Abstract
Avian influenza viruses (AIV), the causative agent of avian flu or bird flu, cause widespread morbidity and mortality in poultry. The symptoms of the disease range from mild flu like symptoms to death. These viruses possess two important surface glycoproteins, namely hemagglutinin (HA) and neuraminidase (NA) against which neutralizing antibodies are produced. Due to the highly mutative nature of the genes which encode these proteins, the viruses often confer resistance to the current anti-viral drugs making the prevention and treatment of infection challenging. In our laboratory, we have recently identified a novel anti-viral peptide (P1) against the AIV H9N2 from a phage displayed peptide library. This peptide inhibits the replication of the virus in ovo and in vitro by its binding to the HA glycoprotein. In the current study, we demonstrate that the peptide inhibits the virus replication by preventing the attachment to the host cell but it does not have any effect on the viral fusion. The reduction in the viral nucleoprotein (NP) expression inside the host cell has also been observed during the peptide (P1) treatment. This novel peptide may have the potential to be developed as a therapeutic agent for the treatment and control of avian influenza virus H9N2 infections.
Keywords: Influenza, peptide inhibitors, fluorescence microscopy, flow cytometry
1. Introduction
Avian influenza virus belongs to the family of Orthomyxoviridae which comprises three genera influenza A, B and C. The influenza A virus has been divided into several subtypes based on the variation in the HA and NA glycoproteins. There are currently 16 HA and 9 NA subtypes circulating among wild birds 1. Although these viruses are present in the wild birds they usually do not cause any disease among them but the poultry birds are severely affected by highly pathogenic form of AIVs 1. Other than culling the birds 2 there are currently two classes of anti-viral drugs being administered for the control and treatment of AIV infection. They are adamantane derivatives (amantadine and rimantadine) and neuraminidase inhibitors (NAI; zanamivir and oseltamivir) 3-5. The increasing rate in the emergence of adamantane and NAI resistant strains stresses the need to develop new class of anti-viral drugs 4, 6-11.
In our laboratory, we have recently identified a peptide based anti-viral molecule against the AIV H9N2 12. The peptide was protective against the virus replication in ovo and in vitro and inhibited the hemagglutination activity of the virus. The peptide's ability to compete with the anti-AIV antibodies proved that they share some common binding sites. From yeast two-hybrid and co-immunoprecipitation experiments, it has been observed that the peptide binds with the HA protein of the virus 12 suggesting that the peptide could either be involved during the attachment of the virus to the host cell receptors or in the fusion of the virus into the infected cells 13.
In an effort to understand the mechanism of action by the P1 peptide during virus replication, we demonstrate here that the P1 inhibits the latter by preventing its attachment to the host cell. We also show that it does not have any effect on the fusion ability of virus.
2. Materials and Methods
2.1 Virus Propagation and Purification
Avian influenza A/Chicken/Iran/16/2000(H9N2), a low pathogenic avian influenza virus was kindly provided by Abdul Rahman Omar. Viruses were propagated in 9-days old specific pathogen free embryonated chicken eggs. The allantoic fluid was clarified and the viruses were purified and concentrated as explained previously 14. The virus titer was determined by hemagglutination test (HA) and the protein concentration of the purified virus was determined by Bradford assay 15.
2.2 Fluorescent labeling of the virus
The sucrose gradient purified viruses were labelled with FITC using the FITC labelling kit (Pierce, USA) as per the instructions given by the manufacturer. The infectious ability of the FITC labelled viruses was confirmed by observing the formation of cytopathic effects (CPE) in MDCK cells.
2.3 Peptides
Peptides were synthesised at GL Biochem, Shanghai, China with more than 98% purity. The peptides contained the sequences as mentioned in Table 1.
Table 1.
Peptides used in this study
| Name of the peptide | Sequence of the peptide |
|---|---|
| P1 | CNDFRSKTC |
| Control Peptide | CSWGEYDMC |
2.4 Immunofluorescence assay
MDCK cells were seeded on glass coverslips (Secureslip™, Sigma, USA) at a density of 5 x 104, were inoculated with medium alone, virus (moi of 0.5) or peptide-treated (0 to 100 µM) virus for 1 h on ice. The unbound viruses were removed by washing with magnesium and calcium free cold PBS. For entry-based assays, untreated virus was allowed to attach on ice for 1 h, followed by washing and addition of medium containing peptide (0 to 50 µM). Cells were shifted to 37°C for 6 h, fixed with ice cold methanol and permeabilised with acetone. They were stained for nucleoprotein with mouse monoclonal anti-nucleoprotein antibodies (AA5H, 1:100; Abcam, USA) for 1 h and FITC-labelled goat polyclonal secondary antibody (1:200; Abcam, USA) diluted in PBS containing 0.1 % Tween-20 and 1 % BSA for 1 h at room temperature. The coverslip was air dried and mounted in ProLong Gold (Molecular Probes, USA), and fluorescence was examined on a microscope (Leica Microsystem, Wetzlar, Germany) at a total magnification of x100. Three randomly selected fields in each experimental group were counted for nucleoprotein positive cells within this group. Data presented are the mean of at least three independent experiments.
