Abstract
The brain has the highest metabolic rate of all organs and depends predominantly on oxidative metabolism as a source of energy. Oxidative metabolism generates reactive oxygen species, which can damage all cellular components, including protein, lipids and nucleic acids. The processes of DNA repair normally remove spontaneous gene damage with few errors. However, cerebral ischemia followed by reperfusion leads to elevated oxidative stress and damage to genes in brain tissue despite a functional mechanism of DNA repair. These critical events occur at the same time as the expression of immediate early genes, the products of which trans-activate late effector genes that are important for sustaining neuronal viability. These findings open the possibility of applying genetic tools to identify molecular mechanisms of gene repair and to derive new therapies for stroke and brain injury.
Brain injury of the ischemia—reperfusion type, which occurs in stroke and cardiac arrest, induces neuronal damage. The mechanisms of neuronal injury include decreased intracellular pH and ATP concentration and increased levels of extracellular glutamate, intracellular Ca2+ and reactive oxygen species (ROS). ROS are generated as byproducts of oxygen metabolism. Under physiological conditions, most ROS are generated in mitochondria, but pathological conditions can cause ROS to form in the cytoplasm as well (Box 1). Mitochondrial DNA is prone to damage1 and mitochondria have functional repair processes2. Because of a short half-life, the hydroxyl radicals generated in the mitochondria under normal conditions might not react with nuclear DNA to a significant extent. In experimental models of ischemia, ROS are produced within 30 minutes of the ischemic episode, predominantly in the penumbral region3. Recent studies show that the radical form of nitric oxide and superoxide anion might participate in nuclear gene damage in the brain (Box 1). The content of nitric oxide increases in the CNS after cerebral ischemia—reperfusion, traumatic head injury and spinal cord injury4–7. The nitric oxide radical has dual effects: first, it combines with the superoxide anion to form peroxynitrite; second, it interferes with superoxide dismutase, whose antioxidant effect is reduced8,9. The mechanisms by which peroxynitrite damages nuclear DNA are unclear, but could involve diffusion from the mitochondria and cytoplasm and cleavage in the nucleus to form hydroxyl radicals or singlet oxygen10,11. Brain injury also results in an elevated extracellular concentration of glutamate, which activates neuronal nitric oxide synthase via Ca2+ influx12,13. Other routes that induce the formation of ROS have been presented in recent reviews14–16. There is evidence that the cellular accumulation of nitric oxide can damage nucleic acidsin the nucleus. For example, mice with either a knockout of neuronal nitric oxide synthase or treated with 7-nitroindazole, an inhibitor of neuronal nitric oxide synthase, are protected against neuronal death in experimental models of brain injury17–22. It has also been reported that 7-nitroindazole reduces damage to nucleic acids in three models of brain injury23–26.
Box 1. The formation of cellular ROS.
At least four routes can generate hydroxyl radicals (•OHs) in a cella.
- Most reactive oxygen species (ROS), including hydrogen peroxide (H2O2), are generated in the mitochondria. ROS genesis in vivo in the mitochondria occurs during oxidative metabolism
- Reduction of oxygen to water:
- The Haber—Weiss reaction:
- The Fenton reaction (catalyzed by Fe2+ and other transition metals):
- NADPH oxidase can generate cytosolic ROS in activated inflammatory cells:
-
Nitric oxide reacts with oxygen, superoxide anion (•O2- ) or thiol compounds, and generates reactive nitrogen species, peroxynitrite or S-nitrosothiol (S-nitroso glutathione)b, respectively. Peroxynitrite has a half-life of 1–2 s and a long diffusion distance of ∼100 μm (Ref. c). Therefore, it readily enters the nuclei or crosses cell membranes and acts in cells other than those in which it is generated.
Arginine→[neuronal nitric oxide synthase, Ca2+, NADPH]→citrulline + NADP + NO - The energy from ionizing radiation causes the formation of cation radical (including H2O+ in all molecules, •OH is formed by the transfer of cation radical to neighboring molecules.
References
Lewen, A. et al. (2000) Free radical pathways in CNS injury. J. Neurotrauma 17, 871–890
Rauhala, P. et al. (1998) Neuroprotection by S-nitrosoglutathione of brain dopamine neurons from oxidative stress. FASEB J. 12, 165–173
Beckman, J.S. et al. (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 87, 1620–1624
ROS, probably the superoxide anion, hydroxyl radical, singlet oxygen and radical nitric oxide can damage nucleic acids, proteins and lipids, and can impair gene expression in bacteria, yeast and mammalian cells grown in culture27–29. In the brain, elevated ROS can cause oxidative damage to all cellular components by chemically modifying nucleic acid bases and causing single- and double-stranded breaks in the DNA backbone and breaks in the glycosylic bond between ribose and individual bases (abasic or AP site)30–33 (Fig. 1). In addition, ROS can cause either DNA strands or protein and DNA to fuse (crosslink). These changes are known as oxidative DNA lesions (ODLs) or oxidative RNA lesions (ORLs) and, if not repaired, they terminate chain elongation or alter the coding properties during transcription (gene expression) or replication. Examples of ORLs/ODLs detected in injured brains are shown in Fig. 2. These lesions mimic those found after ionizing radiation34,35 and some have been demonstrated in CNS diseases36–39. In this communication, we will review our current understanding of ORLs/ODLs in the brain and the consequences of such lesions.
