Abstract
The single polar flagellum of Shewanella oneidensis MR-1 is powered by two different stator complexes, the sodium-dependent PomAB and the proton-driven MotAB. In addition, Shewanella harbors two genes with homology to motX and motY of Vibrio species. In Vibrio, the products of these genes are crucial for sodium-dependent flagellar rotation. Resequencing of S. oneidensis MR-1 motY revealed that the gene does not harbor an authentic frameshift as was originally reported. Mutational analysis demonstrated that both MotX and MotY are critical for flagellar rotation of S. oneidensis MR-1 for both sodium- and proton-dependent stator systems but do not affect assembly of the flagellar filament. Fluorescence tagging of MotX and MotY to mCherry revealed that both proteins localize to the flagellated cell pole depending on the presence of the basal flagellar structure. Functional localization of MotX requires MotY, whereas MotY localizes independently of MotX. In contrast to the case in Vibrio, neither protein is crucial for the recruitment of the PomAB or MotAB stator complexes to the flagellated cell pole, nor do they play a major role in the stator selection process. Thus, MotX and MotY are not exclusive features of sodium-dependent flagellar systems. Furthermore, MotX and MotY in Shewanella, and possibly also in other genera, must have functions beyond the recruitment of the stator complexes.
Flagellum-mediated swimming motility is a widespread means of locomotion among bacteria. Flagella consist of protein filaments that are rotated at the filament's base by a membrane-embedded motor (3, 39). Rotation is powered by electrochemical gradients across the cytoplasmic membrane. Thus far, two coupling ions, sodium ions and protons, have been described as energy sources for bacterial flagellar motors (4, 24, 48). Two major components confer the conversion of the ion flux into rotary motion. The first component forms a rotor-mounted ring-like structure at the base of the flagellar basal body and is referred to as the switch complex or the C ring; it is composed of the proteins FliG, FliM, and FliN. The second major component is the stator system, consisting of membrane-embedded stator complexes that surround the C ring (3). Each stator complex is composed of two subunits in a 4:2 stoichiometry. In Escherichia coli, MotA and MotB constitute the stator complex by forming a proton-specific ion channel; the Na+-dependent counterpart in Vibrio species consists of the orthologs PomA and PomB (1, 5, 49). MotA and PomA both have four transmembrane domains and are thought to interact with FliG via a cytoplasmic segment to generate torque (2, 50). Stator function is presumably made possible by a peptidoglycan-binding motif located at the C-terminal portion of MotB and PomB that anchors the stator complex to the cell wall (1, 8). In E. coli, at least 11 stator complexes can be synchronously involved in driving flagellar rotation (35). However, a single complex is sufficient for rotation of the filament (36, 40). Despite its tight attachment to the peptidoglycan, the stator ring system was found to form a surprisingly dynamic complex. It has been suggested that inactive precomplexes of the stators form a membrane-located pool before being activated upon incorporation into the stator ring system around the motor (13, 45). In E. coli, the turnover time of stator complexes can be as short as 30 s (21).
In Vibrio species, two auxiliary proteins, designated MotX and MotY, are required for motor function of the Na+-driven polar flagellar system (22, 23, 28, 31). Recently, it was shown that the proteins associate with the flagellar basal body in Vibrio alginolyticus to form an additional structure, the T ring (42). MotX interacts with MotY and the PomAB stator complexes, and both proteins are thought to be crucial for the acquisition of the stators to the motor of the polar flagellum. (29, 30, 42). A MotY homolog is also associated with the proton-dependent motor system of the lateral flagella of V. alginolyticus that is induced under conditions of elevated viscosity (41).
We recently showed that Shewanella oneidensis MR-1 uses two different stator systems to drive the rotation of its single polar flagellum, the Na+-dependent PomAB stator and the proton-driven MotAB stator. As suggested by genetic data, the MotAB stator has been acquired by lateral gene transfer, presumably in the process of adaptation from a marine to a freshwater environment (32). The two different stators are recruited to the motor in a way that depends on the sodium ion concentration in the medium. The Na+-dependent PomAB stator is present at the flagellated cell pole regardless of the sodium ion concentration, whereas the proton-dependent MotAB stator functionally localizes only under conditions of low sodium or in the absence of PomAB. It is still unclear how stator selection is achieved and whether additional proteins play a role in this process.
Orthologs of motX and motY have been annotated in S. oneidensis MR-1. We thus hypothesized that MotX and MotY might play a role in stator selection in S. oneidensis MR-1. However, the originally published sequence of motY harbors a frameshift that would result in a drastically truncated protein lacking a functionally relevant putative peptidoglycan-binding domain at its C terminus (16, 18). This situation seemed inconsistent with a role for MotY in S. oneidensis MR-1.
Here we describe a functional analysis of the MotX and MotY orthologs in S. oneidensis MR-1. We found that motY does not, in fact, contain a frameshift mutation, so that MotY is translated in its full-length form. Both MotX and MotY were essential for Na+-dependent and proton-dependent motility. Therefore, these proteins have a role in S. oneidensis MR-1 that differs from their function in Vibrio species. We also used fusions to the fluorescent protein mCherry for functional localization studies of MotX and MotY.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and media.
