Abstract
On the mouse egg, the tetraspanin CD9 is nearly essential for sperm-egg fusion, with another tetraspanin, CD81, playing a complementary role. Based on what is known about these proteins, egg tetraspanins are likely to be involved in regulation of membrane order through associations with other egg membrane proteins. Here we identify a first-level interaction (stable in 1% Triton X-100) between CD9 and the Immunoglobulin Superfamily member IgSF8 (also known as EWI-2), the first evidence in eggs of such an interaction of CD9 with another protein. We next compared the effects of antibody-mediated perturbation of IgSF8 and CD9, evaluating the robustness of these perturbations in in vitro fertilization conditions that heavily favor fertilization and in which fertilization occurs less frequently. These studies demonstrate that IgSF8 participates in mouse gamete interactions, and identify discrete effects of antibody-mediated perturbation of CD9 and IgSF8. An anti-IgSF8 antibody had moderate inhibitory effects on sperm-egg binding, whereas an anti-CD9 antibody significantly inhibited sperm-egg fusion, and in certain assays, had an inhibitory effect on binding as well. This work highlights the critical importance of design of IVF experiments for the detection of different effects of experimental manipulations on gamete interactions.
Keywords: Immunoglobulin Superfamily, tetraspanin, EWI-2, sperm-egg fusion, sperm-egg binding
INTRODUCTION
The interaction of mammalian gametes is mediated by multiple molecules on the egg and sperm, but the mechanistic basis of gamete membrane binding and fusion is still poorly understood. Notable molecules include the immunoglobulin superfamily protein (IgSF) Izumo on the sperm (Inoue et al. 2005) and the tetraspanins CD9 and CD81 on the egg (Kaji et al. 2000; Le Naour et al. 2000; Miyado et al. 2000; Rubinstein et al. 2006a). The tetraspanins are family of membrane proteins that mediate processes such as cell signaling, motility, and adhesion in a wide variety of cell types (Boucheix and Rubinstein 2001; Hemler 2003). CD9 has been shown to play a near-essential role in egg plasma membrane function during fertilization, with CD81 having a complementary role, based on studies of CD9−/− and CD81−/− females (Kaji et al. 2000; Le Naour et al. 2000; Miyado et al. 2000; Rubinstein et al. 2006a). In vitro fertilization (IVF) experiments with zona pellucida (ZP)-free eggs reveal defects in sperm-egg fusion. Combining data from the three original CD9 knockout reports, only 6/135 ZP-free CD9−/− eggs were fertilized in IVF (Kaji et al. 2000; Le Naour et al. 2000; Miyado et al. 2000). In a comparative study with both knockouts, the percentages of two-cell embryos resulting from IVF were 85% for wild type eggs, 1% for eggs from CD9−/− mice, and 11% for eggs from CD81−/− mice (Rubinstein et al. 2006a). Mating trials reveal that only ˜60% of CD9−/− and CD81−/− females became pregnant (versus 100% of wild type females), with time to pregnancy being longer and litter sizes being smaller in the CD9−/− females as compared to wild type and CD81−/− females (Rubinstein et al. 2006a). CD9−/− CD81−/− females are completely infertile (Rubinstein et al. 2006a). The fertilizability of CD9−/− eggs can be partially rescued by expression of CD81, although rescue is more effective with expression of CD9 (Kaji et al. 2002), suggesting that CD9 plays a distinct role in fertilization that is not completely replicated by CD81.
Based on what is known about tetraspanins, the role that egg tetraspanins play is likely linked with their associations in cis with other egg proteins; the functions in eggs include regulating membrane order (Runge et al. 2007) with recent data also suggesting a novel function in transfer of egg membrane vesicles to sperm (Barraud-Lange et al. 2007; Miyado et al. 2008). Tetraspanins interact with other proteins in complexes that have been called tetraspanin webs; tetraspanins appear to function as “organizer” molecules in these networks on the cell surface (Boucheix and Rubinstein 2001; Hemler 2003), and tetraspanin webs could be considered analogous to lipid rafts (Claas et al. 2001; Hemler 2001). Tetraspanins associate with growth factor receptors, G-protein coupled receptors, and various types of cell adhesion molecules, including IgSF proteins and integrins (Andre et al. 2006; Boucheix and Rubinstein 2001; Hemler 2003). Based on gel filtration and SDS-PAGE analyses of tetraspanin complexes isolated in mild detergents such as CHAPS or Brij99, these tetraspanin webs appear to be large (˜10 million Da) and composed of several different proteins (Claas et al. 2001; Skubitz et al. 2000; Stipp et al. 2001a; Stipp et al. 2001b).
The work here focused on the Immunoglobulin Superfamily member IgSF8 (also known as EWI-2, KAI/CD82-associated protein, and prostaglandin-regulatory-like [PGRL] protein). IgSF proteins have extracellular domains with a variable number of immunoglobulin (Ig)-like domains that can participate in cell-cell adhesion (Aricescu and Jones 2007; Barclay 2003). Furthermore, an IgSF member, Izumo, is an essential protein on mouse sperm for gamete fusion (Inoue et al. 2005). Two tetraspanin-associated IgSF members, IgSF8 and Prostaglandin F2 Receptor Negative Regulator (PTGFRN; also known as EWI-F and CD9-P-1) have recently been reported to be expressed by mouse eggs (Rubinstein et al. 2006a; Runge et al. 2007). Preliminary studies of PTGFRN have thus far not provided evidence of a role in fertilization (Rubinstein et al. 2006a), but the role of IgSF8 has not been investigated. IgSF8 associates with CD9 and/or CD81 in several cell types, and functions in cell aggregation, cell motility, and induction of localization of several tetraspanin proteins to filopodia (Charrin et al. 2003; Kolesnikova et al. 2004; Sala-Valdes et al. 2006; Stipp et al. 2001a), and therefore we hypothesized that IgSF8 on the egg could participate in fertilization and gamete membrane interactions.