Fig. 1.

Schematic illustration of the formation of DNA and RNA lesions. Hydroxyl radicals can attack nearby DNA at several places, as indicated by the arrows. Some lesions resulting from the attack are listed in Fig. 2. Numbers represent the position of attack by reactive oxygen species. (1) attack on the base which produces base modifications. (2) attack on the glycosylic bond which produces sites without base (AP sites). (3) attack on the phosphate bond (P), which produces DNA strand breaks, either (3a) with 3′ hydroxyl ends (3′-OH) or (3b) with 3′ phosphate ends (3′-PO4). (4) attack on the ribose which produces 3′ phosphoglycolate ends (3′-PG).
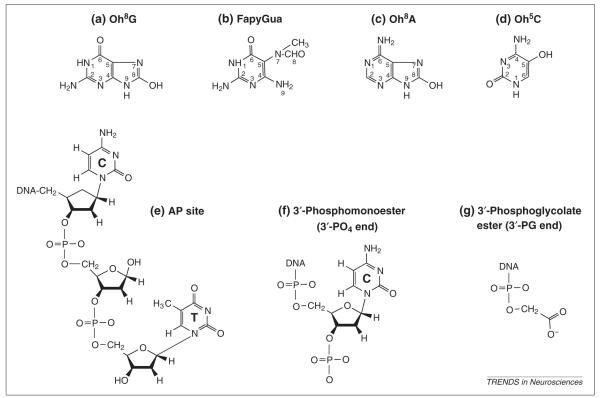
Fig. 2.
Nucleic acid modifications identified in the brain after oxidative stress. (a) 8-hydroxyguanine [Oh8G], the hydrogen on the C-8 carbon of the guanine base (also termed 8-oxo-7,8-dihydroguanine) is replaced with a hydroxyl group. (b) 2,6 diamino-4-hydroxy-5-N-methylformamidopyrimidine (FapyGua), an open ring results from a broken bond between the C-8 and C-9 carbons in the guanine base. (c) 8-hydroxyadenosine (Oh8A), the hydrogen on the C-8 carbon of the adenine base is replaced with a hydroxyl group. (d) 5-hydroxycytosine (Oh5C), the hydrogen on the C-5 carbon of the cytosine base is replaced with a hydroxyl group. (e) AP site in DNA. (f) and (g) DNA breaks with two different termini. Most of these lesions have been reported in brain injury models, including the forebrain ischemia—reperfusion (experimental cardiac arrest) model, the focal cerebral ischemia model (experimental stroke), the seizure model and the traumatic brain injury model1,14,16,23,31-33.
Oxidative lesions
Approximately 70 different types of ORL and ODL, including those of free nucleic acids, can be generated by ROS in vitro40,41 and several assays have been used to detect ORLs/ODLs in the brain (Table 1). Many investigators have detected an increase in single-stranded breaks bearing 3′-OH ends in injured brains. However, the detection of single-stranded breaks with 3′-OH ends might not reliably assay DNA damage, because they could be formed in a number of ways. These include: (1) by a breakage between the ribose and the phosphate group42,43 (Figs 1,3a); (2) as an intermediate of DNA repair (Fig. 3); (3) as Okazaki fragments in the replication fork; and (4) as digestion products of nucleases during DNA fragmentation44. DNA breaks with 3′-OH ends resulting from repair and replication are part of normal cellular processes and generally are not considered to be DNA damage. Alternatively, the presence of 8-hydroxy-2′-deoxyguanosine in DNA (also known as 8-hydroxyguanosine in RNA) is a reliable marker of base modifications, because it is detected consistently after oxidative stress generated by various conditions16,23,26–34. The detection of base modifications are the preferred indicators of ORLs/ODLs (Refs 47, 48) and can be used to investigate some features associated with CNS disorders37,39,47,48. Because of the large number of lesions, we will limit our discussion to the most reliable types of ORLs/ODLs.
Table 1.
Assays that investigate DNA damage and repair in the brain or CNS derived cellsa
| Name of assay | Purpose | Refs |
|---|---|---|
| In vivo mutation assay | The Big Blue transgenic mice with a reporter gene that can be easily isolated for detection of gene mutations. |
31 |
| Single-strand conformation polymorphism (SSCP) |
Mutation screening that detects altered transcript from specific genes. |
31 |
| HPLC or GCMS | Detection of modified nucleosides or bases in purified DNA. |
16,31,32, 39,40 |
| Immunohistochemistry | IgG or IgM against 8-hydroxy- 2′deoxyguanosine/8-hydroxyguanosine. |
23,26,33, 37,49 |
| Gene-specific repair using fragment shift |
The presence of ODLs is detected by the ability of the repair enzyme to remove ODLs in purified DNA. An absence of the gene during hybridization in alkaline blot using gene specific probe signifies the presence of ODLs. |
21,31,56 |
| DNA lesions detected as sensitive sites to DNA repair glycolylases or nucleases in situ |
Detection of specific DNA lesions in brain tissue as sensitive sites to the Fpg protein or exonuclease III (FPGSS or EXOSS, respectively) enzymes followed by post-labeling using DNA polymerase. |
24,50 |
| Repair activity assay | Expression of enzymes that repair base modifications, e.g., 8-hydroxy deoxyguanosine or AP sites. |
2,49 |
| DNA strand-break assay | DNA polymerase-I for detecting SSBs with 3′OH ends. |
42 |
| In vitro PCR | Detection of DNA lesions that do not support PCR in purified DNA. |
1 |
| The comet assay | The presence of a comet tail represents the presence of SSBs. |
35,43 |
Abbreviations: GCMS, gas chromatography and mass spectrometry; HPLC, high-pressure liquid chromatograph; ODL, oxidative DNA lesions; SSB, single-stranded break.
