | CMCP6 ΔflaFBA::cat; constructed using pGTR1120 | This work |
| FLA688 | CMCP6 ΔcheY-3::aph; constructed using pGTR1171 | This work |
| FLA711 | ΔflaCDE::aph ΔflaFBA::cat; constructed in FLA680 using pGTR1136 via conjugation and two-step allelic exchange mutagenesis | This work |
| FLA810 | ΔflaCDE::aph ΔflaFBA::cat; constructed using chitin transformation of genomic DNA from FLA677 into FLA680 | This work |
| FLA900 | CMCP6 ΔrtxA1::aph; constructed using pGTR274 | This work |
| FLA1009 | CMCP6 wza::TnphoA; constructed by chitin transformation using genomic DNA of CVD752 | This work |
DHFR, dihydrofolate reductase.
TABLE 2.
Plasmids used in this work
| Plasmid | Vector | Description | Source and/or reference(s) |
|---|---|---|---|
| pCOS5 | pCOS5 | Cloning vector containing cat, oriT, ColE1, and oriV; Apr Cmr | 10 |
| pCR2.1-TOPO | pCR2.1 | TOPO cloning vector for capturing PCR products using TA cloning | Invitrogen |
| pCVD442 | pCVD442 | R6K ori mob sacB bla; suicide vector for allelic exchange; Apr | 13 |
| pNEB206A | pNEB206A | USER friendly cloning vector; Apr | New England Biolabs; 3 |
| pRK437 | pRK437 | Cloning vector containing tetAR and lacZα; deletion derivative of pRK404 | 38 |
| pUC19 | pUC19 | Cloning vector; lacZα Apr | 51 |
| pUC4K | pUC4K | Cloning vector containing aph and multiple-cloning site of M13mp7 | 43, 44 |
| pGTR265 | pCVD442 | ΔrtxC; rtxC upstream and downstream PCR amplicons digested with NotI and then USER cloned into pGTR1113; Apr | This work |
| pGTR272 | pCVD442 | ΔrtxA1; rtxA1 upstream and downstream PCR amplicons USER cloned into pGTR1129; Apr | This work |
| pGTR274 | pCVD442 | ΔrtxA1::aph; aph-NotI cassette from pGTR1114 made blunt ended with Klenow fragment and inserted into the SmaI site between the rtxA1 upstream and downstream fragments of pGTR272; Apr Kmr | This work |
| pGTR1090 | pCR2.1-TOPO | flaCDE upstream sequences PCR amplified with primers flaCDE-up5′ and flaCDE-up3′ TOPO TA cloned into pCR2.1-TOPO; Apr | This work |
| pGTR1091 | pCR2.1-TOPO | flaCDE downstream sequences PCR amplified with primers flaCDE-down5′ and flaCDE-down3′ TOPO TA cloned into pCR2.1-TOPO; Apr | This work |
| pGTR1092 | pCR2.1-TOPO | flaFBA upstream sequences PCR amplified with primers flaFBA-up5′ and flaFBA-up3′ TOPO TA cloned into pCR2.1-TOPO; Apr | This work |
| pGTR1093 | pCR2.1-TOPO | flaFBA downstream sequences PCR amplified with primers flaFBA-down5′ and flaFBA-down3′ TOPO TA cloned into pCR2.1-TOPO; Apr | This work |
| pGTR1106 | pCR2.1-TOPO | ΔflaFBA; pGTR1093 digested with XhoI and NotI and ligated with flaFBA upstream sequences from pGTR1092 removed with SalI and NotI; Apr | This work |
| pGTR1107 | pCR2.1-TOPO | ΔflaCDE; pGTR1091 digested with XhoI and NotI and ligated with flaCDE upstream sequences from pGTR1090 removed with SalI and NotI; Apr | This work |
| pGTR1108 | pCVD442 | ΔflaFBA sequences from pGTR1106 removed with SstI and XbaI inserted into pCVD442 digested with XbaI and SstI; Apr | This work |
| pGTR1111 | pCVD442 | pCVD442 XbaI-SmaIΩlacZα; lacZα from HaeII fragment from pUC19; Apr | This work |
| pGTR1112 | pCVD442 | ΔflaCDE from pGTR1107 removed with SstI and XbaI inserted into pGTR1111 digested with XbaI and SstI; Apr | This work |
| pGTR1113 | pCVD442 | pGTR1111lacZα::USER; pGTR1111 digested with SphI and SstI and ligated with USER friendly cloning fragment created with oligonucleotides pCVD442-USER Linker top and pCVD442-USER Linker bottom; Apr | This work |
| pGTR1114 | pCVD442 | aph gene PCR amplified from pUC4K with primers aph5′USER and aph3′USER and USER cloned into pGTR1113; Apr Kmr | This work |
| pGTR1116 | pCR2.1 | cat gene PCR amplified from pCOS5 with primers cat-5′ and cat-3′ and TOPO-TA cloned into pCR2.1-TOPO; Apr Kmr Cmr | This work |
| pGTR1117 | pCR2.1 | tetAR genes PCR amplified from pRK437 with primers tetAR5′ and tetAR3′ and TOPO-TA cloned into pCR2.1-TOPO; Apr Kmr Tcr | This work |
| pGTR1120 | pCVD442 | ΔflaFBA::cat; cat gene from pGTR1116 inserted into the NotI site between the flaFBA upstream and downstream sequences of pGTR1108; Apr Cmr | This work |
| pGTR1128 | pCVD442 | pGTR1111 EcoRVΩtetAR; tetAR genes from pGTR1117 removed with NotI, made blunt ended with Klenow fragment, and cloned into pGTR1111 at EcoRV; Apr Tcr | This work |
| pGTR1129 | pCVD442 | pGTR1113 EcoRVΩcat; cat gene from pGTR1116 removed with NotI, made blunt ended with Klenow fragment, and cloned into pGTR1113 at EcoRV; Apr Cmr | This work |
| pGTR1130 | pCVD442 | pGTR1113 EcoRVΩtetAR; tetAR genes from pGTR1117 removed with NotI, made blunt ended with Klenow fragment, and cloned into pGTR1113 at EcoRV; Apr Tcr | This work |
| pGTR1132 | pCVD442 | pGTR1111 EcoRVΩcat; cat gene from pGTR1116 removed with NotI, made blunt ended with Klenow fragment, and cloned into pGTR1111 at EcoRV; Apr Cmr | This work |
| pGTR1133 | pCVD442 | pGTR1111 NdeIΩaph; aph gene removed from pUC4K with BamHI, made blunt ended with Klenow fragment, and inserted into pGTR1111 digested with NdeI and made blunt ended with Klenow fragment | This work |
| pGTR1136 | pCVD442 | ΔflaCDE::aph; aph-NotI cassette from pGTR1114 inserted at the NotI site between the flaCDE upstream and downstream sequences of pGTR1112; Apr Kmr | This work |
| pGTR1137 | pCVD442 | ΔmotAB crossover PCR product USER cloned into pGTR1113; Apr | This work |
| pGTR1141 | pCVD442 | ΔmotAB::aph; aph gene removed from pGTR1114 with NotI and inserted at the NotI site of pGTR1137; Apr Kmr | This work |
| pGTR1144 | pCVD442 | pGTR1113 EcoRVΩaph; aph gene from pGTR1114 removed with NotI, made blunt ended with Klenow fragment, and cloned into pGTR1113 at EcoRV; Apr Kmr | This work |
| pGTR1147 | pNEB206A | ΔcheY-3 crossover PCR product USER cloned into pNEB206A; Apr | This work |
| pGTR1157 | pNEB206A | ΔcheY-3::aph; aph gene from pGTR1114 inserted into the NotI site between the cheY-3 upstream and downstream sequences of pGTR1147 | This work |
| pGTR1160 | pRK437 | pRK437-USER; pRK437 digested with BamHI and HindIII and ligated with a USER friendly cloning fragment created with oligonucleotides pRK437-USER Linker top and pRK437-USER Linker bottom; Tcr | This work |
| pGTR1171 | pCVD442 | ΔcheY-3::aph; ΔcheY-3::aph sequences removed from pGTR1157 with EcoRI, made blunt ended with Klenow fragment, and cloned into pGTR1111 digested with SmaI; Apr Kmr | This work |
| pGTR1200 | pCOS5 | pCOS5 digested with HindIII and ClaI, made blunt ended with Klenow fragment, and religated; Cmr | This work |
| pGTR1202 | pCOS5 | pCOS5::lacZα; lacZα HaeII fragment from pUC19 made blunt ended with Klenow fragment and inserted in a reverse direction relative to those of bla and cat of pGTR1200, digested with BamHI and XbaI, and made blunt ended with Klenow fragment; Cmr | This work |
| pGTR1204 | pCOS5 | pCOS5::lacZα::USER; 47-bp USER friendly sequences from pGTR1113 removed by digestion with HindIII and SstI and ligated into pGTR1202 digested with HindIII and SstI; Cmr | This work |
TABLE 3.
