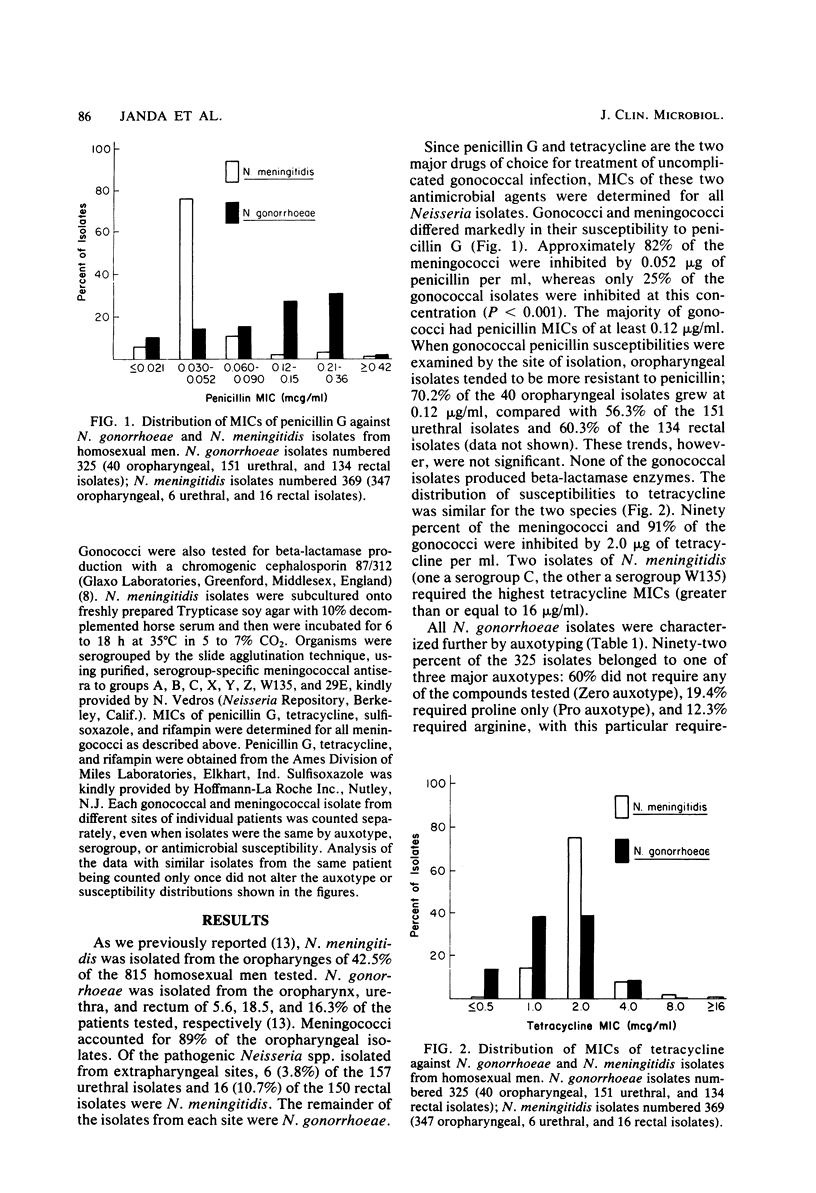
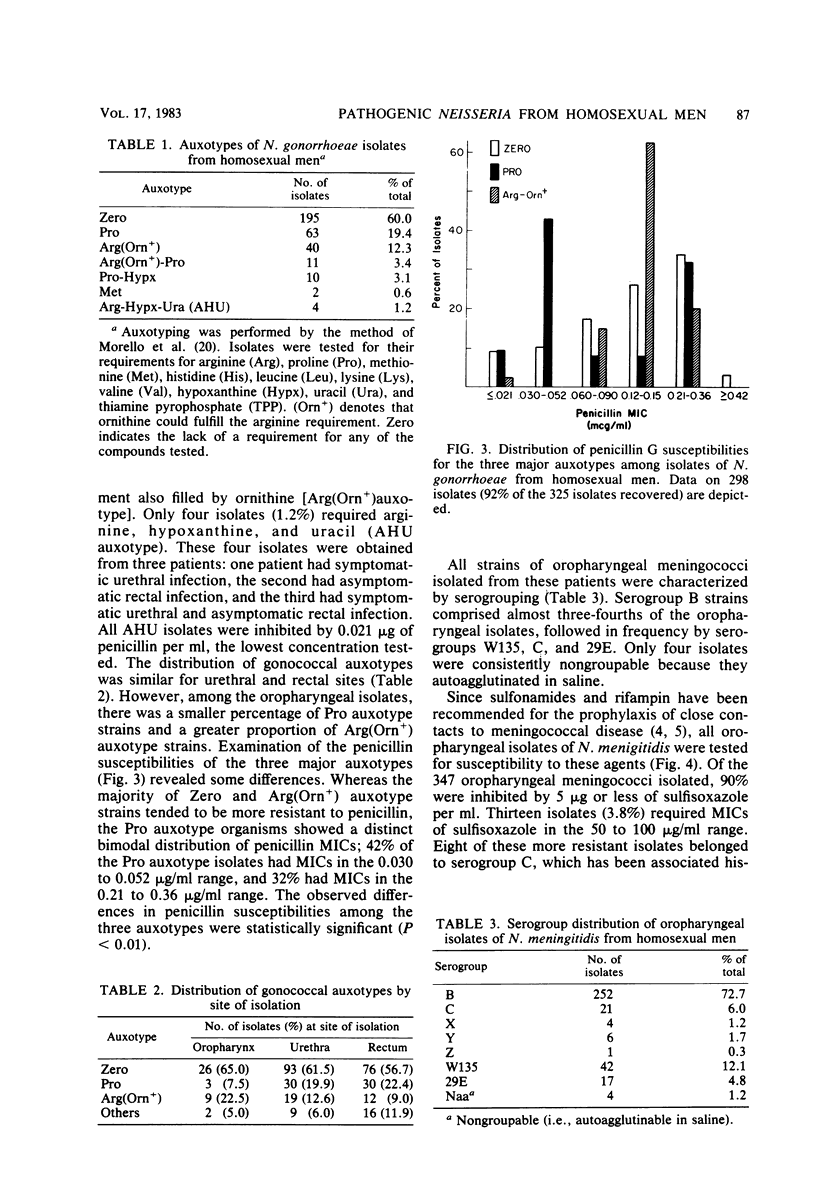
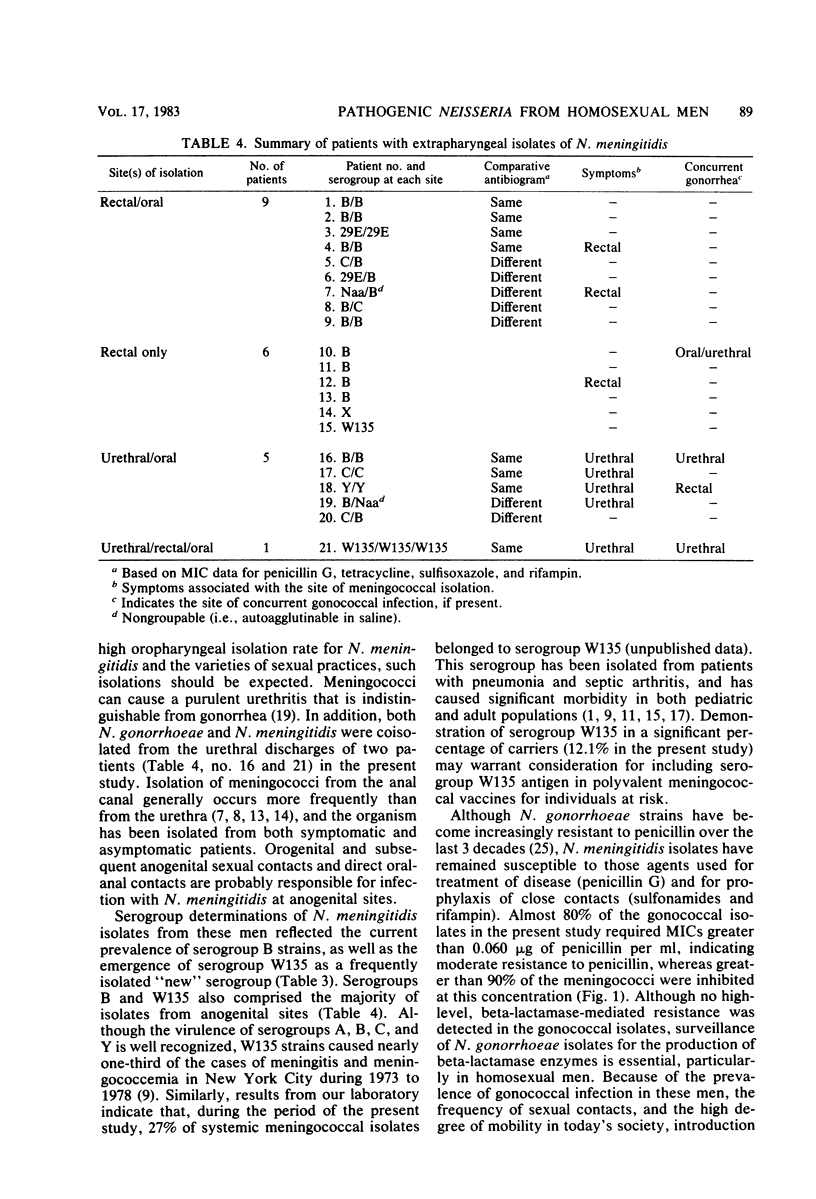
Abstract
Oropharyngeal, urethral, and rectal cultures for pathogenic Neisseria spp. were collected from 815 homosexual men attending a community clinic in Chicago. Meningococci were characterized by serogrouping and antimicrobial susceptibility testing. Gonococci were auxotyped, and susceptibilities to penicillin and tetracycline were determined. Of the 815 men tested, 42.5% carried meningococci in the oropharynx. Gonococci were recovered from the urethra, rectum, and oropharynx of 18.5, 16.3, and 5.6%, respectively. Meningococci were also recovered from the urethra (6 patients) and the rectum (15 patients). Some of these isolates were identical to the isolates from the oropharynges of the same patients, whereas others were distinct from the oropharyngeal isolates by serogroup or antimicrobial susceptibilities. Serogroups B, W135, and C comprised over 90% of the meningococci. Almost 80% of the gonococcal strains required minimal inhibitory concentrations greater than 0.06 micrograms of penicillin per ml, whereas greater than 90% of the meningococci were inhibited at this concentration. Auxotyping demonstrated three major auxotypes: Zero (required none of the nutrients tested), 60%; arginine requiring, 19.4%; and proline requiring, 12.3%. Only four strains (1.2%) required arginine, hypoxanthine, and uracil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandstetter R. D., Blair R. J., Roberts R. B. Neisseria meningitidis serogroup W 135 disease in adults. JAMA. 1981 Nov 6;246(18):2060–2061. [PubMed] [Google Scholar]
- Crawford C., Knapp J. S., Hale J., Holmes K. K. Asymptomatic gonorrhea in men: caused by gonococci with unique nutritional requirements. Science. 1977 Jun 17;196(4296):1352–1353. doi: 10.1126/science.405742. [DOI] [PubMed] [Google Scholar]