In this study, we examine the association of IgSF8 with CD9 and CD81 and assess the relative effects of an anti-IgSF8 and anti-CD9 antibodies on sperm binding and fusion, using in vitro fertilization (IVF) insemination conditions varying the ratio of sperm to the eggs in the insemination drops (from 25:1 up to 500:1). This concept has been applied to assessments of sperm fertilizing ability (Amann and Hammerstedt 2002; Fearon and Wegener 2000), and here we apply it to assessing the ability of eggs to support gamete membrane interactions. Challenging eggs with a sperm:egg ratio of 500:1 heavily favors fertilization, whereas fertilization occurs less frequently when eggs are challenged with a sperm:egg ratio of 25:1; this latter condition allows characterization of experimental manipulations that have somewhat subtle effects.
MATERIALS AND METHODS
Egg collection and zona pellucida (ZP) removal
All work with animals was reviewed and approved by the Johns Hopkins University Animal Care and Use Committee. Metaphase II eggs were collected as previously described (Gardner et al. 2007) from 6- to 8-week-old superovulated CF-1 mice (Harlan, Indianapolis, USA) at 13 hr post-hCG injection. Cumulus cells were removed by incubation (< 5 min) in Whitten's medium ((Whitten 1971); 109.5 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM glucose, 0.23 mM pyruvic acid, 4.8 mM lactic acid hemicalcium salt, 7 mM NaHCO3 and 15 mM HEPES [hereafter referred to as “Whitten's-HEPES”]) containing 0.025% Type IV-S hyaluronidase (Sigma Chemical Co., St. Louis, MO) and 3 mg/ml BSA (Albumax I; Invitrogen, Carlsbad, CA). The ZP were removed by a brief incubation (∼15 sec) in low pH medium (10 mM HEPES, 1 mM NaH2PO4, 0.8 mM MgSO4, 5.4 mM KCl, 116.4 mM NaCl; pH 1.5) and the eggs were then allowed to recover for 60 min in Whitten's medium lacking the 15 mM HEPES and containing 22 mM NaHCO3 and 15 mg/ml BSA (hereafter referred to as Whitten's-Bicarbonate). Eggs were cultured in a humidified atmosphere of 5% CO2 in air.
Immunoblotting and immunoprecipitation
For immunoblot analysis, eggs were lysed in 20 μl of 2X SDS-DTT sample loading buffer (125 mM Tris, pH 6.8, 69 mM SDS, 20% glycerol, and 14.5 μM bromophenol blue) and stored at −20°C until use. Egg lysates were separated by SDS-PAGE under reducing conditions and transferred to an Immobilon membrane. Blots were blocked overnight in PBS-T (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 1% Tween-20) containing 10% cold water fish gelatin (Sigma), and then incubated with the affinity-purified goat anti-mouse IgSF8 polyclonal antibody (4 μg/ml; R&D Systems, Minneapolis, MN) in PBS-T with 3% BSA, followed by HRP-conjugated donkey anti-goat IgG (0.16 μg/ml; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Protein was detected using SuperSignal chemiluminescent substrate (Pierce Chemical Co., Rockford, IL).
For immunoprecipitations, eggs (150-200 eggs per sample) were lysed for 30 min on ice in lysis buffer (150 mM NaCl, 25 mM Hepes, 5 mM MgCl2) containing either 1% Triton X-100 or 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) detergent. For certain experiments (Fig. 1A), ZP-free eggs were surface-biotinylated with EZ-Link Sulfo-NHS-LC-Biotin (Pierce) prior to lysis. Lysates were pre-cleared for 1 hr at 4°C with Protein G-agarose beads and then incubated overnight at 4°C with Protein G-agarose beads that had been previously coupled to the anti-CD9 monoclonal antibody, KMC8.8 (BD Biosciences, San Jose, CA) or the anti-CD81 monoclonal antibody, EAT2 (Biolegend, San Diego, CA). The beads were washed three times in lysis buffer containing the same detergent used for lysis (1% Triton X-100 or CHAPS as indicated), and then boiled in SDS-PAGE sample buffer. Immunoprecipitated samples were resolved by SDS-PAGE and transferred to an Immobilon membrane. IgSF8 or CD9 in these immunoprecipitated samples was detected by immunoblotting; Restore Western Blot Stripping Solution (Pierce) was used for stripping blots that were to be probed with the two different antibodies.
Figure 1. IgSF8 expression and association with CD9 in mouse eggs.
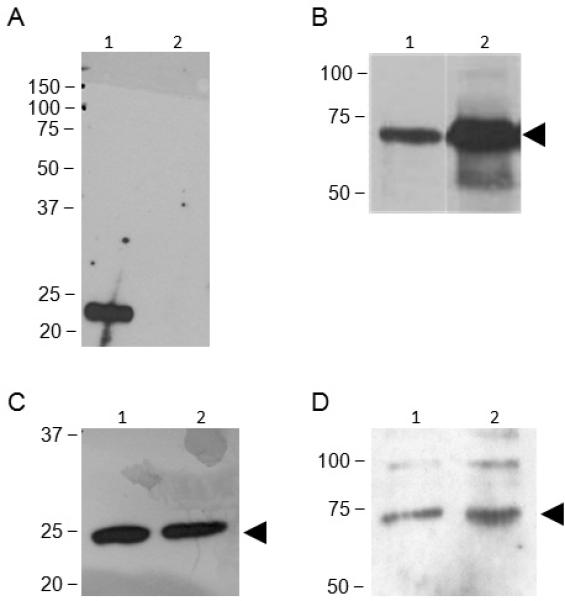
Panel A: ZP-free eggs (150 per lane) were surface-biotinylated and then lysed in buffer containing 1% Triton X-100; immmunoprecipitations were performed with an anti-CD9 antibody (lane 1) or nonimmune rat IgG (lane 2). Detection was performed with avidin-HRP; CD9 is present in immunoprecipitations using the anti-CD9 antibody but not the nonimmune IgG. Panel B: Immunoblot analysis shows IgSF8 protein expression in mouse eggs. Lane 1: Lysate of 75 eggs (1.88 μg). Lane 2: Lysate of mouse brain (0.5 μg). Panels C and D: ZP-free mouse eggs (200 per lane) were lysed in 1% Triton X-100 (lane 1) or CHAPS (lane 2) and immunoprecipitated with an anti-CD9 antibody, and then immunoblotted with an anti-CD9 antibody (Panel C) or an anti-IgSF8 antibody (Panel D). These immunoprecipitations show co-immunoprecipitation of IgSF8 with CD9.
