Abstract
Background:
Persons destined to develop dementia experience an accelerated rate of decline in cognitive ability, particularly in memory. Early life education and participation in cognitively stimulating leisure activities later in life are 2 factors thought to reflect cognitive reserve, which may delay the onset of the memory decline in the preclinical stages of dementia.
Methods:
We followed 488 initially cognitively intact community residing individuals with epidemiologic, clinical, and cognitive assessments every 12 to 18 months in the Bronx Aging Study. We assessed the influence of self-reported participation in cognitively stimulating leisure activities on the onset of accelerated memory decline as measured by the Buschke Selective Reminding Test in 101 individuals who developed incident dementia using a change point model.
Results:
Each additional self-reported day of cognitive activity at baseline delayed the onset of accelerated memory decline by 0.18 years. Higher baseline levels of cognitive activity were associated with more rapid memory decline after that onset. Inclusion of education did not significantly add to the fit of the model beyond the effect of cognitive activities.
Conclusions:
Our findings show that late life cognitive activities influence cognitive reserve independently of education. The effect of early life education on cognitive reserve may be mediated by cognitive activity later in life. Alternatively, early life education may be a determinant of cognitive reserve, and individuals with more education may choose to participate in cognitive activities without influencing reserve. Future studies should examine the efficacy of increasing participation in cognitive activities to prevent or delay dementia.
GLOSSARY
- AD
= Alzheimer disease;
- BL
= baseline;
- CAS
= Cognitive Activity Scale;
- CI
= confidence interval;
- DSM
= Diagnostic and Statistical Manual of Mental Disorders;
- dx
= diagnosis;
- NIA
= National Institute on Aging;
- SRT
= Selective Reminding Test;
- WAIS VIQ
= Wechsler Adult Intelligence Scale Verbal IQ.
The cognitive reserve hypothesis suggests that some individual characteristics result in maintenance of cognitive function in the face of accumulating dementia pathology in the brain. Cognitive reserve may reflect structural or functional brain characteristics that protect against neuropathologic damage caused by the progression of dementia, or compensatory processes that allow the damaged brain to use intact networks or alternative cognitive strategies that offset neuropathologic damage. Cognitive reserve has been proposed to explain delayed onset of clinically diagnosable dementia,1–7 increased levels of brain pathology for given cognitive status,7–10 and later onset of cognitive decline and more rapid post-onset decline11 seen in persons with higher education, a possible marker for cognitive reserve.6
Participation in cognitively stimulating leisure activities has also been associated with reduced rates of dementia12–14 and mild cognitive impairment,15 possibly through some delay in the acceleration of cognitive decline associated with the activity. Such activity has also been shown to be associated with reduced rates of cognitive decline in a cohort of healthy elderly16 but increased rates of cognitive decline in persons with Alzheimer disease (AD),17 further providing evidence that such activities might contribute to cognitive reserve.6 However, not all studies have found associations.18
In this work, we investigate whether self-reported participation in cognitively stimulating leisure activities later in life affects the trajectory of memory decline in persons who ultimately develop dementia. Specifically, we use change point models to ascertain whether the onset of accelerated memory decline (the change point) is delayed in persons with greater participation in such activities, how the rate of decline after the change point is affected, and whether these effects are explained by education early in life. We hypothesized that much of the previously reported effects of early life education might be mediated through participation in cognitively stimulating leisure activities later in life.
METHODS
The Bronx Aging Study cohort included 488 healthy community-dwelling individual volunteers living in Bronx County, New York, enrolled between 1980 and 1983. Study design, methods, and demographics have been previously described.4,12,15,19–22 The study enrolled English-speaking subjects between ages 75 and 85 years. Exclusion criteria included previous diagnoses of idiopathic Parkinson disease, liver disease, alcoholism, or known terminal illness; severe visual and hearing impairment interfering with completion of neuropsychological tests; and presence of dementia. One study participant is still alive and healthy (no dementia) as of July 2008; all others were followed until lost to follow-up or death. The inception cohort was middle class, 90% white, and 64.5% women. The analysis includes 101 study participants who were cognitively normal at baseline, reported their formal education and participation in leisure activities at baseline, and developed dementia during follow-up.
Standard protocol approvals, registrations, and patient consents.
The local institutional review board approved the study protocols. Written informed consent was obtained from all subjects and surrogate decision makers at enrollment.
Cognitive evaluation.
