Abstract
Escherichia coli expressing SOS-inducing mutant topoisomerase I was utilized to demonstrate that covalent protein-DNA complex accumulation results in oxidative damage. Hydroxyl radicals were detected following mutant topoisomerase induction. The presence of the Fe2+ chelator 2,2′-dipyridyl and an iscS mutation affecting Fe-S cluster formation protect against topoisomerase I cleavage complex-mediated cell killing.
DNA topoisomerases are ubiquitous enzymes that carry out catalysis by coupling DNA strand passage with concerted breaking and rejoining of DNA (31). Escherichia coli DNA topoisomerase I, encoded by the topA gene, is the most studied member of the type IA topoisomerase family (40, 42). It is important for regulation of DNA supercoiling (41, 43) and has an essential function for preventing hypernegative supercoiling and R-loop formation during transcription (9, 21). E. coli topA-null mutants are not viable at low temperatures (<30°C) (22, 35). Mutants with a topA deletion have increased sensitivity to high temperatures (>50°C) and oxidative challenges because of defects in transcription of stress response genes needed for survival (6, 29, 30, 39).
Compounds that shift the topoisomerase cleavage-religation equilibrium toward DNA cleavage, resulting in an increased or stabilized covalent complex in vivo, have been found to be effective antibacterial and anticancer therapeutic agents (23, 25, 28). These topoisomerase-targeting compounds are often referred to as topoisomerase poisons (13, 19). However, compounds that act as topoisomerase poisons specific for type IA DNA topoisomerases have not been identified. Every bacterial genome encodes a topoisomerase I (11), which would be vulnerable to perturbation during the catalytic cycle of DNA cleavage and religation. Bacterial topoisomerase I should be utilized as a target for development of new classes of antibacterial drugs to combat multidrug-resistant bacteria (38). The validity of targeting type IA topoisomerases in antibacterial drug development was proven with the identification and characterization of inducible Yersinia pestis and E. coli topoisomerase I mutants that could cause rapid bacterial cell death due to the accumulation of topoisomerase I cleavage complex (4, 5, 34). These cell-killing mutations of bacterial topoisomerase I serve as models for the potential bactericidal drugs that target type IA topoisomerases.
Quinolones achieve killing of bacterial cells by first stabilizing the covalent intermediate complex between bacterial type IIA topoisomerases and the cleaved DNA during the catalytic cycle (7). In E. coli, DNA gyrase is the primary target of fluoroquinolones, while topoisomerase IV is a contributing factor to susceptibility (14, 17). The events that follow the stabilization of the covalent topoisomerase complex, leading to bacterial cell death, remain to be elucidated. Previous studies on the effect of DNA replication and protein synthesis on quinolone-mediated cell death suggest that there may be multiple pathways involved, depending on the individual quinolone structure and growth conditions (8, 20, 27, 44). More recent studies suggest that at least part of the bactericidal action of quinolones can be attributed to oxidative damage from reactive oxygen species (10, 12, 18). It is not clear if this oxidative-damage cell death pathway is dependent on the quinolone structure. In this study, the mutant bacterial topoisomerase I proteins that mediate cell killing through accumulation of cleavage complex were utilized to test whether the induction of the oxidative damage cell death pathway is a direct consequence of stabilized covalent protein-DNA complex on the chromosome.
Detection of hydroxyl radicals following induction of mutant bacterial topoisomerase I.