Immunofluorescence
Germinal vesicle (GV)-intact oocytes and metaphase II eggs were fixed in 3.7% paraformaldehyde, followed by permeabilization with 0.1% Triton X-100 in PBS and blocked for non-specific binding with blocking solution (PBS containing 0.1% BSA and 0.01% Tween 20). Oocytes and eggs were then labeled with a goat anti-IgSF8 polyclonal antibody (40 μg/ml), non-immune control goat IgG, or an rat anti-CD9 monoclonal antibody (50 μg/ml; KMC8.8; BD Biosciences, San Jose, CA) followed by Texas Red-conjugated donkey anti-goat IgG (8 μg/ml) or FITC-conjugated goat anti-rat IgG (8 μg/ml) and 4′,6′-diamidino-2-phenylindole (DAPI; 1.5 μg/ml). Eggs were viewed on a Nikon Eclipse 800 microscope, and images were captured using a Princeton Instruments 5Mhz Cooled CCD camera and IPLabs software (Scanalytics, Fairfax, VA).
In vitro fertilization (IVF)
IVF was performed essentially as previously described (Gardner et al. 2007). Sperm were collected from the epididymides of a CD1 male mouse (retired breeders, Harlan) in 250 μl Whitten's-Bicarbonate. After 15 min, tissue was removed and the sperm were pipetted into the bottom of a tube containing 750 μl Whitten's-Bicarbonate and allowed to swim-up for 45 min. After the swim-up incubation, 220 μl from the top of the tube was removed and placed in a culture dish and covered with light mineral oil. The sperm were cultured for a total of 2 hr for ZP-intact IVF and 3 hr for ZP-free IVF in Whitten's-Bicarbonate to allow the sperm to undergo capacitation and spontaneous acrosome exocytosis prior to incubation with eggs.
Eggs were incubated with antibodies for 60 min prior to insemination. Antibodies used in IVF were pure IgGs dialyzed against Whitten's medium-compatible buffer (100 mM NaCl, 5 mM KCl, 1 mM KH2PO4, 7 mM NaHCO3, pH 7.4); the anti-CD9 antibody KMC8.8 was used in a Na-N3-free, low-endotoxin format (BD Biosciences). Preliminary experiments were performed to determine the concentration dependence of effects of the anti-IgSF8 antibody (ZP-free eggs inseminated for 60 min with a sperm:egg ratio of 25:1). A 59% reduction in sperm binding was detected when the eggs were treated with 400 μg/ml of anti-IgSF8 antibody, whereas no reduction in binding was observed with eggs treated with 200 μg/ml of this antibody (data not shown); therefore, all subsequent experiments used the anti-IgSF8 antibody at 400 μg/ml. The concentration dependence of the effects of anti-CD9 antibodies has been examined in other studies (Chen et al. 1999; Takahashi et al. 2001), and our work here used 100 μg/ml based on our past experience with this antibody (Zhu 2004; Zhu and Evans 2002). Nonimmune goat IgG was included as a negative control for the anti-IgSF8 antibody. In early experiments, a nonimmune rat IgG was also included as a control for the anti-CD9 antibody; no effects were observed, as has been shown in previous studies (Chen et al. 1999; Higginbottom et al. 2003; Miller et al. 2000; Rubinstein et al. 2006a; Takahashi et al. 2001; Zhu and Evans 2002).
Antibody-treated eggs were inseminated for 60 min in the presence of the indicated antibody, with ten ZP-free eggs per 10 μl drop and a sperm:egg ratio of 25:1 (25,000 sperm/ml), 100:1 (100,000 sperm/ml) or 500:1 (500,000 sperm/ml). ZP-intact eggs were inseminated with a sperm:egg ratio of 1,000:1 (106 sperm/ml) for 3 hr. In a subset of experiments, ZP-free eggs were inseminated for 15 min with a sperm:egg ratio of 500:1 (500,000 sperm/ml). After insemination, the eggs were washed through three drops of Whitten's-Bicarbonate containing 15 mg/ml BSA using a thin bore pipet to detach any loosely bound sperm; all washes were done by the same person using the same pipet and washing pressure. Eggs were fixed in 3.7% paraformaldehyde in PBS and stained with 1.5 μg/ml DAPI to assess maternal DNA and to visualize sperm bound to the egg membrane and fused decondensing sperm heads in the egg cytoplasm. The average number of sperm fused per egg and the average number of sperm bound (not including fused sperm) per egg are presented. IVF experiments were performed with a minimum of three replicates, with 20-25 eggs per group per experiment. ANOVA with Fisher's protected least significant difference post-hoc testing was used to analyze the extent of sperm-egg binding and fusion.
RESULTS
We used co-immunoprecipitation studies to investigate the association of IgSF8 with tetraspanins in mouse eggs. Initial studies confirmed that anti-CD9 antibodies immunoprecipitated CD9 from surface-biotinylated eggs, whereas no CD9 band was detected in immunoprecipitation using a nonimmune IgG (Fig. 1A), in agreement with other reports (Chen et al. 1999; Miyado et al. 2000). We determined that IgSF8 is expressed in mouse eggs, using IgSF8 RT-PCR (data not shown) and immunoblotting using an anti-IgSF8 goat polyclonal antibody that cross-reacts with amino acids 25-577 of the mouse IgSF8, although a stronger IgSF8 band was detected in 0.5 μg of the positive control lysate, brain, than in the 1.88 μg of egg lysate (Fig. 1B). Our co-immunoprecipitation studies were based on other studies of protein associations with tetraspanins; these associations can be divided into three categories defined by the protein complexes' resistance to disruption by various detergents (Claas et al. 2001). First-level interactions are direct associations between tetraspanins and other proteins and are resistant to disruption by stringent detergent treatments such as 1% digitonin or Triton-X 100. Second-level and third-level interactions are indirect (i.e., through other proteins). Second-level interactions are disrupted in digitonin or Triton-X 100 but are stable in less hydrophobic detergents such as 1% Brij96 or Brij97. Third-level interactions are resistant to disruption by mild detergents such as 1% Brij99 or CHAPS. CD9 was detected by immunoblotting in immunoprecipitations from egg lysates prepared in 1% Triton X-100 and in 1% CHAPS (Fig. 1C). Furthermore, IgSF8 was detected by immunoblotting of these anti-CD9 immunoprecipitations, revealing the co-immunoprecipitation of IgSF8 with CD9 in egg lysates prepared in buffer containing 1% CHAPS or in 1% Triton X-100 (Fig. 1D). The detection of this IgSF8-CD9 interaction in the presence of Triton X-100 suggests that the interaction of these proteins in eggs is a robust and relatively stable interaction. On the other hand, we were unable to co-immunoprecipitate IgSF8 with CD81 or CD81 with IgSF8 from lysates of 150-200 eggs prepared in either 1% Triton X-100 or 1% CHAPS, despite being able to immunoprecipitate CD81 from egg lysates (data not shown). Immunofluorescence studies revealed that IgSF8 is localized on the microvillar domain of the membrane in metaphase II mouse eggs (in agreement with (Runge et al. 2007)), on the entire surface of GV-intact oocytes, and is colocalized with CD9 at both stages (Fig. 2). The microvillar domain of the mouse egg membrane is the region to which sperm bind and fuse, and develops during progression from prophase I to metaphase II during oocyte maturation.
