Abstract
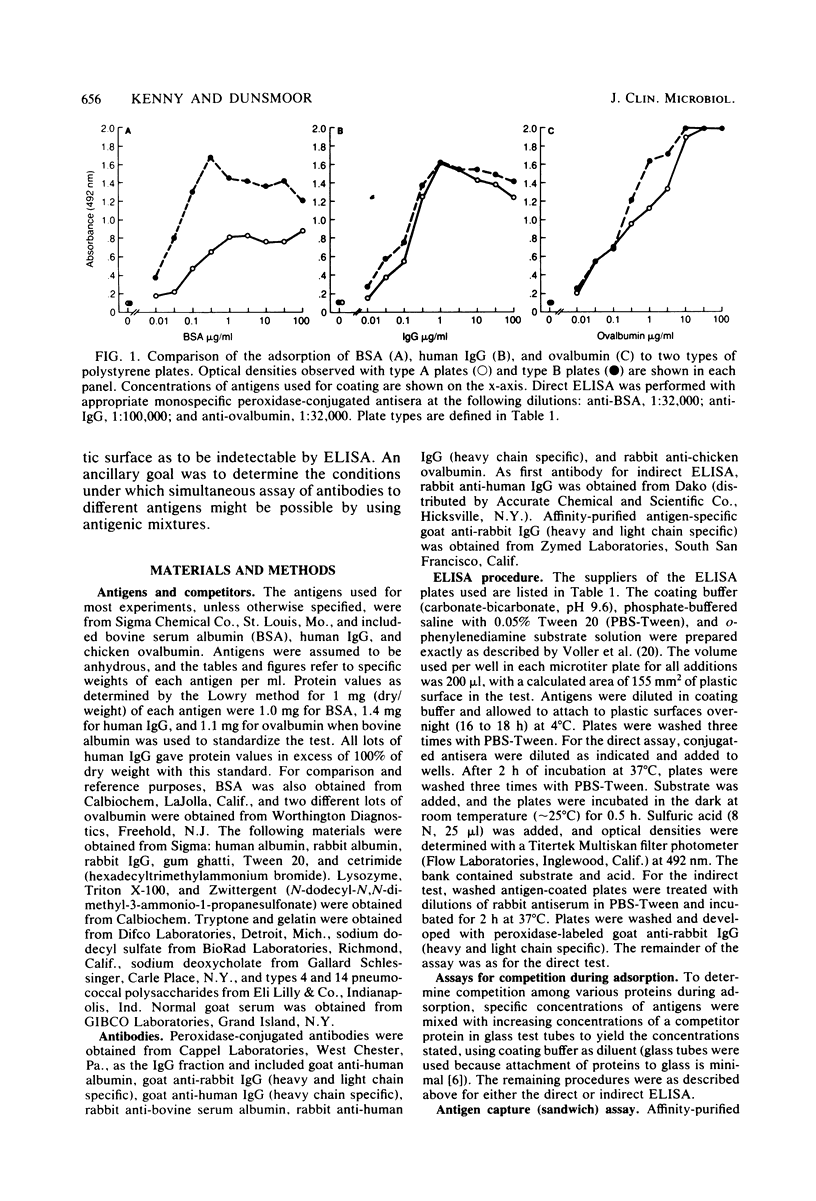
