Abstract
The Vibrio cholerae type II secretion (T2S) machinery is a multiprotein complex that spans the cell envelope. When the T2S system is inactivated, cholera toxin and other exoproteins accumulate in the periplasmic compartment. Additionally, loss of secretion via the T2S system leads to a reduced growth rate, compromised outer membrane integrity, and induction of the extracytoplasmic stress factor RpoE (A. E. Sikora, S. R. Lybarger, and M. Sandkvist, J. Bacteriol. 189:8484-8495, 2007). In this study, gene expression profiling reveals that inactivation of the T2S system alters the expression of genes encoding cell envelope components and proteins involved in central metabolism, chemotaxis, motility, oxidative stress, and iron storage and acquisition. Consistent with the gene expression data, molecular and biochemical analyses indicate that the T2S mutants suffer from internal oxidative stress and increased levels of intracellular ferrous iron. By using a tolA mutant of V. cholerae that shares a similar compromised membrane phenotype but maintains a functional T2S machinery, we show that the formation of radical oxygen species, induction of oxidative stress, and changes in iron physiology are likely general responses to cell envelope damage and are not unique to T2S mutants. Finally, we demonstrate that disruption of the V. cholerae cell envelope by chemical treatment with polymyxin B similarly results in induction of the RpoE-mediated stress response, increased sensitivity to oxidants, and a change in iron metabolism. We propose that many types of extracytoplasmic stresses, caused either by genetic alterations of outer membrane constituents or by chemical or physical damage to the cell envelope, induce common signaling pathways that ultimately lead to internal oxidative stress and misregulation of iron homeostasis.
Vibrio cholerae, a rod-shaped, highly motile, gram-negative bacterium, is the causative agent of the life threatening diarrheal disease cholera (59). The type II secretion (T2S) system plays an important role in the pathogenesis of V. cholerae by secreting cholera toxin (63), which is largely responsible for the symptoms of the disease (33). The T2S system is widespread and well conserved in gram-negative bacteria inhabiting a variety of ecological niches and likely contributes to environmental survival as well as to virulence (11, 21). In V. cholerae, secretion via the T2S machinery is supported by a transenvelope complex of 12 Eps proteins (EpsC to EpsN) and the type 4 prepilin peptidase PilD (VcpD) (25, 44, 63). Transport of exoproteins by the T2S system occurs via a two-step process. The first step, which is either Sec or Tat dependent, requires recognition of the N-terminal signal peptide of the exoproteins and translocation through the inner membrane to the periplasm. Then the folded proteins engage the T2S machinery and are subsequently exported across the outer membrane to the extracellular milieu (23, 29).
Besides periplasmic accumulation of exoproteins, additional phenotypes of T2S mutants are reported for an increasing number of species, possibly indicating involvement of the T2S system in other important cellular processes. For example, alterations in outer membrane protein composition have been described for T2S mutants of V. cholerae, Aeromonas hydrophila, marine Vibrio sp. strain 60, and Shewanella oneidensis (30, 32, 63, 64). The levels of outer membrane porins OmpU, OmpT, and OmpS are decreased in T2S mutants of V. cholerae (63, 65), and likewise, disruption of T2S genes in A. hydrophila leads to diminished quantities of OmpF and OmpS (30). Similarly, the amounts of the c-type cytochromes MtrC and OmcA in the outer membranes of S. oneidensis T2S mutants are reduced (64). Furthermore, we have shown that inactivation of the T2S system in V. cholerae results in a reduced growth rate, compromised outer membrane integrity, and, as a consequence, induction of RpoE activity. In addition, our studies showed that V. cholerae T2S mutants are unable to survive the passage through the infant mouse gastrointestinal tract (65). Growth defects at low temperatures under laboratory conditions as well as in tap water and amoebae were also observed for T2S mutants of Legionella pneumophila (68).
Interestingly, differential abundance of proteins involved in phosphate metabolism and iron uptake has been revealed by proteomic analysis of culture supernatants isolated from wild-type and T2S mutant strains of Pseudoaltermonas tunicata (22). Based on these results, it has been suggested that the T2S system might be involved in iron acquisition. Similarly, certain T2S mutants of Erwinia chrysanthemi exhibit defects indicative of changes in iron homeostasis (17). It has also been noted that the level of aconitate hydratase, an iron-sulfur cluster-containing enzyme, is reduced in L. pneumophila T2S mutants (16).
In this study, in an attempt to explain the phenotypes associated with loss of T2S, we performed microarray gene expression profiling of wild-type and T2S-deficient strains. Our data revealed that inactivation of the T2S machinery results in a metabolic feedback loop leading to oxidative stress and changes in iron metabolism. By analyzing another V. cholerae mutant that shares a similar cell envelope phenotype while remaining competent for T2S, we show that the changes in iron homeostasis and oxidative stress are linked to cell envelope damage and extracytoplasmic stress.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cultures were propagated at 37°C in Luria-Bertani (LB) medium (Fisher Chemicals, Fairlawn, NJ), supplemented as specified in the text. Antibiotics (Sigma-Aldrich, St. Louis, MO) were used at the following concentrations: chloramphenicol, 4 or 10 μg/ml for chromosomal or plasmid expression, respectively, in V. cholerae and 30 μg/ml in Escherichia coli; carbenicillin, 50 or 100 μg/ml for chromosomal or plasmid expression, respectively; kanamycin, 50 μg/ml; polymyxin B sulfate, 100 U/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotypea | Reference or sourceb |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| N16961 | Wild-type El Tor O1 biotype; Smr | Laboratory collection |
| NΔeps | N16961 ΔepsCDEFGHIJKLMN; Cmr | 65 |
| NΔepsD | N16961 ΔepsD; Kanr | This study |
| NtolA | N16961 tolA::pDH149; Ampr | This study |
| E. coli | ||
| MC1061 | F−araD139 Δ(ara-leu)7697 Δ(lac)X74rpsL hsdR2 mcrA mcrB1 | 9 |
| S17.1 | F−recA pro hsdR RP4-2; Tcr::Mu Kanr::Tn7 | 66 |
| SY327 λpir | Δ(lac-pro) argE(Am) recA56 rpoB λpir; Rifr | 51 |
| MM294 (pRK2013) | Donor of transfer function for triparental conjugation | 48 |
| EDL 933 (O157:H7) | Wild type | MSU collection |
| EΔetpM | O157:H7 etpM::kan; Kanr | This study |
| K-12 BW25113 | Wild type | 2 |
| ECK0728 | tolA::kan; Kanr | 2 |
| Plasmids | ||
| pPCR-Script | Cloning vector; Ampr | Stratagene |
| pMMB198 | etxB under ptac control in pMMB66EH; Cmr | 62 |
| pEps | 15-kb XbaI-SphI fragment containing entire eps operon (from epsC to epsN) in pMMB190; Ampr | 65 |
| pMMB-epsD | epsD cloned into pMMB67; Ampr | 42 |
| pMMB207 | Cloning vector, pMMB66; Cmr | 53 |
| pDH149 | Internal fragment of tolA (bp 79-530) cloned into suicide vector pGP704; Ampr | 28 |
| pAES106 | rpoEp2::lux pBBR-lux; Cmr | 65 |
| pAES107 | viuPp::lux pBBR-lux; Cmr | This study |
| pAES108 | hutAp::lux pBBR-lux; Cmr | This study |
| pMMB-tolA | V. choleraetolA cloned into pMMB207; Cmr | This study |
Smr, streptomycin resistance; Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Kanr, kanamycin resistance; Tcr, tetracycline resistance; Rifr, rifampin resistance.
MSU, Michigan State University.
RNA isolation, cDNA preparation, hybridization, and data analysis.
