Abstract
Adeno-associated virus (AAV) has become a leading gene transfer vector for striated muscles. However, the AAV vectors also exhibit broad tropisms after systemic delivery. In an attempt to improve muscle tropism, we inserted a 7-amino-acid (ASSLNIA) muscle-targeting peptide (MTP) in the capsids of AAV2 at residue 587 or 588, generating AAV587MTP and AAV588MTP. In vitro studies showed that both viruses diminished their infectivity on non-muscle cell lines as well as on un-differentiated myoblasts, however, preserved or enhanced their infectivity on differentiated myotubes. AAV587MTP, but not AAV588MTP, also abolished its heparin-binding capacity and infected myotubes in a heparin-independent manner. Furthermore, in vivo studies by intravenous vector administration in mice showed that AAV587MTP enhanced its tropism to various muscles and particularly to the heart (24.3 fold of unmodified AAV2), whereas reduced its tropism to the non-muscle tissues such as the liver, lungs and spleen, etc. This alteration of tissue tropism is not simply due to the loss of heparin-binding, since a mutant AAV2 (AAVHBSMut) containing heparin-binding site mutations lost infectivity on both non-muscle and muscle cells. Furthermore, free MTP peptide, but not the scrambled control peptide, competitively inhibited AAV587MTP infection on myotubes. These results suggest that AAV2 could be re-targeted to the striated muscles by a muscle-targeting peptide inserted after residue 587 of the capsids. This proof of principle study showed first evidence of peptide-directed muscle targeting upon systemic administration of AAV vectors.
Keywords: AAV, capsid modification, muscle targeting, systemic delivery
INTRODUCTION
Diseases of the heart and skeletal muscles affect adults and children worldwide. Gene therapy represents an attractive strategy for a variety of muscle diseases such as muscular dystrophies and heart failure.1,2 Gene transfer vectors, including non-viral and viral vectors, have been shown to accomplish gene delivery to local muscle and heart tissue by direct intramuscular (i.m.) injection or by local vessel perfusion.3,4 The simplest approach of gene therapy is the injection of naked plasmid DNA encoding the therapeutic gene into muscle.5 Although plasmid DNA delivery could achieve long-term gene expression,6 the delivery efficiency of naked plasmid is low following i.m. injection. Viral vectors, especially the AAV vectors,7 offer effective alternative approaches for muscle-directed gene transfer. To functionally correct disorders affecting the heart and skeletal muscles, delivery of gene vectors to a majority of the diseased cells is required. Following direct i.m. injection, AAV vectors can readily saturate individual muscles around the injection sites.8 Gene transfer to large groups of muscles by multiple i.m. injections is feasible9 but ineffective and impractical for muscular dystrophies that affect muscles body-wide. Systemic delivery of AAV vectors to the muscle and heart has been achieved by a single intravenous (i.v.) or intraperitoneal (i.p.) of AAV vectors.10,11 However, the AAV vectors also non-specifically infect a variety of non-muscle tissues. As a result, targeting the AAV vectors to the muscle after systemic delivery is a highly desirable and yet challenging task for muscle-directed gene therapy.
One of the approaches of targeting AAV to the muscle is to alter its native interactions with the cellular receptors and retarget the virus through a different binding ligand to the muscle. Among the currently used AAV serotypes, AAV2 is the best characterized serotype for its viral-cellular interactions. It is also the best documented AAV vector in pre-clinical studies and clinical trials in the past 20 years.12 Therefore, AAV2 can serve as a good candidate for genetic engineering of detargeting and retargeting. AAV2 uses cell membrane-associated heparan sulfate proteoglycan (HSPG) as its primary binding receptor and its transduction can be efficiently competed by free heparin.13 In addition, AAV2 utilizes a number of membrane proteins such as αVβ5 integrin,14 fibroblast growth factor receptor-1 (FGFR1),15 hepatocyte growth factor receptor (c-Met),16 and α5β1 integrin17 as its co-receptors for cell entry. As AAV tropism is determined by a specific interaction between viral capsids and host cellular receptors,13–17 modification of capsid proteins has emerged as a means to alter native vector tropism. In addition, incorporation of targeting peptides selected by either phage display18 or AAV display19 on the surface of AAV capsids has been used to target the vector to specific cell types.20–25 Previously, insertion of an angiogenic vascular targeting motif NGR on AAV2 capsid successfully redirected vector tropism to cells expressing the NGR receptor CD13 that is presented in angiogenic vasculature and in many tumor cell lines.22 Similarly, the incorporation of endothelium-specific peptide SIGYPLP into the AAV2 capsid at position 587 displayed an altered tropism toward human vascular endothelial cells. The infection happened to be independent of HSPG binding.23 A recent study also demonstrated that the insertion of MTPFPTSNEANL peptide into AAV2 capsid after amino acid 587 enhanced gene delivery of AAV vectors into a specific vascular site in vivo and these vectors transduced the vena cava independently of HSPG binding.24 Moreover, Work et al.25 identified peptides homing to the lung and brain by in vivo phage display from rats and they showed that the isolated targeting peptides retargeted AAV vectors to the expected organs in a preferential manner. However, no efforts have been reported on systemic re-targeting of AAV to the muscles, either cardiac and/or skeletal.
In this report we describe the construction and evaluation of AAV2 vectors genetically modified with a muscle-targeting peptide (MTP), which was originally isolated by phage display in differentiated muscle cells in vitro and muscle tissue in vivo.26,27 The MTP-modified AAV2 showed improved tropisms to the striated muscles, and could efficiently and selectively infect differentiated muscle cells in vitro and skeletal and cardiac muscles in vivo.