- Devine L. F., Hagerman C. R. Spectra of susceptibility of Neisseria meningitidis to antimicrobial agents in vitro. Appl Microbiol. 1970 Feb;19(2):329–334. doi: 10.1128/am.19.2.329-334.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T. C. In-vitro and in-vivo studies of resistance to rifampin in meningococci. J Infect Dis. 1971 Apr;123(4):414–420. doi: 10.1093/infdis/123.4.414. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Faur Y. C., Weisburd M. H., Wilson M. E. Isolation of Neisseria meningitidis from the Genito-urinary tract and anal canal. J Clin Microbiol. 1975 Sep;2(3):178–182. doi: 10.1128/jcm.2.3.178-182.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faur Y. C., Wilson M. E., May P. S. Isolation of N. meningitidis from patients in a gonorrhea screen program: a four-year survey in New York City. Am J Public Health. 1981 Jan;71(1):53–58. doi: 10.2105/ajph.71.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaid E. I., Cherubin C. E., Marr J. S., Schaefler S., Barone J., Lee W. Meningococcal disease in New York City, 1973 to 1978. Recognition of groups y and W-135 as frequent pathogens. JAMA. 1980 Nov 14;244(19):2167–2171. [PubMed] [Google Scholar]
- Goldmeier D., Darougar S. Isolation of Chlamydia trachomatis from throat and rectum of homosexual men. Br J Vener Dis. 1977 Jun;53(3):184–185. doi: 10.1136/sti.53.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R., Baltimore R. S. Infections in children due to Neisseria meningitidis serogroup 135. J Pediatr. 1978 Mar;92(3):503–503. doi: 10.1016/s0022-3476(78)80459-4. [DOI] [PubMed] [Google Scholar]