Overnight-grown cultures of wild-type and isogenic NΔeps strain of V. cholerae N16961 were diluted to an optical density at 600 nm (OD600) of 0.03 and grown with aeration in LB medium in three biological replicates. The growth was monitored over time, and 2-ml aliquots of cells were harvested 1 h following entry into stationary phase, when the strains reached an OD600 of 4. The cell pellets were immediately resuspended in 1 ml of TRIzol reagent (Invitrogen) and stored at −80°C. Total cellular RNA was subsequently isolated as described previously (6). The microarrays used in this study are composed of 70-mer oligonucleotides representing the open reading frames present in the V. cholerae N16961 genome and were printed at the University of California, Santa Cruz. Whole-genome expression analysis was performed using a common reference RNA, which was isolated from wild-type cells grown in LB medium as described above. RNA isolated from test and reference samples was used in cDNA synthesis and microarray hybridization, and scanning was performed as described previously using three biological and two technical replicates (6). Normalized signal ratios were obtained with LOWESS print-tip normalization using Bioconductor packages (26) in the R environment. Differentially regulated genes were determined with the SAM (significance analysis of microarrays) program (76) using ≥2.0-fold differences in gene expression and a ≤1% false discovery rate (FDR) as cutoff values.
Genetic techniques.
Chromosomal DNA isolated from V. cholerae N16961 was used as a template for PCR. PCR was carried out with PfuUltra DNA polymerase (Stratagene, La Jolla, CA). Primers were synthesized by IDT Technologies, Inc. (Coralville, IA). The resulting PCR amplification products were purified, subcloned into pPCR-Script Amp SK(+) (Stratagene), and then cloned into the appropriate vector as indicated. Plasmids and suicide vectors were introduced into V. cholerae strains by conjugation with E. coli S17.1 or by triparental conjugation as described previously (65).
Construction of mutant strains.
An epsD deletion mutant of V. cholerae N16961 was constructed as described previously (42). The epsD mutant was complemented with plasmid pMMB-epsD in the presence of 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG). A tolA insertion mutant of V. cholerae N16961 was constructed as described previously (28) using suicide vector pDH149, kindly supplied by Matthew Waldor, Brigham and Women's Hospital, Harvard University. For complementation analysis, the full-length tolA gene was amplified using forward primer 5′-GAGCTCTCCTAAAGTAGGGCTACTCACG-3′ and reverse primer 5′-CTGCAGATAACTAGCTCCAATGCTGC-3′ and was cloned into SacI-PstI-digested pMMB207 (53). Expression of the tolA gene was induced with 10 μM IPTG. An etpM mutant of E. coli O157:H7 strain EDL 933, EΔetpM, was constructed by inserting a kanamycin resistance cassette into the etpM gene using the method of Datsenko and Wanner (15). The following primers were used for the construction of the mutant: etpM01 (5′-CCTTACTGGTTGTTGTTTTTGTCTATTACGTCgtgtaggctggagctgcttc) and etpM02 (5′-TACATCAAGCATTAATCTGTAAATTACGACATCAcatatgaatatcctccttag) (lowercase sequences indicate homology to the kanamycin resistance cassette sequence in the plasmid vector) (15). Mass spectroscopy analysis (M. E. Scott and M. Sandkvist, unpublished data) confirmed that the mutant did not secrete the T2S-dependent metalloprotease StcE (36).
Promoter fusion studies and bioluminescence assays.
The fragment of chromosomal DNA containing the hutA upstream region (220 bp) was PCR amplified as described previously (49) with forward primer 5′-ACTAGTTCTCTCCTATTGATAGTTCACACCGC-3′ and reverse primer 5′-GGATCCGCAATTTCCATGTTGAGTAAAACGC-3′. The viuP upstream region (196 bp) was amplified by using the following primer pair: forward, 5′-ACTAGTGCGGTGTTCGGTGGCT-3′; reverse, 5′-GGATCCCACTGAACAGGCAACCCAAT-3′. The PCR fragments were cloned into SpeI-BamHI-digested pBBRlux (40) upstream of promoterless luxCDABE to yield the transcriptional fusions viuPp::lux and hutAp::lux in pAES107 and pAES108, respectively. Overnight cultures of NΔepsD, NtolA, and isogenic wild-type V. cholerae N16961 carrying pAES106 (65), pAES107, or pAES108 were diluted (1:100) into fresh LB medium alone or supplemented with an iron chelator (2,2′-dipyridyl at a final concentration of 100 or 150 μM for the T2S or NtolA mutants, respectively) or polymyxin B sulfate (100, 200, and 400 U/ml) as indicated in the text. The reporter activities were determined as described previously (65) when the cultures reached the mid-log phase of growth (OD600, about 0.5) and 1 h following entry into stationary phase (at an OD600 of 4). The data represent means and standard errors of the means (SEMs) from three to nine experiments.
Sensitivities to H2O2 and streptonigrin.
Bacterial cultures were grown aerobically in LB medium. One hundred microliters of mid-log-phase cultures (OD600, approximately 0.5) was uniformly distributed on LB agar plates that were either left unsupplemented or supplemented with the iron chelator 2,2′-dipyridyl (100 and 150 μM; Sigma-Aldrich) or with polymyxin B sulfate (100, 200, and 400 U/ml) as indicated in the text. Sterile filter paper discs (diameter, 7 mm) were placed on the surface of the agar and impregnated with 5 μl of H2O2 (44, 88, 176, 440, and 880 mM; Fisher) or streptonigrin (10, 20, 40, 60, 80, and 100 μg/ml; Sigma-Aldrich) for V. cholerae and 5 μl of 8.8 M H2O2 or 100, 200, 400, 600, 800, or 1,000 μg/ml of streptonigrin for E. coli. The zone of growth inhibition around the discs was recorded after 24 h of incubation at 37°C. Means and SEMs for at least three independent experiments are presented.
Detection of intracellular formation of reactive oxygen species (ROS).
The level of intracellular oxidation was measured in vivo by using the molecular probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Cayman Chemical, Ann Arbor, MI) (10, 13). Bacterial cultures were grown in LB medium to the mid-log phase of growth (OD600, approximately 0.5), and the probe was added to a final concentration of 10 μM. The cells were incubated in the dark at 37°C for 1 h, subsequently harvested, washed with 50 mM Tris-HCl buffer (pH 8.0), and disrupted by sonication in the same buffer. Wild-type cells treated with 3 mM H2O2 were used as a positive control in each experiment. The total protein concentration in each sample was measured using a modified Bradford assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. The fluorescence intensity of cell extracts was measured using the excitation and emission wavelengths of 485 and 530 nm, respectively, in a multidetection microplate reader (Synergy HT; Bio-Tek Instruments, Winooski, VT). Each sample was read in technical triplicate. The emission values were normalized by the protein concentration. Means of four independent experiments and corresponding SEMs are presented.
SDS-PAGE, silver staining, and Western blotting.
Samples of whole-cell lysates, culture supernatants, or periplasmic extracts were prepared and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining or Western blotting with anti-EtxB antibodies as described previously (65).
RESULTS AND DISCUSSION
Global analysis of V. cholerae gene expression in response to inactivation of the T2S system.
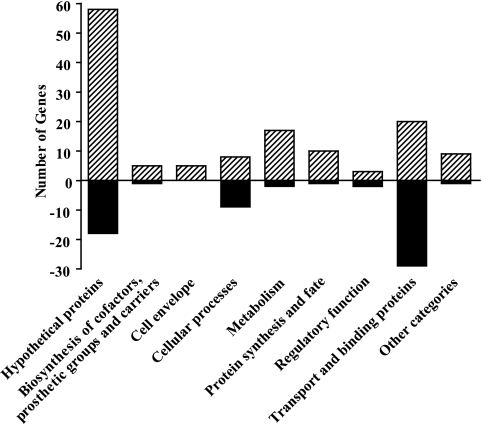
Inactivation of the T2S system, by removal of either single eps genes or the entire eps operon, results not only in the loss of extracellular secretion but also in cell envelope perturbation, upregulation of the σE-mediated stress response, and a reduced growth rate (63, 65). To further investigate the cellular response to inactivation of the T2S system, we performed a global transcriptome analysis of NΔeps, in which the entire eps operon has been removed (65), and the parental wild-type strain, V. cholerae N16961, grown with aeration in LB medium. Because T2S mutants display a reduced growth rate in logarithmically growing cultures, we compared the gene expression profiles at the early stationary phase of growth, when both strains reached a final OD600 of 4.0. The gene expression data were analyzed by applying SAM software (76) using a ≥2.0-fold change in gene expression and a ≤1% FDR as cutoffs. Our analysis identified 198 differentially expressed genes, with 63 genes downregulated and 135 genes upregulated in the T2S-deficient strain relative to the wild-type strain (see Tables S1 and S2 in the supplemental material for a complete list of these genes). The differentially expressed genes belong to several functional groups, as shown in Fig. 1. Inactivation of the T2S machinery resulted in changes in the expression of genes coding for many members of the σE regulon, some of which are outer membrane constituents, and genes encoding proteins participating in chemotaxis/motility, biofilm formation, transport of amino acids and carbohydrates, central metabolic processes, iron physiology, and stress responses that involve heat shock proteins, chaperones, and proteases. Below we discuss a select subset of differentially expressed genes.