2.5 Flow cytometry
MDCK suspension cells (1 x 106) were infected with FITC-labelled peptide treated (0 to 50 µM) or untreated AIV H9N2 virus (16 HA units) for 1 h on ice. After rigorous washing with PBS, the cells were fixed with 1 % paraformaldehyde, and the binding was determined by flow cytometric analysis (Cyan ADP, Dako, Denmark) after 24 hrs. Forward and side scatter characteristics were analysed for 5000 events of mock-infected or infected cells.
2.6 Statistical analysis
All experiments were carried out in triplicate and are representative of at least three separate experiments unless otherwise mentioned. The results represent the means ± standard deviations of triplicate determinations. Statistical significance of the data was determined by student's t test or ANOVA method using SPSS 13.0 software. A P value of < 0.05 was considered statistically significant.
3. Results and Discussion
3.1 Effect of inhibitory peptide on viral attachment
To evaluate the effect of the anti-viral peptide on viral attachment, a flow cytometry based experiment was used. Briefly, MDCK suspension cells were incubated with FITC-labelled, peptide P1 treated (0 to 50 µM) or untreated AIV H9N2 virus for 1 h on ice. The fluorescence of virus infected cells was reduced with increasing concentration of peptide treatment in a dose dependent manner. At 50 µM concentration of the peptide treatment, the fluorescence was reduced significantly (p < 0.05) i.e., more than 50 % (Table 2). The control peptide did not show any significant reduction (P > 0.05) in the fluorescence (Table 2). This result suggests that the peptide prevents the attachment of the virus to the host cell receptor. This result is in consistent with our earlier observation of inhibition of hemagglutination activity of the virus 12 and again ensures that the peptide act by binding with the HA protein of the virus.
Table 2.
Effect of the peptides on viral attachment to host cells
| Peptide (P1) Concentration | % Fluorescence after treatment ^ |
|---|---|
| 0 µM | 100 |
| 10 µM | 70.08 ± 1.94 * |
| 20 µM | 62.12 ± 2.48 * |
| 50 µM | 47.58 ± 1.27 * |
| Control peptide 50 μM | 98.3 ± 1.04 |
^ The results are mean ± standard deviation of three separate experiments. All the values in the above table were normalized to 0 μM treatment which was considered to possess 100 % fluorescence. *, Statistical significance (P < 0.05)
3.2 Effect of inhibitory peptides on viral entry
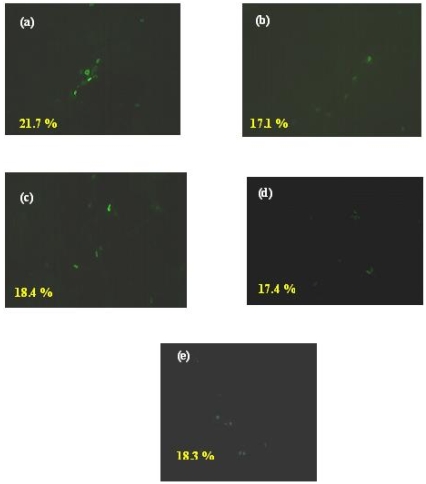
To investigate the effects of peptide on viral entry into the host cells, a fluorescence microscopy based experiment was performed based on the hypothesis that the early protein expression will be inhibited if the virus entry is prevented. Briefly, MDCK cells were incubated with medium alone or infected with the H9N2 virus at 4°C to allow virus attachment to the cells. After one hour incubation period, the cells were incubated with various concentration of (0 to 50 µM) cyclic peptide for 15 min at 4°C. This was followed by incubation at 37°C for 6 h as the entry requires higher temperature (37°C) 16-18. Later, the cells were stained for viral nucleoprotein (NP) to detect the presence of viral particles inside the cell. Three randomly selected fields in each experimental group were counted for nucleoprotein positive cells within this group. The NP protein expression pattern was similar in all cases. Both the anti-viral peptide 'P1' and control peptide do not reduce the NP protein expression significantly (P > 0.05) even at higher concentration (50 µM). The fluorescence stained NP protein expression was around 20 % in all concentrations of peptide treatment (Figure 1). Together with the flow cytometry based experiment explained above, this implies that the peptide does not affect the viral entry but affects only the viral attachment. Although the viral attachment and entry is controlled by the same HA protein of the virus, specific regions on the HA have been shown to be involved in either entry or attachment 16, 19. Therefore, it was possible that only regions which are not involved in entry are involved in the P1 interaction with the host.