Fig. 3.
The two distinct base-excision-repair pathways in mammalian cells. To repair a modified base, the glycosylic bond linking the deoxyribose (dR) and the damaged base (G*) is excised via glycosylase62 (1) to generate an abasic (AP)-site intermediate. The damaged base can also be released spontaneously, without enzyme. The DNA backbone 5′ to the AP site is cleaved by AP endonuclease in Step 2, to generate a single-stranded break with 3′ hydroxyl and 5′-deoxyribose phosphate (dRP) termini that can be repaired by one of two pathways. In the classical pathway, dRP is removed by DNA polymerase-β (DNA pol-β; 3a) followed filling of the one-nucleotide gap (4a) and DNA ligation (5). The alternative pathway (3b and 4b), which occurs in the absence of functional DNA pol-β, involves repair synthesis by proliferating cell nuclear antigen (PCNA), DNA pol-δ or -ε and flip endonuclease (FEN). AP endonuclease, DNA pol-β and DNA ligase I form a multiprotein complex51 in which DNA ligase I can be replaced by DNA ligase III plus complementary factor XRCC1.
Under normal physiological conditions, an ‘average’ human cell with a mass of 10-9 gram and a volume of 10 μm3 uses ∼1 × 1012 molecules of oxygen per cell per day and generates 3 × 109 molecules of hydrogen peroxide per cell per hour34. It has been estimated that ∼2 × 104 ODLs occur in each human genome per day45,46 and that normal brain contains ∼1 × 105 8-hydroxy-2′-deoxyguanosine per genome31,32. This is the burden of repair, or a tolerance of gene damage in each normal living cell and represents a balance between the formation and removal (repair) of ODLs.
Several laboratories have reported a >5-fold increase in ODLs of the brain after a short period of cerebral ischemia16,31,32. The activities of DNA repair increase threefold in ischemic brain and they have the capacity to remove ∼85% of ODLs within three hours of reperfusion1,23,24,31,49,50. However, the load of ODLs may still exceed the capacity for repair. If one also considers other types of ODLs (Fig. 2), the burden of repair may be more than fivefold. For example, there are at least three lesions in each copy of the c-fos gene in animals after cerebral ischemia, compared to one lesion, or fewer, in ten copies of the same gene under normal conditions23,49. The elevated burden is likely to be similar for other nuclear genes31, although different genes may be differentially damaged or repaired29.
DNA repair pathways
Studies using cell cultures reveal the complexity of DNA-repair pathways in normal cells. Repair is believed to occur in DNA only because of its double-stranded structure (to serve as a template) and the presence of DNA-dependent DNA polymerases. The major excision—repair pathways are the nucleotide-excision-repair pathway and the base-excision-repair pathway (Box 2). In very general terms the removal of ODLs by the base-excision-repair pathway (Fig. 3) requires recognition and excision of lesions from the DNA double helix by endonucleases or glycosylases49,51 (Step 1), followed by hydrolysis mediated by AP endonucleases (Step 2) and clipping of the ribosephosphate moiety to generate a gap for repair synthesis using DNA polymerases (Steps 3 and 4). The repair process is completed by the ligase that connects new and old DNA strands (Step 5). In the nucleotide-excision-repair pathway, DNA lesion-specific endonucleases excise the damaged and adjacent nucleotide, followed by repair synthesis of at least 20 nucleotides in one repair patch. However, base-excision repair generally excises the damaged base [(3a) Fig. 3], and incorporates one nucleotide in the presence of DNA polymerase-β (4a), but no more than 10 nucleotides in the absence of functional DNA polymerase-β (3b and 4b). The repair synthesis is mediated by DNA-dependent DNA polymerase-α, -β, -δ, -ε and -γ, with DNA polymerase-β considered to be the most abundant DNA polymerase in the brain. These DNA polymerases act on the 3′-OH end of single-stranded breaks to incorporate the correct dNTP directed by the sequence in the template. In mitochondria, DNA polymerase-γ is responsible for DNA replication and repair synthesis. It is reported that DNA ligation could be dependent on the presence of poly (ADP-ribose) polymerase (PARP) because an inhibition of PARP alters the rate of repair26. Nevertheless, the mechanism of this action is unclear in the brain. Breaks in DNA strands after brain injury activate PARP and cause the depletion of nicotinamide adeninedinucleotide (NAD, a substrate of PARP) and ATP. Base-excision repair is disrupted with PARP-bound DNA (Ref. 52). However, the increase of sister-chromatid exchange in PARP-deficient cells suggests that binding of PARP to DNA is required for orderly ligation of DNA. Activation of PARP enhances necrosis but reduces apoptosis in the brain after injury53,56. Thus, the participation of PARP in gene repair in the brain may be a double-edged sword.
Box 2. DNA-repair pathways in mammals.