FIG. 1.
Functional categories of genes differentially expressed in the NΔeps mutant compared to the wild-type strain. The numbers of genes whose expression is differentially expressed in NΔeps mutant compared to wild-type cells are presented according to the functions assigned by The Institute for Genomic Research. Positive and negative numbers stand for the numbers of genes whose expression is induced and repressed, respectively.
Iron homeostasis is altered in T2S mutants.
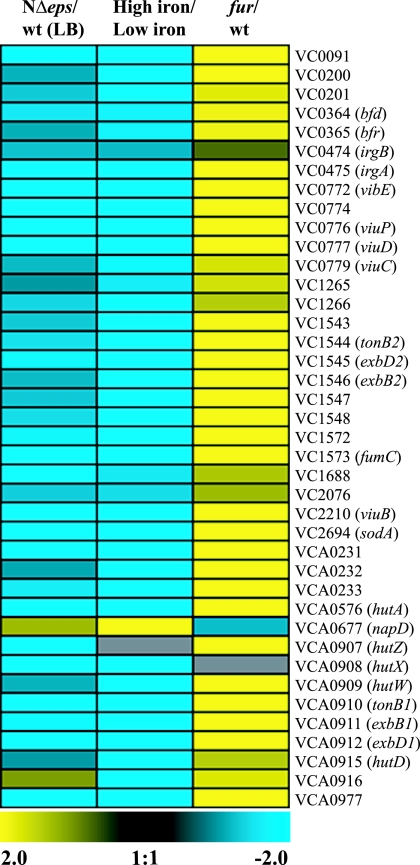
Remarkably, among the differentially expressed genes, the largest cluster consisted of genes that are known to be regulated by the Fe2+-Fur complex in V. cholerae (50). Fur is the major sensor of iron availability, which represses the expression of genes involved in iron acquisition when the level of free iron in the cell is high (1, 50). About 60% (38 out of 63) of the genes downregulated in response to the eps mutation overlapped with those that are negatively regulated by Fur and iron (Fig. 2). Among these, genes encoding enterobactin receptors (irgA, vctA), heme transport proteins (hutA, hutD, hutZ, hutX), proteins involved in vibriobactin biosynthesis and transport (viuC, viuD, viuB, viuP, vibE), and proteins participating in TonB-mediated transport (exbB1, exbB2, exbD1, exbD2, tonB1, tonB2) (81) were the most highly repressed (as much as 13-fold) (Fig. 2; see also Table S1 in the supplemental material). Other Fur-Fe2+-regulated genes of note were those encoding superoxide dismutase (sodA), fumarate hydratase (fumC), bacterioferritin (bfr), and bacterioferritin-associated ferredoxin (bdf) (50), which were 5.3-, 7.6-, 2.2-, and 2.7-fold downregulated, respectively (see Table S1 in the supplemental material). Although our microarray analysis did not reveal significant changes (twofold or more) in fur transcript levels in the T2S mutant, it is known that Fur is produced constitutively in large amounts under normal growth conditions and that its concentration in the cell varies by less than a factor of 2 even after exposure to oxidative stress (79, 84).
FIG. 2.
Global transcriptome analysis reveals that iron homeostasis is affected in the T2S mutant of V. cholerae. Genes that were differentially expressed in the NΔeps mutant compared to the wild-type (wt) strain grown in LB medium were identified by SAM analysis using a ≥2.0-fold change in gene expression and a ≤1% FDR as criteria. Expression profiles of genes that are regulated by iron and Fur and that are differentially expressed in the T2S-deficient strain are presented as a heat map using a log2-based color scale (yellow, induced; blue, repressed). A list of genes regulated by iron and Fur was obtained from previously published gene expression data (50).
In order to validate the data obtained from our microarray analysis, luciferase transcriptional reporter fusions were constructed by cloning the upstream regions of the viuP and hutA genes into plasmid pBBR-lux as described in Materials and Methods. These genes were selected for the following reasons. viuP belongs to a gene cluster, viuPDGC, that is preceded by a promoter containing a Fur-binding box (82), and expression of three genes of this operon differed significantly between the T2S mutant and the wild-type strain according to our microarray analysis (expression was 2.2- to 4.9-fold repressed in the mutant [see Table S1 in the supplemental material]). hutA was the most strongly downregulated gene in the eps mutant (13.1-fold) (see Table S1 in the supplemental material) and has been shown previously to be one of the most responsive Fur-Fe2+-regulated genes (50, 73).
To recapitulate the conditions of our microarray experiments, the activity of each reporter was assessed in wild-type V. cholerae N16961 and its T2S mutant, NΔepsD, 1 h following entry into the stationary phase of growth, when the cultures reached an OD600 of 4. The activity of the viuPp reporter fusion was 7.5-fold lower in the T2S mutant than in the wild-type strain (Fig. 3A). In agreement with previous studies, the overall hutAp reporter fusion activity was significantly higher than that for the viuPp reporter fusion (Fig. 3B) (50, 73). More importantly, however, the hutAp reporter activity in the NΔepsD strain displayed approximately 13-fold downregulation relative to that in the wild type strain (Fig. 3B). Together, the luciferase transcriptional reporter fusion experiments confirmed the microarray data, in which the expression of the viuP and hutA genes was 4.9- and 13.1-fold downregulated, respectively (see Table S1 in the supplemental material).
FIG. 3.
The reporter activities of Fur- and iron-responsive genes confirm the results of microarray analysis. Wild-type (wt) V. cholerae N16961 and its NΔepsD and NtolA mutant strains carrying the luciferase transcriptional fusions viuPp-lux (A) and hutAp-lux (B) were grown in LB medium, and luminescence was assessed in technical triplicate when the cultures were 1 h into the stationary phase of growth (OD600, 4). The bars show average data derived from four independent experiments; error bars represent the corresponding SEMs. The calculated P values displayed above the bars indicate that there were statistically significant differences in reporter activities between the wild-type and mutant strains.