Figure 2.
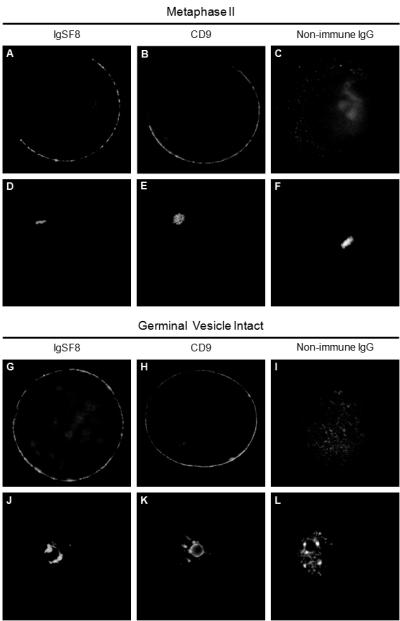
Indirect immunofluorescence of mouse oocytes and eggs show colocalization of IgSF8 with CD9. A-F: Metaphase II egg labeled with anti-IgSF8 antibody (A), anti-CD9 antibody (B), non-immune goat IgG (C), with corresponding DAPI images showing the localization of the metaphase II DNA (D-F). G-L: Germinal vesicle-intact oocyte labeled with anti-IgSF8 antibody (G), anti-CD9 antibody (H), non-immune goat IgG (I), with corresponding DAPI images showing the germinal vesicle (J-L).
We then undertook a series of IVF experiments (a) to determine if IgSF8 plays a role in gamete interactions, and (b) to compare the effects of anti-IgSF8 to anti-CD9 antibodies on gamete membrane interactions. Although studies of anti-CD9 antibodies demonstrate that these antibodies inhibit fertilization (Chen et al. 1999; Higginbottom et al. 2003; Kaji et al. 2000; Le Naour et al. 2000; Miyado et al. 2000; Rubinstein et al. 2006a; Takahashi et al. 2001), a systematic analysis of the robustness of this effect has not been performed. To achieve this goal, our IVF assays used a range of sperm:egg ratios in the inseminations. This is done in relatively few IVF studies, but varying the conditions of IVF (or intrauterine insemination) can be critical to detect differences in fertilizing ability because the incidence of fertilization can be significantly impacted by the number of sperm used (Amann and Hammerstedt 2002). Thus, as has been applied for certain analyses of fertilizing ability of sperm, here we apply the same considerations for analysis of fertilizability of ZP-free eggs, with our IVF assays using different sperm:egg ratios to challenge the eggs.
To assess the effects of challenging eggs with these different sperm:egg ratios, we examined four different IVF outcomes (the numbers of sperm bound per egg and fused per egg [Fig. 3], the percentage of fertilized eggs, and the percentage of polyspermic eggs). This shows that use of a sperm:egg ratio of 500:1 heavily favors the occurrence of fertilization, with high numbers of sperm bound and fused per egg (Fig. 3A,D), and 97% of eggs fertilized and 49% of the eggs having two or more sperm in them. Based on this, inseminations challenging the eggs with a sperm:egg ratio of 500:1 would detect robust inhibitory effects. On the other hand, inseminations with sperm:egg ratios of 25:1 resulted in 79% of eggs fertilized and lower extent of polyspermy (29%) as well as lower numbers of sperm bound and fused per egg (Fig. 3C,F), and thus these conditions would be able to detect more subtle inhibitory effects. Our studies here also used a sperm:egg ratio of 100:1; inseminations with these conditions resulted in 90% of eggs fertilized and 23% of the eggs being polyspermic, and intermediate numbers of sperm bound and fused (Fig. 3B,E).
Figure 3. IVF outcomes from inseminations with sperm:egg ratios of 25:1, 100:1, and 500:1.
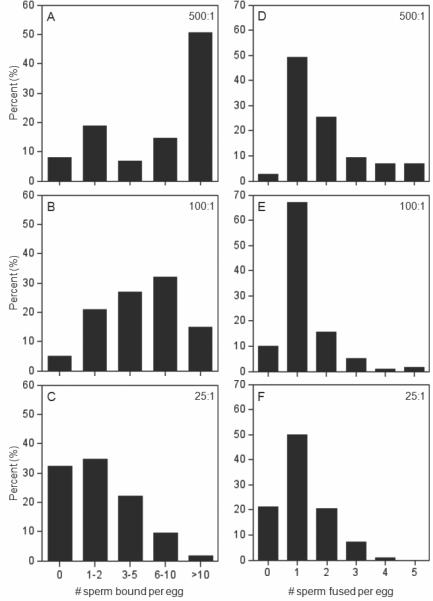
Graphs show the percentages of ZP-free eggs (y-axes) with the indicated numbers of sperm bound (left panels) and fused (right panels) on the x-axes. In these studies, ZP-free eggs were inseminated for 60 min with sperm:egg ratios of 500:1 (Panels A,D; n=75 eggs), 100:1 (Panels B,E; n=200 eggs), or 25:1 (Panels C,F; n=330 eggs).