Oligonucleotides used for PCRs
| Primer and function | Sequence (5′-3′)a |
|---|---|
| Antibiotic resistance | |
| cat-5′ | GGCGGCCGCATGCCTGCAGGTCCAACTTTTGGCG |
| cat-3′ | GGCGGCCGCTTACGCCCCGCCCTGCCACTCATCGC |
| aph5′USER | GGGAAAGUGCGGCCGCTTCTGATTAGAAAAACTCATCGAGC |
| aph3′USER | GGAGACAUGCGGCCGCAAAGCCACGTTGTGTCTCAAAATC |
| tetAR-5′ | GGCGGCCGCGCGCGTTCAATCGGACCAG |
| tetAR-3′ | GGCGGCCGCTAGTGAGTGGGTTGCGCTCC |
| USER friendly constructions | |
| pCVD442-USER Linker top | CTGAGGGAAAGTCTAGATGTCTCCTCAGCATGAGCT |
| pCVD442-USER Linker bottom | CATGCTGAGGAGACATCTAGACTTTCCCTCAGCATG |
| pRK437-USER Linker top | AGCTTGGCTGAGGGAAAGTCTAGATGTCTCCTCAGCGG |
| pRK437-USER Linker bottom | GATCCCGCTGAGGAGACATCTAGACTTTCCCTCAGCCA |
| Flagellin deletion | |
| flaFBAup5′ | GGTCGACAAAAACGCGCGGAAGTGGCG |
| flaFBAup3′ | GGCGGCCGCGGCTTTGGATACTTATCGACCA |
| flaFBAdown5′ | GGCGGCCGCCGGTAGACAGTGAGCGTGCC |
| flaFBAdown3′ | GGAGCTCCTGACGAGCTTGCGCCATT |
| flaCDEup5′ | GGTCGACGTGAACATCGAAGATGTGCC |
| flaCDEup3′ | GGCGGCCGCGCTCACAAAAACAAAAAACGCTG |
| flaCDEdown5′ | GGCGGCCGCTCTCCTTTCGAGTTCGCAAGC |
| flaCDEdown3′ | GGAGCTCGTTGCAAGCTTCGCAGCTCG |
| flaFBAup-out | TGATGGCACTGAAAATCCGAAGC |
| flaFBAdown-out | CATTCATCTTTTCCACCATTCTCAG |
| flaCDEup-out | ATTATCGCACCTTTGTCTGGTGAG |
| flaCDEdown-out | TTGAATTCAATGAGAATTCACGCGC |
| Motility and chemotaxis deletion | |
| motABdown5′ | TGTTTAGCGGCCGCGGATGGGCACTCCTCATGCTATTTTCCG |
| motABdown3′ | GGAGACAUCAGTTGATCCACCAAATTGTCC |
| motABup5′ | GGGAAAGUTGGGTTGTGCAGTTTGACCG |
| motABup3′ | CCCATCCGCGGCCGCTAAACAGGGTACGTCAGTTGAATAAAAG |
| cheYdown5′ | TGTTTAGCGGCCGCGGATGGGAGATTACTTCGTCAGAGCTGC |
| cheYdown3′ | GGAGACAUCAAAGGGCCTTGAATTCACTC |
| cheYup3′ | CCCATCCGCGGCCGCTAAACATGTTTTAATCATTTTGTGTCCAAG |
| cheYup5′ | GGGAAAGUGACGCCTCAAAAGGCGCAAG |
| motABup-out | ATCCACCACTTCAATCTTGATGG |
| motABdown-out | CGTCATCTAAAGCCAGATCACCG |
| cheYup-out | TCCAAGCGGGTATGATTGGCC |
| cheYdown-out | TTGAACTAAAAGATCTTCGATACG |
| RTX deletion | |
| rtxA1-down-3′ | GGGAAAGUCGCTTATGGCAACGGAATTCG |
| rtxA1-down-5′ | ACCCGGGUCAACGAGGCACGAGTATAGAG |
| rtxA1-up-3′ | ACCCGGGUTAAGCCAAACTCTTCTTTAGGAG |
| rtxA1-up-5′ | GGAGACAUGAGCTTGCAGGCGGAGAGTGA |
| rtxC-up5′ | GGAGACAUTTGAGCTTGCAGGCGGAGAG |
| rtxC-up3′ | CTCTTCTTTAGGGCGGCCGCTCTGCCCTATATCAACCAATATG |
| rtxC-down5′ | GATATAGGGCAGAGCGGCCGCCCTAAAGAAGAGTTTGGCTTATG |
| rtxC-down 3′ | GGGAAAGUAGTACGGTCTAGTTTATTGCCC |
| RtxA1-deletion up | GAGCTTGCAGGCGGAGAGTGA |
| RtxA1-deletion down | CGCTTATGGCAACGGAATTCG |
Sequences in boldface type indicate engineered restriction enzyme sites, and underlined sequences indicate USER friendly ends for cloning.
Chemicals, media, and culture.
Unless noted otherwise, components for media were obtained from Difco (Franklin Lakes, NJ), chemicals were obtained from Sigma (St. Louis, MO), DNA extraction and purification kits were obtained from Qiagen (Valencia, CA), molecular genetics enzymes were obtained from New England Biolabs (Ipswich, MA), and oligonucleotides were obtained from IDT (Coralville, IA). Bacteria were grown in Luria-Bertani broth containing 0.85% (wt/vol) NaCl (LB-N) or on LB-N plates containing 1.5% (wt/vol) agar. Strains were stored at −70°C in LB-N with 35% (vol/vol) glycerol. Antibiotics were included in media at the following concentrations: ampicillin at 100 μg/ml, tetracycline at 12.5 μg/ml, chloramphenicol at 30 μg/ml, and kanamycin at 40 μg/ml for E. coli and ampicillin at 10 μg/ml, tetracycline at 6.25 μg/ml, chloramphenicol at 5 μg/ml, and kanamycin at 100 μg/ml for V. vulnificus. To select for V. vulnificus and against donor E. coli cells during filter-mating conjugations, VVM agar (8) or LB-N agar containing 105 U/ml colistin and appropriate antibiotics was used. Counterselection for the loss of suicide plasmids was done using LB-N plates containing 6% (wt/vol) sucrose.
sacB-assisted allelic exchange mutagenesis.
pCVD442-based allelic exchange plasmids were electroporated into E. coli S17-1λpir cells for conjugation into V. vulnificus by filter mating (40). Transconjugants containing single crossovers of the allelic exchange plasmid integrated into the V. vulnificus genome were selected on VVM agar (8) or LB-N agar containing 105 U/ml colistin and appropriate antibiotics. Our current protocol involves colistin and not VVM. The correct insertion of the plasmid was confirmed by PCR. Single-crossover strains were grown overnight for at least one passage in LB-N without selection for the vector but with selection for the antibiotic resistance of the mutation, if applicable. To enrich for cells in which a second crossover event occurred, the bacteria were plated onto LB-N agar containing 6% (wt/vol) sucrose and incubated at 25°C for 24 to 48 h. Sucrose-resistant colonies were passaged on LB-N-sucrose agar and screened for a loss of antibiotic resistance encoded by the vector. It was not uncommon to find colonies on LB-N-sucrose that were not truly sucrose resistant and that had maintained the allelic exchange vector. The correct allelic exchange of the mutant allele for the wild-type allele was confirmed by PCR.
Crab shell (chitin)-based transformation of V. vulnificus.
Chitin-based natural transformation of V. vulnificus was performed as described previously by Meibom et al. (32). Carapaces from blue crabs (Callinectes sapidus) were washed in water, cut into approximately 1-cm2 pieces, and autoclaved. Recipient V. vulnificus cultures were grown statically overnight in LB-N broth at room temperature. The cultures that were grown overnight were diluted 1:20 into fresh LB-N medium and grown with shaking at 37°C until an optical density at 600 nm of 0.4 was attained. The bacteria were washed once with filter-sterilized seawater (University of Florida Whitney Laboratory for Marine Bioscience) diluted to a salinity of 25 ppt, which is typical for estuarine water. The washed bacteria were suspended in a volume of seawater with a salinity of 25 ppt that was twice the starting culture volume. Sterile crab shells were placed into wells of a 12-well tissue culture plate (Costar, Corning, NY) with 2 ml of bacteria in seawater and incubated overnight at 30°C. The following day, the supernatant was aspirated, and 2 ml of fresh seawater with a salinity 25 ppt was added along with 1 μg of linearized plasmid DNA or 2 μg of genomic DNA. Plasmid DNA was linearized either by digestion with an enzyme that cut into the vector opposite the insert sequences (e.g., NdeI for pCVD422-based vectors) or by vortexing in a microcentrifuge tube on a setting of high for 5 min. Genomic DNA was prepared using a Qiagen DNeasy blood and tissue kit, which shears genomic DNA into 15- to 20-kb fragments. The plates were incubated overnight at 30°C. The following day, the supernatants were removed, diluted, and plated onto LB-N agar with and without antibiotics. Crab shells were washed once with phosphate-buffered saline, placed into a 50-ml conical tube containing 2 ml of phosphate-buffered saline, and vortexed to release bacteria, and the supernatant was diluted and plated with and without antibiotics. Transformation frequencies were calculated as the ratio of the numbers of antibiotic-resistant CFU to total CFU.
Construction of pCVD442::lacZα and pCVD442::lacZα::USER.
The 445-bp lacZα gene fragment from pUC19 was excised with HaeII, purified by extraction from an agarose gel, and made blunt ended by treatment with the Klenow fragment of DNA polymerase I (see Fig. S1 in the supplemental material). pCVD442 (13) was digested with XbaI, made blunt ended with the Klenow fragment of DNA polymerase, digested with SmaI to remove sequences containing additional restriction sites (XbaI and SmaI are in the multiple-cloning site of pCVD442), and ligated with the pUC19 lacZα fragment. Ligated DNA was electroporated into E. coli EC100Dpir+ cells (Epicentre, Madison, WI) with selection for ampicillin resistance and screening for blue colonies in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal). The correct insertion of lacZα into the XbaI-SmaI site was confirmed by DNA sequencing (University of Florida Interdisciplinary Center for Biotechnology Research). The resulting plasmid was named pGTR1111.