The bacterial strains used in this study are listed in Table 1. Escherichia coli strains were grown in LB medium at 37°C. For strain WM3064, 2,6-diaminopimelic acid (DAP) was added to a final concentration of 300 μM. S. oneidensis strains were cultivated in LB or LM (32) medium as indicated. When necessary, media were solidified using 1.5% (wt/vol) agar and/or supplemented with 10 μg·ml−1 chloramphenicol, 10 μg·ml−1 gentamicin, and/or 25 μg·ml−1 kanamycin. Protein localization depending on sodium concentration was carried out in LM medium with the indicated amount of sodium chloride added. To obtain a similar ionic strength in the medium, KCl was added to yield an overall concentration of 100 mM.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or phenotypea | Source or reference |
|---|---|---|
| Escherichia coli strains | ||
| DH5α-λpir | φ80dlacZΔM15 Δ(lacZYA-argF)U196 recA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1/λpir | 25 |
| WM3064 | thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::[erm pir(wt)] | W. Metcalf, University of Illinois, Urbana-Champaign |
| Shewanella oneidensis strains | ||
| MR-1 | Wild type | 46 |
| S208 | MR-1 ΔfliF | This work |
| S182 | MR-1 ΔmotX | This work |
| S257 | MR-1 ΔmotY | This work |
| S256 | MR-1 ΔmotX ΔmotY | This work |
| S258 | MR-1 ΔmotX ΔpomAB | This work |
| S255 | MR-1 ΔmotY ΔpomAB | This work |
| S1099 | MR-1 ΔmotX ΔmotAB | This work |
| S1100 | MR-1 ΔmotY ΔmotAB | This work |
| S305 | MR-1 ΔmotX pBBR-motX, complementation of ΔmotX, Gmr | This work |
| S323 | MR-1 ΔmotY pBBR-motY, complementation of ΔmotY, Gmr | This work |
| S292 | MR-1 motX-mCherry, C-terminal fusion of motX to mCherry, Kmr | This work |
| S293 | MR-1 motY-mCherry, C-terminal fusion of motY to mCherry, Kmr | This work |
| S296 | MR-1 ΔfliF motX-mCherry, Kmr | This work |
| S297 | MR-1 ΔfliF motY-mCherry, Kmr | This work |
| S295 | MR-1 ΔmotX motY-mCherry, Kmr | This work |
| S294 | MR-1 ΔmotY motX-mCherry, Kmr | This work |
| S166 | MR-1 pomB-mCherry, C-terminal fusion of pomB to mCherry, Kmr | 32 |
| S265 | MR-1 ΔmotX pomB-mCherry, Kmr | This work |
| S267 | MR-1 ΔmotY pomB-mCherry, Kmr | This work |
| S266 | MR-1 ΔmotXY pomB-mCherry, Kmr | This work |
| S1150 | MR-1 ΔmotAB ΔmotXY pomB-mCherry, Kmr | This work |
| S165 | MR-1 motB-mCherry, C-terminal fusion of motB to mCherry, Kmr | 32 |
| S268 | MR-1 ΔpomAB ΔmotX motB-mCherry, Kmr | This work |
| S269 | MR-1 ΔpomAB ΔmotY motB-mCherry, Kmr | This work |
| S291 | MR-1 ΔmotX ΔmotY motB-mCherry, Kmr | This work |
| S1151 | MR-1 ΔmotY motB-mCherry, Kmr | This work |
| S1152 | MR-1 ΔmotX motB-mCherry, Kmr | This work |
| S1091 | MR-1 ΔpomAB ΔmotAB motX-mCherry, Kmr | This work |
| S1093 | MR-1 ΔpomAB ΔmotAB motY-mCherry, Kmr | This work |
| Plasmids | ||
| pBC SK+ | pUC origin, PtaclacZα, Cmr | Stratagene |
| pBBR1-MCS5 | ori pBBR oriT. broad-host-range vector, Plac, Gmr | 19 |
| pGP704Sac28Km | mobRP4+ori-R6K sacB, suicide plasmid for in frame deletions, Kmr | 44 |
| pJP5603 | mobRP4+ori-R6K, suicide plasmid for mutation by plasmid integration, Kmr | 33 |
| pBBR-motX | motX in pBBR1-MCS5 | This work |
| pBBR-motY | motY in pBBR1-MCS5 | This work |
| pGP704Sac28Km-fliF | fliF in-frame deletion fragment in pGP704Sac28-Km, Kmr | This work |
| pGP704Sac28Km-motX | motX in-frame deletion fragment in pGP704Sac28-Km, Kmr | This work |
| pGP704Sac28Km-motY | motY in-frame deletion fragment in pGP704Sac28-Km, Kmr | This work |
| pJP5603-mCherry | Promoterless mCherry in pJP5603, Kmr | This work |
| pJP5603-motX::mCherry | C-terminal fusion of motX to mCherry in pJP5603, Kmr | This work |
| pJP5603-motY::mCherry | C-terminal fusion of motY to mCherry in pJP5603, Kmr | This work |
| pJP5603-motB::mCherry | C-terminal fusion of motB to mCherry in pJP5603, Kmr | 32 |
| pJP5603-pomB::mCherry | C-terminal fusion of pomB to mCherry in pJP5603, Kmr | 32 |
Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance.
Strain constructions.
DNA manipulations were carried out according to standard techniques (37). Kits for isolation of chromosomal DNA, isolation of plasmids, and purification of PCR products were purchased from Qiagen (Hilden, Germany), Sigma Aldrich GmbH (Taufkirchen, Germany), and/or HISS Diagnostics GmbH (Freiburg, Germany). Enzymes were obtained from New England Biolabs (Frankfurt, Germany) and Fermentas (St. Leon-Rot, Germany). Sequencing was conducted using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Darmstadt, Germany), and the reactions were then analyzed on a 3130 genetic analyzer (Applied Biosystems, Darmstadt, Germany).
For resequencing of motY, the gene region was amplified from chromosomal DNA and, after treatment with BamHI and XhoI, cloned into the vector pBC SK+. Five independent clones were sequenced.
In-frame deletion mutations of motX and motY in S. oneidensis MR-1 strains were constructed as described previously (43, 44). DNA fragments that encompassed only short terminal 5′ and 3′ sections of the target genes were amplified by PCR using corresponding primer pairs (see Table S1 in the supplemental material). After purification and treatment with appropriate restriction enzymes, the fragments were ligated to yield an in-frame fusion product encoding a drastically truncated gene product. Subsequently, the fusion product was amplified using the outer primers, digested with appropriate enzymes, and ligated into suicide vector pGP704-Sac28Km. The resulting plasmid was introduced into the corresponding S. oneidensis MR-1 strain by conjugative mating using E. coli WM3064 as a donor on LB medium containing DAP. Single-crossover integration mutants were selected on LB plates containing kanamycin but lacking DAP. Single colonies were grown overnight in liquid LB without antibiotics and plated on LB containing 10% (wt/vol) sucrose to screen for plasmid excision by double-crossover events. Kanamycin-sensitive colonies were then checked for the targeted deletion by colony PCR using primers bracketing the location of the deletion.