RESULTS
Genetic Modification of AAV2 capsids with a muscle-targeting peptide (MTP)
Ligand insertion at amino acid residues 587 and 588 of AAV2 capsids can be well tolerated.19,22–25 We therefore genetically inserted the muscle-targeting peptide ASSLNIA26,27 after 587 or 588 site of the AAV2 capsid gene, resulting two MTP-modified AAV2 capsid mutants: AAV587MTP and AAV588MTP (Fig. 1a). The MTP insertion after 587 of the AAV2 capsid disrupted the heparin-binding motif and is expected to lose its heparin-binding capability, which is the primary mechanism of AAV2 cell binding and entry.13 In addition, we have generated a third mutant: AAVHBSMut, which had all three arginines mutated (R484E, R585A and R588A) at the heparin-binding site of the AAV2 capsids (Fig. 1a). But further insertion of MTP after 587 of AAVHBSMut caused failure of AAV vector packaging (data not shown). The above three mutant AAV capsids were used to package AAV vectors containing either GFP or luciferase reporter genes. DNA dot-blot assays showed that all three mutant AAVs could yield AAV vector titers comparable to that of the unmodified AAV2 (data not shown). Furthermore, Western analysis using the same quantity of viral particles (viral genomes v.g.) of the three mutants and the unmodified AAV2 did not reveal abnormal quantity or stoichiometry of capsid proteins, VP1, VP2 and VP3 (Fig. 1b), suggesting that the formation of the viral particles and the DNA packaging capacity were not impaired by these mutations.
Figure 1. Construction of AAV mutants.
(a) Schematic representation of modified AAV2 capsid amino acid sequences. The peptide encoding ASSLNIA amino acid sequence flanked by two different linkers was inserted after residue 587 or 588 in the AAV2 capsid. The amino acid changes in AAVHBSMut capsid compared to the wild-type AAV2 are indicated. (b) Capsid protein analysis of modified AAV vectors by Western blotting. Similar numbers of AAV genome-containing particles (2×1010) were separated on 10% SDS-PAGE and analyzed by Western blotting, using anti-AAV2 capsid guinea pig sera.
MTP re-targets AAV2 to myotubes and abolishes infectivity to non-muscle cells in vitro
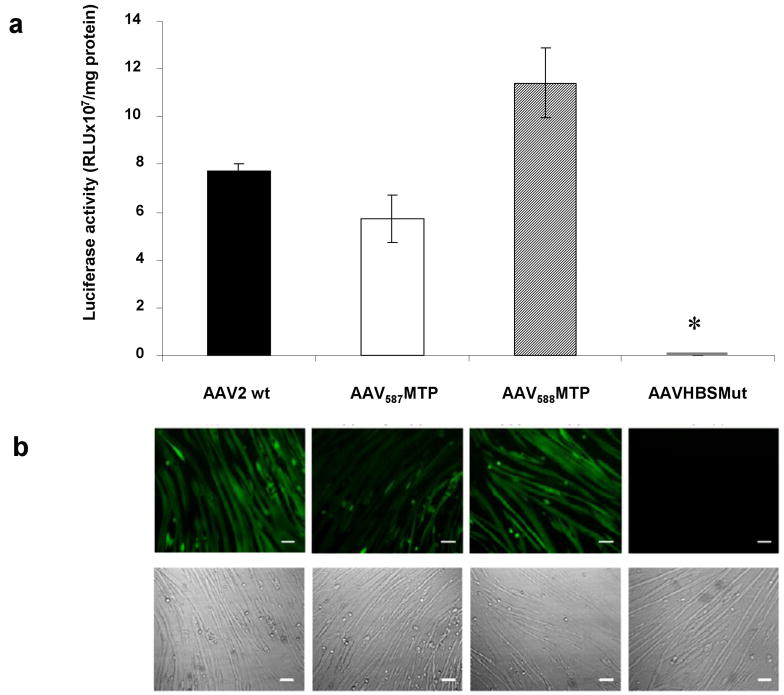
We next examined the effects of MTP insertion on the infectivity of AAV2 in vitro on differentiated muscle cells, the myotubes. Since the ASSLNIA peptide was originally isolated by phage-display selection in murine C2C12 myotubes26 that express many of the proteins presented in skeletal muscles, C2C12 myotubes were used to validate the muscle-targeting efficiencies of peptide-modified AAV vectors in vitro. First, a luciferase (Luc) reporter vector packaged respectively by AAV587MTP, AAV588MTP, AAVHBSMut and unmodified AAV2 was used to infect differentiated C2C12 cells, which formed myotubes after differentiation. As shown in Fig. 2a, both AAV587MTP and AAV588MTP were able to transduce C2C12 myotubes at similar levels as the unmodified AAV2 vector, although AAV587MTP was slightly lower and AAV588MTP was slightly higher (Fig. 2a). As expected, AAVHBSMut with triple mutations on the heparin-binding site dramatically abolished its transduction on C2C12 myotubes by more than 2600 fold (Fig. 2a). To confirm the results obtained by the luciferase reporter vectors, we also used a GFP reporter vector packaged in the same viral capsids and tested again on differentiated C2C12 myotubes in a similar fashion. Fluorescent microscopy showed green fluorescence on myotubes infected with unmodified AAV2, AAV587MTP and AAV588MTP but not on myotubes infected with AAVHBSMut (Fig. 2b), consistent with the results from the luciferase reporter vectors.
Figure 2. Efficiency of modified AAV-mediated gene transfer to targeted C2C12 myotubes.