The first set of IVF experiments used ZP-free eggs in 60 min inseminations with a sperm:egg ratio of 25:1 or 100:1. These assays identified an inhibitory effect with the anti-CD9 antibody in inseminations with 100:1 and with 25:1, while an inhibitory effect with the anti-IgSF8 antibody was only observed in inseminations with 25:1 (Fig. 4). Eggs treated with the anti-IgSF8 antibody and then inseminated with a sperm:egg ratio of 25:1 showed a reduction in sperm-egg binding to 47% of control levels, although this reduction in sperm-egg binding did not lead to a subsequent reduction in sperm-egg fusion (Fig. 4A,B). Eggs treated with the anti-CD9 antibody showed a reduction of sperm-egg fusion in inseminations with sperm:egg ratios of 25:1 and 100:1 (12% and 18% of control levels respectively); no effect of the anti-CD9 antibody on sperm-egg binding was detected in these assays using 60 min inseminations (Fig. 4C,D).
Figure 4. Effects of anti-IgSF8 or anti-CD9 antibodies on sperm-egg binding and fusion in inseminations with sperm:egg ratios of 25:1 or 100:1.
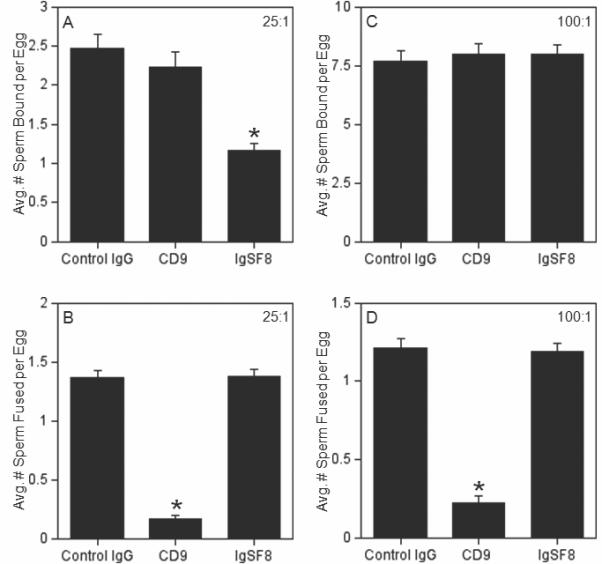
ZP-free eggs were pre-treated with either control IgG (nonimmune goat IgG), anti-CD9, or anti-IgSF8 for 60 min, then inseminated for 60 min with the indicated sperm:egg ratio; the average numbers of sperm bound (Panels A,C) or fused (Panels B,D) per egg were examined. Panels A, B: Eggs inseminated with a sperm:egg ratio of 25:1. Panels C, D: Eggs inseminated with a sperm:egg ratio of 100:1. Data are from 3-6 experiments with 71-220 eggs per treatment group and insemination condition. Asterisks indicate statistically significantly differences (p < 0.05) from the control IgG group.
Since anti-CD9-treated eggs showed a reduction in sperm-egg fusion was seen in inseminations with 25:1 and 100:1, the next studies sought to determine if the inhibitory effect of this anti-CD9 antibody was robust enough to be observed when the eggs were challenged with a sperm:egg ratio of 500:1. In these assays, eggs treated with the anti-CD9 antibody showed a reduction of sperm-egg fusion to 6% of control (non-immune IgG-treated) eggs (Fig. 5). To complement these studies, we also examined anti-CD9-treated and anti-IgSF8-treated ZP-intact eggs, as eggs with intact extracellular vestments are what are fertilized in vivo. On the other hand, one disadvantage of assays with ZP-intact eggs is they are unable to detect differences in sperm-egg membrane binding, which is defined by the sperm that stay adherent to the egg membrane after a specific series of washes, analogous to other cell adhesion assays; it is not possible to perform these washes of sperm within the perivitelline space (Evans 1999; Humphries 2001; Vjugina and Evans 2008). The percentages of eggs fertilized were 80% (60/75) for eggs treated with the nonimmune IgG and 83% (58/70) for the anti-IgSF8-treated eggs, but only 4% (3/72) for anti-CD9-treated eggs. Thus, no effect of the anti-IgSF8 antibody was observed with ZP-intact eggs, whereas a significant decrease in fertilization was observed with ZP-intact anti-CD9 antibody-treated eggs.
Figure 5. Inhibition of sperm-egg fusion by the anti-CD9 antibody in inseminations with sperm:egg ratio of 500:1.
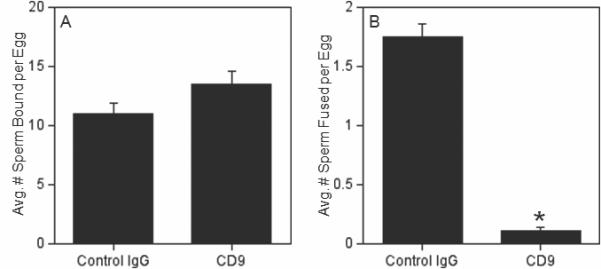
ZP-free eggs were pre-treated with either control IgG (nonimmune rat IgG) or anti-CD9 for 60 min, then inseminated for 60 min with a sperm:egg ratio of 500:1; the average numbers of sperm bound (Panel A) or fused (Panel B) per egg were examined. Data are from three experiments with 75-83 eggs per treatment group. Asterisk indicates statistically significantly differences (p < 0.05) from the control IgG group.
Our observation of no effect of the anti-CD9 antibody on sperm-egg binding (Figs. 4,5) differed from previous studies that showed a reduction of sperm binding to eggs treated with anti-CD9 antibodies (Chen et al. 1999; Takahashi et al. 2001). In comparing these studies to ours, we noted that these experiments differed from ours and had used shorter insemination times (40 min or less), whereas our IVF assays above used 60 min inseminations. Therefore, we tested the hypothesis that an effect on binding would be observed with shorter insemination times; we chose 15 min based on our past experience with an assay specifically designed to examine sperm binding (Redkar and Olds-Clarke 1999; Zhu et al. 2000). These 15 min inseminations used a sperm:egg ratio of 500:1 to increase the likelihood of sperm-egg contacts occurring in the brief insemination time, as has been previously shown (Redkar and Olds-Clarke 1999). We also tested the anti-IgSF8 antibody in these assays, since this antibody inhibited sperm binding in 60 min inseminations (Fig. 4). These experiments revealed that eggs treated with the anti-IgSF8 antibody and eggs treated with the anti-CD9 antibody showed reductions in sperm-egg binding, to 58% and 53% of control levels respectively (Fig. 6).