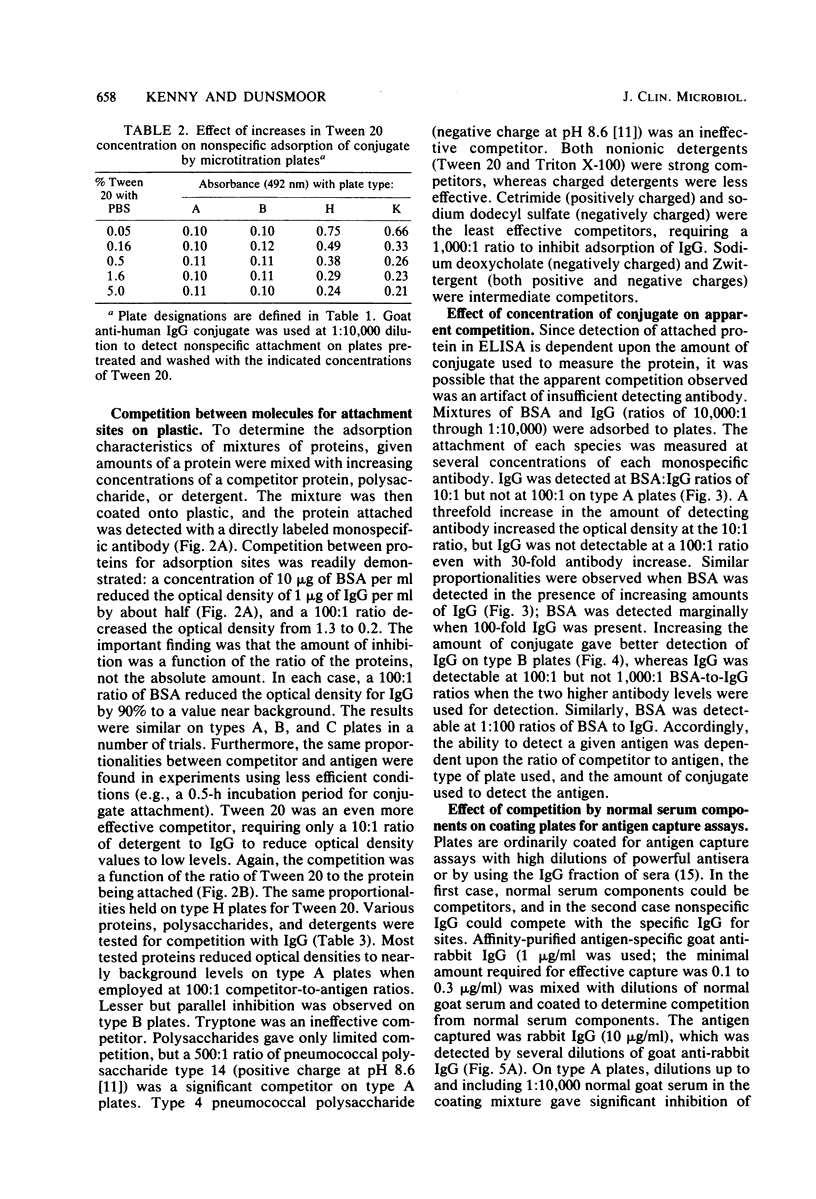
