Abstract
An understanding of the mechanisms that govern pancreatic endocrine cell ontogeny may offer strategies for their somatic replacement in diabetic patients. During embryogenesis, transcription factor FoxO1 is expressed in pancreatic progenitor cells. Subsequently, it becomes restricted to β cells and to a rare population of insulin-negative juxtaductal cells (FoxO1+ Ins−). It is unclear whether FoxO1+ Ins− cells give rise to endocrine cells. To address this question, we first evaluated FoxO1's role in pancreas development using gain- and loss-of-function alleles in mice. Premature FoxO1 activation in pancreatic progenitors promoted α-cell formation but curtailed exocrine development. Conversely, FoxO1 ablation in pancreatic progenitor cells, but not in committed endocrine progenitors or terminally differentiated β cells, selectively increased juxtaductal β cells. As these data indicate an involvement of FoxO1 in pancreatic lineage determination, FoxO1+ Ins− cells were clonally isolated and assayed for their capacity to undergo endocrine differentiation. Upon FoxO1 activation, FoxO1+ Ins− cultures converted into glucagon-producing cells. We conclude that FoxO1+ Ins− juxtaductal cells represent a hitherto-unrecognized pancreatic cell population with in vitro capability of endocrine differentiation.
Diabetes is characterized by complete or relative deficiency of insulin-producing β cells (1). The growing societal and public health toll of the disease provides impetus to isolate or generate β cells for cellular replacement purposes. Moreover, given that most of the newly found diabetes susceptibility genes appear to affect β-cell function rather than insulin action (12, 41, 43), that the two newest classes of antidiabetic medications are β-tropic (2), and that the main therapeutic failures in diabetes are seen in response to β-tropic agents (19, 48), studies of β-cell biology have wide-ranging implications beyond the replacement issue.
Two approaches to β-cell generation have been championed: one endeavors to define culture conditions conducive to embryonic stem cell differentiation into β cells (6), while the other is based on the hypothesis that endocrine cell progenitors, often identified with duct epithelial cells, exist in the adult pancreas and can yield functional β cells (4, 42).
Lineage-tracing studies indicate that pancreatic endocrine cells arise from a neurogenin 3 (Neurog3)-expressing progenitor pool set aside early in embryonic development (13) and that postnatal β-cell turnover is a result of limited β-cell replication and apoptosis (8, 34, 46). These data point to a limited role of pancreatic duct cells in the maintenance of β-cell mass through neogenesis from non-β-cell precursors. Nonetheless, ductal endocrine cell neogenesis can occur following pancreatic duct ligation (17, 49), raising the possibility of generating endocrine cells by commandeering developmental pathways at the genetic level. Along these lines, we made the intriguing observation of a rare population of juxtaductal FoxO1+ cells that do not express insulin. This finding, coupled with the role of FoxOs in governing developmental processes in diverse lineages and in the long-term stability of various tissues (24, 32, 35), prompted us to examine whether these cells are progenitors of duct-associated endocrine cells. In this study, we used a combination of developmental, genetic, and cell biology analyses to identify, isolate, and functionally characterize these cells.
MATERIALS AND METHODS
Antibodies and immunohistochemistry.
We used the following antibodies: antipancytokeratin (Sigma), antivimentin (Santa Cruz), anti-Nkx2.2 (homeodomain protein) (Hybridoma Bank, University of Iowa), anti-FoxO1 (21), anti-pancreas and duodenum homeobox protein 1 (anti-Pdx1) (25), antiglucagon (Sigma), anti-insulin (Dako), antisomatostatin (Chemicon), anti-pancreatic polypeptide (anti-Pp) (Linco), antiamylase (Abcam), anti-green fluorescent protein (anti-GFP) (Santa Cruz), and anti-forkhead box-containing protein A2 (anti-Foxa2) (39). We used fluorescence-conjugated dolichos biflorus agglutinin (DBA) (0.05 mg/ml; Vector Laboratories) for duct cell staining (27). We performed immunostaining using 5-μm-thick paraffin sections and, in some experiments, antigen retrieval, as described previously (25). We visualized immune complexes with fluorescein isothiocyanate- or Cy3-conjugated secondary antibodies. Microscopy was carried out with an Olympus IX-70 inverted fluorescence microscope (Olympus America, Melville, NY) fitted with 10×, 40×, and 100× lenses, and images were captured using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI) and processed using Photoshop 7.0 software. To quantitate insulin-positive juxtaductal cells, we scored ducts with insulin-positive cells as a percentage of the total number of main, interlobular, and intralobular ducts on each section. We scored on average 10 ducts on each of six sections for each mouse and six mice for each genotype. On average, we scored 1,150 duct-associated cells per section, totaling ∼41,400 cells per genotype. One-month-old mice were used for these experiments. We determined the Ki67-labeling index of islet β cells and juxtaductal insulin-positive cells by dividing the number of Ki67/insulin-positive cells by the total number of islet β cells or juxtaductal insulin-positive cells in at least four sections in six Pdx1(cre):FoxO1−/− (the colon indicates the presence of multiple loci) mice and six FoxO1lox/lox controls (31). Ten islets and ten ducts were scored on each section. Two-month-old mice were used for these experiments.
Cells.
βTC3, αTC3 (9), UB (embryonic ureteric bud) (3), M-1 (simian virus 40 [SV40]-transformed kidney cortical collecting duct) (45), TGP-47 (pancreatic acinar carcinoma) (36), and SV40-transformed hepatocytes have been described previously (38).
Animal generation and analysis.
Pdx1(cre) (13), Neurog3(cre) (40), Ins(cre) (15), FoxO1flox (35), and FoxO1+/− (carrying the β-galactosidase [β-gal] knock-in) mice have been described previously (25). Pdx-FoxO1ADA transgenic mice were generated by microinjection into fertilized zygotes of a construct encoding FLAG-tagged FoxO1ADA cDNA (33) driven by the 4.5-kb Pdx1 promoter with a β-globin intron and poly(A) signal (44). Two founder lines were characterized and used for the studies described here. PCR genotyping was carried out with primers GCTTAGAGCAGAGATGTTCTCACATT, CCAGAGTCTTTGTATCAGGCAAATAA, and CAAGTCCATTAATTCAGCACATTGA. We used standard mRNA isolation and real-time reverse transcription-PCR (RT-PCR) techniques. Mice were analyzed at different embryonic and postnatal stages, as indicated in the figure legends.
Primary culture and cloning of pancreatic cells.
We dissected pancreases from 2-month-old mice bearing a β-gal gene targeted to the FoxO1 locus by homologous recombination and fused in frame with the FoxO1-initiating methionine (16). The reporter is active only in cells expressing endogenous FoxO1. Thus, only islet β cells and FoxO1+ Ins− cells should be β-gal positive. We digested the isolated tissue in 1 ml of M199 medium containing 1 mg/ml collagenase P (Roche) and diluted cellular aggregates in 30 ml of the same medium (22). After filtration through a 408-μm Spectra mesh (Spectrum Laboratories), we resuspended cell aggregates in RPMI supplemented with 10% fetal calf serum, 5.5 mM glucose, 100 mg/ml penicillin, 100 mg/ml streptomycin, and 250 ng/ml amphotericin B and cultured them at 37°C in 5% CO2. After 7 days, we replaced the medium with serum-free RPMI supplemented with 8 mM glucose, 1 g/liter ITS (5 mg/liter insulin, 5 mg/liter transferrin, and 5 mg/liter selenium), 2 g/liter bovine serum albumin, 10 mM nicotinamide, and 10 ng/ml keratinocyte growth factor (all from Sigma) and continued selection for 3 weeks until small clusters of cobblestone-shaped cells began to appear. We replaced the serum-free medium every third day during the selection process. The resulting cells were returned to complete medium, expanded, and cloned by limiting dilution in 96-well plates.