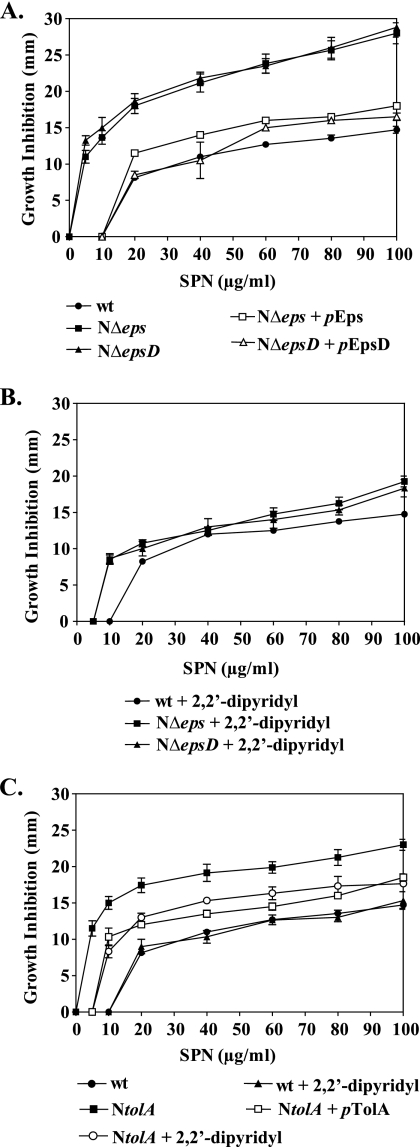
Comparison of our microarray and reporter fusion data with data obtained from transcriptome analysis of iron-depleted V. cholerae cells and fur mutant cells indicated a possible change in iron homeostasis in the T2S mutants, resulting in an iron buildup in these cells (Fig. 2 and 3). In order to investigate this possibility, we employed the iron-activated antibiotic streptonigrin. This antibiotic exerts its bactericidal effect through interaction with iron, and therefore, an increase in the level of free intracellular ferrous iron correlates with an enhancement in sensitivity to streptonigrin (27, 80, 83). The NΔeps and NΔepsD mutants were much more susceptible to the action of streptonigrin than the wild-type strain (Fig. 4A). At a streptonigrin concentration of 5 μg/ml, the sizes of the inhibition zones displayed by the two T2S mutants were comparable to the size of the zone for wild-type cells that formed around discs immersed with 60 μg/ml streptonigrin (Fig. 4A). The sensitivity to streptonigrin was complemented and restored to the wild-type level by ectopic expression of epsCDEFGHIJKLMN and epsD in mutant strains NΔeps and NΔepsD, respectively (Fig. 4A). Moreover, we observed that addition of the iron-specific chelator 2,2′-dipyridyl to the medium of the mutant strains restored resistance to streptonigrin almost to the wild-type level (Fig. 4B). These results, along with the microarray and reporter fusion data, further supported the suggestion that the level of intracellular iron is likely higher in the T2S mutants than in the wild-type strain.
FIG. 4.
Susceptibility to streptonigrin (SPN), an indirect measurement of the intracellular free iron content. Wild-type (wt) V. cholerae N16961 and T2S mutants (NΔeps, NΔepsD) (A and B) or the NtolA mutant (C) were grown in LB medium to mid-log phase (OD600, about 0.5), and 100 μl of each culture was spread onto LB agar medium alone (A) or supplemented with the iron-specific chelator 2,2′-dipyridyl to a final concentration of 100 μM (B) or 150 μM (C). Cultures of NΔeps, NΔepsD, and NtolA containing plasmid pEps, pEpsD, or pTolA, respectively, were also spread onto LB agar alone and tested for complementation in the presence of 10 μM IPTG (A and C). Sterile filter paper discs immersed with different concentrations of streptonigrin, as indicated, were placed immediately on the surface of each plate. The diameter of the inhibition zone was scored after 24 h of incubation at 37°C. Each datum point represents the mean and SEM from three to nine separate experiments. In comparison to wild-type V. cholerae, both T2S mutants and the NtolA strain showed statistically significant increases in susceptibility to streptonigrin at each of the concentrations tested (P < 0.0001).
Oxidative stress is induced in T2S mutants.
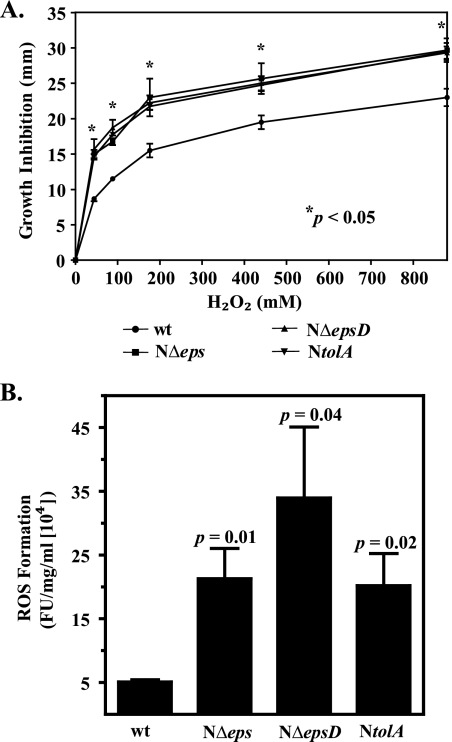
Although iron is crucial for the structure and function of many proteins, excess iron can be deleterious to the cells, because it rapidly reacts with ROS, which are a natural consequence of aerobic metabolism, and generates highly toxic hydroxyl radicals via the Fenton reaction (1, 24). Therefore, tight control of iron metabolism is an integral part of the antioxidant defense response (84). Fur provides protection against oxidative damage by inducing the expression of several genes encoding proteins that confer resistance to oxidative stress (74, 75). Our microarray results revealed that the NΔeps mutant displayed significant upregulation (as much as sevenfold) of genes coding for antioxidant proteins, such as catalase (KatB), alkylhydroperoxidase (AhpC), and superoxide dismutase (SodB) (31) (see Table S2 in the supplemental material). To test the hypothesis that eps mutants suffer from internal oxidative stress, we first examined their sensitivities to H2O2 by measuring the zone of growth inhibition around H2O2-immersed paper discs. The cells of T2S mutants were significantly more sensitive to H2O2 treatment than wild-type cells (Fig. 5A).
FIG. 5.
Sensitivity to hydrogen peroxide and endogenous ROS formation are augmented in T2S- and TolA-deficient strains of V. cholerae. (A) Wild-type (wt) V. cholerae N16961 and its isogenic T2S-deficient and NtolA mutants were grown in LB medium until mid-log phase (OD600, about 0.5). Cultures (100 μl) were uniformly distributed on LB agar plates, followed by immediate placing of sterile filter paper discs impregnated with 5 μl of 44, 88, 176, 440, and 880 mM hydrogen peroxide on the surface of the plate. The diameter of the growth inhibition zone was recorded after 24 h of incubation at 37°C. Error bars represent SEMs for four independent experiments, each performed in duplicate. All mutants displayed statistically significantly enhanced susceptibility to hydrogen peroxide relative to that of the parental strain (P < 0.05). (B) Intracellular ROS formation was measured in cells growing logarithmically in LB medium and incubated with the oxidative-stress-sensitive probe H2DCFDA as described in Materials and Methods. Intracellular ROS formation was expressed in arbitrary units of fluorescence intensity (FU), and the emission values were normalized to the protein concentration. Means for four independent experiments with corresponding SEMs are presented. The calculated P values shown above each bar indicate statistically significant increases in ROS levels in T2S- and TolA-deficient strains over those in the wild-type strain.
Next, we aimed to measure the level of ROS in exponentially growing cells by using the oxidative-stress-sensitive fluorescent probe H2DCFDA (10, 13, 20). The level of ROS-generated fluorescence was four- to sevenfold greater in the T2S mutants than in the wild-type strain (Fig. 5B). The finding that the level of ROS is higher in T2S mutants provides additional evidence that under conditions where the T2S machinery is inactivated, cells are suffering from internal oxidative stress and, as a consequence, display changes in iron homeostasis.
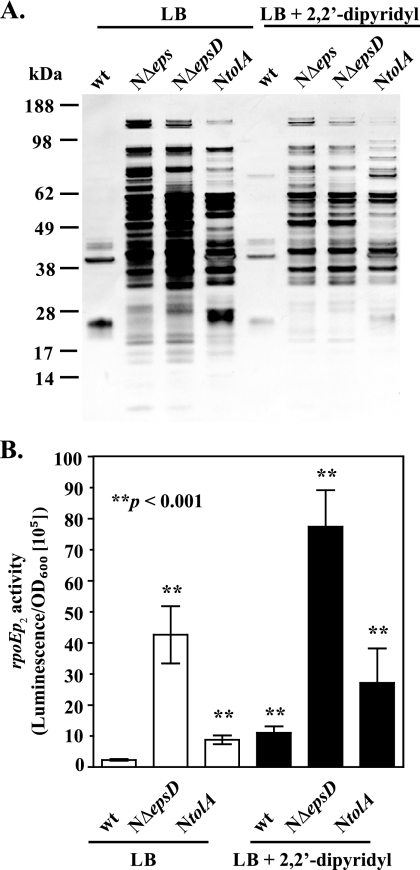
Membrane damage induces oxidative stress.