An extensive battery of validated neuropsychological tests was administered to all subjects at all study visits and was used to inform dementia diagnosis at case conferences. For the purposes of this study, we examined performance on the Buschke Selective Reminding Test (SRT),23 a word list memory test that was not used as part of the diagnostic process. The sum of recall on SRT has been reported to predict incident dementia in this cohort.11,24–26
Dementia diagnosis.
At study visits, subjects with suspected dementia received a clinical workup, including CT scans and blood tests to rule out reversible causes of dementia. Triggers for workup for reversible or underlying causes of dementia during the follow-up visits included reports of new or progressive memory or other cognitive symptoms by the subjects or caregivers during study visits, observations made by study clinicians during the clinical and neurologic evaluations, Blessed test performance27 (decrease of 4 or more points since the previous visit or more than 8 errors on the current visit) and a pattern of worsening scores (cut scores not used) on the neuropsychological test battery were compared to previous visits. As noted above, the SRT score was not used as part of the diagnostic process.
A diagnosis of dementia was assigned at case conferences attended by study neurologist, neuropsychologist, and a geriatric nurse clinician, using the DSM-III, and the DSM-III-R criteria after 1986.28,29 Updated criteria for dementia and subtypes were introduced after the study launch. To ensure uniformity of diagnosis, all cases in the inception cohort were reconferenced in 2001 by a neurologist and a neuropsychologist who did not participate in diagnostic conferences from 1980 to 1998.20 The diagnosticians had access to all available information for each subject at the conference, including results of any investigations performed at or after the study visit when dementia was diagnosed. Disagreements between raters were resolved by consensus after presenting the case to a second neurologist.
Leisure activities.
Leisure activities may be defined as activities that individuals engage in for enjoyment or well-being, independent of work or activities of daily living.12,15 At baseline, subjects were interviewed about participation in 6 leisure time cognitive activities (reading, writing, crossword puzzles, board or card games, group discussions, or playing music). We coded self-reported frequency of participation to generate a scale on which 1 point corresponded to participation in 1 activity for 1 day per week. For each activity, subjects received 7 points for daily participation; 4 points for participating several days per week; 1 point for weekly participation; and 0 points for participating occasionally or never. We summed activity days across activities to generate a Cognitive Activity Scale (CAS) for each participant.12 The intraclass correlation estimated from a random intercept mixed linear model fit via restricted maximum likelihood across all Bronx Aging Study participants responding to the questionnaire was 0.41, meaning that 59% of the total variability in CAS scores was within-subject variability. CAS scores on these scales were not correlated with age.12 These statistics suggest some stability in self-reported participation over time, an imperfect surrogate for cumulative lifetime exposure to cognitively stimulating activity, and therefore a potential predictor of future cognitive decline and incident disease.
Statistical methods.
We modeled scores on the SRT as a function of the subjects’ self-reported participation in cognitively stimulating leisure activities, and time before diagnosis of dementia (measured in years) for each subject contributing observations to the analysis. The basic conceptual model assumes that memory as measured by the SRT declines at a constant (possibly insignificant) rate before some unknown change point (presumed to be several years before dementia diagnosis), after which the decline would be more rapid. This assumption is supported by theory30 and by previously reported findings from this cohort11,19 and elsewhere.31 The rates of decline were also assumed to be constant in time, but random effects were used to allow that rate to vary across individuals. The expected SRT score at the change point, and the rates of decline before and after the change point, were estimated from the data. The change point and the rates of decline before and after were allowed to vary as a function of self-reported participation in the leisure activities as measured by the score on the CAS. Interaction terms were included in the model to allow the rates of cognitive decline before and after the change point to vary as a function of CAS score. Details, including model equations, are available in appendix e-1 on the Neurology® Web site at www.neurology.org. We also fit a similar model in which both CAS score and education were allowed to affect the change point and the rates of cognitive decline to consider the possibility that one of the 2 measures may confound or mediate the effect of the other.
If the change point were known a priori, the model would be a linear model in the unknowns; however, the unknown change point makes this part of a class of statistical models called nonlinear mixed effects models.32 Maximum likelihood, assuming normal distributions for the SRT scores and the random effects, was used to estimate all model unknowns, using the SAS procedure NLMIXED (SAS Institute, Cary, NC, http://www.sas.com). Missing clinic visits were assumed to be at random, and the Akaike information criterion33 was used to assess whether the random effects added to the model fit.