To enable the capture of USER-treated PCR amplicons, pNEB206A-derived DNA sequences were inserted into the lacZα gene fragment of pGTR1111. The USER friendly cloning sequences of pNEB206A (New England Biolabs) (3) were reconstructed by annealing oligonucleotides pCVD442-USER Linker top and pCVD442-USER Linker bottom to create SphI and SstI overhangs. The oligonucleotides were annealed by mixing equal molar concentrations, heating to 95°C for 5 min, and cooling slowly to 25°C. The USER friendly cloning linker contained a single XbaI site with Nt.BbvCI (formerly marketed as N.BbvCIB) sites 8 bases away on each side of the XbaI site. The 8-bp sequences between each Nt.BbvCI-XbaI pair matched the corresponding sequences of USER friendly vector pNEB206A (the left and right sequences are different to enable directional cloning and to prevent self-annealing). The SphI and SstI overhangs were used to clone the fragment into pGTR1111, which was digested with SphI and SstI. This construction inserted the USER friendly vector sequences into lacZα and maintained the lacZα reading frame, exactly as was the case for pNEB206A. The ligation mixture was electroporated into E. coli EC100Dpir+ with selection for ampicillin resistance and screening for blue colonies in the presence of X-gal. The correct insertion of the USER friendly vector sequences into the lacZα site was confirmed by DNA sequence analysis. The resulting plasmid was named pGTR1113.
To increase the usefulness of the pGTR1111 and pGTR1113 allelic exchange vectors in V. vulnificus, we incorporated additional antibiotic resistance genes into unique restriction sites of the plasmids. The cat, aph, and tetAR antibiotic resistance sequences were PCR amplified from pCOS5, pUC4K, and pRK437, respectively, using the oligonucleotides detailed in Table 3. The PCR products were captured into pCR2.1TOPO (Invitrogen) using TA cloning and were electroporated into E. coli TOP10 cells (Invitrogen). The oligonucleotide primers included NotI sites at each 5′ end, so the resulting pCR2.1 clones were digested with NotI, and the antibiotic resistance cassettes were recovered from agarose gels. The NotI overhangs were filled in with the Klenow fragment of DNA polymerase I, and the blunt-ended antibiotic resistance cassettes were cloned into either EcoRV-digested or NdeI-digested Klenow fragment-treated plasmids. Ligated plasmids were electroporated into E. coli EC100Dpir+ cells, plating with selection for the appropriate antibiotic resistance. The following plasmids were created: pGTR1111EcoRVΩtetAR (pGTR1128), pGTR1111EcoRVΩcat (pGTR1132), pGTR1111NdeIΩaph (pGTR1133), pGTR1113EcoRVΩcat (pGTR1129), pGTR1113EcoRVΩtetAR (pGTR1130), and pGTR1113EcoRVΩaph (pGTR1144) (Table 2).
Construction of pRK437::USER and pCOS5::USER vectors.
We constructed USER friendly cloning derivatives of pRK437 (38) and pCOS5 (10), which are conjugable plasmids that we commonly use for cloning and expression for V. vulnificus (see Fig. S1 in the supplemental material). To insert USER friendly vector sequences into the lacZα gene of pRK437, oligonucleotides pRK437-USER Linker top and pRK437-USER Linker bottom (Table 3) were annealed. BamHI and HindIII restriction site overhangs were engineered into the ends to keep the USER friendly vector lacZα sequence in frame with the lacZα gene of pRK437. pRK437 was digested with BamHI and HindIII and ligated with the annealed oligonucleotides. The ligation product was electroporated into E. coli EC100Dpir+ cells with selection for tetracycline resistance and screening for blue colonies in the presence of X-gal. The correct DNA sequence was confirmed by DNA sequence analysis. pRK437::USER was named pGTR1160.
To increase the utility of pCOS5, we added the pUC19 lacZα gene and USER friendly cloning sequences. To destroy the adjacent HindIII and ClaI restriction sites so that HindIII could be used in subsequent derivatives, pCOS5 was sequentially digested with HindIII and ClaI, made blunt ended by treatment with the Klenow fragment of DNA polymerase I, and ligated, yielding pGTR1200. The lacZα HaeII fragment from pUC19 was made blunt ended with Klenow fragment and ligated into pGTR1200, which had been sequentially digested with XbaI and BamHI and made blunt ended with Klenow fragment, thereby destroying both of these sites. The HindIII, ClaI, XbaI, and BamHI sites were not in the coding sequences. This pCOS5::lacZα plasmid with lacZα in the orientation opposite to the flanking bla and cat genes was named pGTR1202. To enable the capture of USER-generated PCR amplicons, pGTR1202 was digested with SstI and HindIII, which cut within the lacZα gene, and ligated with the USER friendly vector sequences removed from the pGTR1113 lacZα gene by digestion with SstI and HindIII. The ligation mixture was electroporated into E. coli EC100Dpir+ cells with selection for chloramphenicol resistance and screening for blue colonies in the presence of X-gal. pCOS5::lacZα::USER was named pGTR1204, and the correct insertion of the USER friendly vector sequences was confirmed by DNA sequence analysis.
Deletion of flaCDE and flaFBA flagellin loci from V. vulnificus CMCP6.
The 0.5-kb upstream and downstream sequences flanking the flaCDE and flaFBA loci were PCR amplified using primers flaCDEup5′ and flaCDEup3′, flaCDE down5′ and flaCDEdown3′, flaFBAup5′ and flaFBAup3′, and flaFBAdown5′ and flaFBAdown3′ (see Fig. S2 in the supplemental material). These 0.5-kb amplicons were individually cloned into pCR2.1TOPO, yielding plasmids pGTR1090, pGTR1091, pGTR1092, and pGTR1093, respectively. The upstream sequences were excised using SalI and NotI, which had been engineered in the oligonucleotide primers, and were cloned next to the respective downstream sequences whose plasmids had been digested with XhoI and NotI, yielding plasmids pGTR1106 for flaFBA and pGTR1107 for flaCDE. The flaFBA upstream-downstream DNA sequence (ΔflaFBA) was excised from pGTR1106 with SstI and XbaI and cloned into pCVD442, which was digested with the same enzymes, yielding pGTR1108. The flaCDE upstream-downstream DNA sequence (ΔflaCDE) was excised from pGTR1107 with SstI and XbaI and cloned into pGTR1111 (pCVD442::lacZα), which was digested with the same enzymes, yielding pGTR1112. pGTR1112 was digested with NotI, separating the upstream and downstream flaCDE sequences, and ligated with the NotI-aph fragment from pGTR1114. Similarly, the NotI cat fragment from pGTR1116 was inserted into the NotI site between the upstream and downstream sequences of ΔflaFBA of pGTR1108. The suicide vectors carrying ΔflaCDE::aph and ΔflaFBA::cat were named pGTR1136 and pGTR1120, respectively, and were electroporated into E. coli S17-1λpir+ cells for conjugation into V. vulnificus. The deletions were recombined into the chromosome of V. vulnificus CMCP6 by using the standard two-step sucrose-resistance-assisted allelic exchange method described above. The use of aph and cat resistance cassettes to label the deletion mutations facilitated the identification of the recombinants. The deletion of the flaCDE and flaFBA loci was confirmed by a series of PCRs using primers flaCDEup-out/flaCDEdown-out and flaFBAup-out/flaFBAdown-out. The ΔflaCDE::aph and ΔflaFBA::cat V. vulnificus mutants were named FLA677 and FLA680, respectively. A double flagellin deletion mutant was made by the deletion of flaCDE from FLA680 using pGTR1136 with the two-step allelic exchange procedure. The ΔflaCDE::aph ΔflaFBA::cat mutant was named FLA711.
USER friendly cloning of single PCR amplicons into USER friendly vectors.
The method described for the USER Friendly cloning kit (New England Biolabs) was followed exactly. Specially constructed vectors, such as pNEB206A (pUC19 containing USER friendly vector sequences in the lacZα gene) (3), or the plasmids that we describe here were treated with XbaI to linearize the vector in the USER friendly cloning sequence, followed by Nt.BbvCI, which created two different 8-bp 3′ overhangs. To create USER friendly compatible ends of PCR amplicons, a single deoxyuridine was included 8 bases from the 5′ end of each oligonucleotide primer, and the DNA sequences preceding the deoxyuridine were complementary to one of the two USER friendly cloning sequences in the vector, either GGAGACAU or GGGAAAGU (Table 3). Taq DNA polymerase was used for PCRs because most other DNA polymerases do not recognize deoxyuridine in the template (3). The PCR product was treated with USER enzyme mix (New England Biolabs), which contains uracil DNA glycosylase to remove the uracil residue, and DNA glycosylase-lyase endonuclease VIII, which nicks the deoxyuridine-containing strand, thereby releasing the terminal single-stranded DNA fragment and creating an 8-bp 3′ overhang designed to be complementary to the vector. Because of the 8-bp complementary overhangs, ligation was performed with T4 DNA ligase for as short as 15 min at room temperature. Ligation reaction mixtures were electroporated into an appropriate E. coli host strain such as EC100Dpir+.