It is well established that accumulation of ROS causes damage to different cellular constituents, including lipids, DNA, and various proteins, particularly iron-sulfur cluster-containing proteins. The oxidative damage of iron-sulfur clusters leads to the release of iron, resulting in changes in iron physiology (31). Therefore, in order to investigate if the alteration in iron homeostasis is a source or a consequence of the compromised membrane integrity of V. cholerae T2S mutants, we used SDS-PAGE and silver staining to examine the culture supernatant profiles of wild-type and T2S-mutants grown in medium supplemented with the iron chelator 2,2′-dipyridyl. In comparison to the situation in the wild-type strain, large amounts of proteins were released to the culture medium when T2S-deficient strains were grown either with or without the iron chelator (Fig. 6A). There was a slight decrease in the amount of proteins leaking out of the mutant strains in the presence of 2,2′-dipyridyl, but the protein profiles remained very similar to those of cultures grown in the absence of the chelator. Leakage of intracellular proteins to the growth medium is observed in cells with membrane damage and during extracellular stress, when the expression of rpoE and the σE regulon is induced (47, 65). Therefore, as another indicator of extracellular stress and membrane damage, we used our rpoEp2 luciferase reporter fusion (65) to examine rpoE expression in the T2S mutant NΔepsD grown in the absence and presence of 2,2′-dipyridyl. Although the presence of the iron-specific chelator rescued the sensitivity to streptonigrin in T2S-deficient cells (Fig. 4B), the expression of rpoE remained considerably higher than that in the wild-type strain, indicating that the membrane perturbation persisted regardless of the iron level (Fig. 6B). In fact, expression of rpoE was increased in both the wild-type and NΔepsD strains when they were grown with the iron chelator. Furthermore, the reduced growth rate of NΔeps in LB medium was not rescued by the presence of different concentrations of 2,2′-dipyridyl (data not shown). In light of these experiments, we conclude that the changes in iron homeostasis in the T2S mutants of V. cholerae most likely occur as a consequence of altered outer membrane integrity. Consistent with this suggestion, in mid-log-phase cultures there was no significant difference in the expression of the Fur-Fe2+ regulated viuP and hutA promoters between wild-type and T2S mutant strains (see Fig. S1 in the supplemental material).
FIG. 6.
Outer membrane integrity is compromised regardless of the iron level. (A) Representative protein profiles of the supernatants isolated from stationary-phase cultures of wild-type (wt) V. cholerae N16961 and its isogenic NΔeps, NΔepsD, and NtolA mutants grown in LB medium without and with the addition of 100 or 150 μM 2,2′-dipyridyl (for the T2S-deficient and NtolA mutants, respectively). Samples were loaded onto SDS-PAGE gels by equivalent OD600 units, and gels were silver stained. The migration of molecular mass markers is indicated on the left. (B) The expression of the rpoEp2-lux fusion gene was determined in wild-type V. cholerae N16961 and its T2S-deficient and NtolA mutants grown at 37°C in LB medium alone or supplemented with 100 or 150 μM 2,2′-dipyridyl (for the T2S-deficient and NtolA mutants, respectively) to mid-log phase (OD600, 0.5). The data are means for three to nine independent experiments (each performed in technical triplicate). Error bars indicate SEMs. The increase in rpoE expression was statistically significant for the NΔeps, NΔepsD, and NtolA strains regardless of the presence or absence of the iron chelator (P < 0.001). Treatment with the iron-specific chelator resulted in the detection of statistically significantly different σE activity in the wild-type strain (P < 0.0001).
To further test the hypothesis that induction of oxidative stress and misregulation of iron homeostasis are direct consequences of outer membrane damage, we decided to examine a V. cholerae mutant that displays outer membrane defects similar to those of eps mutants while retaining a functional T2S machinery. Mutations in genes coding for the Tol-Pal system result in loss of outer membrane integrity, which has been evidenced by extracellular release of periplasmic proteins and increased sensitivity to detergents and other membrane-perturbing agents (7, 18, 28, 41, 77). In addition, inactivation of the Tol system renders cells sensitive to high iron concentrations (72). Therefore, to determine if generalized outer membrane damage induces oxidative stress, we constructed a tolA mutant of V. cholerae N16961 as described previously for strain O395 (28). The newly constructed NtolA mutant exhibited a filamentous phenotype (data not shown) and reduced stability of the outer membrane, as has been reported previously for mutations in the tolA locus (Fig. 6A) (28). Ectopic expression of tolA complemented the defects displayed by NtolA mutant cells (data not shown). As with the T2S mutants (65), rpoE expression was induced >4-fold in the NtolA mutant, as measured by our rpoEp2 reporter gene fusion assay (Fig. 6B). Regardless of the outer membrane alterations, the T2S machinery remained functional in the NtolA cells (see Fig. S2 in the supplemental material, which shows extracellular secretion of the plasmid-encoded heat-labile enterotoxin EtxB in NtolA).
To further explore the role of compromised membrane integrity in the misregulation of iron homeostasis, we first examined the activities of the viuPp and hutAp reporters in the NtolA strain. The luciferase reporter activities were approximately sixfold repressed in the early stationary phase of growth, while no significant changes were detected in mid-log-phase cultures of the NtolA mutant (Fig. 3; see also Fig. S1 in the supplemental material). Next, we tested the sensitivity of the NtolA mutant to streptonigrin in the absence and presence of the iron chelator. Like the eps mutants, cells with an inactivated tolA gene displayed increased sensitivity to streptonigrin, which was partially rescued by the presence of 150 μM 2,2′-dipyridyl (Fig. 4C). In addition, plasmid-expressed tolA complemented the NtolA mutant and restored streptonigrin sensitivity to the wild-type level (Fig. 4C). Together, these experiments suggested that the level of unincorporated iron was also elevated in the NtolA strain. Furthermore, the NtolA mutant cells showed statistically significantly higher sensitivity to H2O2 (Fig. 5A) and an approximately fivefold greater level of ROS formation than wild-type cells (Fig. 5B). As with T2S mutants, the compromised membrane integrity of the NtolA mutant was not suppressed in the presence of 2,2′-dipyridyl, as evidenced by SDS-PAGE and silver staining of supernatants isolated from cultures grown either with or without the iron chelator (Fig. 6A). In agreement with these results, expression of rpoE remained induced in NtolA regardless of the presence or absence of 2,2′-dipyridyl (Fig. 6B). These data support our hypothesis that alterations in the cell envelope contribute to the induction of internal oxidative stress and the increase in the labile iron pool. Moreover, to show that an alteration in iron homeostasis is a response generally observed with cell envelope damage and is not unique to V. cholerae, we also employed an existing tolA mutant of the E. coli laboratory strain K-12 BW25113 (2). As expected, there was an increase in protein quantity in the culture supernatant of the tolA mutant as revealed by SDS-PAGE and silver staining, indicating perturbed outer membrane integrity (see Fig. S3A in the supplemental material), and, in agreement with our hypothesis, the tolA mutant displayed increased susceptibility to the iron-activated antibiotic streptonigrin (see Fig. S3B in the supplemental material).
