Abstract
The presence of various numbers of EPIYA tyrosine phosphorylation motifs in the CagA protein of Helicobacter pylori has been suggested to contribute to pathogenesis in adults. In this prospective study, we characterized H. pylori isolates from symptomatic children, with reference to the diversity of functional EPIYA motifs in the CagA protein and vacA isotypes, and assessed the potential correlation with the histopathological manifestations of the infection. We analyzed 105 H. pylori isolates from 98 children and determined the diversity of EPIYA motifs in CagA by amplification and sequencing of the 3′ variable region of the cagA gene as well as vacA isotypes for the signal, middle, and intermediate regions. CagA phosphorylation and levels of secreted IL-8 were determined following in vitro infection of AGS gastric epithelial cells. Histopathological evaluation of H. pylori colonization, activity, and severity of the associated gastritis was performed according to the updated Sydney criteria. EPIYA A (GLKN[ST]EPIYAKVNKKK), EPIYA B (Q[V/A]ASPEPIY[A/T]QVAKKVNAKI), and EPIYA C (RS[V/A]SPEPIYATIDDLG) motifs were detected in the ABC (46.6%) and ABCC (17.1%) combinations. No isolates harboring more than two EPIYA C motifs in CagA were found. The presence of isogenic strains with variable numbers of CagA EPIYA C motifs within the same patient was detected in seven cases. Occurrence of increasing numbers of EPIYA C motifs correlated strongly with presence of a high-vacuolation (s1 or s2/i1/m1) phenotype and age. A weak positive correlation was observed between vacuolating vacA genotypes and presence of nodular gastritis. However, CagA- and VacA-dependent pathogenicities were not found to contribute to severity of histopathology manifestations in H. pylori-infected children.
Helicobacter pylori infects 50% of the world's population, and wide differences in prevalence of infection appear to exist between countries with different levels of socioeconomic development. Infection usually occurs in childhood and in the majority of cases remains asymptomatic, although major reasons for endoscopy referral can include recurrent epigastric or abdominal pain, with or without vomiting, neither of which correlates with H. pylori infection (17). Antral nodularity is a well-described endoscopic feature of H. pylori-infected children, and histological observations usually include superficial chronic active gastritis with occasional infiltration of eosinophils; in far fewer cases, they include peptic ulcers; and very rarely, they include gastric atrophy and intestinal metaplasia (13, 27). If the infection is left untreated, it persists through adulthood, and although it can still remain asymptomatic in the vast majority of infected hosts, H. pylori infection is now regarded as the most important etiological risk factor for development of gastric cancer in developed countries (28). H. pylori pathogenesis is manifested through a combined effect of bacterial virulence factors, host genetics, and environmental factors, which orchestrate toward the development of distinct phenotypes in adults, namely, superficial asymptomatic gastritis, duodenal ulcer, and gastric cancer (3). The expression and translocation of cytotoxin-associated gene antigen (CagA), a putative H. pylori virulence factor, inside gastric epithelial cells by cagA-positive H. pylori strains harboring a functional type IV secretion system has been suggested to play an important role in H. pylori pathogenesis (22). Early epidemiological studies of adults associated the presence of the cagA gene with development of peptic ulcer disease (31); gastric cancer (14); and increased inflammation (35), cellular proliferation (36), and intestinal metaplasia (20) of the gastric mucosa. However, in infected children, neither cagA status nor any other putative H. pylori virulence factor has been found to correlate with clinical outcome or severity of histological manifestations. However, recent advances into the fascinating cellular biology of CagA inside the gastric epithelial cell have enhanced its reputation as a potential bacterial oncoprotein (22). Following its translocation inside the gastric epithelial cell via the type IV secretion system (32), CagA has been shown to become at least partly tyrosine phosphorylated (5, 11, 41) by Src family kinases (42, 44) on repeating 5-amino-acid glutamic-proline-isoleucine-tyrosine-alanine (EPIYA) motifs present at the C terminus of the protein. Analysis of EPIYA motifs in CagA has revealed considerable type variation, depending on the peptide sequence surrounding it, namely, EPIYA A (EPIYAKVNKKK), EPIYA B (EPIYAQVAKKV), or EPIYA C (EPIYATIDDLG) in isolates from Western populations or EPIYA D (EPIYATIDFD) in isolates of Asian origin. In addition, considerable variation in number of repeating EPIYA C or D motifs at the carboxyl terminus of the protein (10, 44) has been observed, and biological activity of CagA was suggested to be determined by variation in these motifs (25) in phosphorylation-dependent as well as -independent ways (23). Hence, the number and type of EPIYA phosphorylation motifs may be viewed as putative virulence determinants of CagA activity and therefore become useful clinical markers that may predict the degree of individual H. pylori strain virulence potential. In this context, we proposed a PCR amplification and sequencing-based strategy for accurate characterization of the number and type of EPIYA motifs of CagA in H. pylori clinical isolates (34).
A multifactorial role has also been attributed to the secreted VacA virulence factor (16), a protein with multiple cellular activities, as it can disrupt endocytic trafficking of host cells, promote cell death through apoptosis, suppress the local immune system, and possibly potentiate the development of ulcers (6). Although the vacA gene is present in all H. pylori strains, it contains at least three variable parts, the s region, the i region, and the m region, which encode the signal, intermediate, and middle peptides, respectively, which have all been classified as allelic types 1 and 2. The s1-or-s2/i1/m1-or-m2 and s1-or-s2/i1-i2/m1-or-m2 VacA isotypes induce, in general, high and moderate levels of vacuolation, respectively, whereas the s1-or-s2/i2/m1-or-m2 strains induce very little or no vacuolation (39). Consequently, the vacA s/m genotype can also be regarded as a marker of pathogenicity of individual strains (8). Moreover, phylogenetic linkage analysis studies have indicated that there may be a functional basis for the selection of vacA and cagA isotypes (50), although there is substantial distance between vacA loci and cag genes on the bacterial genome. Furthermore, the intermediate region has been associated with development of gastric cancer (39).
In the present study, we investigated the potential association of the CagA and VacA virulence factor polymorphisms with clinicopathological manifestations of the disease in symptomatic Greek children. More specifically, H. pylori clinical strains isolated from symptomatic children were characterized with regard to the number and type of repeating EPIYA phosphorylation motifs in CagA protein and the vacA signal, intermediate, and middle region genotypes. Furthermore, these clinical isolates were carefully assessed for their ability to express phosphorylated CagA as well as induce interleukin-8 (IL-8) secretion following infection of gastric epithelial cells. Finally, the potential association of such functional bacterial determinants with H. pylori-associated histopathology in these patients was assessed.
MATERIALS AND METHODS
Patients.