Deletion of motAB and cheY-3 using crossover PCR.
motAB and cheY-3 deletions were constructed by crossover PCR in a single cloning reaction to create joined upstream-downstream DNA sequences flanking the genes of interest for subsequent deletion by allelic exchange mutagenesis. Crossover PCR was performed essentially as originally described by Horton et al. (18) and as modified by Link et al. (28). Approximately 500-bp upstream and downstream sequences of motAB and cheY-3 were amplified by PCR. The inside primers (motABup3′/motABdown5′ and cheY-3up3′/cheY-3down5′) that were used to join the upstream and downstream sequences had 21 bp of overlap including a NotI site (Table 3). The outside primers (motAB up5′/motAB down3′ and cheY-3 up5′/cheY-3 down3′) for cloning into the vector included the extra 8-base USER friendly cloning sites at their 5′ ends that corresponded to those of vector pNEB206A. The products of the initial PCR amplifications (the respective upstream and downstream sequences) were mixed together and amplified a second time using only the outside primers (the 5′ primer of the upstream sequence and the 3′ primer of the downstream sequence, e.g., motABup5′ and motABdown3′). An annealing temperature was chosen to allow for the annealing of the upstream and downstream overlapping complementary sequences prior to amplification. The resulting combined upstream/downstream fragment was purified by agarose gel electrophoresis and cleaned using Zymo Clean & Concentrator (Zymo Research, Orange, CA). For the deletion of motAB, the crossover PCR product was cloned into pGTR1113 (pCVD442::lacZα::USER) using the USER friendly cloning method, creating pGTR1137. The aph-NotI cassette from pGTR1114 was inserted into the NotI site between the upstream and downstream sequences of motAB in pGTR1137, creating pGTR1141 (ΔmotAB::aph). The cheY-3 deletion was constructed by crossover PCR similarly to the motAB deletion except that the upstream-downstream sequence for ΔcheY-3 was initially constructed in pNEB206A, yielding pGTR1147, and the insertion of an aph-NotI cassette yielded pGTR1157. The cheY-3 upstream-aph downstream sequence was excised from pGTR1157 with EcoRI, made blunt ended with Klenow fragment, and ligated into pGTR1111 (pCVD442::lacZα), which was digested with SmaI. The allelic exchange plasmid for ΔcheY-3::aph was named pGTR1171. pGTR1141 and pGTR1171 were used with sacB-assisted two-step allelic exchange mutagenesis to create CMCP6 ΔmotAB::aph and CMCP6 ΔcheY-3::aph mutants, named FLA674 and FLA688, respectively. The mutants were confirmed by PCR using primers designed to detect the expected changes in size at the motAB or cheY-3 locus (cheYup-out/cheYdown-out and motABup-out/motABdown-out) (Table 3).
Deletion of rtxC using crossover PCR.
The allelic exchange plasmid for the rtxC deletion was designed to be constructed by crossover PCR, as described above. Approximately 500 bp upstream and downstream of rtxC were amplified separately by PCR. Inside primers (rtxC-up3′ and rtxC-down5′) contained 33 bp of overlapping sequences including a NotI site (Table 3). The outside primers (rtxC-up5′ and rtxC-down3′) included the extra 8-base USER friendly cloning sites at the 5′ ends that corresponded to the USER friendly cloning sites of vector pNEB206A. The second PCR, which was supposed to anneal the upstream and downstream fragments to form a single joined fragment, was unsuccessful for unknown reasons. As an alternative to crossover PCR, the upstream and downstream amplicons were digested with NotI to create compatible 5′ overhangs and were cleaned using Zymo Clean & Concentrator. The two NotI-digested PCR products were treated for 15 min at 37°C with USER enzyme mix to create the 3′ overhang sequences at the USER friendly cloning ends and were mixed with allelic exchange vector pGTR1113, which had been treated with XbaI and Nt.BbvCI. The reaction mixture was then incubated at 42°C to allow for annealing of the NotI overhangs and subsequently ligated by adding ligase buffer and T4 DNA ligase and incubating at room temperature for 15 min. The ligation mixture was electroporated into E. coli EC100Dpir+ cultures, which were plated onto LB-N medium containing ampicillin. The resulting plasmid, pGTR265, was used for two-step sacB-assisted allelic exchange mutagenesis to create the CMCP6 ΔrtxC mutant, which was named FLA591. The mutant was confirmed by PCR using outside primers rtxC-up5′ and rtxC-down3′ (Table 3).
Three-fragment USER friendly cloning.
To clone upstream and downstream flanking DNA sequences together in a single step, the vector plasmid containing pNEB206A USER friendly vector sequences was prepared for USER friendly cloning by treatment with XbaI and Nt.BbvCI to create the 8-bp overhangs. The oligonucleotides used to amplify the outside ends of the upstream and downstream flanking sequences included one of the USER friendly vector sequences. The inside ends of the upstream and downstream fragments that were to be joined together were amplified with oligonucleotides that created compatible USER friendly ends containing a restriction enzyme site, either SmaI or NotI (Table 3). For example, to create an SmaI USER friendly cloning end shared between the upstream and downstream sequences, the 5′ ends of the primers were ACCCGGGU, followed by the sequences corresponding to the target DNA. The initial A is complementary to the U that is present in the opposing primer of the other fragment. After PCR amplification, treatment of the upstream and downstream PCR products with the USER enzyme for 15 min at 37°C generated three different pairs of complementary ends: the two different ends of the vector and the end shared between the upstream and downstream fragments. Therefore, there was only one way in which the vector and the two insert PCR amplicons could join to form a closed plasmid. The joining of the upstream and downstream fragments also created an SmaI site between them. The PCR products were mixed with the XbaI-Nt.BbvCI-digested USER friendly vector and were ligated by adding ligase buffer and T4 DNA ligase at room temperature for 15 min. Ligation reaction mixtures were electroporated into E. coli EC100Dpir+ cells with selection for the appropriate antibiotic resistance.
Deletion of rtxA1 using three-fragment USER friendly cloning.
To construct the allelic exchange plasmid to delete the15.6-kb V. vulnificus rtxA1 gene, 1.0-kb upstream and downstream sequences were amplified with the USER friendly cloning sequences complementary to pNEB206A on the outside ends (rtxA1-up-5′ and rtxA1-down-3′) (see Fig. S3 in the supplemental material). The inside ends where the upstream and downstream sequences would join were amplified with ACCCGGGU at the 5′ end to create a common SmaI restriction site (rtxA1-up-3′ and rtxA1-down-5′) (Table 3). These upstream and downstream rtxA1 fragments were cloned into USER friendly allelic exchange vector pGTR1129 exactly as described immediately above for three-fragment USER friendly cloning, yielding pGTR272 (ΔrtxA1). To enable the selection of rtxA1 deletion mutants of V. vulnificus, the aph-NotI kanamycin resistance cassette from pGTR1114 was made blunt ended with Klenow fragment and cloned into the SmaI site between the upstream and downstream sequences of pGTR272, yielding plasmid pGTR274, carrying ΔrtxA1::aph. This allelic exchange plasmid was used to recombine the mutation into V. vulnificus CMCP6 in the two-step sacB-assisted allelic exchange process, yielding strain FLA900. The correct recombination events were confirmed by PCR using oligonucleotides RtxA1-deletion up and RtxA1-deletion down and Southern blot analyses.
Analysis of motility and flagella.
Bacteria from a static culture grown overnight were stabbed into LB-N soft-agar plates containing 0.3% (wt/vol) agar, and the plates were incubated overnight at 37°C. The diameter of the zone of growth was measured after 15 to 18 h of incubation (consistent within each experiment). Triplicate plates were examined for each strain. The presence or absence of flagella was visualized by staining bacterial cells with Nano Orange (Invitrogen). Bacteria were removed from an LB-N soft-agar plate with a toothpick and mixed into 2 μl of phosphate-buffered saline on a microscope slide. A total of 0.5 μl of Nano Orange dye was mixed in with a pipette tip, and a coverslip was placed over the bacteria. The stained cells were examined at a ×1,000 magnification under oil immersion using a Nikon Eclipse 600 fluorescence microscope with a Nikon B-2 E/C filter, which has an excitation filter wavelength of 465 to 495 nm and an emission filter wavelength of 515 to 535 nm. Photomicrographs were made using a Nikon DXM1200C charge-coupled-device camera.
Statistical analysis.
The Student t test was used to determine significant differences between groups. Values were considered significant at P values of <0.05. All quantitative experiments were repeated at least once.
RESULTS
Construction of pCVD442::lacZα and use in deleting flagellin loci from V. vulnificus.
Because V. vulnificus possesses six flagellin genes, an important question is which of them is essential for the production of flagella and motility. We therefore deleted the two unlinked flagellin loci flaCDE and flaFBA separately and combined using the allelic exchange suicide vector pCVD442. To facilitate cloning into pCVD442 by blue-white screening for inserted DNA, we inserted the pUC19 lacZα multiple-cloning site into pCVD442, yielding pGTR1111 (see Fig. S1 in the supplemental material). As described in Materials and Methods (see Fig. S2 in the supplemental material), upstream and downstream sequences flanking the flaCDE and flaFBA loci were individually cloned into pCR2.1TOPO, and the upstream sequences were subsequently combined with the downstream sequences. These ΔflaCDE and ΔflaFBA sequences were then subcloned into pGTR1111 and pCVD442, respectively, and were conferred with different antibiotic resistance genes to facilitate subsequent genetic manipulations, yielding ΔflaCDE::aph in pGTR1136 and ΔflaFBA::cat in pGTR1120. The lacZα multiple-cloning site of pGTR1111 made the cloning of the ΔflaCDE sequences more straightforward. The deletions were recombined into the chromosome of V. vulnificus CMCP6 by using the two-step allelic exchange method involving the selection of a single-crossover integration event followed by sucrose resistance-mediated counterselection for the excision of the vector by a second crossover event, as described in Materials and Methods. V. vulnificus CMCP6 ΔflaCDE::aph and V. vulnificus ΔflaFBA::cat strains were named FLA677 and FLA680, respectively. pGTR1136 was similarly used to create a ΔflaCDE::aph mutation in the ΔflaFBA::cat background of FLA680, yielding a double flagellin locus mutant (ΔflaCDE::aph ΔflaFBA::cat) named FLA711.
