Abstract
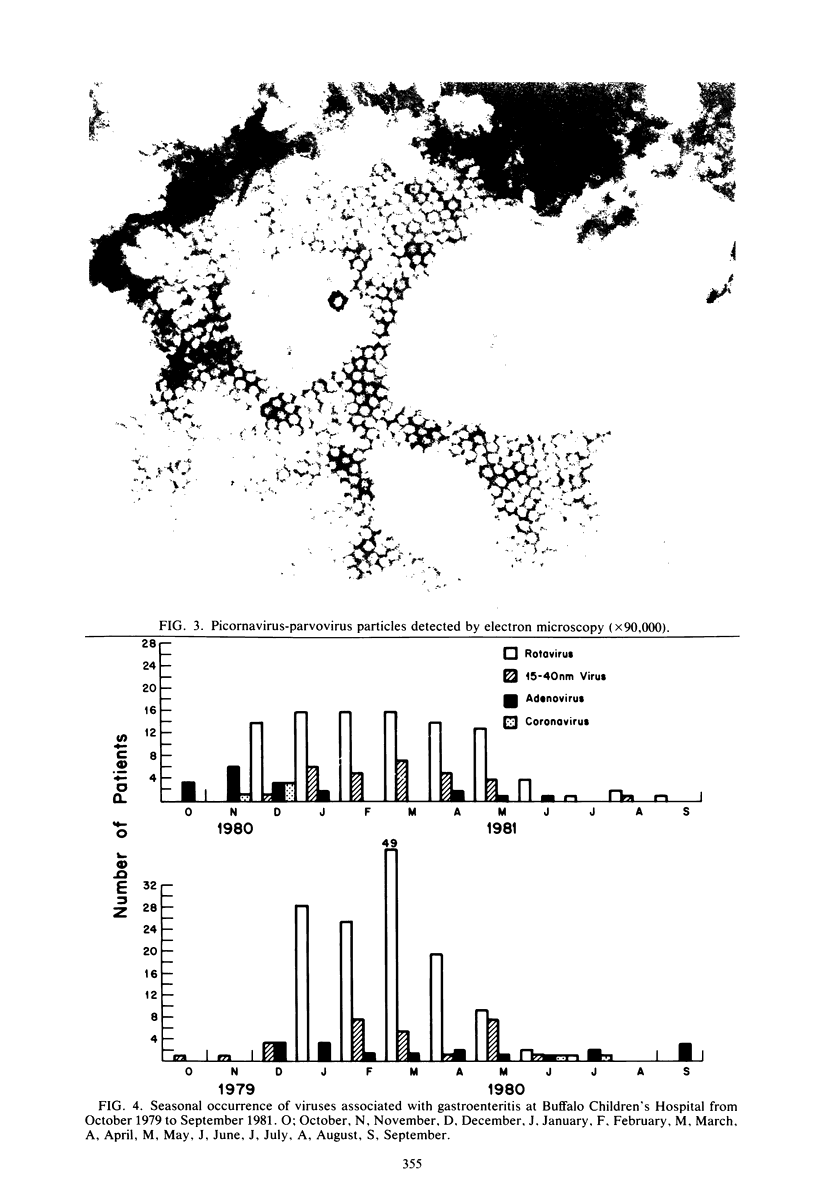
Fecal specimens were obtained from 1,160 infants and young children with acute nonbacterial gastroenteritis over a period of 2 years. A total of 100 specimens were obtained from age-matched asymptomatic controls. The specimens were examined for the presence of viruses by electron microscopy. Viruses or virus-like particles frequently associated with enteritis were detected in 27% (314 of 1,160) of the symptomatic patients. No viruses or virus-like particles were detected in the 100 control subjects. Rotavirus was detected in 73% (230 of 314) of the virus-positive samples. The mean age of rotavirus-positive patients was 11.5 months, although the patients ranged in age from 2 weeks to 5 years. Of the symptomatic patients, 45 (14%) exhibited small virus-like particles (15 to 40 nm) in the feces in the absence of any other detectable pathogen. Some of the virus-like particles observed in these patients appeared to be similar to astrovirus, and some appeared to be similar to the Otofuke agent or possibly minireovirus. Significantly, however, the mean age of infants with enteritis from whom these small virus-like particles were recovered was 4.5 months (range, 10 days to 19 months). Our findings confirmed the already-known fact that rotaviruses constitute the most important cause of viral enteritis in young children. In addition, small viruses may be an important cause of gastroenteritis in infants under 5 months of age.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. R., Caul E. O., Paver W. K. Astrovirus-associated gastroenteritis in children. J Clin Pathol. 1978 Oct;31(10):939–943. doi: 10.1136/jcp.31.10.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Sakuma Y., Kogasaka R., Akihara M., Terashima H., Horino K., Nakao T. Fecal shedding of virus in relation to the days of illness in infantile gastroenteritis due to calicivirus. J Infect Dis. 1980 Aug;142(2):247–249. doi: 10.1093/infdis/142.2.247. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Bishop R. F., Townley R. R., Holmes I. H. Importance of a new virus in acute sporadic enteritis in children. Lancet. 1975 Feb 1;1(7901):242–246. doi: 10.1016/s0140-6736(75)91140-x. [DOI] [PubMed] [Google Scholar]
- Flewett T. H. Electron microscopy in the diagnosis of infectious diarrhea. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):538–543. [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Dolin R., Thornhill T. S., Kalica A. R., Chanock R. M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972 Nov;10(5):1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogasaka R., Nakamura S., Chiba S., Sakuma Y., Terashima H., Yokoyama T., Nakao T. The 33- to 39-nm virus-like particles, tentatively designed as Sapporo agent, associated with an outbreak of acute gastroenteritis. J Med Virol. 