(a) Murine C2C12 myotubes were infected with 2×1010 genomic particles/per well of AAV-CMV-Luc vector which carries either unmodified capsid, peptide-inserted capsid, or heparin-binding mutated capsid in 24-well plates. After 3 days, myotubes were replaced by fresh DMEM containing 2% horse serum and subsequently incubated for 6 days. Luciferase activity was then analyzed to evaluate the transduction efficiencies of modified AAV vectors. Data are shown as a bar graph with mean±standard error of the mean (SEM). *P<0.05 vs. unmodified AAV2 vector. (b) C2C12 myotubes were next transduced with AAV-CB-EGFP vectors at 1×1010 genomic particles/per well in 24-well plates. EGFP expression driven by the CB promoter was then observed under a Nikon TE-300 inverted fluorescent microscope. Pictures were taken at 72 hours after infection. Fluorescent photography is shown in the upper panel and the morphology of C2C12 myotubes on the same field as the fluorescent image is displayed in the lower panel. Scale bar, 100μm.
To further evaluate the targeting specificity of peptide-modified vectors, undifferentiated C2C12 myoblasts were infected for 2 days with the luciferase vectors. AAV587MTP and AAV588MTP showed decreased transduction by 92.59% and 96.95% when compared to the unmodified AAV2, while AAVHBSMut had almost undetectable transduction in undifferentiated C2C12 cells (Table 1). Similar experiments were also done using non-muscle cell lines including HepG2, a human hepatocellular carcinoma cell line, HeLa, a human cervix epitheloid carcinoma cell line, HEK 293, a human embryonic kidney cell line and U-87MG, a human glioblastoma tumor cell line (Table 1). All three mutant AAVs showed dramatic decreases in transduction when compared to the unmodified AAV2, indicating that insertion of MTP could diminish the native infectivity of AAV2 on the permissive non-muscle cells in vitro.
Table 1.
Loss of infectivity on permissive cells by AAV2 after MTP-modification*
| Cell lines | AAV2 wt | AAV587MTP | AAV588MTP | AAVHBSMut |
|---|---|---|---|---|
| C2C12 | 1.94×105±6.11×104 | 1.44×104±7.11×103 | 5.93×103±9.80×102 | 3.47×103±3.08×102 |
| HepG2 | 7.83×106±1.33×106 | 5.92×104±2.42×104 | 7.31×105±1.21×105 | N/D |
| HeLa | 1.44×106±3.17×105 | 2.93×104±1.26×104 | 2.00×104±1.34×104 | 7.01×103±1.17×103 |
| HEK 293 | 2.16×106±9.73×103 | 3.96×104±6.44×103 | 5.80×104±1.95×104 | 9.07×103±3.28×103 |
| U-87MG | 4.04×106±4.11×105 | 1.08×104±1.19×103 | 1.32×105±9.17×103 | N/D |
The cells were infected with AAV-CMV-Luc vectors and luciferase activities were analyzed 48 hour later. AAV infectivity was expressed as mean Luciferase activity (RLU/mg protein) ±SEM.
AAV587MTP infection in myotubes is heparin-independent and MTP dependent
To examine if the insertion of MTP peptide impaired heparin-binding capacity of the mutant AAVs, we performed an in vitro heparin-binding assay. Three mutant AAV vectors and the unmodified AAV2 vector were loaded (5×1011 v.g. each) onto heparin columns for binding. After extensive wash, the bound AAVs were eluted by 1 M NaCl. Fractions from loading flow through, wash and elution were all collected for viral particle analyses. Viruses were monitored by DNA-dot blot using the CMV promoter probe (Fig. 3a) and also by Western blot using a guinea pig anti-AAV2 serum (Fig. 3b). As expected, the unmodified AAV2 showed high affinity for the heparin column, and were only found in the elution fraction (Fig. 3a). AAV588MTP also displayed similar heparin-binding ability to the unmodified AAV2. The majority of the AAV588MTP was found in the elution fraction with negligible amount in the wash fraction. In contrast, AAV587MTP viruses were substantially detected in the wash fraction as well as in the elution faction. As expected, AAVHBSMut was detected in the loading flow-through faction, and mostly in the wash fraction, but undetectable in the elution fraction. These results suggest that the loss of heparin-binding capacities is extensive for AAVHBSMut, substantial for AAV587MTP but minor for AAV588MTP.
Figure 3. Analysis of mutant capsid virus binding to heparin.
(a,b) Heparin-affinity column analysis. 5×1011 of unmodified or peptide-inserted viruses were loaded onto a prepacked and equilibrated 1 ml heparin column. Viral particles appeared in the flow-through, wash, and elution fractions were then detected by DNA dot-blot with CMV probe. The fractions from the heparin-affinity column analysis were also analyzed by Western blot using guinea pig anti-AAV2 serum. The positions of VP1, VP2, and VP3 are indicated. I: Input; F: Flow-through; W: Wash step; E: Elution. (c) Evaluation of HSPG-dependent AAV transduction in C2C12 myotubes. C2C12 myotubes were infected with AAV-CMV-Luc vectors carrying unmodified or peptide-inserted capsids in the absence or presence of 30 μg/ml heparin and analyzed for luciferase expression to examine the HSPG dependence of vectors. Data are shown as mean±SEM. *Indicates P<0.05 vs. transduction in the absence of heparin. (d) Competitive blocking experiment by synthesized MTP in C2C12 myotubes. C2C12 myotubes were infected with AAV-CMV-Luc vectors in the presence or absence of synthesized free peptides. Level of gene transduction efficiency of peptide-modified vectors and unmodified AAV virus were compared by evaluating luciferase expression. Data are mean values±SEM. *P<0.05 vs. value in the absence of peptide.