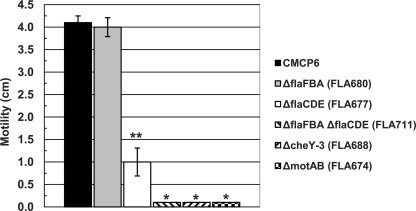
The deletion of flaFBA had no significant effect on motility (Fig. 1) or the presence of flagella (Fig. 2). In contrast, the deletion of flaCDE significantly decreased but did not abolish motility, and the bacteria were still flagellated. As expected, the ΔflaCDE::aph ΔflaFBA::cat double mutant was nonmotile and nonflagellated. Therefore, the flaFBA locus appeared to be completely dispensable for motility in vitro but could partially compensate for the deletion of the flaCDE locus, which had the greater effect on motility in vitro. These flagellin deletion constructions demonstrated the usefulness of incorporating the lacZα-complementing sequences into allelic exchange suicide vector pCVD442 but also exemplified the tedious nature of constructing deletion mutations by separately cloning upstream and downstream fragments into TOPO TA vectors.
FIG. 1.
Motilities of Δfla, ΔmotAB, and ΔcheY-3 mutants. V. vulnificus strains were stabbed into soft LB-N agar, and the diameters of growth from motility after overnight growth were measured as described in Materials and Methods. Strains examined were wild-type strain CMCP6, ΔflaFBA::cat strain FLA680, ΔflaCDE::aph strain FLA677, ΔflaCDE::aph ΔflaFBA::cat strain FLA711, ΔmotAB::aph strain FLA674, and ΔcheY-3::aph strain FLA688. *, P ≤ 1 × 10−5 compared with CMCP6 and FLA680 and P = 0.007 compared with FLA677; **, P ≤ 0.0002 compared with CMCP6 and FLA680 (n = 3).
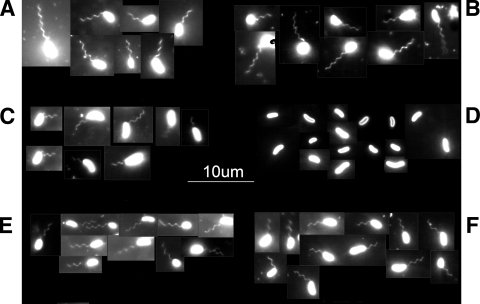
FIG. 2.
Flagellation of Δfla, ΔmotAB, and ΔcheY-3 mutants. Bacteria from LB-N soft-agar plates were stained with Nano Orange and examined using epifluorescence at a ×1,000 magnification as described in Materials and Methods. (A) Wild-type CMCP6; (B) ΔflaFBA::cat strain FLA680; (C) ΔflaCDE::aph strain FLA677; (D) ΔflaCDE::aph ΔflaFBA::cat strain FLA810; (E) ΔmotAB::aph strain FLA674; (F) ΔcheY-3::aph strain FLA688.
Construction of USER friendly vectors for use with V. vulnificus.
TOPO TA cloning and Gateway cloning (Invitrogen) are very useful tools for cloning of PCR-generated DNA sequences. Unfortunately, in our studies of V. vulnificus, none of the commercially available vectors was directly useful because they were not mobilizable. However, the USER Friendly cloning system (New England Biolabs) appeared to be a suitable alternative method of efficiently capturing PCR products. Any plasmid vector can be converted into a USER friendly vector simply by the inclusion of short DNA sequences. Capturing PCR amplicons into USER friendly cloning vectors simply involves the inclusion of 8 nucleotides at the 5′ end of the oligonucleotides for PCR with a deoxyuridine in the place of a thymidine at the eighth nucleotide. USER friendly cloning vector pNEB206A is a pUC19 derivative (3) and is not mobilizable in V. vulnificus. We therefore constructed USER friendly derivatives of the two most useful cloning vectors for our work with V. vulnificus, pCOS5 (10) and pRK437 (38), yielding plasmids pGTR1204 and pGTR1160, respectively (see Materials and Methods; also see Fig. S1 in the supplemental material). Similarly, we constructed a USER friendly derivative of vector pCVD442::lacZα, pGTR1111, yielding pGTR1113 (see Fig. S1 in the supplemental material). Because selection for ampicillin resistance is inefficient for working with V. vulnificus, we also constructed chloramphenicol-, kanamycin-, and tetracycline-resistant derivatives of pGTR1113, named pGTR1129, pGTR1144, and pGTR1130, respectively (Table 2; see Fig. S1 in the supplemental material).
Use of crossover PCR and USER friendly cloning to construct motAB and cheY-3 deletions.
The motAB genes (VV1_0312 and VV1_0311) are the only genes in the V. vulnificus CMCP6 genome annotated as encoding the motor complex for flagella. To confirm that these adjacent genes are essential for motility, we targeted VV1_0312 and VV1_0311 for deletion. In contrast, there are 10 genes annotated as “cheY” or “cheY-like receiver” in the V. vulnificus genome. In the V. cholerae N16961 genome, among the four genes annotated as cheY, the VC2_065 gene, annotated as cheY-3, has been shown to be essential for mediating chemotaxis (19, 27). The protein encoded by the VV1_1953 gene of the V. vulnificus CMCP6 genome has the highest homology to the V. cholerae CheY-3 protein, so we named VV1_1953 cheY-3. To determine if cheY-3 is essential for the chemotaxis of V. vulnificus, we targeted VV1_1953 for deletion.
To delete motAB and cheY-3, rather than cloning the upstream and downstream flanking sequences separately and then combining them into the allelic exchange suicide vector, as was done for the flaCDE and flaFBA loci, we used crossover PCR (28) to amplify the joined upstream and downstream sequences and clone them in a single ligation, as described in Materials and Methods. The ΔmotAB sequences were USER cloned directly into the sacB allelic exchange plasmid pGTR1113, and an aph-NotI cassette was inserted between the upstream and downstream sequences, yielding pGTR1141. In contrast, the ΔcheY-3 sequences from crossover PCR were first USER cloned into pNEB206A, and an aph cassette was then inserted between the upstream and downstream sequences. ΔcheY-3::aph sequences were subcloned into allelic exchange vector pGTR1111, yielding pGTR1171. The ΔmotAB::aph and ΔcheY-3::aph mutations were recombined into the V. vulnificus CMCP6 genome using the two-step allelic exchange sucrose resistance-assisted procedure described in Materials and Methods, yielding strains FLA674 and FLA688, respectively. The mutations were confirmed by PCR (data not shown).
As expected, the ΔmotAB::aph mutation resulted in a complete lack of motility of V. vulnificus (Fig. 1) but did not affect the presence of flagella (Fig. 2). When stabbed into motility agar plates, ΔcheY-3::aph strain FLA688 had the same appearance as ΔmotAB::aph strain FLA674, i.e., no spreading (Fig. 1), which is consistent with a nonchemotactic phenotype (7). To confirm that FLA688 was motile, we examined bacteria from the motility agar in a wet preparation under bright-field microscopy. As opposed to the swimming-and-tumbling behavior of parental strain CMCP6, the ΔcheY-3::aph mutant constantly swam but did not tumble (data not shown). Furthermore, the ΔcheY-3::aph mutant possessed flagella (Fig. 2). These constructions demonstrated the usefulness of USER friendly cloning in capturing PCR-generated DNA sequences into both commercially generated and laboratory-generated vectors as well as the potential usefulness of crossover PCR to clone upstream and downstream flanking sequences in a single step.
Three-part USER friendly cloning is a highly efficient method to produce allelic exchange plasmids.
In using crossover PCR to generate the allelic exchange plasmids described above, we experienced a great difficulty in obtaining sufficient yields of the combined upstream-downstream fragment during the second PCR. As part of a study examining the roles of RTX toxins of V. vulnificus in virulence, we wanted to delete the rtxC gene (VV2_0480) associated with the rtxA1 locus of V. vulnificus CMCP6. As was done for motAB and cheY-3, the upstream and downstream sequences of rtxC were amplified individually and then mixed for crossover PCR. However, we were unable to amplify a crossover PCR product joining the upstream and downstream sequences. Because we had included a NotI restriction site in the common sequences between the upstream and downstream fragments for crossover PCR, we reasoned that we could digest the PCR products with NotI and the USER enzyme mix to create three sets of complementary overhangs (viz., the left and right USER friendly vector sequences and the NotI sequence). This would enable the cloning of the PCR products into USER friendly allelic exchange vector pGTR1129. The three-part cloning reaction yielded allelic exchange plasmid pGTR265, carrying ΔrtxC. pGTR265 was used to recombine ΔrtxC into the genome of V. vulnificus CMCP6 by the two-step sacB-assisted process, yielding FLA591, without the use of an added antibiotic resistance gene cassette between the upstream and downstream sequences. In agreement with data described previously by Liu et al. (30), FLA591 was not attenuated for virulence in subcutaneously inoculated, iron dextran-treated mice (J. L. Joseph et al., unpublished data). This construction demonstrated the usefulness of combining USER friendly cloning with engineered restriction sites in accomplishing three-part cloning.
Considering the success of the three-part cloning by combining USER friendly sequences on the outside ends and a common restriction site on the inside ends of the upstream and downstream sequences, we reasoned that an additional unique USER friendly sequence could be constructed into the sequence common to the upstream and downstream fragments. This is possible because the principal aspect of USER friendly cloning is the incorporation of a deoxyuridine into the oligonucleotides used for PCR. As long as the oligonucleotides to be joined in a USER friendly cloning reaction have a complementary sequence 5′ to the deoxyuridine so as to generate compatible overhangs after USER enzyme treatment, essentially any DNA sequence can be used. The advantage of designing USER friendly sequences at all three junctions (vector-upstream, upstream-downstream, and downstream-vector) is that only a single enzyme treatment, the USER enzyme mix, is required to generate the overhangs. We used this strategy to create a ΔrtxA1::aph mutation in V. vulnificus CMCP6, as described in Materials and Methods (see Fig. S3 in the supplemental material), and a ΔfadR::aph mutation (6). The deletion of the 15.6-kb rtxA1 gene was confirmed by PCR (Fig. 3) and Southern blot (data not shown) analyses. In agreement with the results described previously by others (21, 25, 30), the ΔrtxA1::aph mutant was attenuated for virulence in mice and cytotoxicity in cell culture (Joseph et al., unpublished). The ΔfadR::aph mutant was extremely attenuated for virulence in iron dextran-treated mice (6). In our experience, three-part USER friendly cloning was the most efficient method for generating the allelic exchange plasmids containing the joined upstream-downstream fragments for deletional mutagenesis.