Adenovirus and siRNA transfection.
We described GFP, FoxO1ADA adenovirus, and small interfering RNA (siRNA) previously (24). Following transduction of confluent cells, we cultured them in serum-free medium for 48 h prior to isolation of RNA or fixation for immunohistochemistry. RT-PCR was carried out for 35 cycles in all experiments except in the experiments depicted in Fig. 10D for Pdx1, in which we employed 25 cycles.
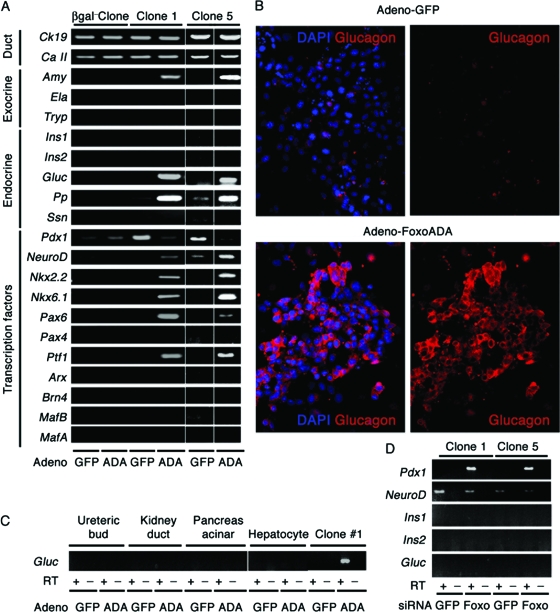
FIG. 10.
Induction of glucagon expression by FoxO1 gain of function in clonal duct cultures. (A) mRNA expression analysis of a β-gal-negative clone and two representative β-gal-positive (FoxO1+ Ins−) clones (1 and 5) transduced with adenovirus expressing constitutively active FoxO1ADA or GFP. Ck19, cytokeratin 19; Ca II, carbonic anhydrase II; Amy, amylase; Ela, elastase; Try, trypsin; Ins1, insulin 1; Ins2, insulin 2; Gluc, glucagon; Ssn, somatostatin. (B) Glucagon immunocytochemistry (red) in cells transduced with adenovirus encoding FoxO1ADA or GFP. (C) We transduced clone 1 and control cells, including embryonic UB cells, M-1 cells, TGP47 cells, and SV40-transformed hepatocytes, with FoxO1ADA adenovirus. After isolating mRNA, we performed semiquantitative RT-PCR with primers for glucagon. (D) Expression of Pdx1, NeuroD, Ins1, Ins2, and Gluc in clones 1 and 5 following transfection of FoxO1 or control siRNA. The RT-PCRs for Pdx1 and NeuroD were carried out for 25 cycles, and Pdx1 was undetectable in control samples under these conditions.
Statistical analyses.
Data are presented as means ± standard errors of the means. Statistical analysis was performed using a two-tailed, unpaired t test.
RESULTS
Juxtaductal FoxO1+ Ins− cells in adult mouse pancreas.
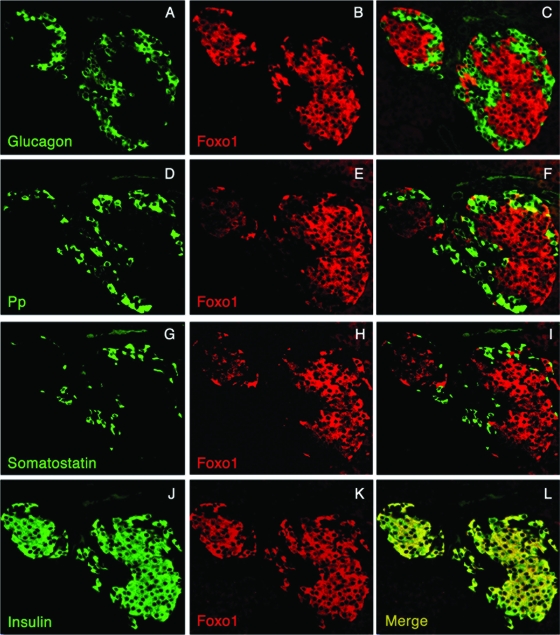
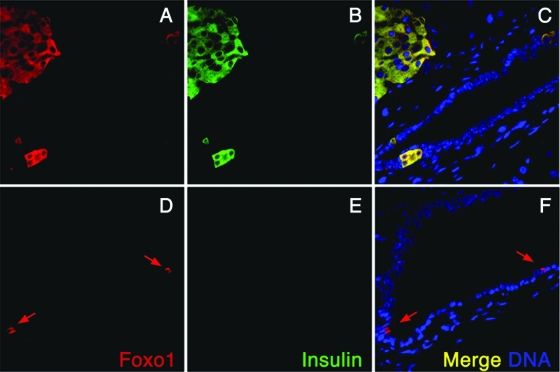
In line with our previous observations (25), FoxO1 expression is restricted to endocrine β cells of the adult pancreas (Fig. 1), including those β cells abutting on ducts (Fig. 2A to C). It is seldom seen in Pp cells (Fig. 1). In addition, consistent with lineage-tracing data (25), there are occasional cells within ducts that express FoxO1 but not insulin (Fig. 2D to F). The cells occur at a frequency of ∼1 in 104 duct cells, and thus represent ∼1 × 10−5 of pancreas cells. We hypothesized that FoxO1 is a marker of a rare subpopulation of adult pancreatic cells with endocrinogenic potential and that FoxO1 is involved in the regulation of pancreatic cell fate specification.
FIG. 1.
FoxO1 localization in adult mouse pancreatic islets. Pancreatic sections from 2-month-old mice were analyzed by immunohistochemistry with antibodies against FoxO1 (B, C, E, F, H, I, K, and L))red), glucagon (A and C) (green), Pp (D and F) (green), somatostatin (G and I) (green), or insulin (J [green] and L [yellow in the merged picture]) and photographed at a magnification of ×40.
FIG. 2.
FoxO1+ cells in pancreatic ducts. Pancreatic immunohistochemistry was performed with anti-FoxO1 (A and D; red) and anti-insulin (B and E; green) antibodies in 3-month-old male WT mice of mixed C57BL × 129sv background. (A to C) All insulin-positive juxtaductal cells coexpress FoxO1 (yellow in the merged channels). (D to F) FoxO1+ Ins− cells (red) are located within ducts (D and F). Arrows indicate occasional cells within ducts that express FoxO1 but not insulin.
Developmental analysis of FoxO1 expression in the pancreas.