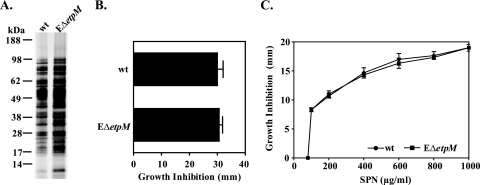
Resolving the role of the T2S system in iron homeostasis was of particular interest, since it has been shown recently that inactivation of the T2S machinery in the marine bacterium P. tunicata results in alterations in the levels of two putative TonB receptors and an ABC transporter involved in iron uptake (22). Moreover, it was reported that certain T2S mutants of E. chrysanthemi lack a periplasmic FbpA-like iron binding protein and display increased susceptibility to oxidants and streptonigrin in the stationary phase of growth (17). Our comparative analysis of different T2S and NtolA mutants exhibiting similarly altered outer membrane integrity, however, suggests that changes in iron homeostasis as well as in oxidative stress are not unique to T2S mutants but rather occur in response to cell envelope perturbation. To investigate the relationship between T2S and iron physiology further, we decided to analyze a T2S mutant of another species, E. coli, which does not display apparently altered outer membrane integrity (Fig. 7 A). We found that the susceptibilities to H2O2 and streptonigrin displayed by the T2S mutant of E. coli O157:H7 strain EDL 933, EΔetpM, were the same as those of the wild-type strain, indicating that a mutation in the T2S system of E. coli O157 does not affect iron homeostasis (Fig. 7B and C). These results add another line of evidence that the T2S system may not be directly involved in iron homeostasis and that the internal oxidative stress and altered iron metabolism observed in T2S mutants of some gram-negative species, including V. cholerae, are a consequence of cell envelope perturbation. The reason why T2S mutants of certain species have altered cell envelopes, however, is not clear at this moment, but the alteration may be the result of uncontrolled activity of periplasmically accumulated T2S substrates, such as proteases and/or chitinases.
FIG. 7.
Iron homeostasis and oxidative stress are not affected in a T2S mutant of E. coli O157:H7. (A) Protein profiles of filtered culture supernatants of the E. coli O157:H7 parental wild-type (wt) strain and its EΔetpM mutant grown in LB medium at 37°C to stationary phase (16 h), revealed by SDS-PAGE and silver staining. Samples were matched by equivalent OD600 units. The migration of molecular mass markers is indicated. (B and C) The susceptibilities of the wild-type and EΔetpM mutant strains to hydrogen peroxide (B) and streptonigrin (SPN) (C) were tested by uniformly distributing 100 μl of mid-log-phase cultures (OD600, about 0.5) on LB agar plates and then immediately placing sterile filter paper discs impregnated with either 8.8 M hydrogen peroxide or different concentrations of streptonigrin, as indicated, on the surfaces of the plates. The diameter of the inhibition zone was recorded after 24 h of incubation at 37°C. Means and corresponding SEMs for three independent experiments are reported.
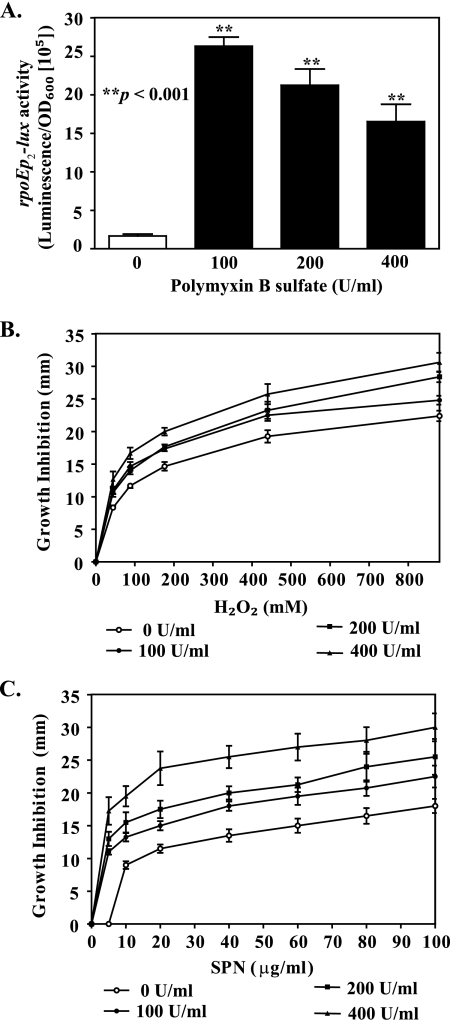
It is known that physical or chemical insults to the cell envelope, such as an elevated temperature, pressure, or treatment with antibiotics and antimicrobial peptides, compromises the integrity of the cell envelope and induces several stress regulons, including RpoE, Cpx, RpoS, and PhoP (3, 35, 46, 71). We hypothesized that, like mutations in the eps and tolA genes, chemical perturbation of the V. cholerae cell envelope would induce extracytoplasmic as well as oxidative stress and cause misregulation of iron homeostasis. To test this hypothesis, we first examined rpoEp2 reporter activity in wild-type V. cholerae N16961 treated with various concentrations of polymyxin B sulfate. We used 100, 200, and 400 U/ml of polymyxin B sulfate, based on our earlier study, which showed that 100 U/ml does not affect the viability of wild-type cells, while treatment with 400 or 800 U/ml results in 26% or 50% inhibition of growth, respectively (65). Wild-type V. cholerae cells responded to polymyxin B treatment with >10-fold upregulation of rpoEp2 activity at all concentrations tested (Fig. 8A), indicating membrane perturbation. These results are consistent with a previously reported increase in rpoE transcription after treatment with sublethal doses of antimicrobial peptides (46). Next, we tested if challenging wild-type cells with polymyxin B affects their sensitivity to H2O2. Like T2S and NtolA mutants, wild-type V. cholerae treated with increasing concentrations of polymyxin B became significantly more vulnerable to H2O2 (P < 0.05) (Fig. 8B). In addition, streptonigrin-mediated growth inhibition of wild-type cells was significantly increased in the presence of polymyxin B (P < 0.01) (Fig. 8C), indicating that chemical perturbation of the cell envelope results in changes in iron metabolism.
FIG. 8.
Extracytoplasmic and oxidative stress responses are triggered in wild-type V. cholerae cells following treatment with polymyxin B sulfate. (A) rpoE activity was monitored for wild-type V. cholerae N16961 grown at 37°C to mid-log phase (OD600, 0.5) in LB medium supplemented with increasing concentrations of polymyxin B sulfate (0, 100, 200, and 400 U/ml). Bars show means and SEMs from four independent experiments. P values indicate that the elevation in the envelope stress response in polymyxin B-treated cultures was statistically significant. (B and C) Wild-type V. cholerae N16961 was grown in LB medium until mid-log phase (OD600, 0.5), and 100 μl of the culture was spread onto LB agar supplemented with different concentrations of polymyxin B sulfate (0, 100, 200, and 400 U/ml). Sterile filter paper discs immersed with various concentrations of hydrogen peroxide (B) or streptonigrin (SPN) (C) were immediately placed on the surfaces of the plates. The diameter of the growth inhibition zone was scored after 24 h of incubation at 37°C. Each datum point represents the mean and SEMs obtained from five independent experiments (P < 0.05).
Concluding remarks.
Our transcriptome profiling and phenotypic analysis demonstrate that inactivation of the T2S system has severe consequences on iron homeostasis and the overall oxidative status of V. cholerae cells. In contrast to previous suggestions for other gram-negative bacteria (17, 22), we show that the T2S system is not directly engaged in iron acquisition in V. cholerae. Instead, misregulation of iron homeostasis in V. cholerae T2S mutants most likely occurs as a secondary effect related to alterations in the extracytoplasmic compartment.
The complexity of the phenotypes associated with mutations that perturb cell envelope homeostasis suggests that several stress pathways may be induced. Our experimental data are consistent with this suggestion and indicate that at least two global regulators, RpoE and Fur, respond to membrane perturbation caused either by mutations in the T2S system or Tol pathway in V. cholerae or by chemical treatment of V. cholerae cells with polymyxin B. Since a loss in membrane stability and increased sensitivity to various membrane-permeating agents are also common phenotypes of mutants deficient in other cell envelope constituents and biogenesis systems, such as major lipoprotein (Lpp), lipopolysaccharide synthesis and assembly (Rfa/Waa), the twin-arginine translocation system, and the membrane-derived oligosaccharides (MDO) biogenesis system (37, 38, 69, 70), we propose that these mutants may also suffer from extracytoplasmic stress and increased ROS formation, which cause an imbalance in the labile iron pool. The extracytoplasmic stress may be of various types and may involve signal transduction pathways such as RpoE, CpxAR, BaeRS, Rcs (phosphorelays), or PSP (phage shock response) (43, 57, 58, 60, 61). In fact, activation of both the RpoE and Rcs phosphorelay systems has been observed in rfa mutants as well as in MDO-, SurA-, and Tol-deficient strains (8, 12, 19, 43, 52, 78), and induction of oxidative stress has been reported for waaL mutants (5). In addition, inactivation of SurA, a periplasmic chaperone required for outer membrane protein biogenesis (67), results in increased sensitivity to iron (72), again suggesting that cell envelope perturbation leads to oxidative stress.