To complement the deletion mutations in motX and motY, the gene regions were amplified from chromosomal DNA, digested with BamHI and EcoRI, cloned into pBBR1-MCS5, and introduced into S. oneidensis MR-1 by electroporation (27).
For C-terminal tagging of proteins to mCherry, the mCherry gene was amplified and cloned into the suicide vector pJP5603 after digestion with BamHI and SalI to yield plasmid pJP5603-mCherry. A derivative of mCherry that was adapted to the codon usage of S. oneidensis MR-1 was used. DNA fragments encoding the C termini of MotX and MotY were amplified and ligated into the EcoRI/BamHI site of pJP5603-mCherry to yield a functional in-frame fusion in which the corresponding target protein is linked to mCherry by a Gly-Ser-Gly-Gly-Gly linker peptide. The resulting constructs were conjugated into S. oneidensis MR-1 from E. coli WM3064, and kanamycin-resistant colonies were checked for the correct insertion by PCR.
Motility assays.
Rapid motility screening was carried out by spotting 3 μl of a liquid culture of the corresponding strain on plates that contained LB medium with an agar concentration of 0.25% (wt/vol). Although these plates are commonly referred to as “swarming plates,” the bacterial movement that was actually scored was flagellum-mediated swimming motility. Strains to be directly compared were always spotted onto the same individual plate. Motility was scored after overnight incubation at 30°C.
To monitor swimming motility in liquid media, cell material was directly transferred from fresh overnight LB plates to LB or LM medium to yield an optical density at 600 nm of 0.2 to 0.4 and incubated for 30 min at room temperature. Swimming motility was then determined by light microscopy.
Fluorescence microscopy.
Prior to microscopy, cells were immobilized on pads composed of LM medium solidified with 1% agarose. Microscopy was performed with an upright Zeiss Image MI (Oberkochen, Germany) equipped with Cascade 1K camera (Visitron Systems, Puchheim, Germany) and a Zeiss Plan Apochromat 100×/1.4 differential interference contrast (DIC) objective. Image processing was carried out using Metamorph 7.1.2, Adobe Photoshop CS2, and Adobe Illustrator CS2. When polar localization was determined, at least 250 cells per data point were observed.
Flagellum staining.
The flagellation state was visualized by the silver impregnation method, which was carried out essentially as described earlier (6). For colocalization of mCherry-tagged stator subunits with the flagellum, the flagellar filaments were visualized by fluorescence labeling using Alexa Fluor 488 carboxylic acid succinimidyl ester (Invitrogen) as previously reported (32). Fluorescence microscopy was carried out as described above. Visualization of the flagellar filament was enhanced by using the “sharpen” function of the Metamorph software.
Immunoblot analysis.
To obtain protein lysates for determining the stability of the MotX-mCherry and MotY-mCherry fusions, cells corresponding to an optical density at 600 nm of 0.25 from exponentially growing LB cultures were harvested by centrifugation. The sediments were resuspended in 25 μl sample buffer (20), heated at 99°C for 5 min, and stored at −20°C. Ten microliters of the sample was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% polyacrylamide gels. Subsequently, proteins were transferred to polyvinylidene difluoride membranes by semidry transfer. For detection of the proteins, polyclonal antibodies raised against dsRed (Clontech) were used at a dilution of 1:500. Secondary anti-rabbit immunoglobulin G-horseradish peroxidase antibody was used at a dilution of 1:20,000, and signals were detected using the Western Lightning chemiluminescence reagent (Perkin-Elmer LAS, Inc.) followed by exposure to autoradiography film.
RESULTS
S. oneidensis MR-1 possesses motX and motY orthologs.
The S. oneidensis MR-1 genome sequence (16) contains two open reading frames that were annotated as motX (SO_3936) and motY (SO_2754) based on homologies to the corresponding genes in Vibrio species. The deduced amino acid sequence of motX exhibits 40% identity and 59% similarity to that of MotX of V. alginolyticus. As in Vibrio, the convergently transcribed gene upstream of motX encodes a protein suggested to be involved in purine ribonucleotide synthesis. MotX is predicted to have a molecular mass of 23.2 kDa and to harbor three Sel1 domains, which are thought to mediate protein-protein interactions (15, 26). Sequence analysis using SignalP (12) strongly indicates that MotX has a signal peptide that should be cleaved between amino acids 19 and 20.
The original sequence of motY in S. oneidensis MR-1 included a frameshift that would create a truncated protein likely to be nonfunctional (16). In order to verify the correctness of that sequence, chromosomal DNA was prepared from an S. oneidensis MR-1 culture exhibiting good swimming motility as determined by light microscopy prior to cell harvest. The gene region of motY was amplified and cloned. The sequences obtained from five independent clones revealed that the originally published sequence contains an insertion of an additional adenosine at position 420, leading to two consecutive stop codons and early termination of translation. However, the open reading frame of motY amplified from motile S. oneidensis MR-1 cells encompasses 871 bp, encoding a protein of 289 amino acids with a calculated molecular mass of 33.0 kDa. All further characterizations carried out in this study will refer to that full-length MotY. Similar to the case for MotX, MotY was predicted to harbor a signal peptide with a cleavage site between amino acids 19 and 20. The protein exhibits 44% identity and 66% similarity to MotY of V. alginolyticus and is predicted to have a putative peptidoglycan-binding domain at the C terminus. As in Vibrio species, the divergently transcribed open reading frame upstream of motY encodes a gene product likely to be involved in RNA processing.
Both motX and motY are highly conserved in all Shewanella species that have been sequenced. Recently, σ28-dependent promoters have been predicted to be located upstream of motX and motY (38), suggesting that both are part of the flagellar regulatory network in Shewanella.
Both MotX and MotY are essential for swimming motility of S. oneidensis MR-1.