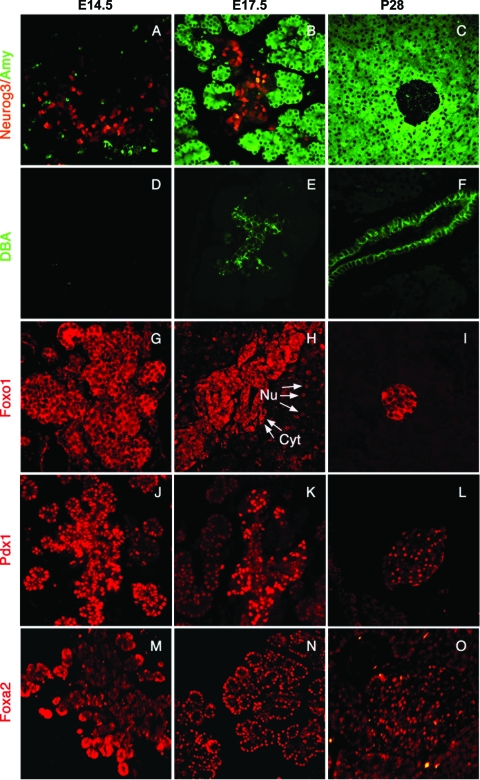
As a first step in assessing FoxO1 in the pancreas developmental program, we examined FoxO1 expression during pancreatogenesis in the mouse (Fig. 3). To define various lineages, we employed immunohistochemistry using well-characterized reagents, including antiamylase antibodies to identify exocrine acinar cells (amylase positive) (Fig. 3A to C, green), anti-GFP antibodies (in Neurog3-GFP transgenic mice) (30) to identify Neurog3-positive endocrine progenitors (Fig. 3A to C, red), and DBA to identify ductal cells (Fig. 3D to F, green). Comparison of expression patterns at embryonic day 14.5 (E14.5), E17.5, and postnatal day 28 (P28) revealed the expected progressive restriction of the different cell type markers. Similarly, FoxO1 was widely expressed at E14.5 (Fig. 3G, red) but became restricted to subset of cells at E17.5 (Fig. 3H) and was confined to β cells postnatally (Fig. 3I). This pattern of expression closely parallels Pdx1 expression, with the notable difference that FoxO1 is cytoplasmic and Pdx1 nuclear (Fig. 3J to L, red). At E17.5, FoxO1 appeared to be nuclear in a subset of cells (Fig. 3H). In contrast, the related forkhead protein Foxa2 (28) was enriched in the tip region of the developing pancreas at E14.5 (Fig. 3M), and remained subsequently expressed in both endocrine and (to a lesser extent) exocrine compartments (Fig. 3N and O).
FIG. 3.
Developmental analysis of FoxO1 expression in embryonic pancreas. Pancreatic sections from Neurog3-GFP transgenic (A to C) and WT (D to O) mice at E14.5, E17.5, and P28 were analyzed by double immunohistochemistry with antibodies against GFP (red) and amylase (green) (A to C), by histochemistry with DBA (D to F) (green), or by immunohistochemistry with antibodies against FoxO1 (G to I) (red), Pdx1 (J to L) (red), and Foxa2 (M to O) (red) and visualized by fluorescence microscopy (magnification, ×100). Arrows indicate representative cells with nuclear (Nu) and cytoplasmic (Cyt) FoxO1. Data are representative of at least six mice for each group and 3 to 10 sections for each mouse.
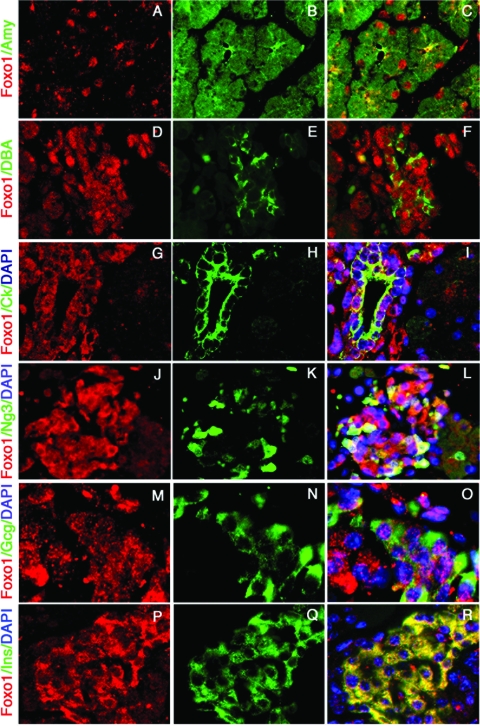
The apparent changes in the distribution and subcellular localization of FoxO1 at E17.5 prompted us to investigate more closely its colocalization with markers of different pancreatic lineages at this stage. In amylase-positive cells (exocrine lineage), FoxO1 was exclusively nuclear (Fig. 4A to C). In a subset of DBA-positive ductal cells, FoxO1 localized to both nucleus and cytoplasm in a punctate pattern that likely reflects targeting to nuclear promyelocytic leukemia-associated protein bodies (26) as well as lysosomal compartments (37) (Fig. 4D to F). We obtained identical results using cytokeratin as a surrogate ductal marker (Fig. 4G-I). We observed a heterogeneous subcellular distribution also in Neurog3-positive endocrine progenitors (Fig. 4J to L). Within the endocrine compartment, FoxO1 showed a punctate nuclear pattern in α cells (Fig. 4M to O) and its signature cytoplasmic pattern in β cells (Fig. 4P to R). The preliminary conclusion is that FoxO1 becomes developmentally restricted to endocrine cells.
FIG. 4.
Subcellular localization of FoxO1 in different pancreatic compartments at E17.5. Immunostaining with anti-FoxO1 (red in panels A, C, D, F, G, I, J, L, M, O, P, and R) and antiamylase (Amy) (B and C), DBA (E and F), cytokeratin (Ck) (H and I), GFP (Neurog3 [Ng3]) (K and L), glucagon (Gcg) (N and O), or insulin (Ins) (Q and R) (all in green) is shown (magnification, × 100). Nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole) in panels I, L, O, and R (blue). Data are representative of six mice analyzed and three sections for each mouse.
Premature activation of FoxO1 impairs exocrine pancreas development.
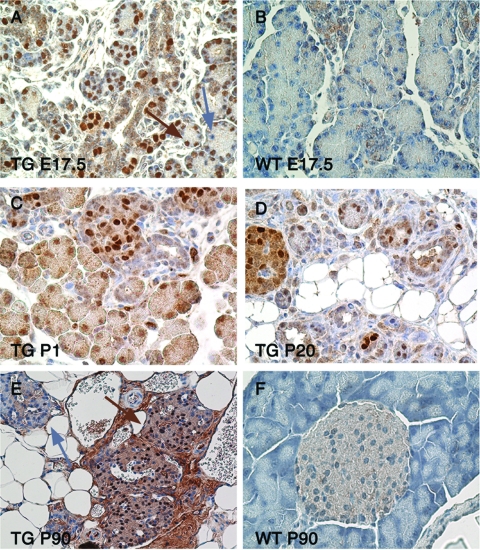
To determine whether FoxO1 nuclear translocation affects pancreatic lineage specification, we generated transgenic mice expressing a FLAG-tagged, constitutively nuclear FoxO1 mutant (FoxO1ADA) (33) from the Pdx1 promoter (30). The expectation was that premature nuclear expression of FoxO1 would affect cell type specification. We observed transgene expression in >90% of pancreatic cells in embryos (Fig. 5A and B) and at birth (Fig. 5C). Postnatally, transgene expression was higher in islets than in exocrine cells (Fig. 5D), consistent with prior reports in which the same Pdx1 promoter had been used (10, 14). In adult mice, we observed an admixture of transgene-positive and -negative cells (Fig. 5E and F), which reflects either loss of expression from the Pdx1 promoter in non-β cells or compensatory growth of transgene-negative cells.
FIG. 5.
Expression of Pdx-FoxO1ADA in transgenic (TG) mice. (A and B) Immunohistochemistry with anti-FLAG antiserum in E17.5 transgenic (A) and control (B) mice. The brown arrow denotes a FLAG-positive cell, and the blue arrow indicates a FLAG-negative cell (n = 12 each). (C to E) Transgene expression at P1 (C), P20 (D), and P90 (E) in transgenic mice. At P20, FLAG expression is higher in endocrine islets than in the exocrine compartment. At P90, most islets consist of a mixture of FLAG-positive and FLAG-negative cells. Occasional islets are entirely FLAG negative (blue arrow). (F) Control WT mice at P90.