Next we performed a competitive inhibition experiment with soluble heparin to see if the loss of heparin binding makes the viral infection in an HSPG-independent manner. Differentiated C2C12 myotubes were infected with unmodified AAV2, AAV587MTP and AAV588MTP containing a luciferase report gene in the presence of soluble heparin, which is known to competitively inhibit AAV2 infection.13 As expected, the transduction of AAV587MTP vector was not inhibited by heparin, whereas both unmodified AAV2 and AAV588MTP vectors significantly diminished their transduction efficiency by 72.03% and 42.25%, respectively, in the presence of heparin sulfate (P<0.05) (Fig. 3c).
Finally we examined if infection of differentiated myotubes by AAV587MTP is mediated by the MTP peptide insertion on the capsids. Unmodified AAV2 and AAV587MTP were compared for their transduction efficiencies (luciferase reporter gene transfer) on C2C12 myotubes in the presence of the specific peptide (ASSLNIA), or a scrambled peptide (LISNSAA). Infection without any competing peptide was used as a positive control. As shown in Fig. 3d, AAV587MTP transduction was significantly inhibited (47.30%) by 2 mg/ml free ASSLNIA peptide (P<0.05), but not by free LISNSAA. The inhibition of unmodified AAV2 by free ASSLNIA peptide was statistically insignificant when compared to the control without peptide (P>0.3). These results suggest that the MTP could re-target AAV2 to differentiated myotubes.
MTP peptide enhances AAV588MTP infectivity to skeletal muscle after local i.m. injection
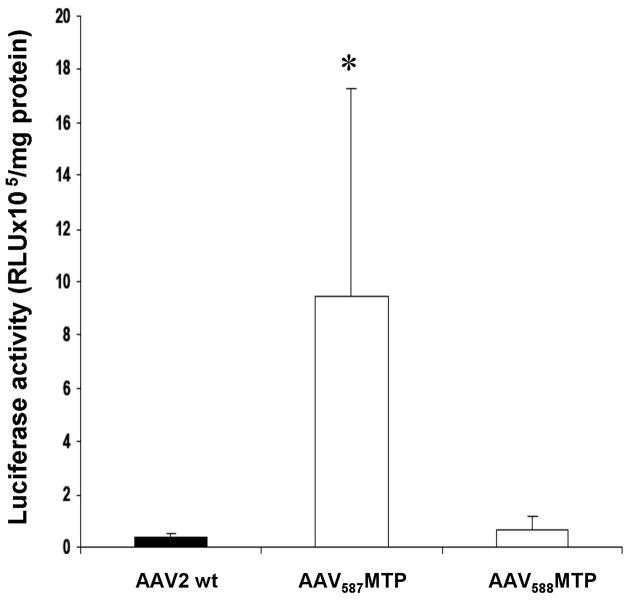
We next investigated if the MTP could enhance AAV2 infectivity to the skeletal muscles. Adult male ICR mice (2 months of age, 4 mice in each group) were injected in the tibialis anterior (TA) muscle of the hind legs with lucifierase vectors respectively packaged in unmodified AAV, AAV587MTP and AAV588MTP (2.5 × 1010 v.g. each). The TA muscles were collected at 4 weeks after i.m. injection of the vectors for luciferase activity assay. When compared to the control AAV2, AAV587MTP had a slightly lower luciferase gene expression while AAV588MTP had a 3.2 fold increase (Fig. 4). This result mirrors the luciferase activity profile obtained in the in vitro assay on differentiated myotubes (Fig. 2), suggesting that AAV587MTP might primarily use the MTP for cell entry, whereas AAV588MTP might use a dual mechanism, both heparin binding and MTP.
Figure 4. Intramuscular delivery (i.m.) of peptide-modified AAV vectors in mice.
Luciferase activities were obtained from the TA muscles of mice injected with 2.5×1010 genomic particles of AAV-CMV-Luc vector carrying wild-type or modified capsids 4 weeks before examination (n=4 TA muscles for each vector tested). Data are expressed as mean±SEM. * P<0.05 vs. unmodified AAV2 vector.
MTP peptide retargets AAV587MTP to heart and muscle after systemic i.v. injection
We next investigated if MTP could enhance the infectivity of AAV2 in vivo to the heart and skeletal muscles after systemic delivery by intravenous injection. Eight-week-old adult ICR mice were administered with unmodified AAV2, AAV587MTP, or AAV588MTP containing the CMV-Luciferase reporter gene cassette via the tail vein at a dose of 9 x 1011 v.g each. Four weeks after i.v. injection, luciferase reporter gene expression was analyzed in various tissues. AAV587MTP achieved higher luciferase gene expression than the unmodified AAV2, and surprisingly, AAV588MTP in various muscles and heart after systemic delivery. The luciferase activity of AAV587MTP in the heart was 24.3 fold of that of the unmodified AAV2, while the activity of AAV588MTP was only 1.82 fold of the unmodified AAV2 (Fig. 5a). In skeletal muscles, the luciferase activity of AAV587MTP was 2.18-fold of the AAV2 in the diaphragm and 2.85-fold in quadriceps (Fig. 5b). Moreover, the AAV587MTP showed reduced transduction in the liver, lungs, spleen, etc than the unmodified AAV2 (Fig. 5c). In contrast, AAV588MTP showed reduced transduction in most tissues, muscle as well as non-muscle, after systemic delivery (Figs. 5b,c).