Figure 6. Effects of anti-IgSF8 and anti-CD9 antibodies on sperm-egg binding in 15 min inseminations.
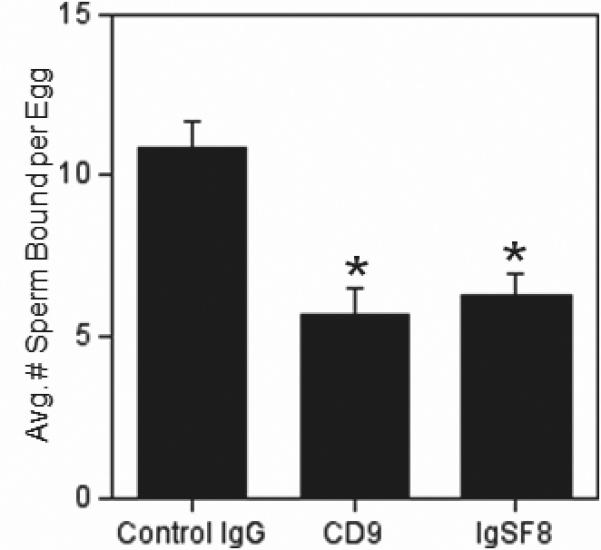
ZP-free eggs were pre-treated with either control IgG (nonimmune goat IgG), anti-CD9, or anti-IgSF8 for 60 min, then inseminated for 15 min with a sperm:egg ratio of 500:1. The average number of sperm bound per egg was examined. Data from three experiments with 86-109 eggs per treatment group. Asterisks indicate statistically significantly differences (p < 0.05) from the control IgG sample.
DISCUSSION
This work provides evidence from co-immunoprecipitation studies that IgSF8 and CD9 are in a first-level interaction (i.e., stable in Triton X-100 (Claas et al. 2001)) in mouse eggs. This is the first data suggestive of first-level interaction of egg CD9 with any egg protein, and of CD9 with IgSF8 in a primary cell type. The other evidence of a first-level CD9-IgSF8 interaction is in the NALM-6 cell line, in lysates prepared in digitonin (Charrin et al. 2003), and through demonstrating close proximity (using a chemical cross-linker with a 12 Å spacer arm) in 293 cells transfected with IgSF8 (Stipp et al. 2001a). The only other reported first-level association of IgSF8 with a tetraspanin in a primary cell type is of an IgSF8-CD81 interaction in hepatocytes (Charrin et al. 2003). We also examined whether IgSF8 is associated with CD81 in eggs, as IgSF8 is in hepatocytes, lymphocytes, and cultured cells (Charrin et al. 2003; Clark et al. 2001; Kolesnikova et al. 2004; Stipp et al. 2001a) and because of the fertilization phenotype of CD81−/− eggs (Rubinstein et al. 2006a). However, we did not detect a co-immunoprecipitation of IgSF8 and CD81 in the conditions tested here. It is possible that IgSF8 does not associate with CD81 in mouse eggs, or that this is requires modified techniques and/or higher numbers of eggs to detect. Tetraspanin-associated proteins in eggs are relatively poorly characterized, and this finding of a first-level interaction of IgSF8 and CD9 in eggs extends recent work that suggests that CD9 has indirect associations with IgSF8, PTGFRN and the α6 integrin subunit in egg lysates prepared in 1% Brij97 or CHAPS (Miyado et al. 2000; Runge et al. 2007). These data set the stage for further analysis of CD9-IgSF8 association and of other tetraspanin-associated proteins in eggs, as well as of IgSF8 expression/localization in eggs lacking tetraspanins such as CD9 and/or CD81.
The IVF studies here provide new insights into sperm-egg membrane interactions, and also demonstrate the importance of design of IVF experiments for detection of various effects. Varying the conditions of IVF has been noted to be crucial to detect differences in fertilizing ability sperm because the incidence of fertilization can be significantly impacted by the number of sperm used (Amann and Hammerstedt 2002). Models have been developed for the relationship between a fertility outcome (e.g., pregnancy rate, fertilized eggs) and the number of sperm used in an insemination (intrauterine or in vitro), with maximum fertility achieved at some number of sperm and higher, creating an asymptote in the curve (Amann and Hammerstedt 2002; Fearon and Wegener 2000). This sets up the premise that the number of sperm used to assess fertilizing ability needs to be in a lower portion of the curve (Amann and Hammerstedt 2002; Fearon and Wegener 2000). Indeed, there are instances in which use of high numbers of sperm in mouse IVF or artificial insemination fails to detect a difference in sperm function, whereas a deficiency is detected when lower sperm numbers are used (Johnson et al. 1995; Sutton et al. 2008). Thus, the same consideration is applied here for analysis of fertilizability of antibody-treated eggs as has been done for analyses of fertilizing ability of sperm.
This is the first report that a member of the IgSF family of cell adhesion molecules on the egg plays a role during fertilization, with the data here indicating that IgSF8 or an IgSF8-containing protein complex participates in sperm binding to the mouse egg membrane. It should also be emphasized that we observe distinctly different effects of anti-IgSF8 and anti-CD9 antibodies on IVF, suggesting that the effects of these antibodies are not simply due to blocking CD9-IgSF8-containing complexes on the egg surface, and that CD9 and this CD9-associated protein have different roles in fertilization. In our multiple experimental series with anti-IgSF8-treated eggs in which the eggs were challenged with a sperm:egg ratio of 25:1 (n= 289 eggs), we observed a reduction in sperm-egg binding to 49% of control levels. However, we did not observe inhibition of sperm-egg binding and fusion when the anti-IgSF8 eggs were challenged with a sperm:egg ratio of 100:1, suggesting that antibody-mediated perturbation of IgSF8 does not lead to robust inhibition of gamete interactions. It is possible that this antibody interacts with IgSF8 with low affinity and/or has a fast off-rate, allowing more opportunity for sperm interaction. Another possible explanation is that it is likely that only a subset of the immunoglobulins in this polyclonal antibody preparation cross-react with a functionally relevant epitope(s) on IgSF8. Structure-function analysis of the IgSF8 extracellular domain may help define an adhesion-mediating motif more precisely. A second explanation (and not mutually exclusive from the other) is that other egg proteins function in an identical or complementary role as IgSF8 does. Such redundancy would mean that even in the presence of antibody-blocked IgSF8, other egg proteins still mediate sperm-egg interactions. Previously suggested candidates include GPI-anchored proteins and integrins; however, the exact members of these groups of proteins that are involved in fertilization have yet to be identified, and three integrin subunits are not essential for fertilization (Alfieri et al. 2003; He et al. 2003; Miller et al. 2000; Rubinstein et al. 2006b; Vjugina and Evans 2008). Yet-to-be-characterized egg proteins are also possible players. It should also be noted that another IgSF member, PTGFRN (EWI-F), is expressed by eggs, although it has been noted that preliminary studies showed no effect of an anti-PTGFRN antibody on mouse fertilization (Rubinstein et al. 2006a). However, it is possible that inhibition of gamete interactions could be detectable in certain IVF conditions with perturbation of PTGFRN, as we observed here for IgSF8.