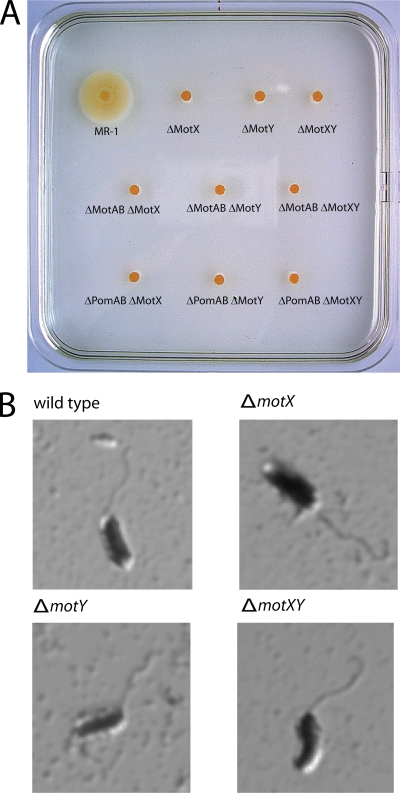
To investigate a possible role for MotX and MotY in the motility of S. oneidensis MR-1, in-frame deletion mutations were created in motX (ΔmotX), motY (ΔmotY), and both (ΔmotXY). To confirm that no further copies of these genes remained in the genome, the deletion was confirmed by Southern analysis (see Fig. S1 in the supplemental material). The mutations did not affect the growth rate (data not shown). Independent of the Na+ concentration, none of the mutants displayed swimming motility as determined by light microscopy and by swimming assays in semisolid agar (Fig. 1). The motility defects conferred by the single mutations were rescued by ectopic expression of plasmid-borne motX and motY in the corresponding mutant (see Fig. S1 in the supplemental material).
FIG. 1.
(A) Swimming phenotype of ΔmotX and ΔmotY mutants on a 0.25% semisolid agar plate. The indicated mutants were spotted on soft agar and incubated overnight at 30°C. (B) Flagellation of motX and motY mutants. Displayed are micrographs of the corresponding strains after flagellar staining.
To rule out that the loss of swimming motility due to deletions in motX and motY was caused by a defect in flagellum assembly, flagellar filament staining was performed on mutant and wild-type cells (Fig. 1C). All mutants possessed a flagellar filament similar to that of wild type cells. Thus, the nonmotile phenotype is the consequence of an inability of the mutants to rotate the filament.
S. oneidensis MR-1 harbors two stator systems, MotAB and PomAB. Under our experimental conditions, flagellar rotation depends mainly on the Na+-driven PomAB stator (32). Thus, the observed phenotype could be due to loss of PomAB function or of both PomAB and MotAB function. To specifically determine whether MotX and MotY are required for PomAB- or MotAB-mediated flagellar function, deletions of motX and motY were introduced into an S. oneidensis MR-1 strain lacking pomAB (ΔpomAB ΔmotX and ΔpomAB ΔmotY) and motAB (ΔmotAB ΔmotX and ΔmotAB ΔmotY), respectively. All of these strains were nonmotile as determined by microscopic observation and motility in semisolid agar. Based on these results, we conclude that both MotX and MotY are required to support flagellar rotation driven by PomAB and MotAB stators.
Localization of MotX and MotY.
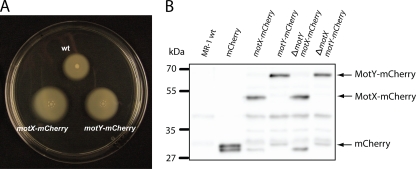
For further functional analysis, the localization of MotX or MotY was determined. Signal peptides were predicted for both proteins, and in V. alginolyticus it has been demonstrated that both are transported into the periplasm via a Sec-dependent mechanism during which the N-terminal signal sequence is cleaved (30). Therefore, mCherry, which is also functional when folding in the periplasm (7), was fused to the C termini of MotX and MotY. Subsequently, the gene fusions were individually integrated into the native loci by single-crossover events. Stability of the fusion proteins was determined by immunoblot analysis using an antibody specific to mCherry (Fig. 2B). In agreement with the estimated molecular masses of the two fusion proteins, single distinct bands at positions corresponding to ∼45 kDa and ∼55 kDa were observed for MotX-mCherry and MotY-mCherry, respectively. Minor degradation was found to occur, except for MotX-mCherry in the absence of MotY (Fig. 2B). To determine whether the fusion proteins were still active, strains harboring MotX-mCherry or MotY-mCherry were tested for motility in semisolid agar. Both fusion proteins supported swimming motility at a higher level than the wild-type proteins, suggesting a stabilizing effect (Fig. 2A). From that we concluded that both fusions of MotX and MotY to mCherry are active and stable enough to be used for localization studies of S. oneidensis MR-1.
FIG. 2.
Properties of MotX and MotY tagged with mCherry. (A) Swimming phenotype of strains harboring motX-mCherry and motY-mCherry. The strains were spotted on a 0.25% semisolid plate. (B) Detection of MotX-mCherry and MotY-mCherry by immunoblotting using antibodies raised against mCherry. Lane 1, wild type (negative control); lane 2, mCherry (positive control); lane 3, S292 (motX::mCherry); lane 4, S293 (motY::mCherry); lane 5, S294 (ΔmotY motX::mCherry); lane 6, S295 (ΔmotX motY::mCherry). Arrows indicate the positions of signals corresponding to MotX-mCherry (54.1 kDa), MotY-mCherry (62.4 kDa), and mCherry (29.3 kDa).