RESULTS
The 101 study participants contributing data to these analyses averaged 79.5 years of age at baseline (range 73.4–87.4 years). Sixty-two percent were women, and 91% were non-Hispanic whites. The mean score on the Buschke SRT23 at baseline was 32.8 (SD 10.8). The mean time to dementia diagnosis was 5.0 years (maximum 15.9 years), a total of 505 person-years of follow-up over 351 clinic visits.
The median score on the cognitive activity scale was 7 activity days (lower/upper quartiles 4, 11). Ten of the 101 participants reported no activities and 11 reported 1 activity day per week; at the other extreme, there was 1 report each of 16, 19, 21, 25, and 28 activity days; the last datum would indicate daily participation in 4 of the 6 queried activities. The correlations of the contributions of the activity days contributed by each of the 6 activities ranged from −0.093 to 0.216, showing that study participants who participate in 1 activity may not necessarily participate in others. Standardized Cronbach alphas ranged from 0.24 to 0.42.
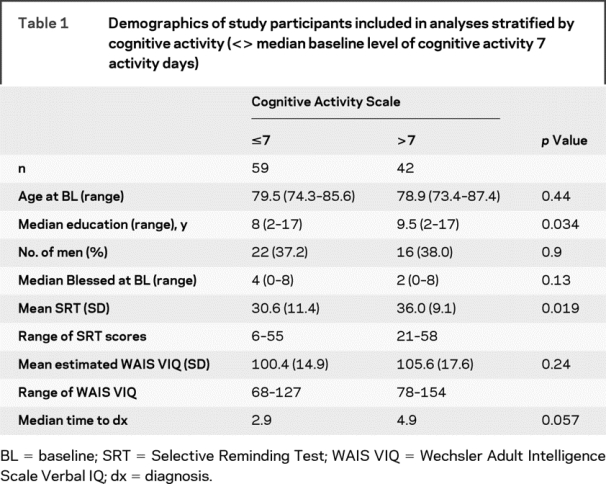
Table 1 lists demographics of the 101 participants by reported baseline cognitive activity.
Table 1 Demographics of study participants included in analyses stratified by cognitive activity (<> median baseline level of cognitive activity 7 activity days)
The Spearman correlation of SRT score at baseline with self-reported years of education was 0.20 (p = 0.048), and of SRT score with CAS score was 0.18 (p = 0.067); the Spearman correlation of CAS score and education was 0.25 (p = 0.012). This suggests that education, cognitive activity, and memory might be at least somewhat independent of each other. Forty-seven of the participants were classified as probable or possible AD, 25 as probable or possible vascular dementia, 23 as mixed dementia, and 6 as other subtypes (2 Lewy body dementia, 1 Pick disease, 2 vitamin B12 deficiency, 1 parkinsonian dementia). The median time from baseline to dementia diagnosis was 4.4 years (lower/upper quartiles 2.0, 6.7 years, maximum 15.9 years). Participation in cognitively stimulating leisure activities was not associated with the age at which dementia was diagnosed.
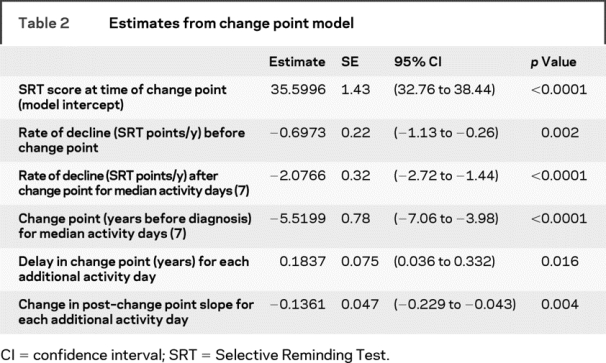
Table 2 shows the results for the change point model. Effect of cognitive activities is reported in terms of activity days (participation in 1 activity for 1 day in a week). Each additional activity day resulted in 0.18 years delay in the beginning of accelerated memory decline, but once the decline began, the rate of decline was 0.14 SRT points per year more rapid for each additional activity day. There was no evidence for heterogeneity in rate of decline either before or after the change point, so those random effects were dropped from the model. Similar results were observed when the analyses were restricted only to participants with a clinical subtype of AD.