Nucleotide-excision-repair (NER) pathwaya,b
The NER excises several nucleotides (20–30 nucleotides) surrounding the lesion in each repair patch. There are two modes: (1) transcript-coupled repair (TCR) mode, which has a preference for repair of the transcribed strand, most likely the exons of active genes. Enzymes of TCR can also be gene activators; or (2) global-excision repair mode, induced by p53, repairs bulky lesions without preference for either strand. Unless there is degeneration of the blood—brain barrier, as in some diseases, chemicals that induce the bulky lesions (e.g. fusion of adjacent bases [dimers] and strands [crosslinks], strand breaks) do not enter the brain.
Base-excision-repair (BER) pathwayc—e
Conventional BER excises and repairs one base (Fig. 3, steps 3a and 4a). DNA polymerase-β is thought to perform repair synthesis during BER. Because DNA polymerase-β is the most abundant DNA polymerase in the brain, BER could be the major repair pathway in this tissue. In the absence of functional DNA polymerase-β an alternative BER pathway that requires several enzymes may operate (Fig. 3, steps 3b and 4b). Thus far, thymine glycol is the only ODL that is preferentially repaired on the transcribed strand.
Mismatch repairf,g
This repair corrects a structure in duplex DNA composed of two unmodified nucleotides in a non-Watson—Crick base pair of mismatched but undamaged bases that are either incorporated during DNA replication and repair synthesis, or that result from deamination of cytosine.
References
Hanawalt, P.C. (1994) Transcription-coupled repair and human disease. Science 266, 1957–1958
Vos, J.M.H. (1995) DNARepair Mechanism: Impact on Human Disease and Cancer. Austin, R.G. Landes Co.
Demple, B. and Harrison, L. (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 63, 915–948
Wilson, D.M. and Thompson, L.H. (1997) Life without DNA repair. Proc. Natl. Acad. Sci. U.S.A. 94, 12754–12757
Croteau, D.L. and Bohr, V.A. (1997) Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J. Biol. Chem. 272, 25409–25412
Belloni, M. et al. (1999) Distribution and kainate-mediated induction of the DNA mismatch repair protein MSH2 in rat brain. Neuroscience, 94, 1323–1331
Brooks, P.J. et al. (1996) DNA mismatch repair and DNA methylation in adult brain neurons. J. Neurosci. 16, 939–945
The removal of mismatched bases or large lesions has been described in differentiated neurons in culture57 (termed mismatch repair or global repair, respectively, Box 2) although it is not known whether all of these repair pathways are active in vivo. Moreover, as some genes are downregulated in cells grown in culture, repair pathways that are absent in culture could be present in vivo.
Gene-repair pathways are probably present and functional in the brain, because several proteins that are involved in all types of repair are induced by brain injury and cerebral ischemia14,49,58–62. Evidence of DNA repair has been presented in the brain after stroke, cerebral ischemia or hypoxia1,23,31. Following cerebral ischemia, the ischemia-induced ODLs in the transcribed strand of the c-fos gene are repaired initially more slowly than the non-transcribed strand, but almost all are repaired within 2–3 hours23,49. Many investigators have reported a difference in the repair capacity in aging animals or in individuals with particular neurological disorders47,48,59. While elevated ODLs are reported in mitochondria from the brains of Alzheimer’s patients39, others observed elevated ORLs but no detectable ODLs in the cytoplasm37. The rate of ODL repair in nuclear and mitochondrial DNA might be similar in the brain1,23,31,49,50.
When the burden of repair in the brain is increased by fivefold after 30 minutes of cerebral ischemia, the frequency of somatic cell mutations resulting from repair errors is increased from the ‘background’ frequency of 1.5 mutations per 105 genes to 7.7 mutations per 105 genes in a lacI reporter gene in a transgenic Big Blue mouse strain31. Therefore, the frequency of mutations in somatic cells in vivo correlates positively with ODL levels in the brain. Mutation is randomly distributed in this reporter gene, but approximately 80% occur at G or C bases, with C to T transitions and frameshift mutations. Errors during DNA synthesis in vitro in the presence of purified DNA polymerase-β consist mainly of one-base deletions or insertions, which result in frameshifts63. The causal role of DNA polymerase in the formation of frameshifts, however, remains to be established in injured brains.
Frameshift mutations are observed in postmitotic neurons64. Mutations have been identified in the gene encoding α-synuclein and the ubiquitin-like parkin gene in autosomal recessive juvenile Parkinsonism65,66, in the gene encoding the amyloid precursor protein in conditions related to premature aging67, and in the gene encoding superoxide dismutase in familial amyotrophic lateral sclerosis68–70. Some of these include nonsense or deletion mutations. Mutagenesis in somatic cells is rare, but might cause a deleterious effect to the cells if it occurs in an essential gene that has already had a germline mutation in one of its two copies of chromosomes (heterozygote or carrier of a mutation). ODLs accumulate in mitochrondria and the frequency of spontaneous mutations in a nuclear gene doubles in the liver of animals lacking the glycosylase that repairs ODLs (oh8G, FapyGua, oh5C and AP sites, see Fig. 2)71,72. In humans deficient in removing 8-hydroxy-2′-deoxyguanosine neurological disorders develop earlier than in the general population39,47. These studies suggest that a deficiency in DNA repair could upset the balance between ODL formation and repair, and lead to neurological dysfunction.