Further support for our model that damage to the cell envelope causes extracytoplasmic stress followed by induction of oxidative stress and alteration in iron homeostasis comes from recent studies of the mechanism of bactericidal antibiotic-mediated cell death. Treatment with penicillin and aminoglycoside antibiotics triggers ROS formation and rapid cell death via a mechanism that appears to involve cross talk between the extracytoplasmic stress regulator Cpx and the redox-responsive Arc system (34, 35). Furthermore, the finding that CpxAR represses the expression of the efeUOB operon and activates the transcription of ftnB, genes that encode proteins involved in iron transport and storage, respectively, provides additional support for our model (55). In addition, induced expression of rpoE and oxidative stress genes has been observed upon exposure of bacterial cells to high temperatures (4, 39, 56), possibly as a result of disruption of electron transport and an increase in ROS formation (56). Finally, an increasing number of studies reveal functional integration and perhaps cross talk between different global regulons, suggesting that bacteria might respond to various cues by triggering common protective stress responses (14, 45, 54). Taken together, many types of insults to the cell envelope, caused either by physical or chemical damage, antibiotic treatment, or genetic modifications, induce a variety of signaling pathways that ultimately lead to common downstream consequences resulting in internal oxidative stress and changes in iron homeostasis.
Supplementary Material
Acknowledgments
We thank Matthew Waldor and Janine Maddock for plasmid pDH149 and wild-type and mutant strains of E. coli K-12 BW25113, respectively. We gratefully acknowledge Tanya L. Johnson for critical reading of the manuscript.
The project described here was supported by award R01AI049294 from the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Published ahead of print on 19 June 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27215-237. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 22006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50219-230. [DOI] [PubMed] [Google Scholar]
- 4.Benov, L., and I. Fridovich. 1995. Superoxide dismutase protects against aerobic heat shock in Escherichia coli. J. Bacteriol. 1773344-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, M. C., G. C. McGhee, Y. Zhao, and G. W. Sundin. 2009. Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora. FEMS Microbiol. Lett. 29180-87. [DOI] [PubMed] [Google Scholar]
- 6.Beyhan, S., A. D. Tischler, A. Camilli, and F. H. Yildiz. 2006. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J. Bacteriol. 1883600-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bina, J. E., and J. J. Mekalanos. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 694681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchart, F., A. Delangle, J. Lemoine, J. P. Bohin, and J. M. Lacroix. 2007. Proteomic analysis of a non-virulent mutant of the phytopathogenic bacterium Erwinia chrysanthemi deficient in osmoregulated periplasmic glucans: change in protein expression is not restricted to the envelope, but affects general metabolism. Microbiology 153760-767. [DOI] [PubMed] [Google Scholar]
- 9.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138179-207. [DOI] [PubMed] [Google Scholar]
- 10.Chávez, F. P., H. Lunsdorf, and C. A. Jerez. 2004. Growth of polychlorinated-biphenyl-degrading bacteria in the presence of biphenyl and chlorobiphenyls generates oxidative stress and massive accumulation of inorganic polyphosphate. Appl. Environ. Microbiol. 703064-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13581-588. [DOI] [PubMed] [Google Scholar]
- 12.Clavel, T., J. C. Lazzaroni, A. Vianney, and R. Portalier. 1996. Expression of the tolQRA genes of Escherichia coli K-12 is controlled by the RcsC sensor protein involved in capsule synthesis. Mol. Microbiol. 1919-25. [DOI] [PubMed] [Google Scholar]
- 13.Crow, J. P. 1997. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1145-157. [DOI] [PubMed] [Google Scholar]
- 14.Cuny, C., M. Lesbats, and S. Dukan. 2007. Induction of a global stress response during the first step of Escherichia coli plate growth. Appl. Environ. Microbiol. 73885-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DebRoy, S., J. Dao, M. Soderberg, O. Rossier, and N. P. Cianciotto. 2006. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. USA 10319146-19151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douet, V., D. Expert, F. Barras, and B. Py. 2009. Erwinia chrysanthemi iron metabolism: the unexpected implication of the inner membrane platform within the type II secretion system. J. Bacteriol. 191795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubuisson, J. F., A. Vianney, N. Hugouvieux-Cotte-Pattat, and J. C. Lazzaroni. 2005. Tol-Pal proteins are critical cell envelope components of Erwinia chrysanthemi affecting cell morphology and virulence. Microbiology 1513337-3347. [DOI] [PubMed] [Google Scholar]
- 19.Ebel, W., G. J. Vaughn, H. K. Peters III, and J. E. Trempy. 1997. Inactivation of mdoH leads to increased expression of colanic acid capsular polysaccharide in Escherichia coli. J. Bacteriol. 1796858-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Echave, P., J. Tamarit, E. Cabiscol, and J. Ros. 2003. Novel antioxidant role of alcohol dehydrogenase E from Escherichia coli. J. Biol. Chem. 27830193-30198. [DOI] [PubMed] [Google Scholar]
- 21.Evans, F. F., S. Egan, and S. Kjelleberg. 2008. Ecology of type II secretion in marine gammaproteobacteria. Environ. Microbiol. 101101-1107. [DOI] [PubMed] [Google Scholar]
- 22.Evans, F. F., M. J. Raftery, S. Egan, and S. Kjelleberg. 2007. Profiling the secretome of the marine bacterium Pseudoalteromonas tunicata using amine-specific isobaric tagging (iTRAQ). J. Proteome Res. 6967-975. [DOI] [PubMed] [Google Scholar]
- 23.Filloux, A. 2004. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694163-179. [DOI] [PubMed] [Google Scholar]
- 24.Fridovich, I. 1995. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 6497-112. [DOI] [PubMed] [Google Scholar]
- 25.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 671393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentleman, R. C., V. J. Carey, D. M. Bates, B. Bolstad, M. Dettling, S. Dudoit, B. Ellis, L. Gautier, Y. Ge, J. Gentry, K. Hornik, T. Hothorn, W. Huber, S. Iacus, R. Irizarry, F. Leisch, C. Li, M. Maechler, A. J. Rossini, G. Sawitzki, C. Smith, G. Smyth, L. Tierney, J. Y. Yang, and J. Zhang. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassett, D. J., B. E. Britigan, T. Svendsen, G. M. Rosen, and M. S. Cohen. 1987. Bacteria form intracellular free radicals in response to paraquat and streptonigrin. Demonstration of the potency of hydroxyl radical. J. Biol. Chem. 26213404-13408. [PubMed] [Google Scholar]
- 28.Heilpern, A. J., and M. K. Waldor. 2000. CTXφ infection of Vibrio cholerae requires the tolQRA gene products. J. Bacteriol. 1821739-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirst, T. R., and J. Holmgren. 1987. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc. Natl. Acad. Sci. USA 847418-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard, S. P., J. Critch, and A. Bedi. 1993. Isolation and analysis of eight exe genes and their involvement in extracellular protein secretion and outer membrane assembly in Aeromonas hydrophila. J. Bacteriol. 1756695-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imlay, J. A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77755-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang, B., and S. P. Howard. 1992. The Aeromonas hydrophila exeE gene, required both for protein secretion and normal outer membrane biogenesis, is a member of a general secretion pathway. Mol. Microbiol. 61351-1361. [DOI] [PubMed] [Google Scholar]
- 33.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 848-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohanski, M. A., D. J. Dwyer, B. Hayete, C. A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130797-810. [DOI] [PubMed] [Google Scholar]
- 35.Kohanski, M. A., D. J. Dwyer, J. Wierzbowski, G. Cottarel, and J. J. Collins. 2008. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lathem, W. W., T. E. Grys, S. E. Witowski, A. G. Torres, J. B. Kaper, P. I. Tarr, and R. A. Welch. 2002. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol. Microbiol. 45277-288. [DOI] [PubMed] [Google Scholar]