Table 2 Estimates from change point model
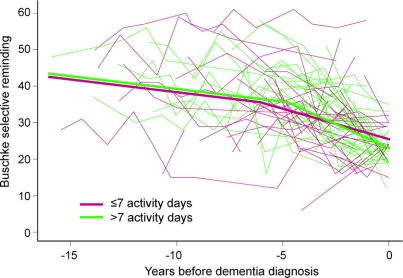
The figure shows the relationship between cognitive activity and the natural history of memory. A typical study participant whose cognitive activity participation was at the upper quartile (75% percentile) of the cohort, with 11 activity days per week, had his or her accelerated decline delayed by 1.29 years compared with a typical study participant whose cognitive activity participation was at the lower quartile (25%th percentile) with 4 activity days per week. The model results also indicate that participants who had reported more cognitive activity at baseline had nonsignificantly lower (poorer) scores on the SRT at the time of diagnosis.
Figure Memory performance as a function of time and cognitive activity
Narrow lines show individual participants’ repeated scores on the Buschke Selective Reminding Test over time; participants reporting more than 7 activity days are shown in green, and participants reporting 7 or fewer are shown in red. The wide lines show the expected trajectories for a hypothetical participant whose reported activity participation is at the 75th percentile (green, 11 activity days) and 25th percentile (red, 4 activity days).
These results show that participation in cognitively stimulating leisure activities has a similar effect on the natural history of memory decline in preclinical stages of dementia as did education in this same cohort.11 We then considered a model in which both education and leisure activity participation could affect the change point and the rate of post–change point decline. Education and cognitive participation were associated with both a delay in the onset of cognitive decline and an increase in the postacceleration rate of decline; however, the addition of education did not improve model fit (likelihood ratio test statistic = 2.0 with 2 degrees of freedom; p = 0.37. Similarly, when interaction terms between education and cognitive activity were added to the model, they proved nonsignificant.
DISCUSSION
We have previously shown that education delayed the onset of accelerated cognitive decline using the Buschke SRT as a measure of memory performance in persons who develop dementia.11 In this study, we show a nearly identical effect for participation in cognitively stimulating leisure activities in a slightly smaller subset of the same cohort. Each additional activity day of participation in cognitively stimulating leisure activities at baseline delayed the onset of accelerated memory decline in subjects who developed dementia by 0.18 years. However, once the decline began, the rate of decline was 0.14 SRT points per year more rapid for each additional activity day in those subjects. Our work is consistent with previous findings that participation in cognitive activities is associated with reduced rates of cognitive decline in healthy elderly16 but with more rapid cognitive decline in persons with AD,17 as well as earlier work that found that cognitive activity mediates the effect of education on the risk of AD.14
Participation in cognitive activity was associated with delayed onset of accelerated memory decline even though activity was measured only at a single point in time. Study volunteers were not asked how long they had been participating in these activities, or whether they had recently increased or decreased them. Thus the self-report at baseline was probably a quite imperfect measure of activity participation later in life, and the actual effect of participation in cognitive activities might be stronger than those we observed. Participants who had reported more cognitive activity at baseline had nonsignificantly lower (poorer) scores on the SRT at the time of diagnosis, possibly an artifact of later diagnosis because of the delayed functional impairment which is required for DSM dementia criteria. Diagnosis using only neuropsychological criteria might have shown an even larger effect of cognitive activity.
Our analysis of the individual activities that contributed information to the cognitive activities scale showed that persons who engage in 1 activity did not necessarily participate in others, resulting in low internal consistency of the CAS. Internal consistency is important when the measures are fallible indicators of an underlying construct, each estimating a latent variable. This was not unsurprising to us; the CAS was designed to cover a variety of measurable cognitive activities rather than an underlying theoretical construct, and the activities themselves are very different from each other. This creates the possibility that some of the activities included in the CAS may have much greater reserve effects than others. In addition, the CAS also did not allow for the potential for less than weekly activity to contribute to cognitive reserve. We plan to examine dose–response effects of individual cognitive activities in this and other cohorts in an attempt to better define the mechanisms by which particular cognitive activities might differentially affect cognitive reserve. The ultimate test of any reserve measure will be its predictive ability, regardless of the internal consistency of its constituent parts. We report our original cognitive activity scale because it was our a priori measure, was used in our previous work, and is similar to cognitive activity scales used elsewhere.14 We plan to report on reserve effects of individual activities in the future.