A major role of DNA repair is to ensure transcription of mRNA from intact genes (Fig. 4). Gene transcription might be pivotal in maintaining neuronal viability after ischemia—reperfusion. Gene activation is induced by oxidative stress73–81, and the genes undergoing transcription contain ODLs (Refs 23–25,31–33,49,50). It is believed that a gene with strand breaks or crosslinks is unable to serve as a template during transcription and that the cell must repair these lesions before transcription can occur29. However, several investigators have reported an elevation of gene transcripts during the first few hours of reperfusion when strand breaks are detected42. Although DNA strand breaks and crosslinks inhibit transcription, there is evidence suggesting that modified bases change the coding properties so that most polymerases insert random nucleotides opposite the base, and that adenine nucleotide is most likely to be inserted opposite the 8-hydroxy-2′-deoxy-guanosine lesion during RNA transcription82,83. This ‘by-pass insertion’ could be another mechanism that generates faulty transcripts with mismatched sequences. What is the probability that a full length mRNA be transcribed from its nuclear gene with ODLs?
Fig. 4.
Proposed mechanism by which ODLs in c-fos could delay trans-activation of late genes by producing faulty gene expression without repair (thin red arrows). Repair processes (green arrows) will enhance the expression of intact mRNA (Ref. 25). Abbreviations: ROS, reactive oxygen species; NGF, nerve growth factor.
One example is the expression of activator protein-1 (AP-1), a transcription factor, which is composed of proteins encoded by c-fos and/or other immediate early genes and trans-activates late effector genes that are either detrimental75,84 or salutary to cell viability80,81,85,86. Under normal physiological conditions, little mRNA encoding FOS is detected in the brain. Levels increase following cerebral ischemia, probably as a result of transcription of its nuclear gene. One hypothesis is, if the nuclear genes contain ODLs, the newly transcribed mRNA could be faulty. This proposal is supported by the finding that it takes over one hour for the increase in FOS/AP-1 activity to become apparent following injury-induced increase in c-fos mRNA (Refs 87,88). It is thought that this delay is caused by an inhibition as a result of translation blockage or a reduced global protein synthesis15. This blockage coincides with the duration of detecting excessive ODLs in c-fos, that is, the blockage expires when the majority of ODLs in nuclear genes are repaired23–25,49,50.
Conclusion
We have discussed some aspects of the emerging field of gene damage and repair in the genetic material of the brain after cerebral ischemia—reperfusion. ORLs/ODLs cause adverse effects on the processes of replication, transcription and translation, therefore they affect every level of cell function. Excessive ORLs/ODLs might cause deleterious effects ranging from mutation to cell death.
Although the effect of ORLs might be transient as a result of the short half-life of RNA, they might have profound effects on the recovery of cells after brain injury. A reduction of ORLs, using 7-nitroindazole, increases neuronal viability suggesting that non-functional mRNA might affect the endogenous response to recovery from the acute conditions of brain injury and stroke25. Reducing non-functional mRNA might increase translation efficacies for functional proteins. Moreover, because RNA is not repaired, the ORL could be stable for the life of RNA. Future direction includes the detection of ORLs as a marker of oxidative damage in cells from various neurological disorders37.
Nuclear genes can be crucial targets for ODLs because they are present in 1–2 copies in each mammalian cell. If the ODL is not repaired, it will alter the transcripts for the remainder of the life of the cell. The repair of ODLs in at least three nuclear genes does not appear to follow the transcription-coupled mechanism23,31. Recent evidence suggests that recovery appears to correlate with the ability of the affected tissue to remove or repair oxidative damage17–25,89. It is clear that during intial reperfusion phase there is a period in which damage exceeds repair. What is the effect on the transcript generated from the gene that is activated but has not yet been completely repaired during oxidative stress? Deciphering the importance of nucleic acid damage and mechanisms of repair could highlight targets for therapeutic limitation of the spread of ischemic damage.
We also discussed that errors in repair appear to occur preferentially at GC sequences and mutations are mostly frameshift mutants in a transgenic animal strain. There is a need to investigate the relationship between frameshift mutations after brain injury and pre-existing heterozygotic germline mutation, especially in those genes that are associated with endogenous responses for neuroprotection, such as ROS scavengers and gene activators, and those genes that are implicated in causing neurological disorders. The identification of damage to genetic material early in the ischemic process might allow development of appropriate therapeutic interventions.
Acknowledgements
The authors wish to acknowledge the contributions of many investigators to this rapidly growing field of CNS repair that we were unable to cite due to space constraints, the National Institute of Neurological Disorders and Stroke, and American Heart Association for research supports.
Contributor Information
Philip K. Liu, Departments of Neurosurgery, Medicine (the Graduate Program of Cardiovascular Sciences) and Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA..
Robert G. Grossman, Department of Neurosurgery.
Chung Y. Hsu, Department of Neurology, Washington University School of Medicine, St Louis, MO 63110, USA.
Claudia S. Robertson, Department of Neurosurgery.