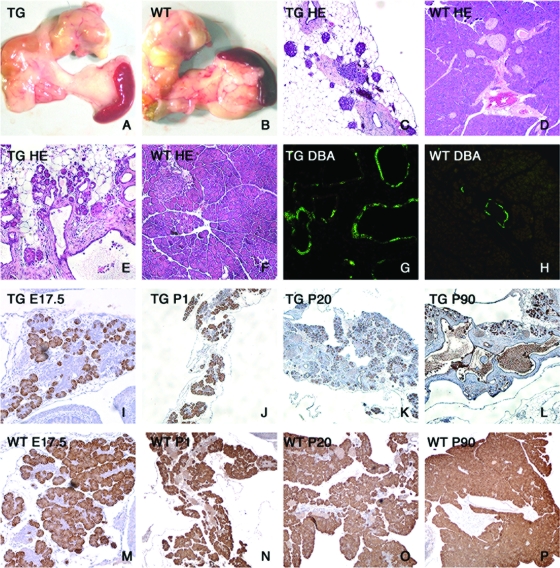
The phenotype of these mice was characterized by marked pancreatic hypoplasia (Fig. 6A and B) and extensive disruption of pancreatic architecture. Only remnants of exocrine tissue could be seen in adult mice (Fig. 6C and D), and extensive ductal hyperplasia occurred, as demonstrated by hematoxylin and eosin staining (Fig. 6E and F), DBA histochemistry (Fig. 6G and H), and histomorphometry (data available upon request). Time course analysis indicated that exocrine hypoplasia was already present in E17.5 embryos and persisted through adulthood (Fig. 6I to L), in marked contrast to the case for wild-type (WT) controls (Fig. 6 M to P).
FIG. 6.
Histomorphometric analysis of transgenic (TG) pancreases. (A and B) Photographs of the gastroduodenal tract in 2-month-old transgenic (A) and control (B) mice. (C to F) Hematoxylin and eosin (HE) staining of pancreatic sections from 2-month-old transgenic (C and E) and control (D and F) mice at a magnification of ×40 (C and D) or ×100 (E and F). (G and H) DBA immunohistochemistry in 3-month-old transgenic (G) and control (H) mice. (I to P) Amylase immunohistochemistry in transgenic (I to L) and control (M to P) mice at E17.5 (I and M), P1 (J and N), P20 (K and O), and P90 (L and P) (magnification, ×40). At each time point, at least six mice of each genotype were analyzed.
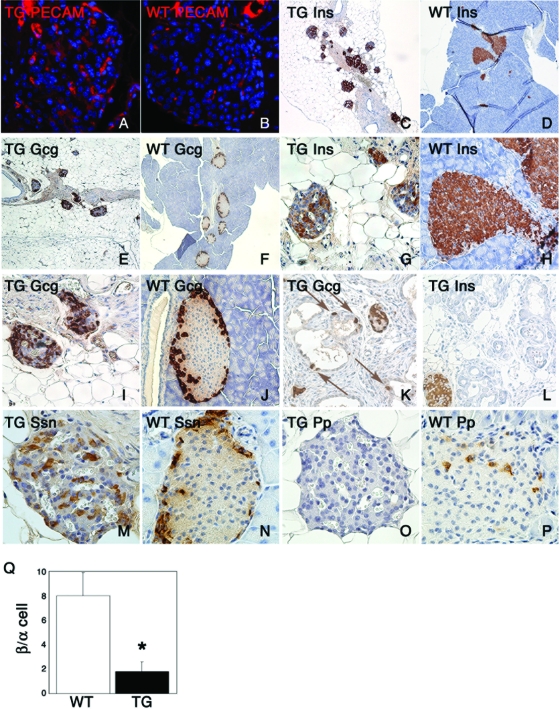
Analysis of endocrine islets showed an expanded vascular bed, as indicated by platelet/endothelial cell adhesion molecule immunohistochemistry (Fig. 7A and B), and decreased islet size (Fig. 7C to F). Accurate estimates of endocrine mass were not possible, owing to the disrupted exocrine pancreas. However, the percentage of islet β cells decreased (Fig. 7G and H), while that of α cells increased (Fig. 7I and J), resulting in a decreased β/α cell ratio from 8 ± 1.8 in WT mice to 2 ± 1.5 in Pdx1-FoxO1ADA transgenic mice (P < 0.001) (Fig. 7Q). Interestingly, we observed numerous ductal α cells but virtually no ductal β cells in transgenic mice (Fig. 7K and L and data available upon request). δ cells were present in normal numbers (Fig. 7M and N), whereas Pp cells were decreased (Fig. 7O and P).
FIG. 7.
Abnormal endocrine islets in 3-month-old Pdx-FoxO1ADA transgenic (TG) mice. (A and B) Platelet/endothelial cell adhesion molecule (PECAM) staining indicating increased vascular bed in transgenic mice (magnification, ×200). (C and D) Insulin (Ins) immunohistochemistry (magnification, ×40). (E and F) Glucagon (Gcg) immunohistochemistry (magnification, ×40). (G and H) Insulin immunohistochemistry (magnification, ×100). (I and J) Glucagon immunohistochemistry (magnification, ×100). (K and L) Glucagon (K) and insulin (L) immunohistochemistry (magnification, ×100). (M and N) Somatostatin (Ssn) immunohistochemistry (magnification, ×200). (O and P) Pp immunohistochemistry (magnification, ×200). (Q) Ratio of β cells to α cells. Six mice of each genotype and six sections for each mouse were analyzed.
These findings indicate that premature nuclear expression of FoxO1 in pancreatic progenitors prevents exocrine cell differentiation and alters the β/α cell ratio and islet vasculature, effectively phenocopying the abnormalities of pancreas development seen in mice lacking both insulin and IGF-1 receptors (20). The similarities between Pdx-FoxO1ADA mice and InsR:Igf1R−/− mice, in which endogenous FoxO1 is nuclear (23, 47), indicate the robustness of the transgenic model.
Generation and analysis of FoxO1 conditional knockouts in pancreas.
The data obtained with transgenic mice indicate that the timing of FoxO1 nuclear translocation is critical for terminal differentiation of pancreatic lineages. When viewed in the context of this study of the role of juxtaductal FoxO1+ Ins− cells, the findings are consistent with the possibility that these cells represent remnants of an uncommitted progenitor population in the adult pancreas. To investigate this point, we sought to determine the effects of loss of FoxO1 function at different stages of pancreatogenesis, using intercrosses of Pdx1(cre), Neurog3(cre), or Ins(cre) transgenic mice with mice bearing floxed FoxO1 alleles (35). In Pdx1(cre):FoxO1−/− offspring, FoxO1 should be ablated in all pancreatic cell types (13), while in Neurog3(cre):FoxO1−/− mice, ablation should occur in all enteroendocrine cells (40) and in Ins(cre):FoxO1−/−mice, it should occur exclusively in β cells (15). Genotyping of DNA extracted from liver, pancreas, and duodenum showed that Cre-mediated excision occurred as planned (Fig. 8A). In addition, the lineage targeting of Cre was confirmed by intercrossing cre transgenic mice with ROSA26-GFP reporter mice (not shown) (21).
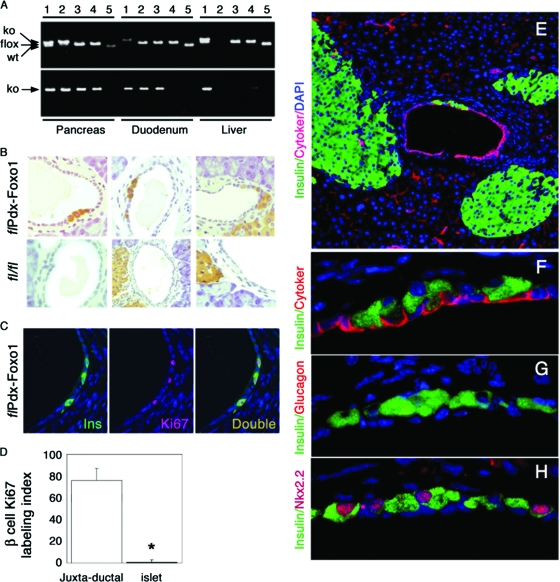
FIG. 8.