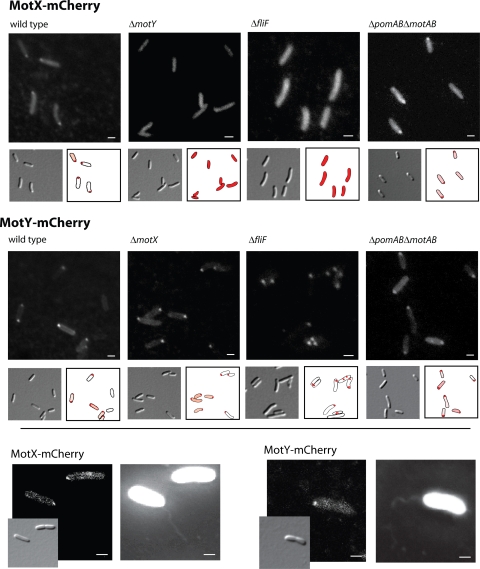
Cells expressing either motX-mCherry or motY-mCherry were grown to exponential growth phase and examined by fluorescence microscopy (Fig. 3). MotX-mCherry was observed to localize to the flagellated cell pole (Fig. 3), which was identified by simultaneous staining of the flagellar filament by staining with Alexa Fluor 488 succinimidyl ester (Fig. 3). Some fluorescence appeared to be associated with the cell envelope away from the pole. In addition, a diffuse fluorescent signal was detected in the cytoplasm, indicating either that a MotX-mCherry moiety is partly degraded or that not all protein synthesized is transported through the cytoplasmic membrane. MotY-mCherry formed a distinct fluorescent locus at the flagellated cell pole. A significant amount of MotY-mCherry also localized to the cell envelope away from the cell pole.
FIG. 3.
Localization of MotX-mCherry and MotY-mCherry in S. oneidensis MR-1. Displayed are DIC and fluorescence micrographs of cells harboring motX-mCherry (upper panel) and motY-mCherry (middle panel) in the wild type, ΔmotY or ΔmotX, ΔfliF, and ΔpomAB ΔmotAB mutant backgrounds (from left to right). To facilitate the visualization, the position of fluorescence signals relative to that of the cells is displayed. The lower panel shows colocalization of the flagellar filament with MotX-mCherry (left) and MotY-mCherry (right). DIC and fluorescence micrographs are displayed. Scale bars, 1 μm.
Since the localization of the MotAB stator in S. oneidensis MR-1 depends on the sodium ion concentration in the medium, we then determined whether a similar dependence exists for MotX and MotY. However, both of the fusion proteins localized equally well at all Na+ concentrations, including in the absence of any added sodium ion (data not shown).
Studies of MotX and MotY of V. alginolyticus have suggested that the two proteins form a complex and associate with the flagellar LP ring (42). Therefore, we investigated whether localization of both proteins is mutually dependent in S. oneidensis MR-1. An mCherry fusion to MotX was introduced into the chromosome of a ΔmotY strain (ΔmotY motX-mCherry), and, correspondingly, an mCherry fusion to MotY was introduced into the chromosome of a ΔmotX strain (ΔmotX motY-mCherry). To determine whether localization depends on the presence of the flagellar complex, both fusions were individually introduced into a ΔfliF mutant that lacks the flagellar basal body. The corresponding strains were then grown to exponential growth phase, and the cells were visualized by fluorescence microscopy (Fig. 3).
In the absence of MotY, no localization of MotX-mCherry was observed, and a diffuse fluorescence signal was distributed throughout the cell. In contrast, when MotX was absent, localization of MotY-mCherry still occurred at the flagellated cell pole. Localization of both proteins was dependent on the presence of the flagellar basal body. In the ΔfliF mutant, MotX-mCherry was distributed as in the ΔmotY strain. In contrast, MotY-mCherry still formed distinct foci in the cell envelope, but these occurred at random positions in the cell rather than exclusively at the flagellated pole. Thus, both MotX and MotY require the flagellar basal body for normal localization. In addition, MotX also requires the correct localization of MotY to be sequestered at the flagellated cell pole.
Stator localization independence on MotX and MotY.
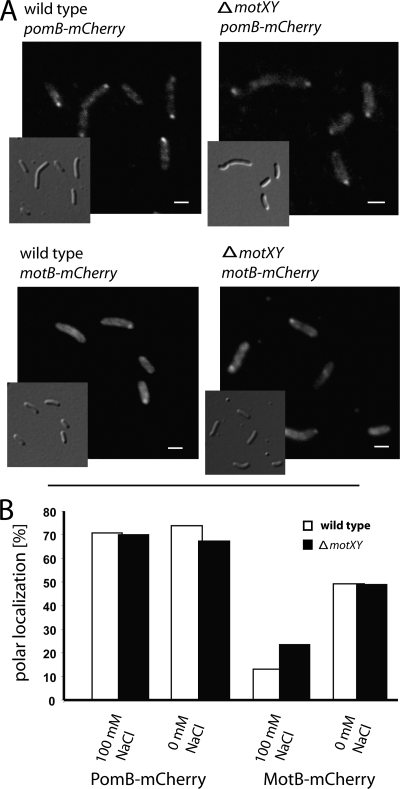
We next determined whether MotX and MotY are crucial for stator recruitment in S. oneidensis MR-1 and whether they are involved in the process by which the appropriate stator system is selected at low and high sodium ion concentrations. To this end, genes encoding PomB-mCherry and MotB-mCherry were individually integrated at the corresponding native loci in ΔmotX, ΔmotY, and ΔmotXY strains. We have previously demonstrated that these fusions are stable and functional (32).
The resulting strains were grown in LM medium supplemented with no or 100 mM sodium chloride, and localization of the stators was determined by fluorescence microscopy (Fig. 4). Only minor effects of the deletions on stator localization were observed. PomB-mCherry localized to the flagellated pole in ∼70% of the cell population of the wild type and all of the mutants, regardless of the presence or absence of added NaCl. At 100 mM NaCl, localization of MotB-mCherry increased from 12% in the wild type to 22% in cells lacking MotX and/or MotY. In the absence of added NaCl, MotB-mCherry localized to the flagellated pole in more than 50% of the cells in each strain. In a Δpom ΔmotXY mutant, MotB-mCherry localized in the same pattern as in a Δpom strain, and the same was observed for PomB-mCherry in a Δmot ΔmotXY mutant compared to a mutant lacking only motAB (data not shown). These results indicate that in S. oneidensis MR-1, MotX and MotY are not mandatory for stator recruitment or stator selection but might have a stabilizing function.