Cognitive activity remained significant after education was added as a predictor of the change point and the rate of decline, and education did not significantly add to the fit of the model in our analyses. One possible explanation is that the effect of early life education is mediated through late life participation in cognitively stimulating activities. If this is so, a cognitively active and engaged lifestyle may mediate the influence of early life education on cognitive reserve. Other studies have found that high-functioning individuals are more likely to participate in cognitively stimulating activities,18 but in our initially healthy cohort the correlation between cognitive activity and memory at baseline was small and nonsignificant, reducing the likelihood that the results are due to self-selection for participation in cognitive activity. A second possibility is that reserve could be primarily the function of late life cognitive activity, with education simply a marker that is both easier to measure and a predictor of late life activity. This would also support the conjecture that the mechanism behind cognitive reserve is the development of compensatory processes in response to participation in cognitive activities that mask the effects of brain damage from dementia pathology until some damage threshold is passed, rendering the compensation ineffective. Further evidence for this is that participation in cognitively stimulating leisure activities was not associated with a delay in the age of diagnosis of dementia. A third possibility is that despite the limitations noted above, the single self-report of participation in cognitive activities might be a better measure of cognitive reserve than the self-report of number of years of formal education. Self-reported education in this study is not a measure of the quality of intensity of early life education, because participants were asked only number of years of formal education and no attempt was made to adjust for quality. A fourth possibility is that there is indeed some shared variance between cognitive activity and education, but that the study lacked power to detect such an effect. A fifth possibility is some unusual characteristic of the Bronx elderly population that affects generalizability, although we have not found such a characteristic over many previous analyses. Nevertheless, the effect of participation in cognitive activities seems to be at least somewhat independent of education, suggesting that engagement in cognitive activities in late life might maintain cognitive vitality regardless of baseline educational attainment. A prospective intervention study could be designed to address these possibilities.
AUTHOR CONTRIBUTIONS
Dr. Hall performed all statistical analyses in the article.
DISCLOSURE
Dr. Hall serves on the editorial board of The Open Neurology Journal; has received honoraria for serving on peer-review panels for the National Cancer Institute; the Breast Cancer Research Program, Congressionally Directed Medical Research Programs, the US Army Department of Defense, the National Institute of Child Health and Human Development; and the National Institute on Aging (NIA); has received salary support from the NIH [P01 AG03949 (Core Leader), P01 AG027734 (Biostatistician), UL1-RR025750-01 (Biostatistician), K30 HL 04110 (Biostatistician), P30 CA13330-35 (Biostatistician), R01 AG017854, National Alzheimer’s Coordinating Center project 2008-01 (Biostatistician), P30 CA13330-33 (Biostatistician)], the US Department of Defense grant BC043301 (Biostatistician), and the US National Institute of Occupational Safety and Health grant U1O-OH008242 (Biostatistician); receives research support from the Breast Cancer Research Foundation, the American Cancer Society, and the NIH [5R01HL070785-05 (Consultant) and 5R01AG022092-05 (Consultant)]; and is a member of the American Statistical Association Media Experts List. Dr. Lipton receives research support from the NIH [PO1 AG03949 (Program Director), PO1AG027734 (Project Leader), RO1AG025119 (Investigator), K23AG030857 (Mentor), K23NS05140901A1 (Mentor), and K23NS47256 (Mentor)], the National Headache Foundation, and the Migraine Research Fund; serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache; has reviewed for the NIA and National Institute of Neurological Disorders and Stroke; holds stock options in Neuralieve Inc. and Minster Inc.; serves as consultant for Advanced Bionics, Allergan, Inc., Boehringer-Ingelheim, Endo, Glaxo Smith Kline, Kowa, Minster, Merck, Neuralieve, and Pfizer; and received honoraria from Allergan, Inc., Glaxo Smith Kline, and Merck, Inc. Dr. Sliwinski serves as Co-Editor-in-Chief of Aging, Neuropsychology and Cognition; has received honoraria for serving on a peer-review panel for the NIH (Cognition and Perception); and receives salary support from the NIH [P01 AG026728 (Principal Investigator) and AG03949 (Investigator)]. Ms. Katz has received research support from the NIH [2P01 AG03949 (Investigator), AG027734-01A1 (Investigator), and AG016976 (Investigator)]. Dr. Derby has received research support from the NIH [AG027734-01-A1 (Investigator) and AG03949 (Investigator)] and from the Thomas Hartman Foundation for Parkinson’s Research [Parkinson’s Research in Genetics, Hormonal Neuroprotection and Biomarkers]. Dr. Verghese serves on the speakers’ bureau of Pfizer; and has received research support from the NIA [PO1 AG03949 (Core Leader), RO1 AG025119 (Principal Investigator), R21 AG029799 (Principal Investigator), and K23 AG024848 (Principal Investigator)], the American Federation for Aging Research, and the Donald W Reynolds Foundation.