Conditional inactivation and ductal immunohistochemistry in mice homozygous for FoxO1 conditional null alleles.(A) Genotyping of DNA isolated from whole pancreas, duodenum, or liver of Hs1(cre):FoxO1−/−(lanes 1), Pdx1(cre):FoxO1−/−(lanes 2), Neurog3(cre):FoxO1−/−(lanes 3), Ins(cre):FoxO1−/−(lanes 4), and FoxO1lox/lox (lanes 5) mice for multiplex detection of WT, floxed, and deleted (ko) alleles (upper panel) or for single detection of the deleted allele (ko) (lower panel). Hs1(cre) transgenic mice are an embryonic deleter strain used as positive control for recombination (7). (B) Immunohistochemistry of representative pancreatic sections from 1-month-old Pdx1(cre):FoxO1−/−(upper panel) and FoxO1lox/lox (lower panel) mice. Six mice of each genotype and six sections per mouse were analyzed. (C and D) Representative image of insulin/Ki67 double immunohistochemistry (C) and Ki67 labeling index of juxtaductal or islet β cells (D) in two-month-old Pdx1(cre):FoxO1−/− mice. Six mice of each genotype and four sections per mouse were analyzed. An asterisk indicates a P value of <0.01 by analysis of variance. (E to G) Double immunohistochemistry with anti-insulin (green) and anticytokeratin (E and F), antiglucagon (G), or anti-Nkx2.2 (H) antibodies (red). Images are shown at a magnification of ×10 (E) or ×100 (F to H).
The following predictions on the outcome of these experiments can be made. If FoxO1+ Ins− cells are progenitors of juxtaductal insulin-positive cells and FoxO1 is a negative regulator of their differentiation (i.e., it must be kept inactive during development to prevent premature differentiation), FoxO1 ablation by Pdx1(cre) should increase the number of juxtaductal insulin-positive cells, whereas ablation at later stages [driven by Neurog3(cre) or Ins(cre)] should not. If the FoxO1+ Ins− population is not a precursor of juxtaductal insulin-positive cells and/or FoxO1 is a bystander in the differentiation process, no changes in the number of juxtaductal insulin-positive cells will be observed. Finally, if juxtaductal insulin-positive cells are like any other β cells and do not derive from FoxO1+ Ins− cells, but FoxO1 affects β-cell differentiation/proliferation, we would expect that changes in juxtaductal insulin-positive cells will mirror those in islet β cells and that the three conditional knockouts will phenocopy each other.
Pancreas morphology and gross anatomical appearance were normal in mice homozygous for the conditional alleles. However, immunohistochemical analyses of pancreases from Pdx1(cre):FoxO1−/− mice showed clusters of two to four insulin-positive Pdx1+ cells in ∼15% of surveyed main, interlobular, and intralobular pancreatic ducts (n = 360) (Fig. 8B and C). Single insulin-positive cells occurred in ∼1% of surveyed ducts in FoxO1lox/lox controls (P = 1.3 × 10−11) and Neurog3(cre):FoxO1−/− and Ins(cre):FoxO1−/− mice (data not shown), but clusters were seen only in Pdx1(cre):FoxO1−/− mice. Insulin-positive cells occurred with frequencies of 5 × 10−3 in ducts from Pdx1(cre):FoxO1−/− mice and 5 × 10−5 in FoxO1lox/lox controls (P = 0.013). It should be emphasized that in control mice, insulin-positive cells were rarely located in the duct proper, even when islets were located near ducts (Fig. 8B). The Ki67 labeling index of juxtaductal insulin-positive cells in 1-month-old Pdx1(cre):FoxO1−/− mice was 200-fold higher than that in islet β cells (Fig. 8C and D), indicating that they replicate at a significantly higher rate than islet β cells. If the juxtaductal insulin-positive cells seen in Pdx(cre):FoxO1−/− mice were simply β cells near ducts, they should be observed in all three conditional knockouts, and their replication rates should be the same as for islet β cells. Further immunohistochemistry with anti-insulin and anticytokeratin antisera indicated that insulin-positive cells were juxtaposed to, but distinct from, duct epithelial cells (Fig. 8E and F). The cells were glucagon negative (Fig. 8G) but Nkx2.2 positive (Fig. 8H), consistent with a β-cell identity.
The presence of relatively large numbers of bona fide juxtaductal insulin-positive cells following FoxO1 ablation in pancreatic progenitors is consistent with the hypothesis that these cells arise from the FoxO1+ Ins− subpopulation. Alternatively, FoxO1 ablation in pancreatic progenitors alters the ductal microenvironment, either generating a ductal homing signal for insulin-positive cells or promoting differentiation of juxtaductal progenitors, distinct from FoxO1+ Ins− cells, into insulin-positive cells.
Isolation and differentiation of FoxO1+ Ins− cells.
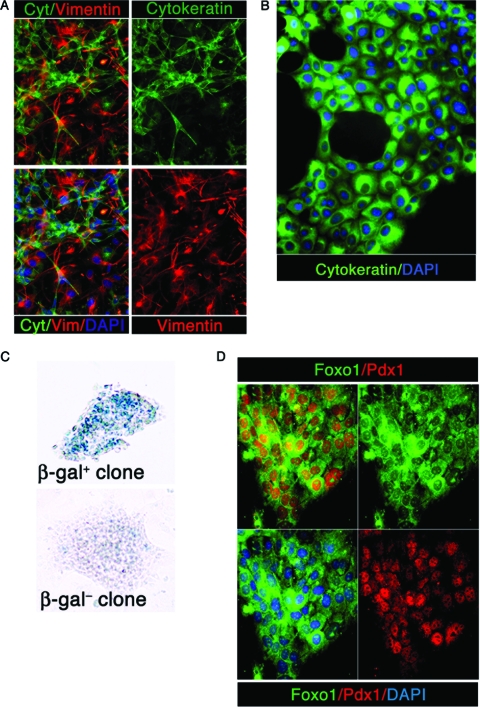
The identification of this unique FoxO1+ Ins− cellular subpopulation, together with the sharp increase of insulin-positive juxtaductal cells seen in Pdx1(cre):FoxO1−/− mice, led us to test whether FoxO1+ Ins− cells possess endocrine progenitor features when cultured. To identify and isolate FoxO1+ Ins− cells, we used a genetic selection approach, relying on a reporter β-gal gene knocked into the FoxO1 locus by homologous recombination and fused in frame with the FoxO1-initiating methionine (16). The reporter is active only in cells expressing endogenous FoxO1. Thus, only islet β cells and FoxO1+ Ins− cells should be β-gal positive, as we showed previously (25). After a 7-day culture, immunocytochemistry with epithelial (cytokeratin) and mesenchymal (vimentin) markers revealed different subpopulations of cells: cytokeratin-positive cells (Fig. 9A, green), vimentin-positive cells (Fig. 9A, red), and cytokeratin-negative, vimentin-negative cells (Fig. 9A, blue nuclei with unstained cytoplasm). These data indicate that the culture is heterogeneous in nature and includes both epithelial and mesenchymal cells. Subsequently, cells were grown in serum-free medium for 3 weeks. Cells that survived growth in serum-free conditions were cobblestone shaped and often appeared to be organized around a central, “duct-like” structure (Fig. 9B, green).
FIG. 9.