Studies that used short insemination times (40 min or less) detect a decrease in sperm binding to anti-CD9-treated eggs (Chen et al. 1999; Takahashi et al. 2001); our studies using 15 min inseminations reinforce this. This phenomenon may be linked with a role of CD9 in an event in early stages of sperm-egg membrane interaction, with one possibility being adhesion strengthening, which has been proposed for CD9 in eggs and for other tetraspanins on other cell types (Feigelson et al. 2003; Lammerding et al. 2003; Zhu and Evans 2002). It is also possible that CD9 associations change following egg activation; for example, an association with an adhesion-mediating protein(s) may be lost in early zygotes by 60 min post-insemination, and thus an effect on sperm-egg binding is only detected before this post-insemination time point. These possibilities will be areas of future investigation.
This work augments previous work on egg molecules participating in gamete binding and fusion, with a hierarchy of molecules emerging. CD9 thus far clearly has the most major role in mouse fertilization, based on studies using a variety of experimental approaches including those here challenging ZP-free eggs with high sperm:egg ratios. The role that CD9 plays is likely to be through CD9-associated proteins, and here we provide evidence that IgSF8 is one member of the tetraspanin network on the mouse egg membrane, and that IgSF8 has a role in fertilization. IgSF8 could be functioning as a binding partner for a protein on the sperm, with one candidate of interest being an IgSF protein, Izumo, a sperm membrane protein that is essential for sperm-egg interaction (Inoue et al. 2005). An alternative/additional role for IgSF8 could be in regulation of tetraspanin-containing and/or integrin-containing complexes, as IgSF8 does in MOLT-4 cells; MOLT-4 cells transfected with IgSF8 have larger membrane complexes containing CD81 and the integrin α4β1 than do untransfected cells (Kolesnikova et al. 2004). Finally, in considering the role of CD9 as an organizer of membrane functionality through CD9-associated proteins, this work also suggests that, in addition to IgSF8, there are likely to be other CD9-associated proteins on the egg that could have roles in murine fertilization.
Acknowledgements
This work was funded by grants from the NIH to JPE (HD037696, HD045671). We thank Chris Hung for critical reading of the manuscript.
Abbreviations
- IgSF
Immunoglobulin Superfamily
- ZP
zona pellucida
- IVF
in vitro fertilization
- GV
germinal vesicle
- PTGFRN
Prostaglandin F2 Receptor Negative Regulator (also known as EWI-F and CD9-P-1)
REFERENCES
- Alfieri JA, Martin AD, Takeda J, Kondoh G, Myles DG, Primakoff P. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J Cell Sci. 2003;116:2149–2155. doi: 10.1242/jcs.00430. [DOI] [PubMed] [Google Scholar]
- Amann RP, Hammerstedt RH. Detection of differences in fertility. J Androl. 2002;23:317–25. [PubMed] [Google Scholar]
- Andre M, Le Caer JP, Greco C, Planchon S, El Nemer W, Boucheix C, Rubinstein E, Chamot-Rooke J, Le Naour F. Proteomic analysis of the tetraspanin web using LC-ESI-MS/MS and MALDI-FTICR-MS. Proteomics. 2006;6:1437–1449. doi: 10.1002/pmic.200500180. [DOI] [PubMed] [Google Scholar]
- Aricescu AR, Jones EY. Immunoglobulin superfamily cell adhesion molecules: zippers and signals. Current Opinion in Cell Biology. 2007;19:543–550. doi: 10.1016/j.ceb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Barclay AN. Membrane proteins with immunoglobulin-like domains--a master superfamily of interaction molecules. Seminars in Immunology. 2003;15:215–223. doi: 10.1016/s1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Barraud-Lange V, Naud-Barriant N, Bomsel M, Wolf JP, Ziyyat A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. Faseb J. 2007;21:3446–9. doi: 10.1096/fj.06-8035hyp. [DOI] [PubMed] [Google Scholar]
- Boucheix C, Rubinstein E. Tetraspanins. Cell.Mol.Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrin S, Le Naour F, Labas V, Billard M, Le Caer JP, Emile JF, Petit MA, Boucheix C, Rubinstein E. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem J. 2003;373:409–21. doi: 10.1042/BJ20030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Tung KSK, Coonrod SA, Takahashi Y, Bigler D, Chang A, Yamashita Y, Kincade PW, Herr JC, White JM. Role of the integrin associated protein CD9 in binding between sperm ADAM 2 and the egg integrin α6β1: Implications for murine fertilization. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11830–11835. doi: 10.1073/pnas.96.21.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas C, Stipp CS, Hemler ME. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J.Biol.Chem. 2001;276:7974–7984. doi: 10.1074/jbc.M008650200. [DOI] [PubMed] [Google Scholar]
- Clark KL, Zeng Z, Langford AL, Bowen SM, Todd SC. PGRL is a major CD81-associated protein on lymphocytes and distinguishes a new family of cell surface proteins. J Immunol. 2001;167:5115–5121. doi: 10.4049/jimmunol.167.9.5115. [DOI] [PubMed] [Google Scholar]
- Evans JP. Sperm disintegrins, egg integrins, and other cell adhesion molecules of mammalian gamete plasma membrane interactions. Front. Biosci. 1999;4:D114–D131. doi: 10.2741/evans. [DOI] [PubMed] [Google Scholar]
- Fearon JM, Wegener PT. Relationship between fertility in cattle and the number of inseminated spermatozoa. J Reprod Fertil. 2000;119:293–308. [PubMed] [Google Scholar]
- Feigelson SW, Grabovsky V, Shamri R, Levy S, Alon R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J Biol Chem. 2003;278:51203–51212. doi: 10.1074/jbc.M303601200. [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Knott JG, Jones KT, Evans JP. CaMKII can participate in but is not sufficient for the establishment of the membrane block to polyspermy in mouse eggs. J. Cell. Physiol. 2007;212:275–280. doi: 10.1002/jcp.21046. [DOI] [PubMed] [Google Scholar]