The study included 98 symptomatic H. pylori-infected children, 2 to 16 years old, who underwent upper endoscopy at the Gastroenterology Clinic of the First Department of Pediatrics of Athens University, Aghia Sophia Children's Hospital. All children were Greek in origin, and their parents had given their consent for participation in the study. The study (protocol number 19321/13.09.2007) was approved by the Hospital Scientific Committee and the Ethical Committee (transcript 23/14.11.2007). None of the patients had received nonsteroidal anti-inflammatory drugs or had recently been prescribed antibiotics for the last 3 months. Three biopsy specimens were collected from the antrum for the histology, culture, and rapid urease test (CLO test), as well as one biopsy specimen from the corpus and two biopsy specimens from the duodenum for histology.
Isolation and culture of H. pylori.
Antral mucosa biopsy specimens collected from the greater curvature were aseptically placed in thioglycolate medium (Oxoid, Basingstoke, United Kingdom) and were processed for H. pylori isolation within 2 to 4 h after endoscopy. Specimens were vigorously vortexed with addition of sterile glass beads and cultured for up to 7 days on Chalgren-Wilkins agar plates containing antibiotics (vancomycin, 10 μg/ml; trimethoprim, 10 μg/ml; polymyxin B, 104 IU/liter; amphotericin B, 2 μg/ml; nalidixic acid, 10 μg/ml; bacitracin, 30 μg/ml; and fluorocytosine, 5 μg/ml) supplemented with 7% (vol/vol) horse blood and 1% (vol/vol) Vitox (Oxoid, Basingstoke, United Kingdom) under microaerophilic conditions (CampyPak Plus; Becton Dickinson, Cockeysville, MD) at 37°C. Plates were inspected daily for the presence of suspected colonies, which were initially screened for by colony morphology analysis and Gram staining and further verified by oxidase, catalase, and urease reactions. Culture sweeps, as well as individual colonies from each patient, were collected. H. pylori clonal relatedness within the same patient was routinely evaluated by randomly amplified polymorphic DNA (RAPD) PCR utilizing primers 1281, D14307, and D11344 (2). Multilocus sequence tagging (MLST) analysis with primers for the atpA, efp, mutY, ppa, trpC, ureI, vacA, and yphC housekeeping genes (19) was used to further characterize isogenic H. pylori strains expressing CagA with the divergent number of EPIYA motifs isolated from the same patient. In total, 105 H. pylori clinical isolates were collected and stored in brain heart infusion broth supplemented with 20% glycerol at −80°C until further analysis.
Characterization of diversity of EPIYA phosphorylation motifs.
Characterization of the EPIYA phosphorylation motifs was accomplished utilizing our strategy as described before (34). Briefly, each clinical isolate was passed twice on Chalgren-Wilkins plates and total bacterial genomic DNA (optical density at 260 nm/optical density at 280 nm ≥ 1.800) was extracted using a DNeasy isolation kit provided by Qiagen AS (Oslo, Norway). The EPIYA-coding regions of the cagA gene were amplified (EPIYA PCR) utilizing primers cagA2530S (5′-GTTAARAATRGTGTRAAYGG-3′, where R represents A or G and Y represents T or C) and cagA3000AS (5′-TTTAGCTTCTGATACCGC-3′), which recognize positions 582453 to 582977 with reference to the H. pylori 26695 genome. Amplicons ranging from 370 to 670 bp (±25 bp) were visualized by agarose gel electrophoresis and sequenced using a GenomeLab DTCS Quick Start sequencing kit (Beckman Coulter, Fullerton, CA) with a CEQ 8000 Beckman Coulter genetic analyzer. The number and type of EPIYA motifs were determined from the deduced peptide sequences following alignment by CLUSTAL W (European Bioinformatics Institute [http://www.ebi.ac.uk/Tools/clustalw2/index.html]). EPIYA PCR-negative cases were confirmed as true cagA-negative isolates by an empty-site-positive PCR assay as described before (1).
Detection of vacA gene diversity.
VacA signal region isotyping was performed by PCR using the VA1-F and VA1-R primers, and middle region isotyping was carried out by multiplex PCR utilizing primers VA4-F, VA4-R, VA7-F, and VA7-R (7). Intermediate region genotypes were determined by PCR according to the instructions of Rhead et al. (39).
In vitro infection of gastric epithelial cells (AGS) with clinical isolates.
Human gastric adenocarcinoma epithelial AGS cells (2 × 106 cells in 25-mm2 flasks or 1 × 106 cells in six-well plates) cultured in F-12 Kaighn's medium (Gibco, Invitrogen, Ltd., Paisley, United Kingdom) containing 10% fetal bovine serum (Gibco) were infected with H. pylori clinical strains at a multiplicity of infection of 100 and incubated in a 5% CO2 atmosphere. Total protein lysates and culture supernatants were collected at selected time points ranging from 1 to 48 h postinfection.
Expression and functional analysis of CagA phosphorylation.
Total protein lysates from H. pylori-infected AGS epithelial cells were obtained in ice-cold lysis radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris-HCl [pH 7.2], 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1% deoxycholate, 5 mM EDTA, 2 mM l-dithiothreitol) containing protease and phosphatase inhibitor cocktails. Lysates with equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (7.5% polyacrylamide) and transferred onto polyvinylidene difluoride (Immobilon P; Millipore Corp., Bedford, MA) membranes. CagA expression was detected by Western blot analysis using an anti-CagA primary polyclonal antibody (Austral Biologicals, San Ramon, CA) followed by a secondary horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G polyclonal antibody (Jackson ImmunoResearch Europe, Ltd., Soham, Cambridgeshire, United Kingdom), detected by the ECL Plus chemiluminescence detection system (Amersham, GE Healthcare UK, Ltd., Buckinghamshire, United Kingdom). CagA tyrosine phosphorylation was evaluated by detection of CagA expression following immunoprecipitation of total cell lysates with an anti-mouse monoclonal pY20 phospho-tyrosine antibody (BD Transduction Laboratories, Franklin Lakes, NJ).
Determination of IL-8 levels.
Culture supernatants collected at selected time points over 48 h from in vitro H. pylori-infected AGS gastric epithelial cells (multiplicity of infection, 100) were centrifuged at 13,000 rpm, and the levels of IL-8 were determined by using a commercial enzyme-linked immunosorbent assay kit (Bender MedSystems GmbH, Vienna, Austria) according to the manufacturer's protocol.
Histological analysis.
Biopsy specimens destined for histopathology evaluation were fixed in 10% neutral buffered formalin solution, processed for histology, and embedded in paraffin.
Several serial longitudinal 4-μm sections from each specimen were cut, two of them were stained with hematoxylin-eosin for evaluation of gastric inflammation, and one was analyzed with the May-Grünwald Giemsa method for assessment of H. pylori colonization. Bacterial density and pathology of gastric mucosa were assessed according to the updated Sydney System (18). Histopathological evaluation was performed by a histopathologist with no prior knowledge of the identity of the samples.