Figure 5. In vivo AAV-mediated gene transduction after intravenous delivery.
9×1011 genomic particles of AAV were delivered to 8-week old male mice via tail vein injection (n=5 for unmodified AAV2, n=6 for AAV587MTP, and n=5 for AAV588MTP vector). Luciferase reporter gene expression in major organs was analyzed one month after delivery. (a) Luciferase activities in cardiac muscle after systemic delivery of peptide-modified vectors. (b) Luciferase activities in striated muscles after systemic AAV administration. (c) Luciferase activities in non-muscle organs after intravenous injection of AAV vectors. *P<0.05 vs. unmodified AAV2 vector. Results are expressed as mean±SEM.
Quantitative PCR was also performed on samples collected from AAV2 and AAV587MTP treated mice for vector tissue distribution. The results showed that AAV587MTP vector DNA copy number in the heart increased by 6.6 fold when compared to the unmodified AAV2 (7.28±3.91 copies vs. 0.96±0.45 copies per μg of tissue DNA, P<0.05) (Fig. 6a). The vector DNA copy numbers also increased in various skeletal muscles, including diaphragm, upper limbs, quadriceps, gastrocnemius, and tibialis anterior muscles when compared to the unmodified AAV2 vector (Fig. 6a). However, the vector DNA copy numbers of AAV587MTP in the liver was less than half (39.4%) of that of unmodified AAV2 (Fig. 6b). In addition, AAV587MTP also showed an approximately 10-fold lower vector DNA distribution in the lung and spleen (Fig. 6b). The real-time PCR results were essentially consistent with the luciferase gene expression profiles, suggesting that MTP could de-target AAV587MTP from non-muscle tissues and re-target it to the striated muscles including the heart and skeletal muscle after systemic delivery.
Figure 6. In vivo vector distribution after intravenous delivery.
9×1011 viral particles of AAV were administered to 2-month old male mice via tail vein injection (n=5 for unmodified AAV2, and n=6 for AAV587MTP). Vector distribution was quantified by real-time PCR. (a) AAV genome distribution in non-muscle and muscle tissues. (b) Quantify hepatic AAV viral genomes after systemic delivery. Data represent means±SEM. * P<0.05 vs. unmodified AAV2 vector.
DISCUSSION
A number of AAV serotype vectors are able to achieve systemic gene delivery into the heart and skeletal muscles, but also show strong tropisms to non-muscle tissues.10,11,28,29 Ideally, systemic delivery of therapeutic genes to striated muscles requires not only efficiency but also tissue specificity. In an attempt to improve the muscle tropism of AAV vectors by ligand-directed gene delivery, we have genetically modified AAV2 capsid surface with a muscle-targeting peptide, MTP, a small 7-mer peptide ASSLNIA 26,27. On cultured cells in vitro, the insertion of MTP not only furnished AAV2 with infectivity to differentiated myotubes but also ablated or impaired its infectivity to the otherwise permissive non-muscle cells. Furthermore, insertion of MTP at capsid position 588 was able to enhance AAV2 infectivity in vivo to the muscle myofibers by 3.2 fold after intramuscular injection. On the otherhand, MTP at position 587 could re-target AAV2 to the heart (an increase of 23.3 fold), and at a less degree, to skeletal muscles after systemic injection. The above phenomena suggest that AAV587MTP may have improved capacity to cross the capillary blood vessel barrier in striated muscles, particularly in the heart. Although much room remains for further improvement and optimization, we believe that our results demonstrate the first example of a ligand-directed targeting of AAV vectors to the striated muscle tissues. Since AAV2 is not a robust virus for either direct local or systemic gene delivery into the heart and muscle, the incorporation of the MTP onto the surface of capsids of more powerful AAV serotype capsids such as AAV7, 8 and 9, which are efficient in crossing the blood vessel barrier to reach muscle myofibers in vivo, could render them more effective in targeting muscles in vivo for systemic gene delivery.
In this study, we chose to modify serotype AAV2 with the muscle-targeting peptide, primarily based on the wealth of information on AAV2 viral capsid structure and functional relationship. Although AAV2 is not the best vector for gene delivery to the muscle, it has been extensively used in mutagenesis studies including point mutations, linker insertions and peptide display on its capsids.19–25,30 Peptide insertions at amino acid residues 587 and 588 are well tolerated because the loop structure is exposed on the surface of the capsids.19,22–25,30 Insertion at 587 also interferes and abolishes viral particle binding to heparan proteoglycans, which are ubiquitous on cell surface of almost all tissues31 as a primary receptor for AAV2 attachment and cell entry.13 Ablation of heparin binding should facilitate tissue-specific targeting. Although both AAV587MTP and AAV588MTP had the same MTP insertion, AAV587MTP largely lost its heparin-binding capacity whereas AAV588MTP had a minor loss. As such, AAV587MTP exhibited better muscle-targeting in vivo after systemic delivery, while AAV588MTP might use a dual mechanism via both heparin and MTP for cell binding and entry. Importantly, the loss of heparin binding alone could not account for the improved muscle-targeting of AAV587MTP, because a heparin-binding deficient mutant AAV2 (AAVHBSMut, R484→E, R585→A and R588→A) not only failed to achieve significant infection on cultured myotubes (Fig. 2) but also failed to achieve luciferase reporter gene expression higher than basal levels in all the organs and tissues tested after systemic tail vein injection (data not shown). Furthermore, free MTP peptide, but not the scrambled peptide, on myotube culture could inhibit the transduction of AAV587MTP but not the unmodified AAV2, suggesting that the MTP is responsible in part for retargeting the AAV2 to the striated muscle tissues, particularly the heart.