Isolation and differentiation of FoxO1+ Ins− cells.(A) Immunocytochemistry with antipancytokeratin antibodies (green), antivimentin antibodies (red), and DAPI (blue) at a magnification of ×100 after a 1-week culture. (B) Typical aspect of the culture after 3 weeks in serum-free medium. Cells were immunostained with antipancytokeratin antibody and photographed at a magnification of ×400. (C) X-Gal staining of representative β-gal-positive and β-gal-negative clones. (D) Immunocytochemistry of a representative clone with anti-FoxO1 (green) and anti-Pdx1 (red) antibodies and DAPI (blue) at a magnification of ×100.
To rule out contamination by β cells, we performed insulin immunocytochemistry and RT-PCR, and we failed to detect residual β cells (data not shown). Moreover, we conducted control experiments in which islets were purified on a Ficoll gradient, plated, and subjected to the same culture conditions in defined medium. At the end of the 3-week culture, no cells survived in the serum-free medium (data not shown).
We performed single-cell cloning by limiting dilution, and stained individual clones with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to identify those derived from FoxO1+ cells. We obtained clonal β-gal-positive and β-gal-negative cells (Fig. 9C). We isolated 24 β-gal-positive clones and four β-gal-negative clones. As expected, all β-gal-positive clones expressed cytoplasmicFoxO1 (Fig. 9D, green), as well as nuclear Pdx1 (Fig. 9D, red) (25). While it is theoretically possible that these FoxO1+ clones are distinct from ductal FoxO1+ Ins− cells, this possibility seems unlikely, as it would require that FoxO1 expression be (i) silenced in FoxO1+ cells and (ii) reactivated in FoxO1− cells during the selection process. Given that FoxO1 function is controlled primarily posttranslationally and not transcriptionally, it is more likely that β-gal-positive clones are direct descendants of ductal FoxO1+ Ins− cells.
Based on the finding that FoxO1 nuclear expression in transgenic mice favors α-cell differentiation, we tested whether the clonal isolates of FoxO1+ cells could be differentiated into endocrine cells by FoxO1 gain of function, using adenoviral transduction of constitutively nuclear FoxO1ADA. Under basal conditions, cells from two representative β-gal-positive clones express ductal markers cytokeratin 19 and carbonic anhydrase II but none of the exocrine (amylase, elastase, and trypsin) and endocrine (insulin 1, insulin 2, glucagon, Pp, and somatostatin) markers. Among pancreas-specific or -enriched transcription factors, they express Pdx1 and neural differentiation-associated transcription factor D1 (NeuroD) (Fig. 10A). Expression of FoxO1ADA had no effect on cytokeratin 19 and carbonic anhydrase II but induced amylase, glucagon, and Pp (Fig. 10A). Importantly, FoxO1ADA did not induce insulin 1, insulin 2, somatostatin, elastase, and trypsin (Fig. 10A). These data are consistent with the increased number of islet α cells (Fig. 7I) and duct-associated α cells (Fig. 7K) in Pdx-FoxO1ADA transgenic mice. We also confirmed glucagon expression by immunocytochemistry (Fig. 10B).
We next surveyed the effect of FoxO1ADA on transcription factors required for pancreatic development and cell type specification. Transduction of FoxO1ADA decreased Pdx1 and increased NeuroD, consistent with our previous findings (25, 26). FoxO1ADA induced expression of Nkx2.2, Nkx6.1, Pax6 (paired-box gene 6) and pancreas transcription factor 1 but not that of Pax4, Arx (homeobox-containing gene on chromosome X), Brn4 (POU homeodomain protein brain 4), MafB (v-Maf cellular ortholog bZIP protein B), MafA (Fig. 10A). The resulting expression pattern is not typical of α cells. It indicates that although FoxO1ADA is able to induce glucagon expression, these cells are unlike bona fide α cells. Interestingly, a FoxO1 consensus binding site is conserved across multiple species in a region of the glucagon promoter that has been shown to direct α cell-specific expression (15). Thus, FoxO1 may be able to activate glucagon expression directly.
To test whether FoxO1-induced glucagon expression is specific to FoxO1+ Ins− pancreatic cells or is commonly seen in other duct-derived murine cell lines, we transduced β-gal-negative clonal ductal cells (Fig. 9C) with FoxO1ADA, but we failed to find an effect on the glucagon gene or any other pancreas-specific gene (Fig. 10A). Likewise, transduction of different types of duct-derived cells, including pancreatic ductal adenocarcinoma TGP47 cells (36), UB cells (3), M-1 cells (45), or SV40-transformed hepatocytes (negative control) (38), shows that FoxO1ADA induced glucagon only in FoxO1+ Ins−-derived clones, indicating that the effect of FoxO1 is specific for these cells (Fig. 10C).
Since FoxO1 ablation in Pdx(cre):FoxO1−/− mice resulted in increased numbers of insulin-positive juxtaductal cells (Fig. 8B), we next asked whether FoxO1 knockdown would promote insulin 1 or insulin 2 expression in FoxO1+ Ins− cells. FoxO1 siRNA resulted in the predicted increase of Pdx1 (25) and decrease of NeuroD (26) but failed to induce insulin 1, insulin 2, and glucagon transcription (Fig. 10D). Pdx1 and NeuroD reactions were carried out for 25 cycles only, to detect quantitative differences between samples transfected with control (GFP) and FoxO1 siRNAs. We tested additional culture conditions that have been employed to differentiate clonal adult pancreatic cells into β-like cells (42), but we were unable to detect insulin 1 or insulin 2 expression.
DISCUSSION
FoxO1's role in pancreatic development.
Our genetic, developmental, and cellular analyses are consistent with a permissive role of FoxO1 in exocrine pancreas differentiation, loosely reminiscent of its role in adipocytes (32), and with a proendocrine role in pancreatic progenitors prior to the divergence of endocrine, exocrine, and ductal lineages. The function of FoxO1 within the endocrine lineage is complex. Constitutive activation, in transgenic mice and in primary cultures of FoxO1+ Ins− cells, preferentially drives the α-cell phenotype. A similar observation has been made in mice with mistimed Neurog3 activation (18). In this instance, early activation of Neurog3 resulted in an increase of glucagon-positive cells, whereas later activation results in a more balanced distribution of different endocrine cell types (18). On the other hand, FoxO1 ablation in pancreatic, but not endocrine, progenitors or in differentiated β cells specifically increases juxtaductal β cells. Thus, the timing of FoxO1 activation appears to be critical for terminal differentiation of specific endocrine cell types. A potential mechanism by which FoxO1 ablation promotes endocrine differentiation is through its interaction with Notch signaling (24). Like FoxO1 ablation, Notch ablation results in higher number of endocrine cells only when the gene is inactivated in pancreatic progenitors and not in differentiated endocrine cells (30). The fact that FoxO1 deletion promotes β-cell formation in cells adjacent to pancreatic duct epithelia suggests that, in this context, β-cell differentiation is dependent on local cues, for example, growth or differentiation factors released from duct-associated cells.
What is the physiologic role of FoxO1+ Ins− adult pancreatic cells?
Considerable controversy surrounds the hypothesis that “ductal” cells undergo endocrine differentiation in the adult pancreas (4, 8, 13, 50). The clones of FoxO1+ Ins− cells characterized in this study seemingly engage in a limited endocrine-like differentiation program in vitro. In view of the many conflicting and dubious claims in this area, we are loath to overinterpret the findings. We are mindful of prior data from us (34) and others (29, 46) that are consistent with a limited role for neogenesis in β-cell turnover. Nonetheless, our study provides proof of principle that FoxO1+ Ins− cells have unique properties that can be exploited for cellular replacement purposes. We do not know whether FoxO1+ Ins− cells are the same population identified by lineage tracing of carbonic anhydrase II-expressing cells (17), but it is interesting that FoxO1+ Ins− cells are carbonic anhydrase II positive. Other possibilities include that FoxO1+ Ins− cells are similar in origin to cells identified in clonal studies of adult pancreatic endocrine precursors (42) and following pancreatic duct ligation (49).