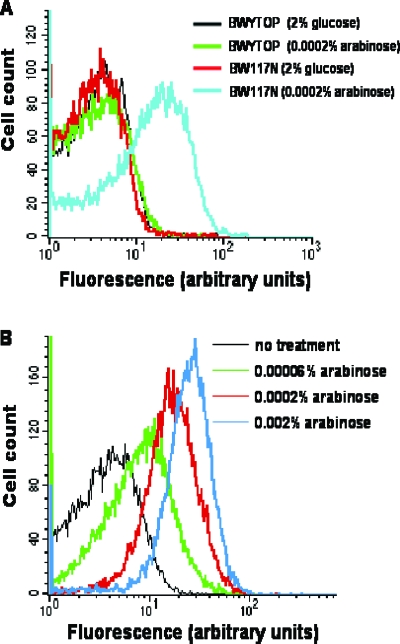
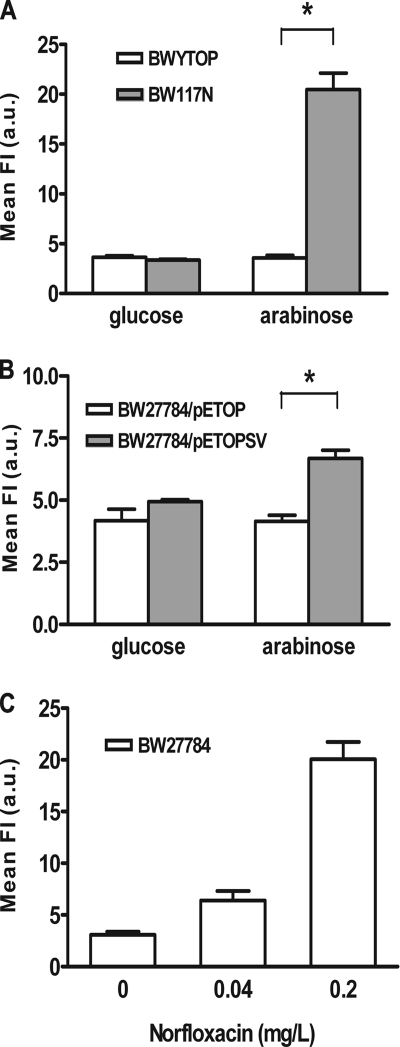
E. coli strain BW117N (Table 1) has a mutant Y. pestis topoisomerase I gene encoding YpTOP-D117N under the control of the BAD promoter inserted into the chromosome (5). Induction of YpTOP-D117N with arabinose resulted in a 104- to 105-fold decrease in viability, while induction of chromosomally integrated wild-type YpTOP in E. coli strain BWYTOP had no significant effect on viability (5). Overnight cultures of BW117N and BWYTOP prepared in LB medium with 2% glucose were inoculated (1:100) into fresh LB medium. At the exponential phase (A600 ∼ 0.4), arabinose (0.0002%) was added to the culture to induce the expression of recombinant proteins. Controls for each strain had 2% glucose to suppress the expression of YpTOP or YpTOP-D117N. After 2.5 h of induction, cells were treated with the fluorescent reporter dye 3′-(p-hydroxyphenyl) fluorescein (HPF) to detect the formation of hydroxyl radicals (33) in a FACScan flow cytometer (Becton Dickinson). Hydroxyl radical formation could be detected following induction of mutant YpTOP-D117N by arabinose for 2.5 h (Fig. 1A). Induction of wild-type YpTOP from E. coli strain BWYTOP did not result in significant hydroxyl radical formation. Hydroxyl radical formation from induction of mutant YpTOP-D117N is dependent on the concentration of arabinose (Fig. 1B). Another mutant bacterial topoisomerase I, E. coli topoisomerase I (EcTOP-G116S/M320V), which contains G116S and M320V mutations (3) and is encoded by pETOPSV, is also bactericidal when overexpressed but is not as lethal as the YpTOP-D117N mutant. The multicopy pETOPSV plasmid could therefore be maintained in the presence of 2% glucose in the growth medium to suppress expression from the BAD promoter (3), while YpTOP-D117N, due to its higher lethality, cannot be expressed and maintained in high-copy-number plasmids in E. coli (5). Induction of the mutant E. coli topoisomerase I EcTOP-G116S/M320V in BW27784 with arabinose also resulted in significant production of hydroxyl radicals detectable by HPF (Fig. 2). Similar results (data not shown) were obtained for induction of mutant Y. pestis topoisomerase I containing analogous G122S and M326V mutations and encoded by plasmid pAYTOP128, the first isolated mutant bacterial topoisomerase I with the cell-killing phenotype (4). Levels of hydroxyl radicals induced by two different concentrations of norfloxacin were also determined for comparison (Fig. 2C).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or construction |
|---|---|---|
| E. coli strains | ||
| BW27784 | DE(araBAD)567 DE(rhaBAD)568 DE(araFGH) Φ(ΔaraEpPCP18-araE) | Yale E. coli Genetic Stock Center (16) |
| BWYTOP | BW27784 with chromosomally integrated Y. pestis topoisomerase I gene (YpTOP), Cmr | 5 |
| BW117N | BW27784 with chromosomally integrated YpTOP-D117N gene, Cmr | 5 |
| JW2514-4 | iscS776(del)::Kan; Keio collection | Yale E. coli Genetic Stock Center (1) |
| TA001 | BW27784 iscS776(del)::Kan | P1(JW2514-4) × BW27784 (this study) |
| Plasmids | ||
| pETOP | Wild-type E. coli topoisomerase I gene (EcTOP) under the control of the BAD promoter in high copy no. | 4 |
| pETOPSV | Mutant derivate of pETOP encoding EcTOP-G116S/M320V | 3 |
| pAYTOP | Wild-type YpTOP under the control of the BAD promoter in medium copy no. | 4 |
| pAYTOP128 | Mutant derivative of pAYTOP encoding YpTOP with G122S, M326V, and A383P mutations | 4 |
FIG. 1.