FIG. 4.
Localization of PomB-mCherry and MotB-mCherry in the ΔmotX ΔmotY mutant. (A) Micrographs (DIC and fluorescence) of cells expressing pomB-mCherry (upper image) and motB-mCherry (lower image) in wild-type and ΔmotX ΔmotY cells. Cells were grown in LM medium with 100 mM NaCl for pomB-mCherry and without added NaCl for motB-mCherry. Scale bars, 1 μm. (B) Percentages of polarly localized PomB-mCherry and MotB-mCherry in wild-type and ΔmotX ΔmotY cells. Each data point represents 250 cells.
Protein interaction studies revealed that in V. alginolyticus, MotX interacts with MotY and PomB (29); however, in S. oneidensis MR-1, MotX and MotY are not crucial for stator acquisition. Thus, polar localization of MotX and MotY might depend on the presence of stator subunits. To test this hypothesis, motX-mCherry and motY-mCherry were integrated at the corresponding native loci in a strain that lacks both stator systems (Δpom Δmot motX-mCherry and Δpom Δmot motY-mCherry). Subsequently, the localization of MotX-mCherry and MotY-mCherry was determined (Fig. 3). Both proteins localized to the flagellated cell pole just as in wild-type cells. Thus, localization of MotX and MotY to the flagellar motor does not depend on the presence of stator complexes.
DISCUSSION
The typical flagellar rotary motor consists of the switch complex components FliM, FliN, and FliG (3, 39). FliG is directly involved in rotation and physically interacts with the stator elements, PomAB for Na+-driven motors and MotAB for proton-driven motors. In some bacteria, auxiliary proteins have been implicated in stator function. In Sinorhizobium meliloti, MotC and MotE are required for flagellar rotation. MotE is thought to serve as a chaperone for MotC, which in turn binds to the stator subunit MotB. It has been speculated that MotC exerts its function by stabilizing the periplasmic domain of MotB (11, 34). The most comprehensive studies of auxiliary components of the bacterial flagellar motors have been conducted on MotX and MotY of Vibrio species. A protein homologous to MotY has also been found associated with the high-speed, proton-driven polar flagellum of Pseudomonas aeruginosa PAO1 (10) and the lateral, proton-driven flagella of Vibrio parahaemolyticus (41). For both systems, a corresponding MotX partner has not been identified. Thus, ancillary stator components in flagellar rotation are not limited to sodium-dependent or polar flagellar systems. We show here that either sodium-driven or proton-driven flagellar rotation in S. oneidensis MR-1 also depends on orthologs of the Vibrio proteins MotX and MotY. Both proteins are highly conserved within the genomes of all Shewanella isolates sequenced to date. Since all Shewanella species also harbor orthologs to the PomAB stator system, it is predicted that, as in Vibrio, MotX and MotY would be required to power their single polar flagellum. However, our results provide direct evidence that the alternative proton-conducting MotAB stator elements of S. oneidensis MR-1 also require MotX and MotY for normal function.
Localization studies of MotX and MotY in Vibrio species were initially carried out with E. coli, where they appear to be associated with the outer membrane (22, 23, 29, 30). However, a more recent model suggests that MotX and MotY form a complex that diffuses in the periplasm before interacting with the flagellar basal body (18). We used fluorescent mCherry fusion proteins to monitor the localization of MotX and MotY in S. oneidensis. As suggested by the presence of a putative N-terminal signal sequence, and in accordance with the Vibrio model, MotY was observed in the cell envelope. However, the fluorescence signal does not distinguish specifically whether the protein is freely diffusing in the periplasmic space or whether it is associated with the inner or outer membrane or the cell wall. As expected, distinct localization of MotY-mCherry occurred at the flagellated cell pole. The signal of MotX-mCherry was more diffuse, and some fluorescence was also detected in the cytoplasmic space. MotX-mCherry localized to the flagellated cell pole, but only when MotY was also present.
In the absence of a flagellar basal body, MotY-mCherry still occurred as distinct fluorescent foci in the cell envelope, indicating unspecific clustering. If MotX and MotY form a precomplex in the periplasm, MotX should also be detected in these clusters, but this was never observed. Instead, in strains lacking the flagellar basal body or MotY, a diffuse fluorescence signal of MotX-mCherry occurred throughout the cell. We conclude that in S. oneidensis MR-1, MotX and MotY do not form a precomplex in the periplasm. We propose instead that MotY is exported into the periplasm, forms a pool in the cell envelope, and interacts with the flagellar basal body before MotX is recruited. If MotY is not localized properly, MotX is degraded. This finding is consistent with the observation that, in Vibrio, MotX is unstable in the absence of MotY (29, 47). However, it remains unknown whether MotY already stabilizes MotX in the cytoplasm. Since stator elements seem to constitute a dynamic complex (21), the abundance of MotX and particularly MotY in the cell envelope away from the flagellated cell pole could indicate that the MotXY complex also undergoes rapid turnover at the basal body.
The exact functions of MotX and MotY remain unclear. For Vibrio species, direct interaction between MotX and PomB has been demonstrated (29), and in V. alginolyticus, the PomAB stator complex does not localize correctly to the flagellated cell pole in the absence of MotX or MotY (42). Therefore, in V. alginolyticus, a major role of MotXY is to recruit the stators to the flagellar motor. Since this organism also expresses a second lateral flagellar system depending on a different proton-dependent stator, this may help to incorporate the correct stator into the corresponding flagellar motors. In S. oneidensis MR-1, two different stator systems are present to drive a single polar flagellum. We have recently demonstrated that selection of the stator system occurs at the level of protein localization in response to the sodium ion concentration (32). At high Na+ concentrations, both stator systems are present but the flagellar motor is occupied almost exclusively by PomAB. MotAB localizes more efficiently under conditions of low Na+ concentration or when a mutation eliminates the PomAB system. Regarding their role in Vibrio, we hypothesized that in Shewanella MotX and MotY may be involved in the stator selection process, e.g., by acquisition and incorporation of the stator complexes in response to changing Na+ levels. Under conditions of high Na+ concentrations, a slight increase of MotAB localization to the flagellated cell pole was observed in the absence of MotX and MotY. This might indicate that PomAB association with the motor is not as effective in that strain. However, in S. oneidensis MR-1, neither MotX nor MotY is absolutely required for localization of PomAB or MotAB to the motor. Hence, in this strain, MotXY must be needed for something involved in generating flagellar rotation after the stator complexes have been recruited.