Figure 1.
Effect of the peptide P1 on viral entry. MDCK cells were infected with H9N2 virus (moi of 0.5) for 1 h on ice. After the unbound viruses were removed by washing peptides were added for 15 min at 4°C; the temperature was shifted to 37°C for 6 hrs and NP expressed was determined. Frame (a) & (c) - Peptide P1; 30 & 50 µM respectively, Frame (b) & (d) - Control peptide [CSWGEYDMC]; 30 & 50 µM respectively; Frame (e) No peptide treatment. The results represent the means of triplicate determinations.
3.3 Effect of inhibitory peptides on viral early protein expression
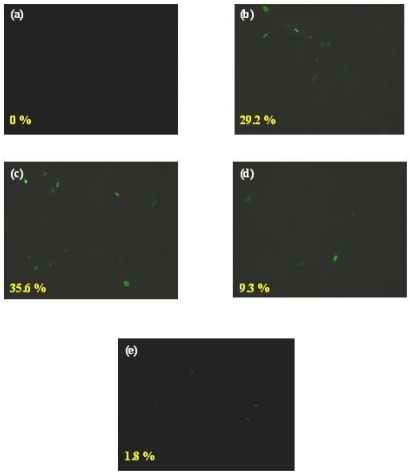
In order to examine whether the P1 peptide inhibited the early or late stage of viral replication, NP expression was monitored by immunofluorescence microscopy. NP is one of the immediate early proteins expressed during the AIV replication inside the host cell. Viruses were treated with various concentrations of P1 as well as control peptides (0 to 50 µM). MDCK cells were incubated with peptide treated or untreated virus H9N2 (moi of 0.5) for 1 h at 4°C to synchronize the infection. Unbound viruses were removed by rigorous washing with PBS. Then the temperature was shifted to 37°C for 6 hrs to allow the internalization of the virus inside the host cells. After fixation and permeabilization, the cells were stained for NP protein expression with fluorescent labelled antibodies as explained above and examined under immunofluorescence microscope. Three different fields from each treatment and each containing 50 cells were counted to determine the percentage of NP positive cells. The level of NP expression was reduced with increasing concentration of peptide P1 treatment. More than 29 % of control peptide treated or untreated (Figure 2; frame b and c) cells were NP positive when compared to mock infected cells (Figure 2; frame a). The NP expression was reduced at a dose dependent manner. At 50 µM peptide treatment, the positive cells were 9 % (Figure 2; frame d) whereas at 100 µM peptide treatment, it was further reduced to less than 2 % (Figure 2; frame e). It was a statistically significant reduction (p < 0.05). The observation shows that the peptide P1 inhibits the early stage of the viral infection independent of the prevention of virus entry. There was a small reduction in the early gene expression with control peptide treatment. This reduction is not statistically significant. Besides, this reduction was not dose dependant.
Figure 2.
Effect of anti-viral peptide on early gene expression. MDCK cells were incubated with medium alone (a), control peptide [CSWGEYDMC] (30 µM) treated (b) or peptide P1 treated [0 µM - (c); 50 µM - (d); 100 µM - (e)] viruses H9N2 (moi of 0.5) for 1 h on ice. After shifting the temperature to 37°C, the NP protein expression was monitored by immunofluorescence microscopy. The results represent the means of triplicate determinations. *, Statistical significance (P < 0.05)
Viruses should penetrate the host cells in order to cause infection. Like most of the enveloped viruses, the influenza viruses use receptor binding and fusion as principal route of entry. The HA protein of the virus interacts with the host cell sialic acid receptors and enters by receptor-mediated endocytosis 20. There are two important binding regions in the HA protein of the influenza virus which are responsible for the attachment and entry of the virus to the host cell. The attachment region is present in the HA1 domain whereas the binding or fusion region is present in the HA2 domain of the protein and their functions are independent of each other 13. Although the virus can attach to the host cell receptors even at 4° C, the fusion protein requires 37° C for its activity 16, 21, 22. Based on this fact, the study was designed to investigate whether the anti-viral peptides inhibited the attachment or the entry of virus to the cell. The observation showed us that the peptide inhibit the virus replication by blocking its attachment but not the entry.
Prevention of viral entry is an attractive anti-viral strategy as it can minimize the chance of virus evolution and subsequent drug resistant strain development. It can inactivate the virus at an early stage and block the cell-cell spread 23. Besides, since the entry protein is present extracellularly, the drug molecules can reach the target easier than intracellular viral proteins.