- Handsfield H. H., Knapp J. S., Diehr P. K., Holmes K. K. Correlation of auxotype and penicillin susceptibility of Neisseria gonorrhoeae with sexual preference and clinical manifestations of gonorrhea. Sex Transm Dis. 1980 Jan-Mar;7(1):1–5. doi: 10.1097/00007435-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Janda W. M., Bohnoff M., Morello J. A., Lerner S. A. Prevalence and site-pathogen studies of Neisseria meningitidis and N gonorrhoeae in homosexual men. JAMA. 1980 Nov 7;244(18):2060–2064. [PubMed] [Google Scholar]
- Judson F. N., Ehret J. M., Eickhoff T. C. Anogenital infection with Neisseria meningitidis in homosexual men. J Infect Dis. 1978 Apr;137(4):458–463. doi: 10.1093/infdis/137.4.458. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Hoffpauir C. W., Janney A. Meningitis caused by Neisseria meningitidis type W-135. J Pediatr. 1978 Aug;93(2):326–326. doi: 10.1016/s0022-3476(78)80567-8. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Bacteriocin production by strains of Neisseria meningitidis. J Bacteriol. 1966 May;91(5):1696–1699. doi: 10.1128/jb.91.5.1696-1699.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman M. B., Reynolds J., Steinfeld J., Allen S. D., Smith J. W. Meningitis caused by Neisseria meningitidis serogroup 135. J Clin Microbiol. 1978 Nov;8(5):621–622. doi: 10.1128/jcm.8.5.621-622.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Millikin P., Griffin P. S., Sexton R. A., Yousuf M. Neisseria meningitidis urethritis. A case report. JAMA. 1979 Oct 12;242(15):1656–1657. [PubMed] [Google Scholar]
- Morello J. A., Lerner S. A., Bohnhoff M. Characteristics of atypical Neisseria gonorrhoeae from disseminated and localized infections. Infect Immun. 1976 May;13(5):1510–1516. doi: 10.1128/iai.13.5.1510-1516.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands M. Nonsymptomatic urethral gonorrhea in homosexual men. Sex Transm Dis. 1980 Oct-Dec;7(4):206–206. [PubMed] [Google Scholar]