The well-characterized proton-driven flagella of E. coli do not require any stator components beyond the MotAB complex (3). However, when the E. coli MotAB complex was ectopically produced in mutants of V. cholerae and V. alginolyticus lacking PomAB and MotXY, cells became motile using a proton-driven motor (2, 14). When PomA was expressed in V. alginolyticus in concert with a chimeric protein containing the cytoplasmic and transmembrane portions of PomB fused to the periplasmic segment of E. coli MotB, these two proteins formed an active, Na+-dependent stator complex that worked independently from MotXY. This same pair of proteins also powered Na+-dependent rotation of flagella in E. coli (2, 40). The overall conclusion is that the requirement for MotXY arises from the periplasmic domain of PomB. An analysis of MotB mutants revealed that its periplasmic region contains a segment that has been proposed to act as a plug to prevent premature proton flow until the MotAB complex encounters a flagellar motor. A conformational change triggered by contact with the basal body might both open the proton-conducting channel and enable tight binding of the periplasmic domain to peptidoglycan in the cell wall (17, 45). Electron microscopy performed on basal complexes isolated from V. alginolyticus revealed an additional structure, termed the “T ring,” attached below the P and L rings, which function as the bushing for the rotating flagellar rod (9, 42). This structure, which presumably contains MotX and MotY, might be required for conformational changes in the PomAB and MotAB stator complexes of S. oneidensis MR-1 that open the ion-conducting channels and enable binding to peptidoglycan.
Prior studies of MotX and MotY have focused almost exclusively on the flagellar motor of Vibrio species. Characterization of these two components in S. oneidensis MR-1 complements the Vibrio studies and suggests possible activities carried out by ancillary motor proteins. Future experiments will be directed toward elucidating those functions.
Supplementary Material
Acknowledgments
We are grateful to Lotte Søgaard-Andersen and Penelope Higgs for critically reading the manuscript and for helpful remarks and discussions as well as to three reviewers whose comments helped to improve the paper.
This work was supported by the Max-Planck-Gesellschaft.
Footnotes
Published ahead of print on 5 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Asai, Y., S. Kojima, H. Kato, N. Nishioka, I. Kawagishi, and M. Homma. 1997. Putative channel components for the fast-rotating sodium-driven flagellar motor of a marine bacterium. J. Bacteriol. 179:5104-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, Y., T. Yakushi, I. Kawagishi, and M. Homma. 2003. Ion-coupling determinants of Na+-driven and H+-driven flagellar motors. J. Mol. Biol. 327:453-463. [DOI] [PubMed] [Google Scholar]
- 3.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19-54. [DOI] [PubMed] [Google Scholar]
- 4.Blair, D. F. 2003. Flagellar movement driven by proton translocation. FEBS Lett. 545:86-95. [DOI] [PubMed] [Google Scholar]
- 5.Blair, D. F., and H. C. Berg. 1990. The MotA protein of Escherichia coli is a proton-conducting component of the flagellar motor. Cell 60:439-449. [DOI] [PubMed] [Google Scholar]
- 6.Blenden, D. C., and H. S. Goldberg. 1965. Silver impregnation stain for Leptospira and flagella. J. Bacteriol. 89:899-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J. C., P. H. Viollier, and L. Shapiro. 2005. A membrane metalloprotease participates in the sequential degradation of a Caulobacter polarity determinant. Mol. Microbiol. 55:1085-1103. [DOI] [PubMed] [Google Scholar]
- 8.De Mot, R., and J. Vanderleyden. 1994. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 12:333-334. [DOI] [PubMed] [Google Scholar]
- 9.DeRosier, D. J. 1998. The turn of the screw: the bacterial flagellar motor. Cell 93:17-20. [DOI] [PubMed] [Google Scholar]
- 10.Doyle, T. B., A. C. Hawkins, and L. L. McCarter. 2004. The complex flagellar torque generator of Pseudomonas aeruginosa. J. Bacteriol. 186:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggenhofer, E., M. Haslbeck, and B. Scharf. 2004. MotE serves as a new chaperone specific for the periplasmic motility protein, MotC, in Sinorhizobium meliloti. Mol. Microbiol. 52:701-712. [DOI] [PubMed] [Google Scholar]
- 12.Emanuelsson, O., S. Brunak, G. von Heijne, and H. Nielsen. 2007. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2:953-971. [DOI] [PubMed] [Google Scholar]
- 13.Fukuoka, H., T. Yakushi, A. Kusumoto, and M. Homma. 2005. Assembly of motor proteins, PomA and PomB, in the Na+-driven stator of the flagellar motor. J. Mol. Biol. 351:707-717. [DOI] [PubMed] [Google Scholar]
- 14.Gosink, K. K., and C. C. Häse. 2000. Requirements for conversion of the Na+-driven flagellar motor of Vibrio cholerae to the H+-driven motor of Escherichia coli. J. Bacteriol. 182:4234-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant, B., and I. Greenwald. 1996. The Caenorhabditis elegans sel-1 gene, a negative regulator of lin-12 and glp-1, encodes a predicted extracellular protein. Genetics 143:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 17.Hosking, E. R., C. Vogt, E. P. Bakker, and M. D. Manson. 2006. The Escherichia coli MotAB proton channel unplugged. J. Mol. Biol. 364:921-937. [DOI] [PubMed] [Google Scholar]
- 18.Kojima, S., A. Shinohara, H. Terashima, T. Yakushi, M. Sakuma, M. Homma, K. Namba, and K. Imada. 2008. Insights into the stator assembly of the Vibrio flagellar motor from the crystal structure of MotY. Proc. Natl. Acad. Sci. USA 105:7696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 166:175-176. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Leake, M. C., J. H. Chandler, G. H. Wadhams, F. Bai, R. M. Berry, and J. P. Armitage. 2006. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature 443:355-358. [DOI] [PubMed] [Google Scholar]