Hydroxyl radical formation in E. coli following accumulation of topoisomerase I cleavage complex. (A) Representative fluorescence population distributions of cultures of BWYTOP and BW117N in the presence of 2% glucose or after addition of 0.0002% arabinose. (B) Effect of different arabinose concentrations on hydroxyl radical formation from induction of YpTOP-D117N in BW117N.
FIG. 2.
Quantitation of hydroxyl radicals in BW117N (A) and BW27784/pETOPSV (B) cells, expressing Y. pestis and E. coli mutant topoisomerase I, and in BW27784 cells treated with norfloxacin (C). Hydroxyl radicals from 20,000 cells were measured by HPF following accumulation of cleavage complex by 0.0008% arabinose or in the presence of 2% glucose as a control. Fluorescence measurements of the cells containing wild-type topoisomerase I (BWYTOP and BW27784/pETOP) are also shown for comparison. Mean fluorescence intensity, expressed in arbitrary units (a.u.), is the level of hydroxyl radical at 2.5 h (BW117N and BW27784) or 3 h (BW27784/pETOPSV) after addition of arabinose or norfloxacin. Data are means plus standard errors of the means from at least triplicate experiments. *, P < 0.05.
Suppression of cell killing by an iron chelator and an iscS mutation.
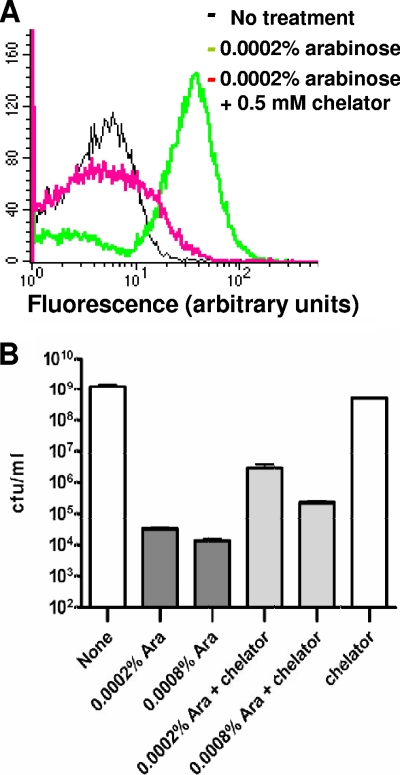
Hydroxyl radicals are generated in the Fenton reaction between free ferrous ions and hydrogen peroxide (37). To demonstrate that hydroxyl radical formation contributes to the cell killing events initiated by accumulation of bacterial topoisomerase I covalent complex, the iron chelator 2,2′-dipyridyl was added to the growth medium along with arabinose when YpTOP-D117N was induced in BW117N. This resulted in suppression of hydroxyl radical formation after induction of mutant YpTOP-D117N in E. coli BW117N (Fig. 3A). The reduction of hydroxyl radical formation due to the presence of the iron chelator correlated with an up-to-100-fold increase in cell survival rate (Fig. 3B). Western blot analysis (see Fig. S1 in the supplemental material) showed that the effect of the iron chelator was not due to change in the level of mutant topoisomerase I protein.
FIG. 3.
Effect of the iron chelator 2,2′-dipyridyl on hydroxyl radical formation (A) and relative cell viability (B) following induction of mutant Y. pestis topoisomerase I. E. coli BW117N was treated with arabinose in the absence and presence of 0.5 mM 2,2′-dipyridyl. CFU per ml were determined at 3 h after addition of arabinose. The results (B) are means and standard errors from triplicate experiments.