While our characterization of FoxO1+ Ins− cells should be considered preliminary, it is reassuring to note that FoxO1 gain of function results in increased glucagon expression both in cultured cells and in transgenic mice and that the latter phenotype dovetails with the observation that premature activation of endocrine differentiation preferentially yields glucagon-positive cells (18). Similarly, coactivation of glucagon and Pp expression by FoxO1 is reminiscent of the phenotype due to Arx gain of function (5). We propose that the failure to yield β cells reflects a critical requirement for mesenchymal/epithelial interactions, as observed in normal pancreatic development (11). Coculture experiments and isolation of FoxO1+ Ins− cells from embryonic pancreases may shed light on this area.
In conclusion, our data demonstrating that FoxO1 ablation increases β-cell formation at a specific anatomical location and during a narrow developmental window should rekindle efforts to generate β cells from sources other than embryonic stem cells.
Acknowledgments
This work was supported by NIH grants DK64819 and DK63608 (Columbia Diabetes & Endocrinology Research Center) and by the Russell Berrie Program in Cell-Based Therapies at Columbia University. R.A.D. is an American Cancer Society Research Professor and an Ellison Medical Foundation Senior Scholar and is supported by the Robert A. and Renee E. Belfer Family Institute for Innovative Cancer Science.
We thank J. Barasch (Columbia University) for UB cells, B. L. Hogan (Duke University) for anti-Foxa2 antibodies, A. Leiter (Tufts University) for Neurog3(cre) mice, D. Melton (Harvard University) for Pdx1(cre) mice, and C. Osawa (Gunma University) for skilled technical assistance with histochemistry. We thank members of the Accili laboratory for useful discussions and L. Sussel for critical reading of the manuscript.
Footnotes
Published ahead of print on 8 June 2009.
REFERENCES
- 1.Accili, D. 2004. The struggle for mastery in insulin action: from triumvirate to republic. Diabetes 531633-1642. [DOI] [PubMed] [Google Scholar]
- 2.Baggio, L. L., and D. J. Drucker. 2006. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu. Rev. Med. 57265-281. [DOI] [PubMed] [Google Scholar]
- 3.Barasch, J., L. Pressler, J. Connor, and A. Malik. 1996. A ureteric bud cell line induces nephrogenesis in two steps by two distinct signals. Am. J. Physiol. 271F50-61. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir, S., M. Taneja, G. C. Weir, K. Tatarkiewicz, K. H. Song, A. Sharma, and J. J. O'Neil. 2000. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA 977999-8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collombat, P., J. Hecksher-Sorensen, J. Krull, J. Berger, D. Riedel, P. L. Herrera, P. Serup, and A. Mansouri. 2007. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J. Clin. Investig. 117961-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Amour, K. A., A. G. Bang, S. Eliazer, O. G. Kelly, A. D. Agulnick, N. G. Smart, M. A. Moorman, E. Kroon, M. K. Carpenter, and E. E. Baetge. 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 241392-1401. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich, P., I. Dragatsis, S. Xuan, S. Zeitlin, and A. Efstratiadis. 2000. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm. Genome 11196-205. [DOI] [PubMed] [Google Scholar]
- 8.Dor, Y., J. Brown, O. I. Martinez, and D. A. Melton. 2004. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 42941-46. [DOI] [PubMed] [Google Scholar]
- 9.Efrat, S., S. Linde, H. Kofod, D. Spector, M. Delannoy, S. Grant, D. Hanahan, and S. Baekkeskov. 1988. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc. Natl. Acad. Sci. USA 859037-9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon, M., P. L. Herrera, and C. V. Wright. 2000. Mosaic Cre-mediated recombination in pancreas using the pdx-1 enhancer/promoter. Genesis 26143-144. [DOI] [PubMed] [Google Scholar]
- 11.Gittes, G. K., P. E. Galante, D. Hanahan, W. J. Rutter, and H. T. Debase. 1996. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development 122439-447. [DOI] [PubMed] [Google Scholar]
- 12.Grant, S. F., G. Thorleifsson, I. Reynisdottir, R. Benediktsson, A. Manolescu, J. Sainz, A. Helgason, H. Stefansson, V. Emilsson, A. Helgadottir, U. Styrkarsdottir, K. P. Magnusson, G. B. Walters, E. Palsdottir, T. Jonsdottir, T. Gudmundsdottir, A. Gylfason, J. Saemundsdottir, R. L. Wilensky, M. P. Reilly, D. J. Rader, Y. Bagger, C. Christiansen, V. Gudnason, G. Sigurdsson, U. Thorsteinsdottir, J. R. Gulcher, A. Kong, and K. Stefansson. 2006. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 38320-323. [DOI] [PubMed] [Google Scholar]
- 13.Gu, G., J. Dubauskaite, and D. A. Melton. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 1292447-2457. [DOI] [PubMed] [Google Scholar]
- 14.Heller, R. S., D. A. Stoffers, T. Bock, K. Svenstrup, J. Jensen, T. Horn, C. P. Miller, J. F. Habener, O. D. Madsen, and P. Serup. 2001. Improved glucose tolerance and acinar dysmorphogenesis by targeted expression of transcription factor PDX-1 to the exocrine pancreas. Diabetes 501553-1561. [DOI] [PubMed] [Google Scholar]
- 15.Herrera, P. L. 2000. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 1272317-2322. [DOI] [PubMed] [Google Scholar]
- 16.Hosaka, T., W. H. Biggs III, D. Tieu, A. D. Boyer, N. M. Varki, W. K. Cavenee, and K. C. Arden. 2004. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. USA 1012975-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inada, A., C. Nienaber, H. Katsuta, Y. Fujitani, J. Levine, R. Morita, A. Sharma, and S. Bonner-Weir. 2008. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA 10519915-19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson, K. A., U. Dursun, N. Jordan, G. Gu, F. Beermann, G. Gradwohl, and A. Grapin-Botton. 2007. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 12457-465. [DOI] [PubMed] [Google Scholar]
- 19.Kahn, S. E., S. M. Haffner, M. A. Heise, W. H. Herman, R. R. Holman, N. P. Jones, B. G. Kravitz, J. M. Lachin, M. C. O'Neill, B. Zinman, and G. Viberti. 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 3552427-2443. [DOI] [PubMed] [Google Scholar]