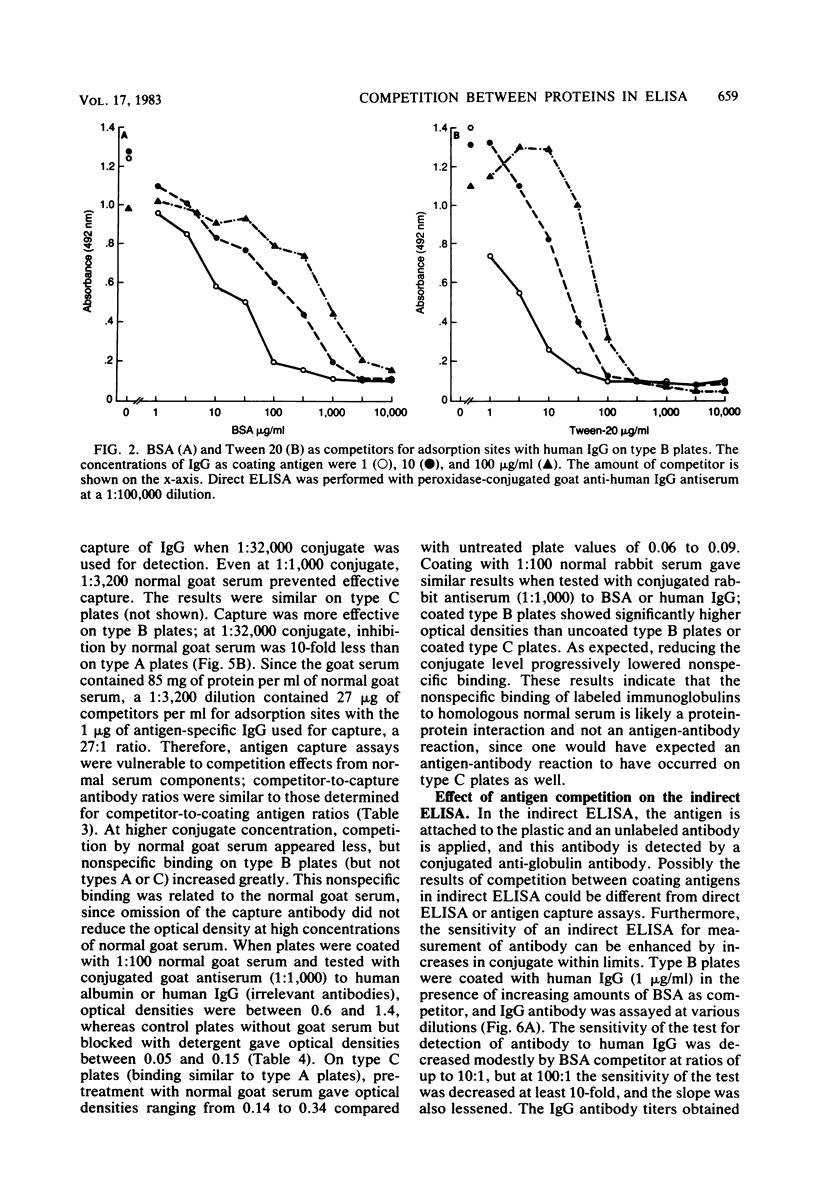
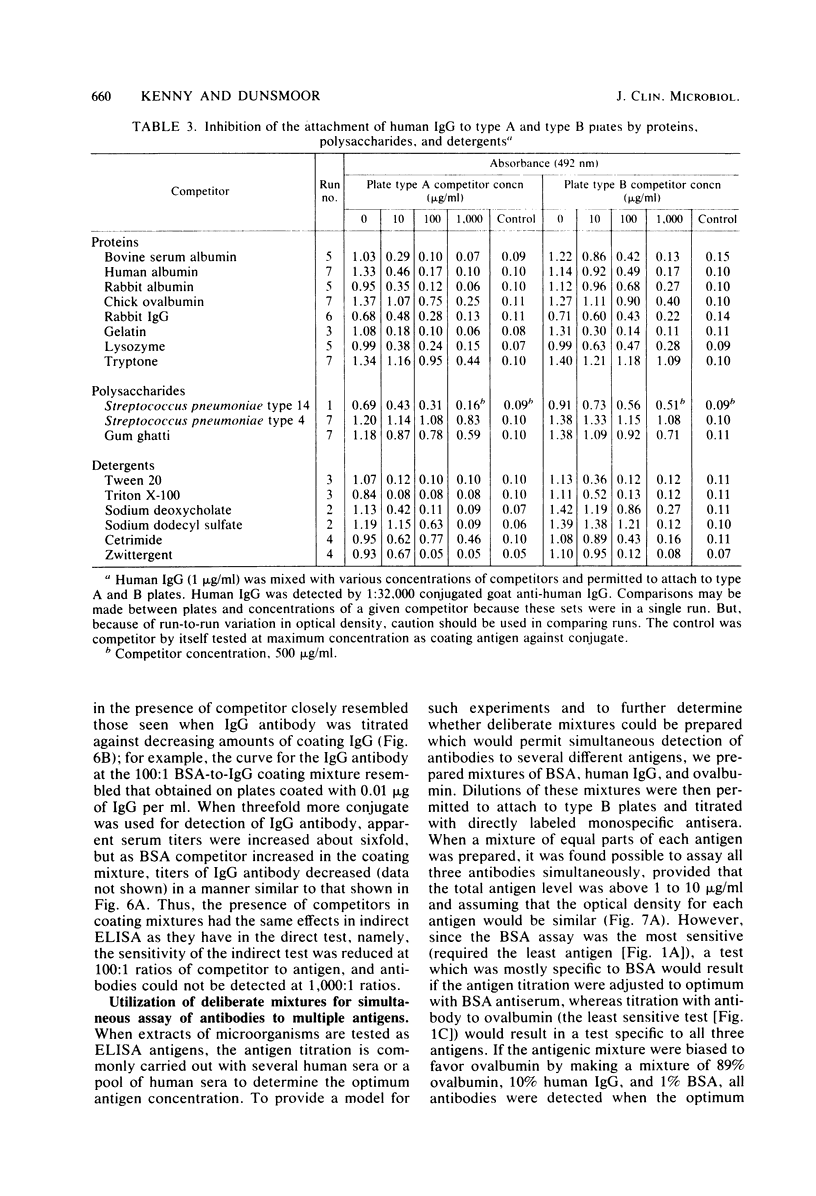