References
- 1.Englander EW, et al. Hypoxia-induced mitochondrial and nuclear DNA damage in the rat brain. J. Neurosci. Res. 1999;58:262–269. [PubMed] [Google Scholar]
- 2.Chen D, et al. Detection of DNA base-excision repair activity for oxidative lesions in adult rat brain mitochondria. J. Neurosci. Res. 2000;61:225–236. doi: 10.1002/1097-4547(20000715)61:2<225::AID-JNR13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Solenske NJ, et al. Differential hydrylation of salicylate in core and penumbra regions during focal reversible cerebral ischemia. Stroke. 1997;28:2545–2551. doi: 10.1161/01.str.28.12.2545. [DOI] [PubMed] [Google Scholar]
- 4.Cherian L, et al. Nitric oxide concentrations in the brain after controlled cortical impact injury in rats. J. Neurophysiol. 2000;83:2171–2178. doi: 10.1152/jn.2000.83.4.2171. [DOI] [PubMed] [Google Scholar]
- 5.Kumura E, et al. Generation of nitric oxide and superoxide during reperfusion after focal cerebral ischemia in rats. Am. J. Physiol. 1996;270:C748–C752. doi: 10.1152/ajpcell.1996.270.3.C748. [DOI] [PubMed] [Google Scholar]
- 6.Smith MA, et al. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott GS. Peroxynitrite production and activation of poly(adenosine diphosphate-ribose) synthetase in spinal cord injury. Ann. Neurol. 1999;45:120–124. [PubMed] [Google Scholar]
- 8.Lipton SA, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of NO and related nitroso compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 9.Estevez AG, et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 10.Khan AU, et al. The decomposition of peroxynitrite to nitroxyl anion (NO-) and singlet oxygen in aqueous solution. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2984–2989. doi: 10.1073/pnas.050587297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghafourifar P, et al. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J. Biol. Chem. 1999;274:31185–31188. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- 12.Sheng M, Lee SH. Growth of the NMDA receptor industrial complex. Nat. Neurosci. 2000;3:633–635. doi: 10.1038/76576. [DOI] [PubMed] [Google Scholar]
- 13.Dawson VL, et al. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewen A, et al. Free radical pathways in CNS injury. J. Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 15.Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 16.Floyd RA. Neuroinflammatory processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic. Biol. Med. 1999;26:1346–1355. doi: 10.1016/s0891-5849(98)00293-7. [DOI] [PubMed] [Google Scholar]
- 17.Eliasson ML, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J. Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T, et al. The NOS inhibitor, 7-nitroindazole, decreases focal infarct volume but not the response to topical acetylcholine in pial vessels. J. Cereb. Blood Flow Metab. 1994;14:924–929. doi: 10.1038/jcbfm.1994.123. [DOI] [PubMed] [Google Scholar]
- 19.Iadecola C, et al. Delayed reduction of ischemia brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997;23:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill MJ, et al. Neuroprotective effects of 7-nitroindazole in the gerbil model of global cerebral ischemia. Eur. J. Pharmacol. 1996;310:115–122. doi: 10.1016/0014-2999(96)00387-1. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 22.Kamii H, et al. Effects of nitric oxide synthase inhibition on brain infraction in SOD-1-transgenic mice following transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1994;16:1153–1157. doi: 10.1097/00004647-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Cui JK, et al. Oxidative damage to the c-fos gene and reduction of its transcription after focal cerebral ischemia. J. Neurochem. 1999;73:1164–1174. doi: 10.1046/j.1471-4159.1999.0731164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang D, et al. In situ detection of AP sites and DNA strand breaks with 3′-phosphate ends in ischemic mouse brain. FASEB J. 2000;14:407–417. doi: 10.1096/fasebj.14.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui JK, Liu PK. Neuronal NOS inhibitor that reduces oxidative DNA lesions and neuronal sensitivity increases the expression of intact c-fos transcripts after brain injury. J. Biomed. Sci. 2001;8:336–341. doi: 10.1007/BF02258375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz JB, et al. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J. Neurosci. 1995;15:8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindahl T, Wood RD. Frontiers in cell biology: quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 28.Sekiguchi M, Hayakawa H. Mammalian enzymes for preventing mutations caused by oxidative of guanine nucleotides. In: Nikoloff JA, Hoekstra MF, editors. DNA Damage and Repair: DNA Repair in Higher Eukaryotes. Vol. 2. Humana Press; 1998. [Google Scholar]
- 29.Hanawalt PC. Overview. In: Nikoloff JA, Hoekstra MF, editors. DNA Damage and Repair: DNA Repair in Higher Eukaryotes. Vol. 2. Humana Press; 1998. [Google Scholar]
- 30.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PK, et al. Damage, repair and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J. Neurosci. 1996;16:6795–6806. doi: 10.1523/JNEUROSCI.16-21-06795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan J, et al. Formation of the base modification 8-hydroxyl-2′-deoxyguanosine and DNA fragmentation following seizures induced by systemic kainic acid in the rat. J. Neurochem. 2000;74:302–309. doi: 10.1046/j.1471-4159.2000.0740302.x. [DOI] [PubMed] [Google Scholar]