Statistical analysis.
Statistical analysis with reference to potential associations between virulence factor variability and histopathological manifestations was conducted by using the SPSS package.
Nucleotide sequence accession numbers.
The partial cagA nucleotide sequences generated in the present study were submitted to the GenBank/EMBL/DDBJ databases (accession numbers AM292556 and -7, AM292559 to -76, AM292579 to -95, AM295786, AM295789, and FM957544 to -62).
RESULTS
Patient demographics, clinical outcomes, and histopathologies.
In total, 98 symptomatic children (49 male), aged 2 to 16 years (mean age, 10.7 ± 0.3 years), were included in the study. Symptoms included epigastric or abdominal pain (n = 73), pain coupled with vomiting (n = 10), or just vomiting (n = 15). Upon endoscopic evaluation, antral nodular gastritis was evident in 72 out of 98 patients (73.5%) and edema and erythema were evident in the corpus in 42 cases (42.7%), while in 6 children (5 male), duodenal ulcer was observed. Upon histological examination, all patients developed chronic active gastritis in the antrum, and 41 patients also developed it in the corpus. Neutrophil infiltration was assessed as mild (n = 9 patients), moderate (n = 80), or marked (n = 7). Mild (n = 5), moderate (n = 51), and marked (n = 40) lymphocytic infiltration was also observed. The presence of eosinophils was apparent in 43 patients (mild, n = 26; moderate, n = 17). H. pylori colonization was classified as absent (n = 9), mild (n = 10), moderate (n = 67), and marked (n = 10). Only one patient presented with atrophy and none with intestinal metaplasia. Lymphoid follicle formation was observed in 27 patients (18 male). Finally, no formation of ectopic gastric mucosa in the duodenum was observed in our sample population.
Determination of EPIYA diversity in CagA protein.
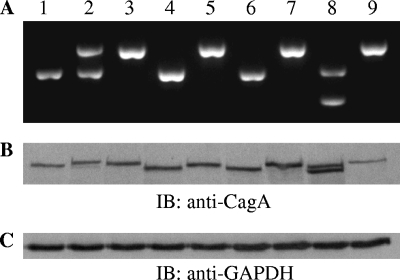
Ninety-eight H. pylori clinical isolates were collected from our patients, and the bacterial DNA was subjected to EPIYA PCR for determination of EPIYA diversity. Thirty-five isolates (33.3%) were found to be negative in EPIYA PCR amplification (Table 1). These were all confirmed as true cagPAI-negative strains by empty-site positive PCR (1), which generates an amplicon in the absence of the whole pathogenicity island (data not shown). They were further verified by the absence of an immunospecific band in Western blot determination of CagA expression in individual protein lysates. In 56 isolates, single-band amplification by EPIYA PCR (Fig. 1A) and CagA protein expression (Fig. 1B) was observed. However, in the isolates from seven patients, we observed two amplified bands in the EPIYA-PCR (Fig. 1A). We verified by isolation and sequencing that these were cagA-specific gene sequences, indicating the presence of at least two infecting strains within the same patient. The expression of such dual CagA protein species was further verified by Western blot analysis of total protein lysates of AGS cells infected with the corresponding H. pylori strains (Fig. 1B), indicative of the simultaneous presence of two infecting strains expressing CagA protein with divergent numbers of EPIYA motifs. We proceeded to separate these pairs of isolates by following the methodology described in our earlier publication (34), bringing the total number of strains to 105. More specifically, we successfully separated those subclones by limiting dilution, H. pylori colony selection, and screening of individual colonies for a single PCR amplicon by our EPIYA PCR. RAPD PCR analysis on genomic DNA derived from the isolated pairs of strains revealed identical profiles (see Fig. S1 in the supplemental material), an indication that these strains were clonally closely related. The subclones were further subjected to MLST analysis, which afforded identical sequences for all seven genes (data not shown), suggesting that the isolated clones were isogenic. For six out of seven patients, sequence analysis of the EPIYA PCR amplicons revealed the simultaneous presence of CagA protein species with either three or four EPIYA domains (ABC/ABCC combination). Upon comparison, sequences were found to be identical on a nucleotide basis outside the 102-bp sequence repeat coding for the 34-amino-acid peptide segment containing the additional EPIYA C motif. The remaining isolate contained two subclones expressing CagA with either an AB or an ABC combination of EPIYA motifs.
TABLE 1.
CagA protein diversity with regard to EPIYA phosphorylation motifs in H. pylori isolates from symptomatic children
| Strain group and EPIYA statusa | No. (%) of strains |
|---|---|
| cagPAI negative | 35 (33.3) |
| cagPAI defective | |
| ABC | 9 (8.5) |
| ABCC | 3 (2.8) |
| cagPAI functional | |
| AB | 1 (1.0) |
| ABC | 40 (38.1) |
| ABBC | 1 (1.0) |
| ABCC | 15 (14.3) |
| ACC | 1 (1.0) |
| Total | 105 (100.0) |
A, B, and C refer to the EPIYA A (EPIYAKVNKKK[A/T/V/S]GQ), EPIYA B (EPIY[A/T][Q/K]VAKKVNAKI), and EPIYA C (EPIYATIDDLG) motifs, respectively.
FIG. 1.
CagA protein polymorphism with reference to EPIYA motifs. DNA from representative H. pylori clinical strains (lanes 1 to 9) was amplified by EPIYA PCR, and the PCR products were analyzed on a 1.5% agarose gel (A). Analysis of the expressed CagA proteins by immunoblotting (IB) (B) with anti-CagA rabbit polyclonal antibody, following in vitro infection of AGS gastric epithelial cells with the corresponding isolates shown in panel A. Note the presence of two amplified bands in samples 2 and 8 in panel A, denoting the presence of two distinct amplicons and the expression of the respective two different CagA protein species (B). The immunoblot in panel C depicts expression of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) protein as a total protein loading control for panel B.
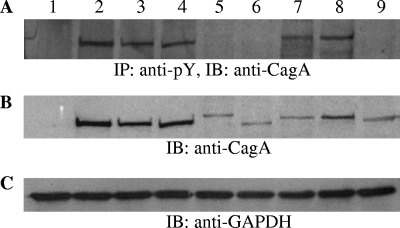
The nucleotide sequences of the EPIYA-PCR amplicons from all 105 H. pylori isolates were aligned, and the deduced peptide sequences revealed the presence of EPIYA motifs, namely, EPIYA A (GLKN[ST]EPIYAKVNKKK), EPIYA B (Q[V/A]ASPEPIY[A/T]QVAKKV), and EPIYA C (RS[V/A]SPEPIYATIDDLG). The ST dipeptide preceding the EPIYA A motif was observed in only 15 patients. The majority of isolates contained alanine (A) (n = 46) within the EPIYA B motif; however, equal distributions of valine and alanine were observed for EPIYA C. No significant variations in the individual peptide sequences surrounding the EPIYA motifs were observed in our sample from children and those already published for adult populations in Greece (34). In all, 49 out of 105 (46.7%) isolates were found to possess three EPIYA domains in the ABC conformation and 18 (17.1%) isolates four domains in the ABCC conformation (Table 1). Rare cases of CagA proteins with EPIYA domains in combinations such as ABBC (n = 1) and ACC (n = 1) were also observed, whereas no cases with more than two EPIYA C repeats were detected. For the cagA-positive isolates, cagPAI functionality was assessed by determination of CagA protein phosphorylation (Fig. 2) and levels of secreted IL-8 in the supernatant (Fig. 3), following incubation of H. pylori strains with AGS gastric epithelial cells. In this way, we identified 12 isolates with defective cagPAI within our cagA-positive population, 9 with the ABC and 3 with the ABCC combination of EPIYA (Table 1).