Supplementary Material
Address correspondence and reprint requests to Dr. Charles B. Hall, Department of Epidemiology and Population Health, and Department of Neurology, Albert Einstein College of Medicine, 1300 Morris Park Ave., Bronx, NY 10461 chall@aecom.yu.edu
Supplemental data at www.neurology.org
Supported in part by a program project grant from the National Institute on Aging (P01 AG03949). Original data collection in the Bronx Aging Study had been funded by earlier grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The sponsors played no role in the study design; nor in the collection, analysis, and interpretation of data; nor in the writing of the report; nor in the decision to submit the paper for publication. Dr. Hall had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Disclosure: Author disclosures are provided at the end of the article.
Received October 23, 2008. Accepted in final form April 22, 2009.
REFERENCES
- 1.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 2.Callahan MC, Hall KS, Hui SL, Musick BS, Unverzagt FW, Hendrie HC. Relationship of age, education, and occupation with dementia among a community-based sample of African Americans. Arch Neurol 1996;53:134–140. [DOI] [PubMed] [Google Scholar]
- 3.Qiu C, Backman L, Winblad B, Aguero-Torres H, Fratiglioni L. The influence of education on clinically diagnosed dementia incidence and mortality data from the Kumgsholmen project. Arch Neurol 2001;58:2034–2039. [DOI] [PubMed] [Google Scholar]
- 4.Katzman R, Aronson M, Fuld P, et al. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol 1989;25:317–324. [DOI] [PubMed] [Google Scholar]
- 5.Katzman R. A neurologist’s view of Alzheimer’s disease and dementia. Int Psychogeriatr 2004;16:259–273. [DOI] [PubMed] [Google Scholar]
- 6.Stern Y, Albert S, Tang M-X, Tsai W-Y. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 1999;53:1942–1947. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology 2000;55:370–376. [DOI] [PubMed] [Google Scholar]
- 8.Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol 2000;57:713–719. [DOI] [PubMed] [Google Scholar]
- 9.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–1095. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 2007;69:1657–1664. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348:2508–2516. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Mendes del Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–1914. [DOI] [PubMed] [Google Scholar]
- 15.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006;66:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghisletta P, Bickel JF, Lovden M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol Psychol Sci 2006;61:253–261. [DOI] [PubMed] [Google Scholar]
- 17.Helzner EP, Scarmeas N, Cosentino S, Porter F, Stern Y. Leisure activity and cognitive decline in incident Alzheimer disease. Arch Neurol 2007;64:1749–1754. [DOI] [PubMed] [Google Scholar]
- 18.Aartsena MJ, Smitsa CHM, van Tilburga T, Knipscheer KCPM, Deeg DJH. Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol Psychol Sci 2002;57:P153–P162. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Ying J, Kuo L, Lipton RB. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Computational Statistics and Data Analysis 2003;42:91–109. [Google Scholar]
- 20.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of Non-Alzheimer’s dementia. N Engl J Med 2002;347:1761–1768. [DOI] [PubMed] [Google Scholar]
- 21.Aronson MK, Ooi WL, Morgenstern H, et al. Women, myocardial infarction, and dementia in the very old. Neurology 1990;40:1102–1106. [DOI] [PubMed] [Google Scholar]
- 22.Hall CB, Verghese J, Sliwinski M, et al. Dementia incidence may increase more slowly after age 90: results from the Bronx Aging Study. Neurology 2005;65:882–886. [DOI] [PubMed] [Google Scholar]
- 23.Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior 1973;12:543–550. [Google Scholar]
- 24.Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology 1994;44:1427–1432. [DOI] [PubMed] [Google Scholar]
- 25.Masur DM, Fuld PA, Blau AD, Crystal H, Aronson MK. Predicting development of dementia in the elderly with the Selective Reminding Test. J Clin Exp Neuropsychol 1990;12:529–538. [DOI] [PubMed] [Google Scholar]
- 26.Masur DM, Fuld PA, Blau AD, Thal LJ, Levin HS, Aronson MK. Distinguishing normal and demented elderly with the selective reminding test. J Clin Exp Neuropsychol 1989;11:615–630. [DOI] [PubMed] [Google Scholar]
- 27.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114:797–811. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 30.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology 2003;60:1782–1787. [DOI] [PubMed] [Google Scholar]
- 32.Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics 1990;46:673–687. [PubMed] [Google Scholar]
- 33.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control 1974;19:716–723. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.