- 37.Lazzaroni, J. C., N. Fognini-Lefebvre, and R. Portalier. 1989. Cloning of the excC and excD genes involved in the release of periplasmic proteins by Escherichia coli K12. Mol. Gen. Genet. 218460-464. [DOI] [PubMed] [Google Scholar]
- 38.Lazzaroni, J. C., and R. Portalier. 1992. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL). Mol. Microbiol. 6735-742. [DOI] [PubMed] [Google Scholar]
- 39.Lee, P. C., B. R. Bochner, and B. N. Ames. 1983. AppppA, heat-shock stress, and cell oxidation. Proc. Natl. Acad. Sci. USA 807496-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 11869-82. [DOI] [PubMed] [Google Scholar]
- 41.Llamas, M. A., J. L. Ramos, and J. J. Rodriguez-Herva. 2000. Mutations in each of the tol genes of Pseudomonas putida reveal that they are critical for maintenance of outer membrane stability. J. Bacteriol. 1824764-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lybarger, S. R., T. L. Johnson, M. D. Gray, A. E. Sikora, and M. Sandkvist. 2009. Docking and assembly of the type II secretion complex of Vibrio cholerae. J. Bacteriol. 1913149-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majdalani, N., and S. Gottesman. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59379-405. [DOI] [PubMed] [Google Scholar]
- 44.Marsh, J. W., and R. K. Taylor. 1998. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol. Microbiol. 291481-1492. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-Antonio, A., and J. Collado-Vides. 2003. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6482-489. [DOI] [PubMed] [Google Scholar]
- 46.Mathur, J., B. M. Davis, and M. K. Waldor. 2007. Antimicrobial peptides activate the Vibrio cholerae σE regulon through an OmpU-dependent signalling pathway. Mol. Microbiol. 63848-858. [DOI] [PubMed] [Google Scholar]
- 47.McBroom, A. J., A. P. Johnson, S. Vemulapalli, and M. J. Kuehn. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 1885385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meselson, M., and R. Yuan. 1968. DNA restriction enzyme from E. coli. Nature 2171110-1114. [DOI] [PubMed] [Google Scholar]
- 49.Mey, A. R., and S. M. Payne. 2001. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol. Microbiol. 42835-849. [DOI] [PubMed] [Google Scholar]
- 50.Mey, A. R., E. E. Wyckoff, V. Kanukurthy, C. R. Fisher, and S. M. Payne. 2005. Iron and fur regulation in Vibrio cholerae and the role of fur in virulence. Infect. Immun. 738167-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 1702575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Missiakas, D., J. M. Betton, and S. Raina. 1996. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol. Microbiol. 21871-884. [DOI] [PubMed] [Google Scholar]
- 53.Morales, V. M., A. Bäckman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 9739. [DOI] [PubMed] [Google Scholar]
- 54.Partridge, J. D., G. Sanguinetti, D. P. Dibden, R. E. Roberts, R. K. Poole, and J. Green. 2007. Transition of Escherichia coli from aerobic to micro-aerobic conditions involves fast and slow reacting regulatory components. J. Biol. Chem. 28211230-11237. [DOI] [PubMed] [Google Scholar]
- 55.Price, N. L., and T. L. Raivio. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 1911798-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Privalle, C. T., and I. Fridovich. 1987. Induction of superoxide dismutase in Escherichia coli by heat shock. Proc. Natl. Acad. Sci. USA 842723-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 451599-1611. [DOI] [PubMed] [Google Scholar]
- 58.Raivio, T. L. 2005. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 561119-1128. [DOI] [PubMed] [Google Scholar]
- 59.Reidl, J., and K. E. Klose. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol. Rev. 26125-139. [DOI] [PubMed] [Google Scholar]
- 60.Rowley, G., M. Spector, J. Kormanec, and M. Roberts. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4383-394. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz, N., and T. J. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8122-126. [DOI] [PubMed] [Google Scholar]
- 62.Sandkvist, M., T. R. Hirst, and M. Bagdasarian. 1990. Minimal deletion of amino acids from the carboxyl terminus of the B subunit of heat-labile enterotoxin causes defects in its assembly and release from the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 26515239-15244. [PubMed] [Google Scholar]
- 63.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, V. J. DiRita, and M. Bagdasarian. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 1796994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi, L., S. Deng, M. J. Marshall, Z. Wang, D. W. Kennedy, A. C. Dohnalkova, H. M. Mottaz, E. A. Hill, Y. A. Gorby, A. S. Beliaev, D. J. Richardson, J. M. Zachara, and J. K. Fredrickson. 2008. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J. Bacteriol. 1905512-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sikora, A. E., S. R. Lybarger, and M. Sandkvist. 2007. Compromised outer membrane integrity in Vibrio cholerae type II secretion mutants. J. Bacteriol. 1898484-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 67.Sklar, J. G., T. Wu, D. Kahne, and T. J. Silhavy. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 212473-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Söderberg, M. A., J. Dao, S. R. Starkenburg, and N. P. Cianciotto. 2008. Importance of type II secretion for survival of Legionella pneumophila in tap water and in amoebae at low temperatures. Appl. Environ. Microbiol. 745583-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanley, N. R., K. Findlay, B. C. Berks, and T. Palmer. 2001. Escherichia coli strains blocked in Tat-dependent protein export exhibit pleiotropic defects in the cell envelope. J. Bacteriol. 183139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki, H., Y. Nishimura, S. Yasuda, A. Nishimura, M. Yamada, and Y. Hirota. 1978. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol. Gen. Genet. 1671-9. [DOI] [PubMed] [Google Scholar]
- 71.Tam, C., and D. Missiakas. 2005. Changes in lipopolysaccharide structure induce the σE-dependent response of Escherichia coli. Mol. Microbiol. 551403-1412. [DOI] [PubMed] [Google Scholar]
- 72.Tamayo, R., S. S. Ryan, A. J. McCoy, and J. S. Gunn. 2002. Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella enterica serovar Typhimurium. Infect. Immun. 706770-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tashima, K. T., P. A. Carroll, M. B. Rogers, and S. B. Calderwood. 1996. Relative importance of three iron-regulated outer membrane proteins for in vivo growth of Vibrio cholerae. Infect. Immun. 641756-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 3731-6. [DOI] [PubMed] [Google Scholar]
- 75.Touati, D., M. Jacques, B. Tardat, L. Bouchard, and S. Despied. 1995. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 1772305-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 985116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vianney, A., T. M. Lewin, W. F. Beyer, Jr., J. C. Lazzaroni, R. Portalier, and R. E. Webster. 1994. Membrane topology and mutational analysis of the TolQ protein of Escherichia coli required for the uptake of macromolecules and cell envelope integrity. J. Bacteriol. 176822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinés, E. D., C. L. Marolda, A. Balachandran, and M. A. Valvano. 2005. Defective O-antigen polymerization in tolA and pal mutants of Escherichia coli in response to extracytoplasmic stress. J. Bacteriol. 1873359-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watnick, P. I., T. Eto, H. Takahashi, and S. B. Calderwood. 1997. Purification of Vibrio cholerae fur and estimation of its intracellular abundance by antibody sandwich enzyme-linked immunosorbent assay. J. Bacteriol. 179243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White, J. R., and H. N. Yeowell. 1982. Iron enhances the bactericidal action of streptonigrin. Biochem. Biophys. Res. Commun. 106407-411. [DOI] [PubMed] [Google Scholar]
- 81.Wyckoff, E. E., A. R. Mey, and S. M. Payne. 2007. Iron acquisition in Vibrio cholerae. Biometals 20405-416. [DOI] [PubMed] [Google Scholar]
- 82.Wyckoff, E. E., A. M. Valle, S. L. Smith, and S. M. Payne. 1999. A multifunctional ATP-binding cassette transporter system from Vibrio cholerae transports vibriobactin and enterobactin. J. Bacteriol. 1817588-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeowell, H. N., and J. R. White. 1982. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob. Agents Chemother. 22961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 1814639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.