The iscS gene in E. coli codes for cysteine desulfurase and is required for the biosynthesis of Fe-S clusters (32). Previous studies showed that E. coli strains with ΔiscS have increased resistance to norfloxacin, supporting the role of ferrous iron from Fe-S clusters in generating hydroxyl radicals for the cell death pathway (10). The ΔiscS mutation was introduced into strain BW27784 by transduction, resulting in strain TA001. Induction with arabinose showed that viability following expression of mutant YpTOP from pAYTOP128 (viability [ratio of counts of viable cells treated with 0.0002% arabinose to counts of untreated cells], 0.50 ± 0.05 [mean ± standard error]) and of mutant EcTOP from pETOPSV (0.24 ± 0.06) was greatly enhanced in TA001 compared with BW27784 (viability, [2.2 ± 1.3] × 10−4 and [2.9 ± 0.3] × 10−5, respectively). This further supported the role of hydroxyl radicals generated from ferrous ions of Fe-S clusters in the cell killing mechanism of topoisomerase I cleavage complex accumulation.
Synergistic cell killing by topoisomerase I and gyrase cleavage complex.
In order to test whether targeting bacterial topoisomerase I and gyrase simultaneously would enhance the bactericidal action, low levels of arabinose (to induce expression of recombinant mutant YpTOP) and the fluoroquinolone norfloxacin (to induce cleavage complex accumulation by DNA gyrase) were used to treat E. coli BW117N expressing the mutant YpTOP-D117N. The fractional inhibitory concentration index cannot be calculated with the arabinose treatment for evaluation of synergy. Nevertheless, synergy from two compounds can be determined if there is a ≥2-log10-CFU/ml decrease in viable counts compared to counts with the more active compound alone in a time-kill analysis (2, 15). In contrast, the effect of two treatments will be additive only if there is a <10-fold increase in killing (15). The results of time-kill studies (Table 2) showed that the combination treatment of relatively low concentrations of arabinose to induce the topoisomerase I cleavage complex and norfloxacin to induce the gyrase cleavage complex resulted in a >2-log10-CFU/ml decrease in viability of E. coli BW117N compared with viability after each treatment alone.
TABLE 2.
Synergistic effects of gyrase inhibitor and topoisomerase I cleavage complex on E. coli viability determined in time-kill study
| Treatment | Log CFU/mla
|
|
|---|---|---|
| 1 h | 2 h | |
| None | 8.30 ± 0.04 | 8.95 ± 0.04 |
| 0.00005% arabinose | 6.56 ± 0.16 | 5.94 ± 0.08 |
| 0.2 mg/liter norfloxacin | 6.78 ± 0.16 | 5.96 ± 0.11 |
| 0.00005% arabinose + 0.2 mg/liter norfloxacin | 4.14 ± 0.08 | 3.46 ± 0.09 |
Viable counts of BW117N at 1 or 2 h after the addition of 0.2 mg/liter norfloxacin and/or induction of topoisomerase I cleavage complex were determined by serial dilutions and plating on LB plates with 2% glucose and chloramphenicol. The averages and standard errors of results from three experiments are shown.
Oxidative damage is a consequence of covalent protein-DNA complex accumulation.
From genetic studies, it has been shown that quinolone treatment of E. coli induces the SOS response via the double-strand break recombination repair pathway with RecBC-dependent loading of RecA (24, 26). Our previous work showed that bacterial topoisomerase I cleavage complexes accumulated on chromosomal DNA are also converted to double-strand breaks to be processed by RecBC (36). This study demonstrates that besides the similarity in processing of the covalent protein-DNA complex, type IA and type IIA topoisomerase cleavage complexes also share a common bactericidal pathway. Based on studies of the effects of DNA replication and protein synthesis inhibition, it was proposed that multiple pathways of cell killing are involved in quinolone action, and the individual quinolone structure may be a factor for certain pathways (8, 20, 27, 44). Hydroxyl radical formation has recently been implicated in the cell death caused by bactericidal antibiotics, including quinolones (10). The results obtained here with the mutant Y. pestis and E. coli topoisomerase I models suggest that the oxidative damage pathway of cell killing induced by quinolones is likely to be a direct consequence of covalent protein-DNA complex accumulation and is not dependent on the individual quinolone structure. The events that occur after covalent protein-DNA complex accumulation that result in hydroxyl radical formation from the Fenton reaction remain to be fully elucidated. The Y. pestis and E. coli topoisomerase I mutants employed in this study could provide a useful model for further studies of this bactericidal pathway.
Supplementary Material
Acknowledgments
We thank Carl Hamby for use of the FACScan flow cytometer.
This work was supported by Public Health Service grant R01 AI 069313 from the National Institutes of Health.