FIG. 2.
Phosphorylation and expression of CagA protein following infection of gastric epithelial cells with representative H. pylori clinical isolates. CagA tyrosine phosphorylation (A) was evaluated by immunoblotting (IB) with anti-CagA rabbit polyclonal antibody following immunoprecipitation (IP) of total cell lysates with an anti-mouse monoclonal pY20 phospho-tyrosine antibody. CagA expression (B) was determined by immunoblotting with anti-CagA rabbit polyclonal antibody on total cell lysates. Lane 1, cagPAI-negative isolate. Lanes 2 to 4 and 7 and 8, isolates with functional cagPAI and CagA protein with three (ABC) and four (ABCC) EPIYA motifs, respectively. Lanes 5, 6, and 9, cagA-positive isolates with defective cagPAI resulting in the absence of CagA protein phosphorylation. The immunoblot in panel C depicts expression of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) protein as a total protein loading control for panel B. Anti-CagA rabbit polyclonal antibody and anti-GAPDH mouse monoclonal antibody were utilized at 1:4,000 and 1:10,000 dilutions, respectively, in the presence of 5% nonfat dry milk. Protein separation by SDS-polyacrylamide gel electrophoresis was realized using 6% polyacrylamide gels.
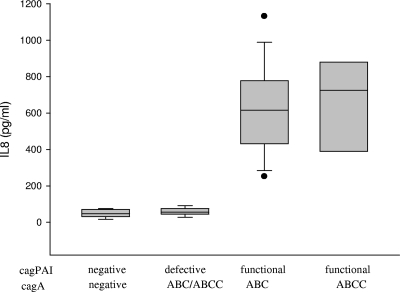
FIG. 3.
Levels of IL-8 secreted by gastric epithelial cells following 48-hour in vitro infection with H. pylori clinical isolates. H. pylori strains are classified as cagPAI negative, cagPAI defective, and cagPAI functional, with the CagA protein harboring EPIYA motifs in the ABC and ABCC combinations.
Classification of strains according to cagA EPIYA diversity and cagPAI status.
In view of the critical role of cagPAI-encoded proteins in successful translocation of CagA protein inside the gastric epithelial cell and the putative role of phosphorylated EPIYA C motifs in increased deregulation of SH2 domain-containing protein-tyrosine phosphatase-2 (SHP2 phosphatase) (24), we grouped our isolates according to the presence of phosphorylated CagA protein as well as the number of EPIYA C motifs. Consequently, cagA-negative strains, as well isolates for which we could not detect phosphorylated CagA, were grouped together (n = 48 isolates) (Tables 2, 3, and 4). In this group, a cagA-positive strain without EPIYA C motifs (AB) was also included. Forty-one isolates bearing three EPIYA motifs in an ABC combination and one isolate with the ABBC combination of EPIYA motifs, all with functional cagPAI, were classified in the group with one EPIYA C motif. Accordingly, 16 isolates with CagA bearing four EPIYA motifs (ABCC) as well as 1 isolate with the ACC combination and functional cagPAI were classified in the group with two EPIYA C motifs. No correlation was observed between EPIYA C diversity and gender of host (Pearson's χ2 = 0.787; P = 0.675). Finally, no correlation was observed between EPIYA C status and reason for referral (Pearson's χ2 = 2.773; P = 0.597), as the overwhelming majority of our patients were referred for recurrent abdominal pain.
TABLE 2.
CagA protein diversity with regard to EPIYA-C motifs and vacA signal, intermediate, and middle region isotypes
| VacA isotype | No. of isolates with indicated no. of EPIYA C repeatsa in CagA protein (%)
|
||
|---|---|---|---|
| Zero | One | Two | |
| s1 | |||
| i1/m1 | 3 (2.9) | 11 (10.5) | 6 (5.7) |
| i1/m2 | 4 (3.8) | 3 (2.9) | 2 (1.9) |
| i1-i2/m1 | 2 (1.9) | 7 (6.7) | 0 |
| i1-i2/m2 | 6 (5.7) | 7 (6.7) | 2 (1.9) |
| i2/m1 | 0 | 1 (1.0) | 0 |
| i2/m2 | 4 (3.8) | 9 (8.6) | 4 (3.8) |
| s2 | |||
| i1/m2 | 0 | 1 (1.0) | 1 (1.0) |
| i1-i2/m2 | 10 (9.5) | 2 (1.9) | 1 (1.0) |
| i2/m2 | 19 (18.1) | 0 | 0 |
The presence of the characteristic EPIYA C motif (EPIYATIDDLG) is indicated. “Zero” refers to the absence of tyrosine-phosphorylated CagA.
TABLE 3.
VacA and CagA diversity in relation to patient age
| Patient age | No. of isolates with indicated vacuolation levela (%)
|
No. of isolates with indicated no. of EPIYA C repeatsb in CagA protein (%)
|
||||
|---|---|---|---|---|---|---|
| None | Low | High | Zero | One | Two | |
| ≤10 yr | 19 (19.4) | 16 (16.3) | 5 (5.1) | 20 (20.4) | 15 (15.3) | 5 (5.1) |
| >10 yr | 17 (17.3) | 18 (18.4) | 23 (23.5) | 26 (26.5) | 20 (20.4) | 12 (12.2) |
VacA isotypes were classified into high-vacuolation (s1/i1), low-vacuolation (s1 or s2/i1-i2), and nonvacuolation (s1 or s2/i2) groups, irrespective of middle region status.
The presence of the characteristic EPIYA C motif (EPIYATIDDLG) is indicated. “Zero” refers to the absence of tyrosine-phosphorylated CagA.
TABLE 4.