In this study, we have examined two MTP-modified AAV2 capsids on cultured cells in vitro and in mice in vivo by direct vector injection. In the in vitro experiments, both AAV587MTP and AAV588MTP dramatically lost their infectivities (~ 100 fold) to all the cell types tested including the undifferentiated myoblast C2C12, except the differentiated muscle cells (myotubes). It suggested that the MTP insertion played a major role in de-targeting AAV2 from non-muscle cells and re-targeting it to myotubes in vitro. However, the in vivo de-targeting and re-targeting results by the MTP-modified AAV2 were much less impressive than the results obtained in vitro. These discrepancies could be attributed primarily to the profound in vitro and in vivo differences in the environment throughout the process of AAV infection. It is well documented that AAV2 vectors are robust in vitro but less efficient in vivo, while numerous new AAV serotypes are exactly the opposite. The viruses might favor different receptors or co-receptors in vivo. Furthermore, the serum proteins and a barrage of different cell types in vivo could also alter the behavior of the vectors. Another reason could be the high complexity and variability of in vivo experiments. In our study, reporter gene expression in some muscle groups showed statistic differences between the wt AAV2 and MTP-modified AAV2, while other groups did not. We have also observed discrepancies between reporter gene expression and vector DNA copy numbers in some tissues, which again could reflect the complex situations in vivo. For example, it was demonstrated that AAV2 and AAV8 had similar vector copy numbers in the liver shortly after intravenous vector delivery. However, AAV8 achieved dramatically high levels of gene expression due to more efficient intracellular trafficking and uncoating of the viral particles41. Therefore, direct in vivo screening and evaluation of new vectors should be a more reliable and preferred method.
Finally, we believe that minor differences on MTP insertion sites (587 vs. 588) and the linker sequences flanking the MTP made a significant difference on the behavior between AAV587MTP and AAV588MTP. These differences could not be simply explained by the alteration of heparin binding. It is conceivable to see a near complete loss of infectivity of AAV587MTP on the permissive non-muscle cell lines for AAV2 because of the loss of heparin binding. But AAV588MTP also showed a near complete loss of infectivity on those cells, although its heparin- binding motif RXXR (X is any amino acid) is still intact 32. Again unexpectedly, AAV588MTP was significantly more resistant than AAV587MTP to anti-AAV2 antibody neutralization using anti-sera from guinea pig, mouse and pooled human IVIG (data not shown), despite the presence of intact heparin-binding motif. A previous report showed that linker sequences flanking the peptide insertion could significantly influence the configuration and display of the engineered peptide epitope.33 Insertion of an integrin-specific peptide ligand (L14) at residue 587 of AAV2 capsid enabled the vector to escape antibody neutralization33. However, it was not true for AAV587MTP, which was as sensitive to human IVIG as the unmodified AAV2. Molecular modeling34,35,36 of the putative 3-dimensional structures of those capsids (data not shown) revealed that the loop containing the heparin-binding RXXR motif is missing on AAV587MTP, but still exists on AAV588MTP, supporting the in vitro heparin-binding and inhibition data (Fig. 3). On the other hand, the MTP loop is extended and protruding on AAV587MTP but takes a fold-back configuration on AAV588MTP. This may make the MTP on AAV588MTP less effective for muscle targeting, but more effective on stereotactic blockade of neutralizing antibody binding to key components on AAV2. Thus, different linker contexts could assign different properties on the displayed peptide. It also echoes a previous study that showed that the choices of linker sequences could make a big difference on AAV2 targeting and resistance to neutralizing antibodies 30, a very important issue for in vivo gene delivery with AAV vectors in human patients 37, 38.
MATERIALS AND METHODS
Cell culture
C2C12 murine myoblasts (American Type Culture Collection, Rockville, Maryland), human hepatocellular carcinoma HepG2 cells, human cervical carcinoma HeLa cells, human embryonic kidney 293 cells (HEK 293 cells), and human glioblastoma tumor U-87MG cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). To induce the differentiation of C2C12 myoblasts into myotubes, the cells were switched from growth medium (DMEM with 10% FBS) to differentiation medium (DMEM with 2% horse serum) and were incubated in differentiation medium for up to 9 days. After 4 days of differentiation, the C2C12 myotubes were fully formed.
Plasmid construction
The plasmid pBSKS-AAV2Cap containing AAV2 cap gene was used as the template for the construction of all modified capsids by using PCR. The mutations of heparin-binding sites on AAV2 capsid were introduced by the mutagenic primers which contain the desired mutation. For the peptide-modified capsids, we designed the primers encoding amino acids ASSLNIA flanked by peptide linker. This muscle-specific peptide (ASSLNIA) was then inserted into the site after residue 587/or 588 of AAV2 capsid by PCR. The synthesized PCR products were digested with DpnI endonuclease to eliminate the parental plasmid template and further added phosphates at 5′ of oligonucleotides by T4 polynucleotide kinase (New England BioLabs) to allow subsequent ligation. The sequences of primers are the following: R484E+, 5′AGC AGC AGC GAG TAT CAA AG 3′; R484E−, 5′CGT AAC AGG GTC AGG AAG C 3′; R585A−, 5′TGC CTG GAG GTT GGT AGA TAC AGA ACC AT 3′; R588A+, 5′GGC AAC GCA CAA GCA GCT ACC GCA GAT GTC 3′; 587 TG MTP+, 5′AAC ATC GCC GGA TTA AGT AGA CAA GCA GCT ACC GCA 3′; 587 TG MTP−, 5′GAG GGA GGA AGC TCC TGT GTT GCC TCT CTG GAG GTT 3′; 588 HB MTP+, 5′AAC ATC GCC GCC GCC CAA GCA GCT ACC GCA GAT 3′; 588 HB MTP−, 5′GAG GGA GGA GGC GCG GCG GTT GCC TCT CTG GAG GTT 3′. The modified cap gene was then subcloned from pBSKS-AAV2Cap to pXX239 by EcoRV and XcmI.