Footnotes
Published ahead of print on 12 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajaksouzian, S., M. A. Visalli, M. R. Jacobs, and P. C. Appelbaum. 1997. Activities of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with amikacin, against acinetobacters as determined by checkerboard and time-kill studies. Antimicrob. Agents Chemother. 41:1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, B., E. P. Sorokin, and Y. C. Tse-Dinh. 2008. Mutation adjacent to the active site tyrosine can enhance DNA cleavage and cell killing by the TOPRIM Gly to Ser mutant of bacterial topoisomerase I. Nucleic Acids Res. 36:1017-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, B., S. Shukla, S. Vasunilashorn, S. Mukhopadhyay, and Y. C. Tse-Dinh. 2005. Bacterial cell killing mediated by topoisomerase I DNA cleavage activity. J. Biol. Chem. 280:38489-38495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, B., T. Annamalai, E. Sorokin, M. Abrenica, S. Aedo, and Y. C. Tse-Dinh. 2009. Asp-to-Asn substitution at the first position of the DxD TOPRIM motif of recombinant bacterial topoisomerase I is extremely lethal to E. coli. J. Mol. Biol. 385:558-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, B., S. Rui, C. Ji, V. W. Gong, T. K. Van Dyk, M. Drolet, and Y. C. Tse-Dinh. 2003. RNase H overproduction allows the expression of stress-induced genes in the absence of topoisomerase I. FEMS Microbiol. Lett. 221:237-242. [DOI] [PubMed] [Google Scholar]
- 7.Drlica, K., and M. Malik. 2003. Fluoroquinolones: action and resistance. Curr. Top. Med. Chem. 3:249-282. [DOI] [PubMed] [Google Scholar]
- 8.Drlica, K., M. Malik, R. J. Kerns, and X. Zhao. 2008. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 52:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drolet, M. 2006. Growth inhibition mediated by excess negative supercoiling: the interplay between transcription elongation, R-loop formation and DNA topology. Mol. Microbiol. 59:723-730. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer, D. J., M. A. Kohanski, B. Hayete, and J. J. Collins. 2007. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forterre, P., S. Gribaldo, D. Gadelle, and M. C. Serre. 2007. Origin and evolution of DNA topoisomerases. Biochimie 89:427-446. [DOI] [PubMed] [Google Scholar]
- 12.Goswami, M., S. H. Mangoli, and N. Jawali. 2006. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob. Agents Chemother. 50:949-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heddle, J. G., S. J. Blance, D. B. Zamble, F. Hollfelder, D. A. Miller, L. M. Wentzell, C. T. Walsh, and A. Maxwell. 2001. The antibiotic microcin B17 is a DNA gyrase poison: characterisation of the mode of inhibition. J. Mol. Biol. 307:1223-1234. [DOI] [PubMed] [Google Scholar]
- 14.Hooper, D. C. 1995. Quinolone mode of action. Drugs 49(Suppl. 2):10-15. [DOI] [PubMed] [Google Scholar]
- 15.Jung, R., M. Husain, M. K. Choi, and D. N. Fish. 2004. Synergistic activities of moxifloxacin combined with piperacillin-tazobactam or cefepime against Klebsiella pneumoniae, Enterobacter cloacae, and Acinetobacter baumannii clinical isolates. Antimicrob. Agents Chemother. 48:1055-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khlebnikov, A., K. A. Datsenko, T. Skaug, B. L. Wanner, and J. D. Keasling. 2001. Homogeneous expression of the P(BAD) promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology 147:3241-3247. [DOI] [PubMed] [Google Scholar]
- 17.Khodursky, A. B., E. L. Zechiedrich, and N. R. Cozzarelli. 1995. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:11801-11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohanski, M. A., D. J. Dwyer, B. Hayete, C. A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797-810. [DOI] [PubMed] [Google Scholar]
- 19.Liu, L. F. 1989. DNA topoisomerase poisons as antitumor drugs. Annu. Rev. Biochem. 58:351-375. [DOI] [PubMed] [Google Scholar]
- 20.Malik, M., S. Hussain, and K. Drlica. 2007. Effect of anaerobic growth on quinolone lethality with Escherichia coli. Antimicrob. Agents Chemother. 51:28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masse, E., and M. Drolet. 1999. Relaxation of transcription-induced negative supercoiling is an essential function of Escherichia coli DNA topoisomerase I. J. Biol. Chem. 274:16654-16658. [DOI] [PubMed] [Google Scholar]