CagA and VacA diversity among H. pylori isolates in relation to colonization levels and associated gastritis in the antrum
| VacA isotype and no. of EPIYA C repeatsa | No. of patientsb with:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
H. pylori colonization
|
Chronic gastritis activity
|
Chronic inflammatory infiltration
|
||||||||
| Normal | Mild | Moderate | Marked | Mild | Moderate | Marked | Mild | Moderate | Marked | |
| High vacuolation | ||||||||||
| 0 | 1 | 1 | 4 | 0 | 0 | 6 | 0 | 0 | 4 | 2 |
| 1 | 1 | 1 | 7 | 3 | 0 | 10 | 2 | 0 | 5 | 7 |
| 2 | 1 | 3 | 2 | 0 | 0 | 6 | 0 | 0 | 6 | 0 |
| Low vacuolation | ||||||||||
| 0 | 0 | 3 | 13 | 1 | 2 | 15 | 0 | 0 | 10 | 7 |
| 1 | 0 | 0 | 11 | 2 | 0 | 13 | 0 | 0 | 8 | 5 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No vacuolation | ||||||||||
| 0 | 2 | 2 | 16 | 3 | 4 | 17 | 2 | 3 | 12 | 8 |
| 1 | 1 | 0 | 8 | 0 | 2 | 6 | 1 | 1 | 1 | 7 |
| 2 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 3 |
VacA isotypes were classified into high-vacuolation (s1/i1), low-vacuolation (s1 or s2/i1-i2), and no-vacuolation (s1 or s2/i2) groups, irrespective of middle region status. For each isotype, the presence of the characteristic EPIYA C motif (EPIYATIDDLG) is indicated. “0” refers to the absence of tyrosine-phosphorylated CagA.
The severity levels (normal, mild, moderate, and marked) are defined in reference 18.
Determination of vacA diversity and correlation with cagA genotype.
We characterized all isolates according to the vacA signal, intermediate, and middle region isotypes (Table 2). In the absence of functional EPIYA C motifs (Table 2), the majority of isolates (41 out of 48) were found to possess presumed nonvacuolation (s2/i2/m2 [n = 19; 18.1%] and s1/i2/m2 [n = 4; 3.8%]) or low-vacuolation (s1/i1-i2/m1 or m2 [n = 8; 7.6%] and s2/i1-i2/m2 [n = 10; 9.5%]) isotypes, whereas only 7 (6.7%) isolates were of high-vacuolation (s1/i1/m1 or m2) isotypes. No isolates bearing the s2/m1 isotype, irrespective of intermediate region type, were observed. In the overwhelming majority of CagA-positive strains with functional cagPAI, the presence of EPIYA C motifs was positively correlated (Pearson chi-square test; P < 0.001) with high-vacuolation (s1/i1/m1 or m2 [n = 22; 21.0%]) or lower-vacuolation (s1/i2 or i1-i2/m1 or m2 [n = 30; 28.8%]) isotypes. Only five (4.9%) among the cagA-positive isolates were found to be of nonvacuolation (s2/i1 or i2 or i1-i2/m2) isotypes. A positive correlation between presence of high-vacuolation isotypes and increasing age was observed (Pearson chi-square test; P = 0.026) (Table 3). Interestingly, in 12 out of 17 cases where two EPIYA C motifs were present (including cases of mixed ABC/ABCC infection), the patients were over 10 years old (Table 3). Therefore, a positive correlation between higher number of EPIYA C motifs in the CagA protein and increasing age may also exist, but analyses of more cases are required for determination of this.
Association of EPIYA diversity in CagA protein and vacA isotypes with histopathology in the antrum.
To facilitate statistical analysis, isolates were classified according to (i) the numbers of EPIYA C motifs in the groups, namely none, one, or two, and (ii) the levels of vacuolating potential, namely, high for s1/i1/m1-or-m2 isotypes, low for s1-or-s2/i1-i2 isotypes, and none for s1-or-s2/i2 isotypes, irrespective of the middle region isotype. This classification was utilized for the assessment of possible associations with the corresponding histological manifestations in the 91 patients where infection by a single H. pylori strain was detected (Table 4). No correlation was observed between number of repeating EPIYA motifs and either chronic gastritis activity, chronic inflammatory infiltration, or levels of H. pylori colonization in the antrum in the children in our study (P = 0.571, P = 0.193, and P = 0.074, respectively) (Table 4). Also, no correlation with grade of eosinophil infiltration (P = 0.957) or presence of lymphoid follicle formation (P = 0.912) was observed (Table 5). Furthermore, with reference to the vacA genotype, we did not observe any correlation with chronic gastritis activity, chronic inflammatory infiltration, or levels of H. pylori colonization in the antrum (P = 0.054, P = 0.499, and P = 0.230, respectively) (Table 4) or with eosinophil infiltration or formation of lymphoid follicles (P = 0.341 and P = 0.283, respectively) (Table 5). Interestingly, the only significant correlation was the one between vacA genotype and presence of nodular gastritis (Pearson chi-square test; P = 0.034) (Table 6), an endoscopic observation quite common in cases of H. pylori-associated gastritis in children. All correlations between EPIYA C status and different vacA isotypes and clinical and histopathological manifestations in the antrum were found to be nonsignificant. Collectively, these results suggest that the number of repeating EPIYA C motifs in CagA protein, coupled with the distinct vacA isotypes, does not seem to correlate with severity of gastric inflammation in H. pylori-infected children.
TABLE 5.
CagA and VacA diversity among H. pylori isolates in relation to eosinophil infiltration and lymph node hyperplasia in the antrum
| VacA isotype and no. of EPIYA C repeatsa | No. of patients with:
|
||||
|---|---|---|---|---|---|
| Eosinophil infiltrationb
|
Lymph node hyperplasia
|
||||
| Mild | Moderate | Marked | No | Yes | |
| High vacuolation | |||||
| 0 | 2 | 2 | 2 | 5 | 1 |
| 1 | 6 | 5 | 1 | 8 | 4 |
| 2 | 2 | 2 | 2 | 6 | 0 |
| Low vacuolation | |||||
| 0 | 9 | 6 | 2 | 14 | 4 |
| 1 | 7 | 4 | 2 | 10 | 3 |
| 2 | 0 | 0 | 0 | 0 | 0 |
| No vacuolation | |||||
| 0 | 14 | 5 | 4 | 15 | 8 |
| 1 | 7 | 0 | 2 | 6 | 3 |
| 2 | 2 | 1 | 0 | 1 | 2 |
VacA isotypes were classified into high-vacuolation (s1/i1), low-vacuolation (s1 or s2/i1-i2), and nonvacuolation (s1 or s2/i2) groups, irrespective of middle region status. For each isotype, the presence of the characteristic EPIYA C motif (EPIYATIDDLG) is indicated. “0” refers to the absence of tyrosine-phosphorylated CagA.
The severity levels (mild, moderate, and marked) are defined in reference 18.
TABLE 6.