AAV vector production and evaluation of AAV titers
To produce AAV virus, the three-plasmid cotransfection method was applied.40 The plasmids used in transfection were the following: i) AAV-CMV-Luc plasmid with the luciferase (Luc) gene driven by the CMV promoter, or AAV-CB-EGFP plasmid with the enhanced green fluorescent protein (EGFP) gene controlled by the CB promoter (CMV enhancer/chicken beta-actin promoter). Both plasmids carry the promoter-driven transgene flanked by AAV ITRs; ii) the pXX6 plasmid, which contains the helper genes from adenovirus; iii) the modified pXX2 plasmid, which supplies AAV2 rep protein and modified capsid protein. As a control, wild-type AAV capsid was also prepared with unmodified pXX2 plasmid. The vector production and purification were performed according to previously published method with two rounds of CsCl centrifugation.40 AAV genomic titers were determined by DNA dot-blot assay. Briefly, 2 μl of the purified AAV stock was digested with DNase I (10 μg/ml) in DMEM at 37°C for one hour and then 200 μl of 2x proteinase K buffer (20 mM Tris.Cl pH 8.0, 20 mM EDTA pH 8.0, 1% SDS) was added. Next, proteinase K was added to reactions at a final concentration of 1 mg/ml and the samples were incubated at 55°C for one hour. Viral DNA was precipitated by ethanol and the DNA pellet was dissolved in an alkaline buffer (0.4 M NaOH, 10 mM EDTA pH 8.0). DNA samples were applied to Nylon membranes and probed with a horseradish-peroxidase-labeled CMV or EGFP probe. Signals were detected by the North2South® chemiluminescence kit (Pierce). In order to detect if these mutant virions were composed of three capsid proteins, 2×1010 viral particles of unmodified or modified AAV-CMV-Luc virus were subjected to Western blotting with anti-AAV2 capsid guinea pig sera (purchased from ATCC).
In vitro transduction assay
C2C12 myotubes were grown in 24-well plates and infected with AAV-CMV-Luc vectors at 2×1010 genomic particles/per well and continued to incubate at 37°C for 6 days. Then, myotubes were lysed for luciferase assay. In addition, the C2C12 myotubes were transduced with AAV-CB-EGFP at 1×1010 genomic particles/per well. EGFP expression was observed under a Nikon TE-300 inverted fluorescent microscope. Images were taken at 100x magnification at 72 hours after infection.
For transduction in undifferentiated myoblasts and non-muscle cell lines, the cells were seeded in 12-well plate at the following cell densities per well: C2C12 at 2×104 ; HepG2 at 1×105 ; HeLa at 6×104 ; HEK 293 at 3×105; and U-87MG at 3×104. One day later the cells were infected with various AAV-CMV-Luc vectors at 103 v.g./cell except C2C12 and U-87MG, which were infected at 104 v.g./cell. The cells were also co-infected with Ad5 at 5 m.o.i. for expedited transgene expression. Two days after vector infection, luciferase activity assay was performed. The harvested cell pellets were washed with 1x PBS and then lysed in 100 μl of luciferase lysis buffer (0.05% Triton X-100, 0.1 M Tris-HCl pH 7.8, 2 mM EDTA). The lysate was centrifuged at 12,000 rpm for 15 minutes in 4°C and 20 μl or 40 μl of supernatant was measured for light activity using the luciferase kit (Promega) with a luminometer. Protein content in each sample was determined by Bradford protein assay (BioRad). Luciferase activities were expressed as relative light units per milligram of protein (RLU/mg protein). AAV-mediated in vitro transduction assays were repeated independently at least two or three times in triplicate.
Determination of AAV heparin binding
In heparin column chromatography, 5×1011 genomic particles of AAV-CMV-Luc were suspended in 0.5 ml of viral suspension buffer (50 mM NaH2PO4, 2 mM MgCl, 2.5 mM KCl, 50 mM Hepes, 150 mM NaCl, pH 8.0) and were then loaded onto a 1-ml HiTrap heparin column (Amersham Bioscience) preequilibrated with 0.15 M NaCl and 50 mM Tris at pH 7.5. The column was further washed twice with 5 ml of binding buffer (10 mM NaH2PO4 pH 7.0) and eluted twice with 5 ml of elution buffer (10 mM NaH2PO4, 1 M NaCl pH 7.0). The flow-through, wash, and elution fractions were collected. 20 μl of each fraction was analyzed by DNA dot-blot assay with a CMV probe and was also subjected to Western blotting with guinea pig anti-AAV2 capsid sera. Heparin dependence was verified by estimating viruses present in wash or elution fraction. For in vitro heparin competition assay, a total of 2×1010 genomic particles of AAV-CMV-Luc vectors were first incubated with or without 30 μg/ml heparin (from porcine intestinal mucosa; Sigma) in DMEM containing 2% HS for 1 h at 37°C. AAV alone or AAV-heparin mixture was added into C2C12 myotubes for 72 hours. Cell were next given fresh DMEM with 2% HS and were subsequently incubated at 37°C for 6 days. The infected myotubes were then harvested for the luciferase assay. The competitive blocking experiment by the synthetic peptides was carried out on C2C12 myotubes. AAV-CMV-Luc and C2C12 myotubes were preincubated with 2 mg/ml of the synthetic muscle-specific peptide (NH2-ASSLNIA-CONH2) or the scrambled peptide (NH2-LISNSAA-CONH2) as control at 37°C for an hour. Then, AAV vectors were added onto C2C12 myotubes for 24 hours. C2C12 myotubes were washed and changed with fresh DMEM containing 2% HS. After 96 hours of continuous incubation, the cells were harvested and analyzed by luciferase assay.