- 22.McCarter, L. L. 1994. MotX, the channel component of the sodium-type flagellar motor. J. Bacteriol. 176:5988-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarter, L. L. 1994. MotY, a component of the sodium-type flagellar motor. J. Bacteriol. 176:4219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarter, L. L. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittl, P. R., and W. Schneider-Brachert. 2007. Sel1-like repeat proteins in signal transduction. Cell. Signal. 19:20-31. [DOI] [PubMed] [Google Scholar]
- 27.Myers, C. R., and J. M. Myers. 1997. Replication of plasmids with the p15A origin in Shewanella putrefaciens MR-1. Lett. Appl. Microbiol. 24:221-225. [DOI] [PubMed] [Google Scholar]
- 28.Okabe, M., T. Yakushi, Y. Asai, and M. Homma. 2001. Cloning and characterization of motX, a Vibrio alginolyticus sodium-driven flagellar motor gene. J. Biochem. 130:879-884. [DOI] [PubMed] [Google Scholar]
- 29.Okabe, M., T. Yakushi, and M. Homma. 2005. Interactions of MotX with MotY and with the PomA/PomB sodium ion channel complex of the Vibrio alginolyticus polar flagellum. J. Biol. Chem. 280:25659-25664. [DOI] [PubMed] [Google Scholar]
- 30.Okabe, M., T. Yakushi, M. Kojima, and M. Homma. 2002. MotX and MotY, specific components of the sodium-driven flagellar motor, colocalize to the outer membrane in Vibrio alginolyticus. Mol. Microbiol. 46:125-134. [DOI] [PubMed] [Google Scholar]
- 31.Okunishi, I., I. Kawagishi, and M. Homma. 1996. Cloning and characterization of motY, a gene coding for a component of the sodium-driven flagellar motor in Vibrio alginolyticus. J. Bacteriol. 178:2409-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulick, A., A. Koerdt, J. Lassak, S. Huntley, I. Wilms, F. Narberhaus, and K. M. Thormann. 2009. Two different stator systems drive a single polar flagellum in Shewanella oneidensis MR-1. Mol. Microbiol. 71:836-850. [DOI] [PubMed] [Google Scholar]
- 33.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutants in bacteria. Gene 118:145-146. [DOI] [PubMed] [Google Scholar]
- 34.Platzer, J., W. Sterr, M. Hausmann, and R. Schmitt. 1997. Three genes of a motility operon and their role in flagellar rotary speed variation in Rhizobium meliloti. J. Bacteriol. 179:6391-6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid, S. W., M. C. Leake, J. H. Chandler, C. J. Lo, J. P. Armitage, and R. M. Berry. 2006. The maximum number of torque-generating units in the flagellar motor of Escherichia coli is at least 11. Proc. Natl. Acad. Sci. USA 103:8066-8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu, W. S., R. M. Berry, and H. C. Berg. 2000. Torque-generating units of the flagellar motor of Escherichia coli have a high duty ratio. Nature 403:444-447. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, K., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Song, W., F. S. Juhn, D. Q. Naiman, K. T. Konstantinidis, T. S. Gardner, and M. J. Ward. 2008. Predicting sigma28 promoters in eleven Shewanella genomes. FEMS Microbiol. Lett. 283:223-230. [DOI] [PubMed] [Google Scholar]
- 39.Sowa, Y., and R. M. Berry. 2008. Bacterial flagellar motor. Q. Rev. Biophys. 41:103-132. [DOI] [PubMed] [Google Scholar]
- 40.Sowa, Y., A. D. Rowe, M. C. Leake, T. Yakushi, M. Homma, A. Ishijima, and R. M. Berry. 2005. Direct observation of steps in rotation of the bacterial flagellar motor. Nature 437:916-919. [DOI] [PubMed] [Google Scholar]
- 41.Stewart, B. J., and L. L. McCarter. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 185:4508-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terashima, H., H. Fukuoka, T. Yakushi, S. Kojima, and M. Homma. 2006. The Vibrio motor proteins, MotX and MotY, are associated with the basal body of Na+-driven flagella and required for stator formation. Mol. Microbiol. 62:1170-1180. [DOI] [PubMed] [Google Scholar]
- 43.Thormann, K. M., S. Duttler, R. M. Saville, M. Hyodo, S. Shukla, Y. Hayakawa, and A. M. Spormann. 2006. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J. Bacteriol. 188:2681-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thormann, K. M., R. M. Saville, S. Shukla, and A. M. Spormann. 2005. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 187:1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Way, S. M., E. R. Hosking, T. F. Braun, and M. D. Manson. 2000. Mot protein assembly into the bacterial flagellum: a model based on mutational analysis of the motB gene. J. Mol. Biol. 297:7-24. [DOI] [PubMed] [Google Scholar]
- 46.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 2:705-724. [DOI] [PubMed] [Google Scholar]
- 47.Yagasaki, J., M. Okabe, R. Kurebayashi, T. Yakushi, and M. Homma. 2006. Roles of the intramolecular disulfide bridge in MotX and MotY, the specific proteins for sodium-driven motors in Vibrio spp. J. Bacteriol. 188:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yorimitsu, T., and M. Homma. 2001. Na(+)-driven flagellar motor of Vibrio. Biochim. Biophys. Acta 1505:82-93. [DOI] [PubMed] [Google Scholar]
- 49.Yorimitsu, T., M. Kojima, T. Yakushi, and M. Homma. 2004. Multimeric structure of the PomA/PomB channel complex in the Na+-driven flagellar motor of Vibrio alginolyticus. J. Biochem. 135:43-51. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, J., S. A. Lloyd, and D. F. Blair. 1998. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc. Natl. Acad. Sci. USA 95:6436-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.