- 33.Won MH, et al. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70–78. doi: 10.1016/s0006-8993(99)01611-x. [DOI] [PubMed] [Google Scholar]
- 34.Newcomb TG, Loeb LA. In: DNA Damage and Repair: DNA Repair in Prokarypotes and Lower Eukaryotes. Nikoloff JA, Hoekstra MF, editors. Vol. 1. Humana Press; 1998. pp. 65–84. [Google Scholar]
- 35.Gobbel GT, et al. Response of postmitotic neurons to x-irradiation: Implications for the role of DNA damage in neuronal apoptosis. J. Neurosci. 1998;18:147–155. doi: 10.1523/JNEUROSCI.18-01-00147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward JF. Nature of lesions formed by ionizing radiation. In: Nikoloff JA, Hoekstra MF, editors. DNA Damage and Repair: DNA Repair in Higher Eukaryotes. Vol. 2. Humana Press; 1998. [Google Scholar]
- 37.Nunomura A, et al. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovell MA, et al. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease. J. Neurochem. 1999;72:771–776. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- 39.Mecocci P, et al. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 40.Dizdaroglu M. Oxidative damage to DNA in mammalian chromatin. Mutat. Res. 1992;275:331–342. doi: 10.1016/0921-8734(92)90036-o. [DOI] [PubMed] [Google Scholar]
- 41.Rink SM, et al. Creation of RNA molecules that recognize the oxidative lesion 7,8-dihydro-8-hydroxy-2′-deoxyguanosine (8-oxodG) in DNA. Proc. Natl. Acad. Sci. U. S. A. 1998;95:11619–11624. doi: 10.1073/pnas.95.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: Implications for the role of DNA damage in apoptosis and neuronal cell death. J. Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 43.Olive PL, et al. Hypoxic fractions measured in murine tumors and normal tissues using the comet assay. Int. J. Radiat. Oncol. Biol. Phys. 1994;29:487–491. doi: 10.1016/0360-3016(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 44.Gavrieli Y, et al. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckman KB, Ames BN. Oxidative decay of DNA. J. Biol. Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 46.Park E-M, et al. Assay of excised oxidative DNA lesions: Isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3375–3379. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reardon JT, et al. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: Possible explanation for neurodegeneration in Xeroderma pigmentosum patients. Proc. Natl. Acad. Sci. U. S. A. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parshad R, et al. Fluorescence light-induced chromatid breaks distinguish Alzheimer disease cells from normal cells in tissue culture. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5146–5150. doi: 10.1073/pnas.93.10.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin L, et al. Up-regulation of base excision repair activity for 8-hydroxy-2′ deoxyguanosine in the mouse brain after forebrain ischemia-reperfusion. J. Neurochem. 2000;74:1098–1105. doi: 10.1046/j.1471-4159.2000.741098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui JK, et al. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000;14:955–967. doi: 10.1096/fasebj.14.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava DK, et al. Mammalian abasic site base excision repair: identification of the reaction sequence and rate-determining steps. J. Biol. Chem. 1998;273:21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- 52.Stevnsner T, et al. Inhibition of gene-specific repair of alkylation damage in cells depleted of poly (ADP-ribose) polymerase. Nucleic Acids Res. 1994;22:4620–4624. doi: 10.1093/nar/22.22.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaPlaca MC, et al. Temporal patterns of poly(ADP-ribose) polymerase activation in the cortex following experimental brain injury in the rat. J. Neurochem. 1999;73:205–213. doi: 10.1046/j.1471-4159.1999.0730205.x. [DOI] [PubMed] [Google Scholar]
- 54.Eliasson MJ, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 55.Endres M, et al. Ischemia brain injury is mediated by the activation of poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Nagayama T, et al. Activation of poly (ADP-ribose) polymerase in the rat hippocampus may contribute to cellular recovery following sublethal transient global ischemia. J. Neurochem. 2000;74:1636–1645. doi: 10.1046/j.1471-4159.2000.0741636.x. [DOI] [PubMed] [Google Scholar]
- 57.Nouspikel T, Hanawalt PC. Terminially differentiated human neurons repair transcribed genes but display attenuated global DNA repair and modulation of repair gene expression. Mol. Cell. Biol. 2000;20:1562–1570. doi: 10.1128/mcb.20.5.1562-1570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardozo-Pelaez F, et al. DNA damage, repair, and antioxidant systems in brain regions: a correlative study. Free Radic. Biol. Med. 2000;28:779–785. doi: 10.1016/s0891-5849(00)00172-6. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz C, et al. Age-related changes of DNA repair and mitochondrial DNA synthesis in the mouse brain. Acta Neuropathol. 1999;97:71–81. doi: 10.1007/s004010050957. [DOI] [PubMed] [Google Scholar]
- 60.Belloni M, et al. Distribution and kainate-mediated induction of the DNA mismatch repair protein MSH2 in rat brain. Neuroscience. 1999;94:1323–1331. doi: 10.1016/s0306-4522(99)00380-2. [DOI] [PubMed] [Google Scholar]
- 61.Jin K, et al. Focal ischemia induces expression of the DNA damage-inducible gene GADD45 in the rat brain. NeuroReport. 1996;7:1797–1802. doi: 10.1097/00001756-199607290-00022. [DOI] [PubMed] [Google Scholar]