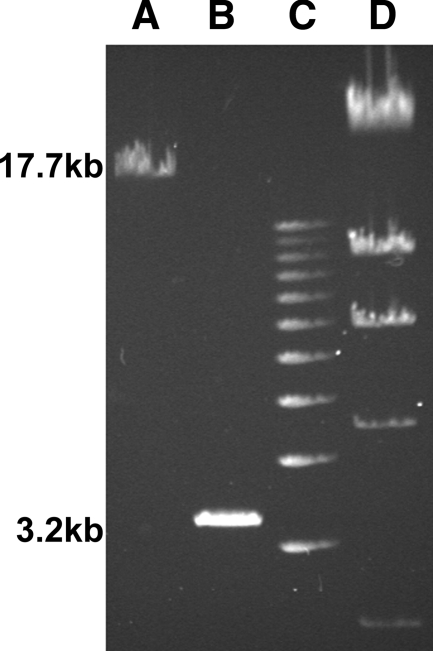
FIG. 3.
PCR confirmation of the deletion of 15.6-kb rtxA1 and creation of ΔrtxA1::aph. Genomic DNAs from V. vulnificus CMCP6 (lane A) and ΔrtxA1::aph strain FLA900 (lane B) were PCR amplified using oligonucleotide primers rtxA1-deletion-up and rtxA1-deletion-down, which amplify 1 kb upstream and downstream of the start and stop codons of rtxA1. The sizes of the products are shown to the left, based on the 1-kb ladder (lane C) and lambda phage-HindIII digestion (lane D). Southern blot analysis also confirmed the absence of rtxA1 sequences (data not shown).
Crab shell (chitin)-mediated natural transformation of V. vulnificus.
The studies described above detail our development of methods to capture PCR-generated DNA sequences to construct allelic exchange plasmids to create deletions and insertions in V. vulnificus using pCVD442-derived vectors. A central aspect of using such vectors is the sacB-encoded susceptibility to sucrose in gram-negative bacteria as a counterselection against the vector to select for the second crossover event (13). However, we and our colleagues have found that sucrose selection is not as effective for V. vulnificus as it is for V. cholerae and other gram-negative bacteria (e.g., in our experience, E. coli and Salmonella enterica serovar Typhimurium) (46). Most often, in spite of our attempts at optimizing the system, colonies growing on the initial sucrose-containing plates were not truly sucrose resistant. An alternative to plasmid-mediated two-step allelic exchange would be to have the recipient bacteria take up linear sequences of DNA encoding the mutation labeled with a selectable marker so that the only way that the marker could be acquired would be by a double-crossover event and marker rescue. Meibom et al. (32) previously identified and characterized the ability of V. cholerae to become competent for transformation with linear DNA during growth in the presence of chitin. Chitin could be provided as a soluble substrate or by growing the bacteria in the presence of crab shells. We therefore attempted to reproduce the results from V. cholerae with V. vulnificus.
As described in Materials and Methods, V. vulnificus CMCP6 was grown in diluted seawater in the presence of a fragment of autoclaved crab shell. We initially tested the chitin transformation system by reconstructing a ΔflaCDE::aph ΔflaFBA::cat double mutant strain. FLA680, which contains the ΔflaFBA::cat mutation, was chosen as the recipient strain, and allelic exchange plasmid pGTR1136 was the donor of the ΔflaCDE::aph mutation. When intact pGTR1136 was added to the V. vulnificus culture growing with the crab shell, no kanamycin-resistant, chloramphenicol-resistant recombinants were obtained. However, when pGTR1136 was sheared by sonication or linearized by digestion with EcoRV, the addition of 1 μg of DNA to the crab shell culture yielded kanamycin-resistant, chloramphenicol-resistant recombinants with a frequency of 1.2 × 10−7. Apparently, only linear DNA is capable of being taken up by the chitin-competent V. vulnificus recipients. We confirmed this hypothesis by attempting to transform uncut vector plasmids pUC19, pRK437, and pCOS5 into V. vulnificus, and we failed to obtain any antibiotic-resistant isolates. We also examined if the linear fragment of DNA encoding the homologous flanking sequences and internal antibiotic resistance cassette for chitin transformation could be obtained directly by PCR amplification without first being cloned into a plasmid vector. When we used the same PCR amplicons that had been cloned into the suicide allelic exchange vectors for chitin-based transformation, the frequencies of recombinants were much lower and sometimes were undetectable (data not shown). Given that the lengths of the homologous flanking sequences were the same in the linearized allelic exchange plasmids and the PCR-generated fragments, it appeared that the length of total flanking DNA is critical for determining transformation efficiency, possibly because of the exonuclease activity of V. vulnificus (50).
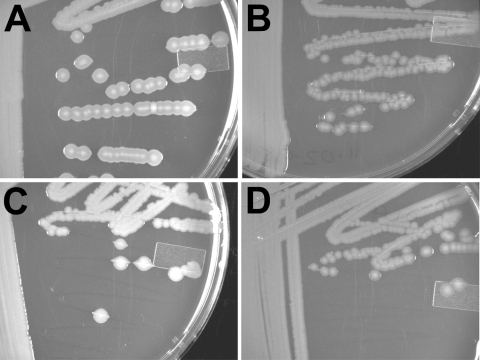
Given the optimal efficiency of chitin transformation with longer linear pieces of DNA, we considered the possibility that chitin-based transformation could be used to move mutations with selectable markers between V. vulnificus strains. We tested this by moving the ΔflaCDE::aph mutation of FLA677 into ΔflaFBA::cat strain FLA680 to create a double deletion of the flagellin loci. Two micrograms of genomic DNA from FLA677, either untreated or sheared by sonication, was added to a FLA680 culture growing on a crab shell. Kanamycin-resistant recombinants were readily obtained and were confirmed by PCR. The phenotype of the double flagellin deletion strain FLA810 was confirmed by the lack of motility (data not shown) and the lack of flagella (Fig. 2). This genomic DNA-mediated recombination was performed using the same strain CMCP6 background for the donor and recipient strains. To determine if chitin-based transformation could be accomplished between different strain backgrounds, we moved the wza::TnphoA mutation of V. vulnificus M06-24/O derivative CVD752 (48, 49) into CMCP6. The wza gene encodes an essential component of capsule biosynthesis, and the mutation of wza would be expected to yield a translucent-colony morphotype. Genomic DNA from CVD752 was added to CMCP6 cells growing on a crab shell, and recombinants were selected by plating onto LB-N agar with kanamycin. The wza::TnphoA phenotype of the resulting strain, FLA1009, was obvious by the translucent colonies, which no longer expressed capsular polysaccharide (Fig. 4). Furthermore, FLA1009 was significantly attenuated for virulence in subcutaneously inoculated iron dextran-treated mice (6a). These results demonstrated that chitin transformation is useful for moving selectable mutations between V. vulnificus strains.
FIG. 4.
Chitin transformation of wza::TnphoA from V. vulnificus CVD752 into CMCP6 yields a translucent-colony morphotype. Genomic DNA from CVD752 (M06-24/O wza::TnphoA) was chitin transformed into CMCP6, selecting for kanamycin resistance and yielding FLA1009. Kanamycin-resistant colonies acquired the translucent-colony morphotype (B and D). (A) M06-24/O; (B) CVD752; (C) CMCP6; (D) FLA1009.
Cocultivation of two V. vulnificus strains on crab shell enables chitin-based transformation.
Meibom et al. (32) previously reported that chitin-based transformation could be accomplished by simply mixing two V. cholerae strains together during growth on crab shells. Apparently, sufficient DNA is released in the coculture to enable chitin-based transformation. To examine if coculture could mediate the chitin-based transformation of V. vulnificus, ΔflaCDE::aph strain FLA677 and ΔflaFBA::cat strain FLA680 were mixed for growth overnight on crab shells. The next day, the frequency of kanamycin- and chloramphenicol-resistant CFU was 2.3 × 10−9. Eighteen colonies were verified to be nonmotile by stabbing into motility soft-agar plates. To determine if increasing the incubation time could increase transformation frequencies by possibly causing a greater release of genomic DNA and/or an increase in competency, we sampled the coculture for up to 3 days. The transformation frequencies on days 2 and 3 were 6.0 × 10−8 and 3.4 × 10−7, respectively. Therefore, it appears that V. vulnificus can exchange genomic DNA during growth in the presence of chitin, and an increased incubation time increases the frequency of recombination.
DISCUSSION
We report here a progression of the development of genetic methods that have vastly improved our ability to manipulate the genome of V. vulnificus. Many of the methods and certainly most of the plasmid vectors should be useful to others examining other gram-negative bacteria. Most trivially, we produced derivatives of commonly used plasmid vectors to improve their usefulness, such as the inclusion of lacZα into pCVD442 (13) and pCOS5 (10) to enable blue-white screening of the insertion of cloned DNA sequences using appropriate E. coli hosts and the inclusion of alternative antibiotic resistance markers (Table 2; see Fig. S1 in the supplemental material). However, since our preferred method of constructing mutations in specific genes or loci is allelic exchange mediated by cloning and joining sequences upstream and downstream of the targeted locus, we examined methods to improve the efficiency of cloning of dual fragments of DNA.