Once the virus enters the cell, the ribonucleoproteins are released into the cytoplasm and then transported to the nucleus. It is one of the early proteins expressed upon the onset of viral infection 24. So, the investigation of the expression level of influenza virus nucleoprotein (NP) is a good indicator to check the efficiency of the viral inhibitor. This experiment shows that the entry inhibitor blocks the early viral replication independent of whether the virus fusion or entry is inhibited or not.
4. Conclusion
In conclusion, the novel peptide identified in our previous study inhibits the avian influenza virus replication by blocking its attachment to the host cell and thereby preventing the early viral genes expression. Given the importance to the public health problems caused by these viruses, the understanding of virus-cell interaction and the prevention of this early step of infection is necessary to combat the disease. We believe that the P1 peptide has the potential to study the host-pathogen interaction and also possess therapeutic qualities.
Acknowledgments
This project is supported by the Ministry of Science, Technology and Innovation (MOSTI) of Government of Malaysia grant No.01-02-04-009 BTL/ER/38. Rajik is supported by the Universiti Putra Malaysia graduate research fellowship. The authors thank Ms. Hamidah binti ali kamarulzaman for her help in statistical analysis.
References
- 1.Fouchier RA, Munster V, Wallensten A. et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79(5):2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenbeck JE. An Avian Connection as a Catalyst to the 1918-1919 Influenza Pandemic. Int J Med Sci. 2005;2(2):87–90. doi: 10.7150/ijms.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353(13):1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362(9397):1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C, Takeuchi K, Pinto LH. et al. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67(9):5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bright RA, Shay DK, Shu B. et al. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA. 2006;295(8):891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- 7.de Jong MD, Tran TT, Truong HK. et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 8.Hayden FG. Respiratory viral threats. Curr Opin Infect Dis. 2006;19(2):169–178. doi: 10.1097/01.qco.0000216628.51563.b1. [DOI] [PubMed] [Google Scholar]
- 9.Hurt AC, Selleck P, Komadina N. et al. Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Res. 2007;73(3):228–231. doi: 10.1016/j.antiviral.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Le QM, Kiso M, Someya K. et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 11.Puthavathana P, Auewarakul P, Charoenying PC. et al. Molecular characterization of the complete genome of human influenza H5N1 virus isolates from Thailand. J Gen Virol. 2005;86(2):423–433. doi: 10.1099/vir.0.80368-0. [DOI] [PubMed] [Google Scholar]
- 12.Rajik M, Jahanshiri F, Omar AR. et al. Identification and characterisation of a novel anti-viral peptide against avian influenza virus H9N2. Virol J. 2009;6(1):74. doi: 10.1186/1743-422X-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb RA, Krug RM. Orthomyxoviridae: The viruses and their replication. In: Fields BN, Knipe DM, Howley PM, eds, Fields virology, Philadelphia: Lippincott-Raven Publishers; 1996. pp. 1353–1395. [Google Scholar]
- 14.Ramanujam P, Tan WS, Nathan S. et al. Novel peptides that inhibit the propagation of Newcastle disease virus. Arch Virol. 2002;147(5):981–993. doi: 10.1007/s00705-001-0778-y. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 16.Hahon N, Booth JA, Eckert HL. Cell attachment and penetration by influenza virus. Infect Immun. 1973;7(3):341–351. doi: 10.1128/iai.7.3.341-351.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haywood AM, Boyer BP. Time and temperature dependence of influenza virus membrane fusion at neutral pH. J Gen Virol. 1986;67( Pt 12):2813–2817. doi: 10.1099/0022-1317-67-12-2813. [DOI] [PubMed] [Google Scholar]
- 18.Jones JC, Turpin EA, Bultmann H. et al. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J Virol. 2006;80(24):11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy BR, Webster RG. Orthomyxoviruses. In: Fields BN, Knipe DM, Howley PM, eds, Fields Virology, Philadelphia: Lippincott-Raven; 1996. pp. 1397–1445. [Google Scholar]
- 20.Lakadamyali M, Rust MJ, Zhuang X. Endocytosis of influenza viruses. Microbes Infect. 2004;6(10):929–936. doi: 10.1016/j.micinf.2004.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones JC, Turpin EA, Bultmann H. et al. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J Virol. 2006;80(24):11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramalho-Santos J, Nir S, Duzgunes N. et al. A common mechanism for influenza virus fusion activity and inactivation. Biochemistry. 1993;32(11):2771–2779. doi: 10.1021/bi00062a006. [DOI] [PubMed] [Google Scholar]
- 23.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herz C, Stavnezer E, Krug R. et al. Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]