- 22.Masse, E., and M. Drolet. 1999. R-loop-dependent hypernegative supercoiling in Escherichia coli topA mutants preferentially occurs at low temperatures and correlates with growth inhibition. J. Mol. Biol. 294:321-332. [DOI] [PubMed] [Google Scholar]
- 23.Mitscher, L. A. 2005. Bacterial topoisomerase inhibitors: quinolone and pyridone antibacterial agents. Chem. Rev. 105:559-592. [DOI] [PubMed] [Google Scholar]
- 24.Newmark, K. G., E. K. O'Reilly, J. R. Pohlhaus, and K. N. Kreuzer. 2005. Genetic analysis of the requirements for SOS induction by nalidixic acid in Escherichia coli. Gene 356:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitiss, J. L. 2002. DNA topoisomerases in cancer chemotherapy: using enzymes to generate selective DNA damage. Curr. Opin. Investig. Drugs 3:1512-1516. [PubMed] [Google Scholar]
- 26.Phillips, I., E. Culebras, F. Moreno, and F. Baquero. 1987. Induction of the SOS response by new 4-quinolones. J. Antimicrob. Chemother. 20:631-638. [DOI] [PubMed] [Google Scholar]
- 27.Piddock, L. J., R. N. Walters, and J. M. Diver. 1990. Correlation of quinolone MIC and inhibition of DNA, RNA, and protein synthesis and induction of the SOS response in Escherichia coli. Antimicrob. Agents Chemother. 34:2331-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pommier, Y. 2006. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 6:789-802. [DOI] [PubMed] [Google Scholar]
- 29.Qi, H., R. Menzel, and Y. C. Tse-Dinh. 1999. Increased thermosensitivity associated with topoisomerase I deletion and promoter mutations in Escherichia coli. FEMS Microbiol. Lett. 178:141-146. [DOI] [PubMed] [Google Scholar]
- 30.Rui, S., and Y. C. Tse-Dinh. 2003. Topoisomerase function during bacterial responses to environmental challenge. Front. Biosci. 8:d256-d263. [DOI] [PubMed] [Google Scholar]
- 31.Schoeffler, A. J., and J. M. Berger. 2008. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q. Rev. Biophys. 41:41-101. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz, C. J., O. Djaman, J. A. Imlay, and P. J. Kiley. 2000. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setsukinai, K., Y. Urano, K. Kakinuma, H. J. Majima, and T. Nagano. 2003. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 278:3170-3175. [DOI] [PubMed] [Google Scholar]
- 34.Sorokin, E. P., B. Cheng, S. Rathi, S. J. Aedo, M. V. Abrenica, and Y. C. Tse-Dinh. 2008. Inhibition of Mg2+ binding and DNA religation by bacterial topoisomerase I via introduction of an additional positive charge into the active site region. Nucleic Acids Res. 36:4788-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stupina, V. A., and J. C. Wang. 2005. Viability of Escherichia coli topA mutants lacking DNA topoisomerase I. J. Biol. Chem. 280:355-360. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland, J. H., B. Cheng, I. F. Liu, and Y. C. Tse-Dinh. 2008. SOS induction by stabilized topoisomerase IA cleavage complex occurs via the RecBCD pathway. J. Bacteriol. 190:3399-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 38.Tse-Dinh, Y. C. 2009. Bacterial topoisomerase I as a target for discovery of antibacterial compounds. Nucleic Acids Res. 37:731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tse-Dinh, Y. C. 2000. Increased sensitivity to oxidative challenges associated with topA deletion in Escherichia coli. J. Bacteriol. 182:829-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tse-Dinh, Y. C. 1998. Bacterial and archaeal type I topoisomerases. Biochim. Biophys. Acta 1400:19-27. [DOI] [PubMed] [Google Scholar]
- 41.Tse-Dinh, Y. C. 1985. Regulation of the Escherichia coli DNA topoisomerase I gene by DNA supercoiling. Nucleic Acids Res. 13:4751-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viard, T., and C. B. de la Tour. 2007. Type IA topoisomerases: a simple puzzle? Biochimie 89:456-467. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J. C. 2002. Cellular roles of DNA topoisomerases: a molecular perspective. Nat. Rev. Mol. Cell Biol. 3:430-440. [DOI] [PubMed] [Google Scholar]
- 44.Zhao, X., M. Malik, N. Chan, A. Drlica-Wagner, J. Y. Wang, X. Li, and K. Drlica. 2006. Lethal action of quinolones against a temperature-sensitive dnaB replication mutant of Escherichia coli. Antimicrob. Agents Chemother. 50:362-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.