1981;8(3):187–193. doi: 10.1002/jmv.1890080305. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Lee T. W., Craig J. W., Reed S. E. Astrovirus infection in volunteers. J Med Virol. 1979;3(3):221–230. doi: 10.1002/jmv.1890030308. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Lee T. W., Pickering D. Astrovirus associated gastroenteritis in a children's ward. J Clin Pathol. 1977 Oct;30(10):948–952. doi: 10.1136/jcp.30.10.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeley C. R., Cosgrove B. P. Letter: 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975 Sep 6;2(7932):451–452. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- Madeley C. R., Cosgrove B. P. Letter: Caliciviruses in man. Lancet. 1976 Jan 24;1(7952):199–200. doi: 10.1016/s0140-6736(76)91309-x. [DOI] [PubMed] [Google Scholar]
- McSwiggan D. A., Cubitt D., Moore W. Calicivirus associated with winter vomiting disease. Lancet. 1978 Jun 3;1(8075):1215–1215. doi: 10.1016/s0140-6736(78)91012-7. [DOI] [PubMed] [Google Scholar]
- Middleton P. J., Szymanski M. T., Petric M. Viruses associated with acute gastroenteritis in young children. Am J Dis Child. 1977 Jul;131(7):733–737. doi: 10.1001/archpedi.1977.02120200015004. [DOI] [PubMed] [Google Scholar]
- Rodriguez W. J., Kim H. W., Arrobio J. O., Brandt C. D., Chanock R. M., Kapikian A. Z., Wyatt R. G., Parrott R. H. Clinical features of acute gastroenteritis associated with human reovirus-like agent in infants and young children. J Pediatr. 1977 Aug;91(2):188–193. doi: 10.1016/S0022-3476(77)80810-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt H. C., Marks M. I., Gomersall M., Gill P., Pai C. H. Nosocomial infantile gastroenteritis associated with minirotavirus and calicivirus. J Pediatr. 1978 Dec;93(6):922–926. doi: 10.1016/S0022-3476(78)81212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Konno T., Kutsuzawa T., Imai A., Tazawa F., Ishida N., Katsushima N., Sakamoto M. The occurrence of calicivirus in infants with acute gastroenteritis. J Med Virol. 1979;4(4):321–326. doi: 10.1002/jmv.1890040410. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Virus-like particle, 35 to 40 nm, associated with an institutional outbreak of acute gastroenteritis in adults. J Clin Microbiol. 1979 Nov;10(5):730–736. doi: 10.1128/jcm.10.5.730-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindling A. M., Walker-Smith J. A., Bird R. Micro-organisms in outpatient infantile gastroenteritis. Arch Dis Child. 1980 Mar;55(3):185–188. doi: 10.1136/adc.55.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]