- He Z-Y, Brakebusch C, Fassler R, Kreidberg JA, Primakoff P, Myles DG. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm–egg binding and fusion. Dev Biol. 2003;254:226–237. doi: 10.1016/s0012-1606(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Specific tetraspanin functions. J.Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Ann Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Higginbottom A, Takahashi Y, Bolling L, Coonrod SA, White JM, Partridge LJ, Monk PN. Structural requirements for the inhibitory action of the CD9 large extracellular domain in sperm/oocyte binding and fusion. Biochem Biophys Res Commun. 2003;311:208–214. doi: 10.1016/j.bbrc.2003.09.196. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Cell adhesion assays. Mol.Biotechnol. 2001;18:57–61. doi: 10.1385/MB:18:1:57. [DOI] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Pilder SH, Bailey JL, Olds-Clarke P. Sperm from mice carrying one or two t haplotypes are deficient in investment and oocyte penetration. Dev.Biol. 1995;168:138–149. doi: 10.1006/dbio.1995.1067. [DOI] [PubMed] [Google Scholar]
- Kaji K, Oda S, Miyazaki S, Kudo A. Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev.Biol. 2002;247:327–334. doi: 10.1006/dbio.2002.0694. [DOI] [PubMed] [Google Scholar]
- Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of CD9-deficient mice. Nat. Genet. 2000;24:279–282. doi: 10.1038/73502. [DOI] [PubMed] [Google Scholar]
- Kolesnikova TV, Stipp CS, Rao RM, Lane WS, Luscinskas FW, Hemler ME. EWI-2 modulates lymphocyte integrin α4β1 functions. Blood. 2004;103:3012–3019. doi: 10.1182/blood-2003-07-2201. [DOI] [PubMed] [Google Scholar]
- Lammerding J, Kazarov AR, Huang H, Lee RT, Hemler ME. Tetraspanin CD151 regulates a6b1 integrin adhesion strengthening. Proc Natl Acad Sci U S A. 2003;100:7616–7621. doi: 10.1073/pnas.1337546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Georges-Labouesse E, Primakoff P, Myles DG. Normal fertilization occurs with eggs lacking the integrin α6β1 and is CD9-dependent. J.Cell Biol. 2000;149:1289–1295. doi: 10.1083/jcb.149.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E. Requirement of CD9 on the egg plasma membrane for fertilization. Science. 2000;287:321–324. doi: 10.1126/science.287.5451.321. [DOI] [PubMed] [Google Scholar]
- Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci U S A. 2008;105:12921–6. doi: 10.1073/pnas.0710608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redkar AA, Olds-Clarke PJ. An improved mouse sperm-oocyte plasmalemma binding assay: Studies on characteristics of sperm binding in medium with or without glucose. J.Androl. 1999;20:500–508. [PubMed] [Google Scholar]
- Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, Le Naour F, Boucheix C. Reduced fertility of female mice lacking CD81. Dev Biol. 2006a;290:351–358. doi: 10.1016/j.ydbio.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C. The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol. 2006b;17:254–263. doi: 10.1016/j.semcdb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Runge KE, Evans JE, He Z-Y, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol. 2007;304:317–325. doi: 10.1016/j.ydbio.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Sala-Valdes M, Ursa A, Charrin S, Rubinstein E, Hemler ME, Sanchez-Madrid F, Yanez-Mo M. EWI-2 and EWI-F link the tetraspanin web to the actin Cytoskeleton through their direct Association with ezrin-radixin-moesin proteins. J. Biol. Chem. 2006;281:19665–19675. doi: 10.1074/jbc.M602116200. [DOI] [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Skubitz AP. CD63 associates with CD11/CD18 in large detergent-resistant complexes after translocation to the cell surface in human neutrophils. FEBS Lett. 2000;469:52–56. doi: 10.1016/s0014-5793(00)01240-0. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J.Biol.Chem. 2001a;276:40545–40554. doi: 10.1074/jbc.M107338200. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Orlicky D, Hemler ME. FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J Biol Chem. 2001b;276:4853–4862. doi: 10.1074/jbc.M009859200. [DOI] [PubMed] [Google Scholar]
- Sutton KA, Jungnickel MK, Florman HM. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc Natl Acad Sci U S A. 2008;105:8661–8666. doi: 10.1073/pnas.0800603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Bigler D, Ito Y, White JM. Sequence-specific interaction between the disintegrin domain of mouse ADAM3 and murine eggs: Role of the β1 integrin-associated proteins CD9, CD81, and CD98. Mol.Biol.Cell. 2001;12:809–820. doi: 10.1091/mbc.12.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vjugina U, Evans JP. New insights into the molecular basis of mammalian sperm-egg membrane interactions. Front Biosci. 2008;13:462–476. doi: 10.2741/2693. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Nutrient requirements for the culture of preimplantation embryos in vitro. Adv.Bio.Sci. 1971;6:129–139. [Google Scholar]
- Zhu X. The roles of fertilin β, egg integrins, and tetraspanin CD9 in sperm-egg interactions. Johns Hopkins University; 2004. [Google Scholar]
- Zhu X, Bansal NP, Evans JP. Identification of key functional amino acids of the mouse fertilin β (ADAM2) disintegrin loop for cell-cell adhesion during fertilization. J. Biol. Chem. 2000;275:7677–7683. doi: 10.1074/jbc.275.11.7677. [DOI] [PubMed] [Google Scholar]
- Zhu X, Evans JP. Analysis of the roles of RGD-binding integrins, α4/α9 integrins, α6 integrins, and CD9 in the interaction of the fertilin β (ADAM2) disintegrin domain with the mouse egg membrane. Biol. Reprod. 2002;66:1193–1202. doi: 10.1095/biolreprod66.4.1193. [DOI] [PubMed] [Google Scholar]