The greatest obstacle in our molecular genetic manipulation is the fact that the transformation and electroporation of intact plasmids into V. vulnificus are difficult, if not impossible. Therefore, all of the plasmids that we use for genetic manipulations must be mobilizable for transfer by conjugation. One of the most efficient ways to clone PCR-amplified DNA fragments is TA cloning coupled with topoisomerase ligation (Invitrogen). Unfortunately, none of the commercially available TOPO TA vectors, such as pCR2.1 TOPO, are mobilizable. Therefore, as described above for the deletions of flaCDE and flaFBA, we TOPO TA cloned the upstream and downstream sequences separately into pCR2.1 TOPO, followed by the joining of the fragments into a pCR2.1-vectored clone. This was cumbersome and time-consuming (see Fig. S2 in the supplemental material). These difficulties led us to refine cloning methods for PCR-generated products. Converting our commonly used vectors to TOPO TA vectors was not feasible. Upon investigating alternative means to capture PCR amplicons, we found USER friendly cloning from New England Biolabs (3). USER friendly cloning had several advantages for our use. First, by simply incorporating specific deoxyuridine-containing nucleotide sequences into PCR primers, we generated 3′ overhangs compatible with vector pNEB206A for efficient cloning. Second, we constructed USER friendly cloning lacZα derivatives of our most useful expression and allelic exchange vectors, pRK437, pCOS5, and pCVD442 (Table 2; see Fig. S1 in the supplemental material). Therefore, we could capture upstream and downstream sequences for mutagenesis and entire genes for complementation or reversion.
To create the upstream-downstream deletion cassettes in a single cloning step, we first examined crossover PCR (18, 28). We had some success by coupling crossover PCR with USER friendly cloning to create deletions of motAB and cheY-3; however, the low fidelity of obtaining the joined upstream and downstream fragments by crossover PCR was problematic. We were unsuccessful in using crossover PCR to generate the deletion construct for the rtxC gene, which encodes a putative acyl transferase that is required in other bacteria for the modification and functional activity of associated RtxA toxins (45). We do not know why crossover PCR failed in this attempt, but the inconsistency of the method was noted previously by others (3). Therefore, we took advantage of the fact that we had engineered NotI sites into the overlapping sequences for crossover PCR between the upstream and downstream rtxC sequences. By treating the fragments with NotI and then performing a three-part ligation using the USER friendly ends of the fragments to join to the vector and the NotI ends between the insert fragments, we constructed the desired upstream-downstream deletion clone for rtxC in our USER friendly vector pGTR1129. We used sacB-assisted allelic exchange to recombine the ΔrtxC mutation into the V. vulnificus CMCP6 genome. In agreement with results described previously by Liu et al. (30), our V. vulnificus rtxC mutant exhibited wild-type levels of virulence in our animal model of disease (Joseph et al., unpublished).
Given the ease of the three-part cloning used for constructing the ΔrtxC mutation with a shared NotI site between the insert fragments, we created the common overlapping sequence with USER friendly cloning sites to construct a deletion of the rtxA1 gene. Up to this point, we had used only the two USER friendly sequences that were complementary to cloning vector pNEB206A and the USER friendly vectors that we had constructed with the pNEB206A sequences. However, USER enzyme mix can be used to create 3′ overhangs by simply incorporating a deoxyuridine within the PCR primer used to generate the fragment. To create a deletion of the rtxA1 gene, we amplified 1-kb upstream and downstream flanking sequences with a SmaI restriction site shared between them. By flanking the SmaI site with a deoxyadenine residue and a deoxyuridine residue, treatment with USER enzyme generated TGGGCCCA overhangs. By incorporating the USER friendly sequences complementary to pNEB206A at the left end of the upstream sequence and the right end of the downstream sequence, the fragments were captured into our USER friendly allelic exchange vector pGTR1129 very efficiently (see Fig. S3 in the supplemental material). The SmaI restriction site was used to insert an aph kanamycin resistance gene cassette between the upstream and downstream fragments to enable the selection of the deletion mutations during sacB-assisted allelic exchange into the V. vulnificus genome. In agreement with results described previously by others (21, 25, 30), the ΔrtxA1::aph mutant V. vulnificus strain was defective for cytotoxicity to epithelial cells and was attenuated for virulence in iron dextran-treated mice (Joseph et al., unpublished). We similarly used three-part USER friendly cloning and sacB-assisted allelic exchange to rapidly construct a ΔfadR::aph mutant and determined that FadR-mediated regulation of fatty acid metabolism is critical for the virulence of V. vulnificus (6).
Three-part USER friendly cloning coupled with our modified USER friendly vectors used to create the ΔrtxA1::aph and ΔfadR::aph mutations enabled the creation of allelic exchange plasmids for the unlabeled deletion of genes in a single day, including PCRs, USER enzyme treatment, ligation, and electroporation into E. coli. The inclusion of the antibiotic resistance cassette into the deletion mutation plasmids added a couple of days to the plasmid constructions. The three-part USER friendly cloning system for creating allelic exchange plasmids is highly efficient and should be useful to many others working with gram-negative bacteria. As opposed to the difficulties in creating TOPO TA cloning vectors, the ability to incorporate USER friendly sequences into any vector is particularly useful. The only limitation to creating one's own USER friendly cloning site is that a restriction enzyme site that leaves an A residue at the 5′ end (such as XbaI) is used in conjunction with the Nt.BbvCI nicking site. This A is complementary to the T that takes the place of the U in the USER friendly primer.
After the relative ease of creating the allelic exchange plasmids with USER friendly cloning, working with two-step sacB-assisted allelic exchange was problematic. After conjugation into V. vulnificus and selection for the single-crossover integration event, counterselection against the sacB gene of our pCVD442-based allelic exchange vectors in V. vulnificus was inefficient. Blomfield et al. (5) previously reported that eliminating sodium chloride from the sucrose-containing plates improved counterselection in E. coli; however, the strict requirement for V. vulnificus to have salt in the medium precludes this modification of the procedure. We examined numerous concentrations of sucrose, different growth media, and different growth temperatures to optimize counterselection; however, we still found it necessary to screen over 100 putative sucrose-resistant colonies to find a true excision event that had lost the allelic exchange vector, and in some cases, we never isolated truly sucrose-resistant excision events. The use of an antibiotic resistance gene cassette between the upstream and downstream sequences to label the mutation enabled selection for the mutant genotype; however, when no antibiotic resistance marker was used, we had to screen considerably more sucrose-resistant isolates, since many of them had reverted to the wild type.
A solution to this problem was found by applying the results described previously by Meibom et al. (32), who demonstrated that V. cholerae becomes naturally transformable upon growth in the presence of chitin, to our studies of V. vulnificus. Those authors examined both purified chitin and crab shells; we used only crab shells in our studies. By simply growing the recipient V. vulnificus strain in seawater with a salinity of 25 ppt with a small piece of sterile crab shell, we could readily obtain transformants with linear DNA. Intact plasmids, including cloning vectors pCOS5 and pRK437, could not be taken up and/or maintained by the recipient V. vulnificus cells. Even if the plasmids encoded V. vulnificus DNA sequences to enable the integration of the plasmids into the recipient genome, no antibiotic-resistant transformants were obtained. However, if the vectors containing V. vulnificus sequences flanking an antibiotic resistance cassette were linearized by restriction enzyme digestion or shearing (sonication or vortexing), single-step allelic exchange events were obtained with usable frequencies, on the order of 1.2 × 10−7. The use of the PCR amplicons consisting of V. vulnificus DNA sequences flanking an antibiotic resistance cassette was not efficient or reliable for creating recombinants. However, if the same sequences were incorporated into a plasmid vector, followed by linearization, the transformation and recombination were efficient. Apparently, PCR product-based allelic exchange via transformation suffers from the ends of the transformed DNA fragment being too short, possibly being degraded by the potent nuclease of V. vulnificus (50). However, the presence of vector sequences outside of the cloned upstream and downstream V. vulnificus sequences apparently protects the ends long enough to enable recombination into the homologous sequences of the recipient V. vulnificus strain. The use of chitin-based transformation for allelic exchange has obviated the need to use sucrose resistance as a counterselectable marker when a selectable genetic marker is included with the mutation.
Until now, if one had created an antibiotic resistance-labeled mutation in one V. vulnificus strain and wanted to create the same mutation in a different strain, either to combine mutations (as was the case for the ΔflaCDE::aph and ΔflaFBA::cat mutations described here) or to examine mutations in different strain backgrounds, the allelic exchange plasmid and the tedious two-step sacB-assisted allelic exchange had to be used again for each strain. However, the fact the chitin-based natural transformation of V. vulnificus works best with relatively long pieces of linear DNA led us to examine the possibility of using genomic DNA of strains with antibiotic resistance-labeled mutations to move the mutations from one strain to another or to replace the mutation in the same strain background to preclude the effects of unlinked secondary mutations that could occur during strain construction. We used chitin-based transformation with genomic DNA to combine the ΔflaCDE::aph and ΔflaFBA::cat mutations and showed that this method was an effective means of moving established mutations within the CMCP6 strain background. Similarly, we transformed the wza::TnphoA mutation, which abolishes capsular polysaccharide synthesis (49), from the V. vulnificus M06-24/O background into the CMCP6 background in a single step by selecting for the acquisition of kanamycin resistance. As expected, the kanamycin-resistant transformants acquired the translucent-colony morphotype typical of capsule mutants and were attenuated for virulence in our subcutaneously inoculated iron dextran-treated mouse model. Therefore, once an antibiotic resistance-labeled mutation has been created in one V. vulnificus strain, there is no further need for the difficult two-step sacB-assisted counterselection allelic exchange procedure to move the mutation into different strains.