- 62.Shackelford DA, et al. Changes in expression of the DNA repair protein complex DNA-dependent protein kinase after ischemia and reperfusion. J. Neurosci. 1999;19:4727–4738. doi: 10.1523/JNEUROSCI.19-12-04727.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feig DI, Loeb LA. Mechanisms of mutation by oxidative DNA damage reduced fidelity of mammalian DNA polymerase β. Biochemistry. 1993;32:4466–4473. doi: 10.1021/bi00067a040. [DOI] [PubMed] [Google Scholar]
- 64.Evans DAP, et al. Frameshift mutations at two hotspots in vasopressin transcripts in post-mitotic neurons. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6059–6063. doi: 10.1073/pnas.91.13.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 66.Solano SM, et al. Expression of α-synclein and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: gene associated with familial parkinson’s disease. Ann. Neurol. 2000;47:201–210. [PubMed] [Google Scholar]
- 67.van Leeuwen FW, et al. Frameshift mutants of beta amyloid precursor protein and ubiquitin-B in Alzheimer’s and Down patients. Science. 1989;279:242–247. doi: 10.1126/science.279.5348.242. [DOI] [PubMed] [Google Scholar]
- 68.Cha CI, et al. Immunocytochemical study on the distribution of nitrotyrosine in the brain of the transgenic mice expressing a human Cu/Zn SOD mutation. Brain Res. 2000;853:156–161. doi: 10.1016/s0006-8993(99)02302-1. [DOI] [PubMed] [Google Scholar]
- 69.Guo Z, et al. ALS-linked Cu/Zn-SOD mutation impairs cerebral synaptic glucose and glutamate transport and exacerbates ischemic brain injury. J. Cereb. Blood Flow Metab. 2000;20:463–468. doi: 10.1097/00004647-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Liu D, et al. The roles of free radicals in amyotrophic lateral sclerosis: reactive oxygen species and elevated oxidation of protein, DNA, and membrane phospholipids. FASEB J. 1999;13:2318–2328. doi: 10.1096/fasebj.13.15.2318. [DOI] [PubMed] [Google Scholar]
- 71.de Souza-Pinto NC, et al. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Can. Res. 2001;61:5378–5381. [PubMed] [Google Scholar]
- 72.Klungland A, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitfield PC, Pickard JD. Expression of the immediate early genes c-Fos and c-Jun after head injury in man. Neurol. Res. 2000;22:138–144. doi: 10.1080/01616412.2000.11741050. [DOI] [PubMed] [Google Scholar]
- 74.Davis TW, et al. Transcriptional responses to damage created by ionizing radiation, molecular sensors. In: Nikoloff JA, Hoekstra MF, editors. DNA Damage and Repair: DNA Repair in Higher Eukaryotes. Vol. 2. Humana Press; 1998. [Google Scholar]
- 75.Gillardon F, et al. Activation of c-Fos contributes to amyloid β-peptide-induced neurotoxicity. Brain Res. 1996;706:169–172. doi: 10.1016/0006-8993(95)01332-6. [DOI] [PubMed] [Google Scholar]
- 76.Sharp FR, et al. Heat-shock protein protection. Trends Neurosci. 1999;22:97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- 77.Raghupathi R, McIntosh TK. Regionally and temporally distinct patterns of induction of c-fos, c-jun and junB mRNAs following experimental brain injury in the rat. Brain Res. Mol. Brain Res. 1996;37:134–144. doi: 10.1016/0169-328x(95)00289-5. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, et al. Posttranslesional mechanism leading to mammalian gene activation in response to genotoxic stress. In: Nikoloff JA, Hoekstra MF, editors. DNA Damage and Repair: DNA Repair in Higher Eukaryotes. Vol. 2. Humana Press; 1998. [Google Scholar]
- 79.Tamatani M, et al. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat. Med. 2001;7:317–323. doi: 10.1038/85463. [DOI] [PubMed] [Google Scholar]
- 80.Hengerer B, et al. Lesion-induced increase in nerve growth factor mRNA is mediated by c-fos. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui JK, et al. Suppression of postischemic hippocampal nerve growth factor expression by a c-fos antisense oligodeoxynucleotide. J. Neurosci. 1999;19:2784–2893. doi: 10.1523/JNEUROSCI.19-04-01335.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 83.Viswanathan A, Doetsch PW. Effects of nonbulky DNA base damages on Escherichia coli RNA polymerase-mediated elongation and promoter clearance. J. Biol. Chem. 1998;273:21276–21281. doi: 10.1074/jbc.273.33.21276. [DOI] [PubMed] [Google Scholar]
- 84.Camandola S, et al. The lipid peroxidation product 4-hydroxy-2, 3-nonenal increases AP-1-binding activity through caspase activation in neurons. J. Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang YJ, et al. Suppression of post-ischemic-induced fos protein expression by an antisense oligonucletide to c-fos mRNA leads to increased tissue damage. Brain Res. 1999;19:112–117. doi: 10.1016/s0006-8993(99)01459-6. [DOI] [PubMed] [Google Scholar]
- 86.Deng X, et al. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J. Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang K, et al. Increased expression of c-fos mRNA and AP-1 transcription factor after cortical impact injury in rats. Brain Res. 1994;664:141–147. doi: 10.1016/0006-8993(94)91964-x. [DOI] [PubMed] [Google Scholar]
- 88.An G, et al. Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann. Neurol. 1993;33:457–464. doi: 10.1002/ana.410330508. [DOI] [PubMed] [Google Scholar]
- 89.Kawase M, et al. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: implication of the failure of DNA repair in neuronal apoptosis. Stroke. 1999;30:441–449. doi: 10.1161/01.str.30.2.441. [DOI] [PubMed] [Google Scholar]