VacA isotypes and endoscopic observation of nodular gastritis
| Nodular gastritis status | No. (%) of isolates with indicated vacuolation levela
|
||
|---|---|---|---|
| High | Low | None | |
| Absent | 9 (9.9) | 3 (3.3) | 12 (13.2) |
| Present | 16 (17.6) | 28 (30.8) | 23 (25.3) |
VacA isotypes were classified into high-vacuolation (s1/i1), low-vacuolation (s1 or s2/i1-i2), and nonvacuolation (s1 or s2/i2) groups, irrespective of middle region status.
Induction of IL-8 levels and relation to virulence factor variability.
In order to evaluate whether potential differences in IL-8 level depended upon EPIYA diversity, we infected AGS cells in vitro over a 48-h period with H. pylori isolates (i) without cagPAI (n = 18), (ii) with cagPAI present but defective (n = 10), and (iii) with CagA positivity and functional cagPAI and EPIYA motifs in the ABC (n = 19) or ABCC (n = 7) combination and determined the levels of IL-8 protein secreted in the supernatant. No significant difference in IL-8 levels was observed between cagA-positive isolates with one and two EPIYA C motifs in the CagA protein (mean levels of 615.5 ± 51.6 pg/ml and 724.9 ± 89.7 pg/ml, respectively) (Fig. 3). As expected CagA-negative isolates lacking functional cagPAI induced only basal IL-8 levels (n = 12 isolates; 48.4 ± 7.7 pg/ml). Within the CagA-positive population tested, 62.8% (n = 22) of the isolates were positive for the presence of phosphorylated CagA protein and induction of IL-8 secretion. Finally, we sought to determine the potential difference in level of secreted IL-8 between the individual isogenic subclones expressing CagA protein with variable numbers of EPIYA C repeats isolated from the same patient. AGS cells were infected over a 48-h period with the individual H. pylori isogenic subclones expressing CagA with EPIYA repeats in the AB or ABC combination (GenBank accession numbers AM292594 and AM292593) and a second pair of subclones with the ABC or ABCC combination (GenBank accession numbers AM292577 and AM292578). No differences in level of secreted IL-8 were observed between the two isogenic subclones expressing CagA with EPIYA in the AB or ABC combination (1,331.5 ± 129.0 versus 1,319.0 ± 128.2 pg/ml) or between the two subclones with the ABC or ABCC combination (1,384.3 ± 101.6 pg/ml versus 1,242.7 ± 112.2 pg/ml).
Consequently, no correlation between level of secreted IL-8 and EPIYA combination was observed. Also, no correlation was observed between IL-8 level and chronic gastritis activity, chronic inflammatory infiltration, or level of H. pylori colonization in the antrum (data not shown).
DISCUSSION
In the present study, we have examined the potential correlation between H. pylori virulence factor diversity and endoscopic and histopathologic manifestations in symptomatic children. With relation to the CagA protein, EPIYA phosphorylation motif diversity has been suggested to be a major contributor to H. pylori pathogenesis in adults (12). From a mechanistic point of view, CagA has been suggested to interact in a phosphorylation-dependent manner with and perturb the normal activity of the tyrosine phosphatase SHP2 (26), the C-terminal Src kinase Crk (47), and the Crk adaptor proteins (45). Deregulation of SHP2 and its downstream effector focal adhesion kinase (48) was suggested to be mediated via the EPIYA C motif and, for the C-terminal Src kinase (Csk), via the EPIYA A or EPIYA B motif (29). In this respect, careful characterization of clinical isolates with reference to their ability to express functional CagA and determination of EPIYA diversity could provide useful predictive tools for H. pylori pathogenesis. Nearly 47% of cagA-positive isolates from the population of children analyzed in this study harbored three EPIYA motifs in the ABC combination, and only 17% harbored four EPIYA domains in the ABCC combination. Furthermore, among cagA-positive strains, about 19% (12 out of 63) were found to lack the capacity to express functional CagA protein and induce IL-8 secretion following infection of gastric epithelial cells. No significant variations in the individual peptide sequences surrounding the EPIYA motifs were observed in our sample from children and those already published for adult patients in Greece (34). However, although EPIYA phosphorylation motifs of the CagA protein in H. pylori strains isolated from Greek children do not present structural differences compared to those prevailing in adults, some subtle variations were noted. We observed a trend toward higher prevalence of cagA negativity in strains isolated from children than in those isolated from adults, an observation that has already been reported for populations with much higher prevalences of H. pylori infection (40). The higher prevalences of cagA- and cagPAI-negative H. pylori strains among children may reflect an evolutionary modification permitting successful colonization during transmission. Indeed, studies involving murine models (37) have shown that H. pylori strains in which cagPAI is absent or in which there is loss of functions required for activation of NF-κB exhibit a selective advantage for colonization of the murine host. Therefore, there may be a selective pressure for colonizing H. pylori strains to lose such features in vivo. Furthermore, we observed a positive correlation between number of EPIYA C motifs in CagA and age; in 12 out of 17 cases where two EPIYA C motifs were present (including mixed ABC/ABCC), the patients were over 10 years old. In contrast to what was found for the H. pylori strains isolated from adults (34), we did not detect any CagA-positive isolates harboring more than two EPIYA C repeats in children, and this may be related to reduced rates of survival of such strains in acidic conditions (49). As far as the contribution of CagA to H. pylori pathogenesis is concerned, this fact may offer a good explanation for the reduced severity levels of clinical and pathological manifestations associated with H. pylori infection in children compared to the levels observed in adults. Interestingly, we detected simultaneously present microevolving strains in the antrum with identical RAPD and MLST profiles, expressing CagA proteins with variable numbers of EPIYA motifs, including one pair with AB/ABC combinations and six pairs with ABC/ABCC combinations of EPIYA motifs in CagA. This may suggest that children when first acquiring the infection may be colonized by multiple H. pylori variants which, over time, through selection influenced by host genetics and bacterial factors, result in various H. pylori genotypes that predominate in adulthood. Similar results suggesting that, after infection in childhood, individual strains will undergo evolutionary changes during the course of infection have been reported before (33).
With relation to vacA status, we have observed an association between cagA-negative genotype and nonvacuolation (s2/i1 or i2/m2) vacA isotypes. Also, cagA-positive strains with one or two EPIYA C repeats were mostly associated with high-vacuolation (s1/i1/m1) or lower-vacuolation (s1 or s2/i1-i2) isotypes, suggesting that cagA and vacA genotypes may not be regarded as independent variables in H. pylori pathogenesis. Indeed, there is increasing evidence that a functional association between vacA and cagA virulence factors may exist (4). In line with this observation, isolates from our pediatric population with high vacuolation potential and higher numbers of EPIYA C motifs in the CagA protein were more prevalent among children over 10 years old.