In their studies of chitin-based transformation of V. cholerae, Meibom et al. (32) previously showed that simply growing different V. cholerae strains together in the presence of crab shells enabled horizontal gene transfer between the strains. We similarly asked if the coculture of V. vulnificus strains on crab shells could enable horizontal gene transfer. By growing the ΔflaCDE::aph and ΔflaFBA::cat strains together on a crab shell in seawater, we readily obtained double mutants that were confirmed by PCR and by their nonmotile phenotype. Horizontal gene transfer between V. vulnificus cells growing on chitin has implications for population genetics of V. vulnificus. There are two clades of V. vulnificus based on multilocus sequence typing, ribotyping, and other PCR-based measures (2, 9, 33). However, in their multilocus sequence typing analysis, Bisharat et al. (2) recently detailed a mosaic pattern between the clades, suggesting that horizontal gene transfer was occurring in nature. Similarly, we have examined relationships among diverse genotyping strategies examining different loci in the V. vulnificus genome, and we have observed strains that appear to have acquired sequences from different clades or genotypes (P. C. Thiaville et al., unpublished data). We have examined 19 V. vulnificus phages for their abilities to transduce a spontaneous rifampin resistance mutation between V. vulnificus strains, but we never identified a generalized transducing phage (J. L. Martin et al., unpublished data). There is very little known about plasmids of biotype 1 V. vulnificus strains and even less so about the possible Hfr-mediated exchange of chromosomal DNA sequences. However, conserved conjugative and mobilizable plasmids have been identified for biotype 2 V. vulnificus strains (24). Because V. vulnificus is believed to grow as biofilms on chitin-containing planktonic organisms in the environment (16, 35, 36, 47), it is conceivable that chitin-based transformation occurs in the wild and could significantly contribute to the mosaic pattern of the genomic structure of V. vulnificus.
In summary, we believe that the vectors that we have described here will be useful to others for capturing PCR amplicons for both expression and allelic exchange mutagenesis. Similarly, our adaption of USER friendly three-part cloning for the single-step creation of allelic exchange sequences should facilitate the construction of such plasmids for use with a variety of bacteria. USER friendly cloning can be adapted to essentially any plasmid vector. Finally, with relevance to V. vulnificus and likely other species of Vibrio, chitin-based transformation and recombination should make allelic exchange mutagenesis, at least that employing antibiotic resistance cassettes to label mutations, considerably more rapid and efficient than the standard two-step selection for single-crossover and counterselection for second crossover events.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 AI056056 to P.A.G. J.L.J. was supported by NIH training grant T32 AI060527.
We thank Jorge Girón for critical review of the manuscript.
Footnotes
Published ahead of print on 5 June 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Akerley, B. J., E. J. Rubin, A. Camilli, D. J. Lampe, H. M. Robertson, and J. J. Mekalanos. 1998. Systematic identification of essential genes by in vitro mariner mutagenesis. Proc. Natl. Acad. Sci. USA 95:8927-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisharat, N., D. I. Cohen, M. C. Maiden, D. W. Crook, T. Peto, and R. M. Harding. 2007. The evolution of genetic structure in the marine pathogen, Vibrio vulnificus. Infect. Genet. Evol. 7:685-693. [DOI] [PubMed] [Google Scholar]
- 3.Bitinaite, J., M. Rubino, K. H. Varma, I. Schildkraut, R. Vaisvila, and R. Vaiskunaite. 2007. USER friendly DNA engineering and cloning method by uracil excision. Nucleic Acids Res. 35:1992-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake, P. A., M. H. Merson, R. E. Weaver, D. G. Hollis, and P. C. Heublein. 1979. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N. Engl. J. Med. 300:1-5. [DOI] [PubMed] [Google Scholar]
- 5.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 5:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Brown, R. N., and P. A. Gulig. 2008. Regulation of fatty acid metabolism by FadR is essential for Vibrio vulnificus to cause infection of mice. J. Bacteriol. 190:7633-7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Brown, R. N., and P. A. Gulig. Roles of RseB, σE, and DegP in virulence of colony morphotype of Vibrio vulnificus. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 7.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 101:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerda-Cuellar, M., J. Jofre, and A. R. Blanch. 2000. A selective medium and a specific probe for detection of Vibrio vulnificus. Appl. Environ. Microbiol. 66:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, A. L., J. D. Oliver, A. DePaola, E. J. Feil, and E. F. Boyd. 2007. Emergence of a virulent clade of Vibrio vulnificus and correlation with the presence of a 33-kilobase genomic island. Appl. Environ. Microbiol. 73:5553-5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connell, T. D., A. J. Martone, and R. K. Holmes. 1995. A new mobilizable cosmid vector for use in Vibrio cholerae and other gram-negative bacteria. Gene 153:85-87. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geu-Flores, F., H. H. Nour-Eldin, M. T. Nielsen, and B. A. Halkier. 2007. USER fusion: a rapid and efficient method for simultaneous fusion and cloning of multiple PCR products. Nucleic Acids Res. 35:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulig, P. A., K. L. Bourdage, and A. M. Starks. 2005. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 43:118-131. [PubMed] [Google Scholar]
- 16.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the γ-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlady, W. G., and K. C. Klontz. 1996. The epidemiology of Vibrio infections in Florida, 1981-1993. J. Infect. Dis. 173:1176-1183. [DOI] [PubMed] [Google Scholar]
- 18.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 19.Hyakutake, A., M. Homma, M. J. Austin, M. A. Boin, C. C. Hase, and I. Kawagishi. 2005. Only one of the five CheY homologs in Vibrio cholerae directly switches flagellar rotation. J. Bacteriol. 187:8403-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, Y. R., S. E. Lee, C. M. Kim, S. Y. Kim, E. K. Shin, D. H. Shin, S. S. Chung, H. E. Choy, A. Progulske-Fox, J. D. Hillman, M. Handfield, and J. H. Rhee. 2003. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 71:5461-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, Y. R., S. E. Lee, H. Kook, J. A. Yeom, H. S. Na, S. Y. Kim, S. S. Chung, H. E. Choy, and J. H. Rhee. 2008. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell. Microbiol. 10:848-862. [DOI] [PubMed] [Google Scholar]
- 22.Kim, Y. R., and J. H. Rhee. 2003. Flagellar basal body flg operon as a virulence determinant of Vibrio vulnificus. Biochem. Biophys. Res. Commun. 304:405-410. [DOI] [PubMed] [Google Scholar]
- 23.Kleckner, N., J. Bender, and S. Gottesman. 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol. 204:139-180. [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. T., C. Amaro, K. M. Wu, E. Valiente, Y. F. Chang, S. F. Tsai, C. H. Chang, and L. I. Hor. 2008. A common virulence plasmid in biotype 2 Vibrio vulnificus and its dissemination aided by a conjugal plasmid. J. Bacteriol. 190:1638-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, J. H., M. W. Kim, B. S. Kim, S. M. Kim, B. C. Lee, T. S. Kim, and S. H. Choi. 2007. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45:146-152. [PubMed] [Google Scholar]
- 26.Lee, J. H., J. B. Rho, K. J. Park, C. B. Kim, Y. S. Han, S. H. Choi, K. H. Lee, and S. J. Park. 2004. Role of flagellum and motility in pathogenesis of Vibrio vulnificus. Infect. Immun. 72:4905-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 30.Liu, M., A. F. Alice, H. Naka, and J. H. Crosa. 2007. The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 75:3282-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manoil, C., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. USA 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meibom, K. L., M. Blokesch, N. A. Dolganov, C. Y. Wu, and G. K. Schoolnik. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824-1827. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson, W. B., R. N. Paranjpye, A. DePaola, and M. S. Strom. 2003. Sequence polymorphism of the 16S rRNA gene of Vibrio vulnificus is a possible indicator of strain virulence. J. Clin. Microbiol. 41:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okujo, N., T. Akiyama, S. Miyoshi, S. Shinoda, and S. Yamamoto. 1996. Involvement of vulnibactin and exocellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol. Immunol. 40:595-598. [DOI] [PubMed] [Google Scholar]
- 35.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1982. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl. Environ. Microbiol. 44:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1983. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl. Environ. Microbiol. 45:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paranjpye, R. N., and M. S. Strom. 2005. A Vibrio vulnificus type IV pilin contributes to biofilm formation, adherence to epithelial cells, and virulence. Infect. Immun. 73:1411-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott, H. N., P. D. Laible, and D. K. Hanson. 2003. Sequences of versatile broad-host-range vectors of the RK2 family. Plasmid 50:74-79. [DOI] [PubMed] [Google Scholar]
- 39.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 40.Starks, A. M., K. L. Bourdage, P. C. Thiaville, and P. A. Gulig. 2006. Use of a marker plasmid to examine growth and death of Vibrio vulnificus in infected mice. Mol. Microbiol. 61:310-323. [DOI] [PubMed] [Google Scholar]
- 41.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. 2000. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron dextran-treated mice. Infect. Immun. 68:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 43.Taylor, L. A., and R. E. Rose. 1988. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 16:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing synthetic primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 45.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, J. A., and P. A. Gulig. 1998. Regulation of the spvR gene of the Salmonella typhimurium virulence plasmid during exponential phase growth in intracellular salts medium and at stationary phase in L broth. Microbiology 144:1823-1833. [DOI] [PubMed] [Google Scholar]
- 47.Wright, A. C., R. T. Hill, J. A. Johnson, M. C. Roghman, R. R. Colwell, and J. G. J. Morris. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright, A. C., L. M. Simpson, J. D. Oliver, and J. G. Morris, Jr. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, S. I., S. K. Lo, C. P. Shao, H. W. Tsai, and L. I. Hor. 2001. Cloning and characterization of a periplasmic nuclease of Vibrio vulnificus and its role in preventing uptake of foreign DNA. Appl. Environ. Microbiol. 67:82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanisch Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 52.Zakaria-Meehan, Z., G. Massad, L. M. Simpson, J. C. Travis, and J. D. Oliver. 1988. Ability of Vibrio vulnificus to obtain iron from hemoglobin-haptoglobin complexes. Infect. Immun. 56:275-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.