- 20.Kido, Y., J. Nakae, M. L. Hribal, S. Xuan, A. Efstratiadis, and D. Accili. 2002. Effects of mutations in the insulin-like growth factor signaling system on embryonic pancreas development and beta-cell compensation to insulin resistance. J. Biol. Chem. 27736740-36747. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura, T., Y. Feng, Y. I. Kitamura, S. C. Chua, Jr., A. W. Xu, G. S. Barsh, L. Rossetti, and D. Accili. 2006. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 12534-540. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura, T., Y. Kido, S. Nef, J. Merenmies, L. F. Parada, and D. Accili. 2001. Preserved pancreatic beta-cell development and function in mice lacking the insulin receptor-related receptor. Mol. Cell. Biol. 215624-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura, T., Y. Kitamura, J. Nakae, A. Giordano, S. Cinti, C. R. Kahn, A. Efstratiadis, and D. Accili. 2004. Mosaic analysis of insulin receptor function. J. Clin. Investig. 113209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamura, T., Y. I. Kitamura, Y. Funahashi, C. J. Shawber, D. H. Castrillon, R. Kollipara, R. A. Depinho, J. Kitajewski, and D. Accili. 2007. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Investig. 1172477-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura, T., J. Nakae, Y. Kitamura, Y. Kido, W. H. Biggs III, C. V. Wright, M. F. White, K. C. Arden, and D. Accili. 2002. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J. Clin. Investig. 1101839-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura, Y. I., T. Kitamura, J. P. Kruse, J. C. Raum, R. Stein, W. Gu, and D. Accili. 2005. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2153-163. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi, H., T. L. Spilde, Z. Li, J. K. Marosky, A. M. Bhatia, M. J. Hembree, K. Prasadan, B. L. Preuett, and G. K. Gittes. 2002. Lectin as a marker for staining and purification of embryonic pancreatic epithelium. Biochem. Biophys. Res. Commun. 293691-697. [DOI] [PubMed] [Google Scholar]
- 28.Lee, C. S., N. J. Sund, R. Behr, P. L. Herrera, and K. H. Kaestner. 2005. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev. Biol. 278484-495. [DOI] [PubMed] [Google Scholar]
- 29.Lin, X., A. Taguchi, S. Park, J. A. Kushner, F. Li, Y. Li, and M. F. White. 2004. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J. Clin. Investig. 114908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murtaugh, L. C., B. Z. Stanger, K. M. Kwan, and D. A. Melton. 2003. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 10014920-14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakae, J., W. H. Biggs, T. Kitamura, W. K. Cavenee, C. V. Wright, K. C. Arden, and D. Accili. 2002. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 32245-253. [DOI] [PubMed] [Google Scholar]
- 32.Nakae, J., T. Kitamura, Y. Kitamura, W. H. Biggs, K. C. Arden, and D. Accili. 2003. The forkhead transcription factor foxo1 regulates adipocyte differentiation. Dev. Cell 4119-129. [DOI] [PubMed] [Google Scholar]
- 33.Nakae, J., T. Kitamura, D. L. Silver, and D. Accili. 2001. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 1081359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto, H., M. L. Hribal, H. V. Lin, W. R. Bennett, A. Ward, and D. Accili. 2006. Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J. Clin. Investig. 116775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paik, J. H., R. Kollipara, G. Chu, H. Ji, Y. Xiao, Z. Ding, L. Miao, Z. Tothova, J. W. Horner, D. R. Carrasco, S. Jiang, D. G. Gilliland, L. Chin, W. H. Wong, D. H. Castrillon, and R. A. Depinho. 2007. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128309-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettengill, O. S., V. A. Memoli, T. Brinck-Johnsen, and D. S. Longnecker. 1994. Cell lines derived from pancreatic tumors of Tg(Ela-1-SV40E)Bri18 transgenic mice express somatostatin and T antigen. Carcinogenesis 1561-65. [DOI] [PubMed] [Google Scholar]
- 37.Plas, D. R., and C. B. Thompson. 2003. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 27812361-12366. [DOI] [PubMed] [Google Scholar]
- 38.Rother, K. I., Y. Imai, M. Caruso, F. Beguinot, P. Formisano, and D. Accili. 1998. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J. Biol. Chem. 27317491-17497. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki, H., and B. L. Hogan. 1994. HNF-3 beta as a regulator of floor plate development. Cell 76103-115. [DOI] [PubMed] [Google Scholar]
- 40.Schonhoff, S. E., M. Giel-Moloney, and A. B. Leiter. 2004. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 270443-454. [DOI] [PubMed] [Google Scholar]
- 41.Scott, L. J., K. L. Mohlke, L. L. Bonnycastle, C. J. Willer, Y. Li, W. L. Duren, M. R. Erdos, H. M. Stringham, P. S. Chines, A. U. Jackson, L. Prokunina-Olsson, C. J. Ding, A. J. Swift, N. Narisu, T. Hu, R. Pruim, R. Xiao, X. Y. Li, K. N. Conneely, N. L. Riebow, A. G. Sprau, M. Tong, P. P. White, K. N. Hetrick, M. W. Barnhart, C. W. Bark, J. L. Goldstein, L. Watkins, F. Xiang, J. Saramies, T. A. Buchanan, R. M. Watanabe, T. T. Valle, L. Kinnunen, G. R. Abecasis, E. W. Pugh, K. F. Doheny, R. N. Bergman, J. Tuomilehto, F. S. Collins, and M. Boehnke. 2007. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 3161341-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seaberg, R. M., S. R. Smukler, T. J. Kieffer, G. Enikolopov, Z. Asghar, M. B. Wheeler, G. Korbutt, and D. van der Kooy. 2004. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 221115-1124. [DOI] [PubMed] [Google Scholar]
- 43.Steinthorsdottir, V., G. Thorleifsson, I. Reynisdottir, R. Benediktsson, T. Jonsdottir, G. B. Walters, U. Styrkarsdottir, S. Gretarsdottir, V. Emilsson, S. Ghosh, A. Baker, S. Snorradottir, H. Bjarnason, M. C. Ng, T. Hansen, Y. Bagger, R. L. Wilensky, M. P. Reilly, A. Adeyemo, Y. Chen, J. Zhou, V. Gudnason, G. Chen, H. Huang, K. Lashley, A. Doumatey, W. Y. So, R. C. Ma, G. Andersen, K. Borch-Johnsen, T. Jorgensen, J. V. van Vliet-Ostaptchouk, M. H. Hofker, C. Wijmenga, C. Christiansen, D. J. Rader, C. Rotimi, M. Gurney, J. C. Chan, O. Pedersen, G. Sigurdsson, J. R. Gulcher, U. Thorsteinsdottir, A. Kong, and K. Stefansson. 2007. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat. Genet. 39770-775. [DOI] [PubMed] [Google Scholar]
- 44.Stoffers, D. A., R. S. Heller, C. P. Miller, and J. F. Habener. 1999. Developmental expression of the homeodomain protein IDX-1 in mice transgenic for an IDX-1 promoter/lacZ transcriptional reporter. Endocrinology 1405374-5381. [DOI] [PubMed] [Google Scholar]
- 45.Stoos, B. A., A. Naray-Fejes-Toth, O. A. Carretero, S. Ito, and G. Fejes-Toth. 1991. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 391168-1175. [DOI] [PubMed] [Google Scholar]
- 46.Teta, M., S. Y. Long, L. M. Wartschow, M. M. Rankin, and J. A. Kushner. 2005. Very slow turnover of beta-cells in aged adult mice. Diabetes 542557-2567. [DOI] [PubMed] [Google Scholar]
- 47.Ueki, K., T. Okada, J. Hu, C. W. Liew, A. Assmann, G. M. Dahlgren, J. L. Peters, J. G. Shackman, M. Zhang, I. Artner, L. S. Satin, R. Stein, M. Holzenberger, R. T. Kennedy, C. R. Kahn, and R. N. Kulkarni. 2006. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat. Genet. 38583-588. [DOI] [PubMed] [Google Scholar]
- 48.United Kingdom Prospective Diabetes Study Group. 1995. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 441249-1258. [PubMed] [Google Scholar]
- 49.Xu, X., J. D'Hoker, G. Stange, S. Bonne, N. De Leu, X. Xiao, M. Van de Casteele, G. Mellitzer, Z. Ling, D. Pipeleers, L. Bouwens, R. Scharfmann, G. Gradwohl, and H. Heimberg. 2008. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132197-207. [DOI] [PubMed] [Google Scholar]
- 50.Yatoh, S., R. Dodge, T. Akashi, A. Omer, A. Sharma, G. C. Weir, and S. Bonner-Weir. 2007. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes 561802-1809. [DOI] [PubMed] [Google Scholar]