Induction of IL-8 secretion following H. pylori infection of gastric epithelial cells is largely regarded to be a cagPAI-dependent phenomenon (43). However, there is mounting evidence that translocated CagA protein may also contribute to IL-8 induction through a Ras/Raf/Mek/extracellular signal-regulated kinase/NF-κB signaling pathway (15). We determined levels of secreted IL-8 in the supernatant of gastric epithelial cells, infected in vitro with a variety of strains, including cagA-negative as well as cagA-positive H. pylori isolates bearing one or two EPIYA C repeats in the CagA protein, with or without functional cagPAI. In this context, apart from IL-8 secretion, cag pathogenicity island functionality was further established by simultaneously screening for the presence of phosphorylated CagA protein in protein lysates from H. pylori-infected AGS cells. We observed no correlation between number of repeating EPIYA C motifs and level of proinflammatory IL-8 secreted by gastric epithelial cells. IL-8 levels were markedly different between patients, irrespective of the number of EPIYA motifs in CagA, an observation also reported by others (30, 38). Furthermore, to account for unknown variability between the different clinical strains, we utilized pairs of isogenic strains with variable numbers of EPIYA C, one of which totally lacked EPIYA C motifs. We observed no differences in level of secreted IL-8 between those isogenes with zero, one, or two EPIYA C repeats, suggesting that the contribution of the number of EPIYA C repeats in the CagA protein to IL-8 secretion may be marginal.
Although our analysis was considerably thorough, in order to characterize strains with reference to the functional status of CagA and VacA virulence factors and not just the genotypic status, no correlation between virulence factor diversity and clinical symptoms was observed, as the overwhelming majority of our patients were referred for endoscopy with symptoms of recurrent abdominal pain. Also, no correlation between virulence factor diversity and developing histopathology in the antrum and the fundus in children was observed. Similar conclusions with reference to the presence of CagA and the signal and middle region genotypic variation of VacA have been reported before in a number of studies involving H. pylori-infected children (9, 21, 33), but never associating functional characteristics which contribute to the pathogenic role of CagA, cagPAI functionality, or the more recently reported intermediate region VacA isotypes. Interestingly, the only positive, yet weak, correlation was the one observed between vacuolating vacA genotypes and presence of nodular gastritis, an endoscopic observation quite common in cases of H. pylori-associated gastritis in children. Such positive correlation was not observed in earlier studies involving H. pylori bacterial genotypes and endoscopic observations in children and may warrant further verification. H. pylori prevalence in biopsied symptomatic children in Greece is estimated around 14% on the basis of detection by culture or histology (E. Roma-Giannikou, unpublished data), which is in line with what is observed in other northern and western European countries (46). The lack of correlation between H. pylori virulence factor diversity and severity of histopathology in symptomatic children may correlate with differences in the course of infection, possibly with correlations becoming stronger as the duration of infection increases and potential increments in the number of repeating EPIYA motifs in CagA occur throughout adulthood. This is further supported by our observations that potentially more virulent forms of H. pylori were found to be present in older children and that CagA species with three or even four EPIYA C repeats are exclusively observed in strains isolated from adults and not from children.
Supplementary Material
Acknowledgments
This study was supported by an Internal Grant of The Hellenic Pasteur Institute (HPI-922616) and in part by an Actions Concertées Inter Pasteuriennes (ACIP) program entitled Etude de la fréquence de l'infection par Helicobacter pylori (Hp) des patients présentant une pathologie gastro-duodénale, de la sensibilité des souches aux antibiotiques et de la diversité de la région 3′ du gène cagA.
There are no potential conflicts of interest.
Footnotes
Published ahead of print on 17 June 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 2837-53. [DOI] [PubMed] [Google Scholar]
- 2.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 205137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amieva, M. R., and E. M. El-Omar. 2008. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 134306-323. [DOI] [PubMed] [Google Scholar]
- 4.Argent, R. H., R. J. Thomas, D. P. Letley, M. G. Rittig, K. R. Hardie, and J. C. Atherton. 2008. Functional association between the Helicobacter pylori virulence factors VacA and CagA. J. Med. Microbiol. 57145-150. [DOI] [PubMed] [Google Scholar]
- 5.Asahi, M., T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 27017771-17777. [DOI] [PubMed] [Google Scholar]
- 7.Atherton, J. C., T. L. Cover, R. J. Twells, M. R. Morales, C. J. Hawkey, and M. J. Blaser. 1999. Simple and accurate PCR-based system for typing vacuolating cytotoxin alleles of Helicobacter pylori. J. Clin. Microbiol. 372979-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atherton, J. C., R. M. Peek, Jr., K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 11292-99. [DOI] [PubMed] [Google Scholar]
- 9.Azuma, T., S. Kato, W. Zhou, S. Yamazaki, A. Yamakawa, M. Ohtani, S. Fujiwara, T. Minoura, K. Iinuma, and T. Kato. 2004. Diversity of vacA and cagA genes of Helicobacter pylori in Japanese children. Aliment. Pharmacol. Ther. 20(Suppl. 1)7-12. [DOI] [PubMed] [Google Scholar]
- 10.Azuma, T., A. Yamakawa, S. Yamazaki, K. Fukuta, M. Ohtani, Y. Ito, M. Dojo, Y. Yamazaki, and M. Kuriyama. 2002. Correlation between variation of the 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J. Infect. Dis. 1861621-1630. [DOI] [PubMed] [Google Scholar]
- 11.Backert, S., S. Moese, M. Selbach, V. Brinkmann, and T. F. Meyer. 2001. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol. Microbiol. 42631-644. [DOI] [PubMed] [Google Scholar]
- 12.Basso, D., C. F. Zambon, D. P. Letley, A. Stranges, A. Marchet, J. L. Rhead, S. Schiavon, G. Guariso, M. Ceroti, D. Nitti, M. Rugge, M. Plebani, and J. C. Atherton. 2008. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 13591-99. [DOI] [PubMed] [Google Scholar]
- 13.Blank, C., M. Sabri, and C. Di Lorenzo. 2004. Pediatric gastric and duodenal disorders. Curr. Opin. Gastroenterol. 20551-555. [DOI] [PubMed] [Google Scholar]
- 14.Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 552111-2115. [PubMed] [Google Scholar]
- 15.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 1029300-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cover, T. L., and S. R. Blanke. 2005. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Microbiol. 3320-332. [DOI] [PubMed] [Google Scholar]
- 17.Czinn, S. J. 2005. Helicobacter pylori infection: detection, investigation, and management. J. Pediatr. 146S21-S26. [DOI] [PubMed] [Google Scholar]