Vector biodistribution studies in vivo
Eight-week-old adult male ICR-CD1 mice (4 to 6 per group) were injected intravenously via tail vein with a viral solution containing 9×1011 genomic particles of AAV-CMV-Luc. After four weeks, the mice were sacrificed and representative organs (brain, heart, liver, skeletal muscles, kidney, testis, and spleen) were harvested for luciferase assay. Luciferase activity was expressed as relative light units (RLU) per milligram of protein. Genomic DNA was extracted from organs using DNeasy kit (Qiagen Inc). Relative numbers of vector genome were determined using real-time PCR. A luciferase DNA standard curve was generated from serial dilutions of the pAAV CMV-Luc plasmid by use of SYBR green with 100 pmol/μl sense 5′-GACGCCAAAAACATAAAGAAAG-3′ and antisense 5′-AGGAACCAGGGCGTATCTCT-3′ Luc primers. 200 ng genomic DNA was used for PCR amplification and the PCR products were quantified using TaqMan data analysis software (Applied Biosystems). All data were expressed as vector copies per ng genomic DNA. The following PCR reaction conditions were used: denaturation, 95°C for 2 min; 40 cycles of amplification, 95°C for 15 sec, 60°C for 1 min.
Statistical analysis
In vitro data were tested by unpaired Student’s t test with one- or two-tailed test. In vivo data were analyzed using the nonparametric Mann-Whitney U test. Data were considered significant when P < 0.05.
Acknowledgments
We thank Dr. Zhong Wang for helpful advice and Ms. Chunlian Chen for technical assistance. This work is part of C. Yu’s Ph.D. thesis at the University of Pittsburgh. It is supported by NIH grants AR45967 and AR50595 to X. Xiao.
References
- 1.Cox GA, Cole NM, Matsumura K, Phelps SF, Hauschka SD, Campbell KP, et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 1993;364:725–729. doi: 10.1038/364725a0. [DOI] [PubMed] [Google Scholar]
- 2.Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A. 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci U S A. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greelish JP, Su LT, Lankford EB, Burkman JM, Chen H, Konig SK, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- 5.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 6.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 7.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 10.Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 12.Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther. 2000;7:24–30. doi: 10.1038/sj.gt.3301109. [DOI] [PubMed] [Google Scholar]
- 13.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 15.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 19.Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat Biotechnol. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 20.Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T, et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol. 2000;74:8635–8647. doi: 10.1128/jvi.74.18.8635-8647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loiler SA, Conlon TJ, Song S, Tang Q, Warrington KH, Agarwal A, et al. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Ther. 2003;10:1551–1558. doi: 10.1038/sj.gt.3302046. [DOI] [PubMed] [Google Scholar]
- 22.Grifman M, Trepel M, Speece P, Gilbert LB, Arap W, Pasqualini R, et al. Incorporation of tumor-targeting peptides into recombinant adeno-associated virus capsids. Mol Ther. 2001;3:964–975. doi: 10.1006/mthe.2001.0345. [DOI] [PubMed] [Google Scholar]
- 23.Nicklin SA, Buening H, Dishart KL, de Alwis M, Girod A, Hacker U, et al. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol Ther. 2001;4:174–181. doi: 10.1006/mthe.2001.0424. [DOI] [PubMed] [Google Scholar]
- 24.White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- 25.Work LM, Buning H, Hunt E, Nicklin SA, Denby L, Britton N, et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13:683–693. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Samoylova TI, Smith BF. Elucidation of muscle-binding peptides by phage display screening. Muscle Nerve. 1999;22:460–466. doi: 10.1002/(sici)1097-4598(199904)22:4<460::aid-mus6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.Samoylov AM, Samoylova TI, Hartell MG, Pathirana ST, Smith BF, Vodyanoy V. Recognition of cell-specific binding of phage display derived peptides using an acoustic wave sensor. Biomol Eng. 2002;18:269–272. doi: 10.1016/s1389-0344(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 28.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 29.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi W, Arnold GS, Bartlett JS. Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors targeted to alternative cell-surface receptors. Hum Gene Ther. 2001;12:1697–1711. doi: 10.1089/104303401750476212. [DOI] [PubMed] [Google Scholar]
- 31.Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J Biol Chem. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- 32.Margalit H, Fischer N, Ben-Sasson SA. Comparative analysis of structurally defined heparin binding sequences reveals a distinct spatial distribution of basic residues. J Biol Chem. 1993;268:19228–19231. [PubMed] [Google Scholar]
- 33.Huttner NA, Girod A, Perabo L, Edbauer D, Kleinschmidt JA, Buning H, et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther. 2003;10:2139–2147. doi: 10.1038/sj.gt.3302123. [DOI] [PubMed] [Google Scholar]
- 34.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 37.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 38.Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Samulski RJ, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–22. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]