- 18.Dixon, M. F., R. M. Genta, J. H. Yardley, P. Correa, et al. 1996. Classification and grading of gastritis: the updated Sydney System. Am. J. Surg. Pathol. 201161-1181. [DOI] [PubMed] [Google Scholar]
- 19.Falush, D., T. Wirth, B. Linz, J. K. Pritchard, M. Stephens, M. Kidd, M. J. Blaser, D. Y. Graham, S. Vacher, G. I. Perez-Perez, Y. Yamaoka, F. Megraud, K. Otto, U. Reichard, E. Katzowitsch, X. Wang, M. Achtman, and S. Suerbaum. 2003. Traces of human migrations in Helicobacter pylori populations. Science 2991582-1585. [DOI] [PubMed] [Google Scholar]
- 20.Figura, N., C. Vindigni, A. Covacci, L. Presenti, D. Burroni, R. Vernillo, T. Banducci, F. Roviello, D. Marrelli, M. Biscontri, S. Kristodhullu, C. Gennari, and D. Vaira. 1998. cagA positive and negative Helicobacter pylori strains are simultaneously present in the stomach of most patients with non-ulcer dyspepsia: relevance to histological damage. Gut 42772-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold, B. D., L. J. van Doorn, J. Guarner, M. Owens, D. Pierce-Smith, Q. Song, L. Hutwagner, P. M. Sherman, O. L. de Mola, and S. J. Czinn. 2001. Genotypic, clinical, and demographic characteristics of children infected with Helicobacter pylori. J. Clin. Microbiol. 391348-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatakeyama, M. 2004. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4688-694. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama, M. 2008. SagA of CagA in Helicobacter pylori pathogenesis. Curr. Opin. Microbiol. 1130-37. [DOI] [PubMed] [Google Scholar]
- 24.Higashi, H., A. Nakaya, R. Tsutsumi, K. Yokoyama, Y. Fujii, S. Ishikawa, M. Higuchi, A. Takahashi, Y. Kurashima, Y. Teishikata, S. Tanaka, T. Azuma, and M. Hatakeyama. 2004. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J. Biol. Chem. 27917205-17216. [DOI] [PubMed] [Google Scholar]
- 25.Higashi, H., R. Tsutsumi, A. Fujita, S. Yamazaki, M. Asaka, T. Azuma, and M. Hatakeyama. 2002. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc. Natl. Acad. Sci. USA 9914428-14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295683-686. [DOI] [PubMed] [Google Scholar]
- 27.Horvitz, G., and B. D. Gold. 2006. Gastroduodenal diseases of childhood. Curr. Opin. Gastroenterol. 22632-640. [DOI] [PubMed] [Google Scholar]
- 28.Lochhead, P., and E. M. El-Omar. 2008. Gastric cancer. Br. Med. Bull. 8587-100. [DOI] [PubMed] [Google Scholar]
- 29.Naito, M., T. Yamazaki, R. Tsutsumi, H. Higashi, K. Onoe, S. Yamazaki, T. Azuma, and M. Hatakeyama. 2006. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology 1301181-1190. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson, C., A. Sillen, L. Eriksson, M. L. Strand, H. Enroth, S. Normark, P. Falk, and L. Engstrand. 2003. Correlation between cag pathogenicity island composition and Helicobacter pylori-associated gastroduodenal disease. Infect. Immun. 716573-6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nomura, A. M., G. I. Perez-Perez, J. Lee, G. Stemmermann, and M. J. Blaser. 2002. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. Am. J. Epidemiol. 1551054-1059. [DOI] [PubMed] [Google Scholar]
- 32.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2871497-1500. [DOI] [PubMed] [Google Scholar]
- 33.Oleastro, M., M. Gerhard, A. I. Lopes, P. Ramalho, J. Cabral, A. Sousa Guerreiro, and L. Monteiro. 2003. Helicobacter pylori virulence genotypes in Portuguese children and adults with gastroduodenal pathology. Eur. J. Clin. Microbiol. Infect. Dis. 2285-91. [DOI] [PubMed] [Google Scholar]
- 34.Panayotopoulou, E. G., D. N. Sgouras, K. Papadakos, A. Kalliaropoulos, G. Papatheodoridis, A. F. Mentis, and A. J. Archimandritis. 2007. Strategy to characterize the number and type of repeating EPIYA phosphorylation motifs in the carboxyl terminus of CagA protein in Helicobacter pylori clinical isolates. J. Clin. Microbiol. 45488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, X. Zhao, J. C. Atherton, and M. J. Blaser. 1995. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab. Investig. 73760-770. [PubMed] [Google Scholar]
- 36.Peek, R. M., Jr., S. F. Moss, K. T. Tham, G. I. Perez-Perez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt, and M. J. Blaser. 1997. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 89863-868. [DOI] [PubMed] [Google Scholar]
- 37.Philpott, D. J., D. Belaid, P. Troubadour, J. M. Thiberge, J. Tankovic, A. Labigne, and R. L. Ferrero. 2002. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylory isolates. Cell. Microbiol. 4285-296. [DOI] [PubMed] [Google Scholar]
- 38.Reyes-Leon, A., J. C. Atherton, R. H. Argent, J. L. Puente, and J. Torres. 2007. Heterogeneity in the activity of Mexican Helicobacter pylori strains in gastric epithelial cells and its association with diversity in the cagA gene. Infect. Immun. 753445-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhead, J. L., D. P. Letley, M. Mohammadi, N. Hussein, M. A. Mohagheghi, M. Eshagh Hosseini, and J. C. Atherton. 2007. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133926-936. [DOI] [PubMed] [Google Scholar]
- 40.Rocha, G. A., A. M. Oliveira, D. M. Queiroz, A. S. Carvalho, and A. M. Nogueira. 2000. Immunoblot analysis of humoral immune response to Helicobacter pylori in children with and without duodenal ulcer. J. Clin. Microbiol. 381777-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 9614559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selbach, M., S. Moese, C. R. Hauck, T. F. Meyer, and S. Backert. 2002. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 2776775-6778. [DOI] [PubMed] [Google Scholar]
- 43.Sharma, S. A., M. K. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 631681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43971-980. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, M., H. Mimuro, T. Suzuki, M. Park, T. Yamamoto, and C. Sasakawa. 2005. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 2021235-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres, J., G. Perez-Perez, K. J. Goodman, J. C. Atherton, B. D. Gold, P. R. Harris, A. M. la Garza, J. Guarner, and O. Munoz. 2000. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch. Med. Res. 31431-469. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 2783664-3670. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsumi, R., A. Takahashi, T. Azuma, H. Higashi, and M. Hatakeyama. 2006. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 26261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaoka, Y., H. M. El-Zimaity, O. Gutierrez, N. Figura, J. G. Kim, T. Kodama, K. Kashima, and D. Y. Graham. 1999. Relationship between the cagA 3′ repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology 117342-349. [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki, S., A. Yamakawa, T. Okuda, M. Ohtani, H. Suto, Y. Ito, Y. Yamazaki, Y. Keida, H. Higashi, M. Hatakeyama, and T. Azuma. 2005. Distinct diversity of vacA, cagA, and cagE genes of Helicobacter pylori associated with peptic ulcer in Japan. J. Clin. Microbiol. 433906-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.