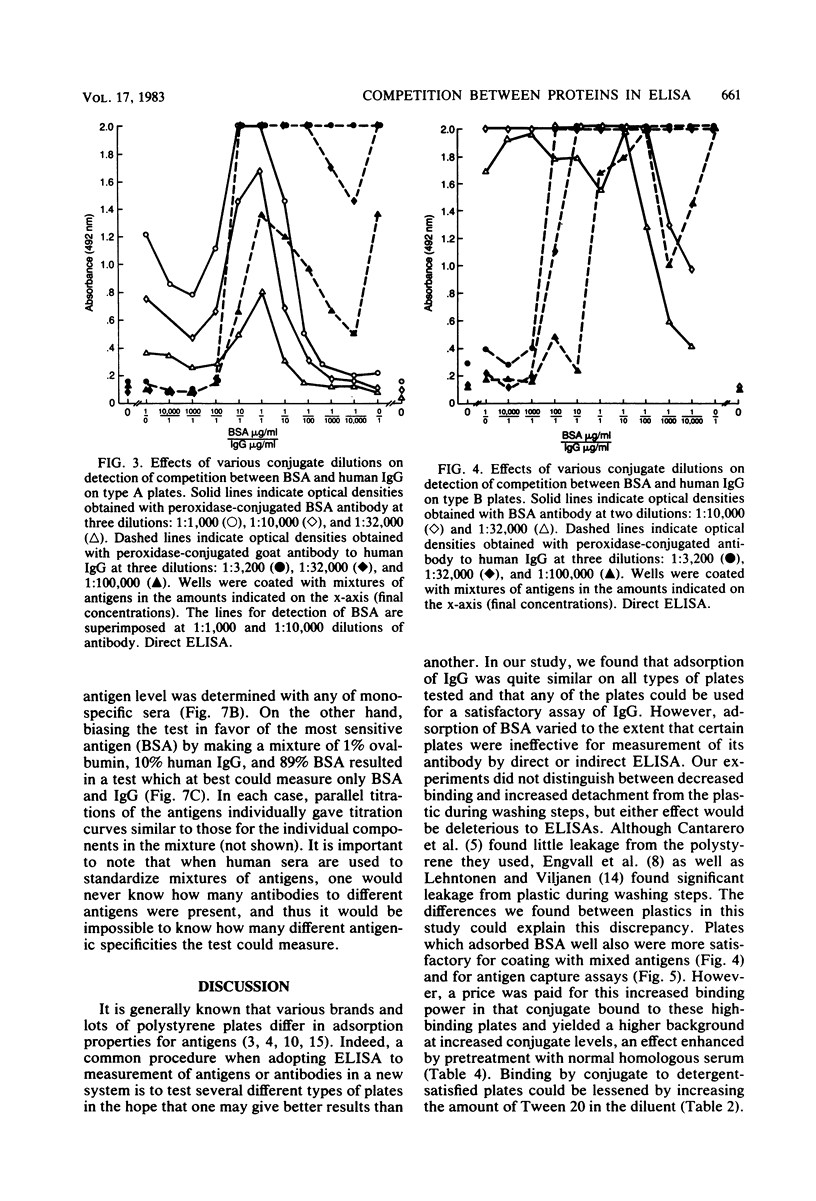
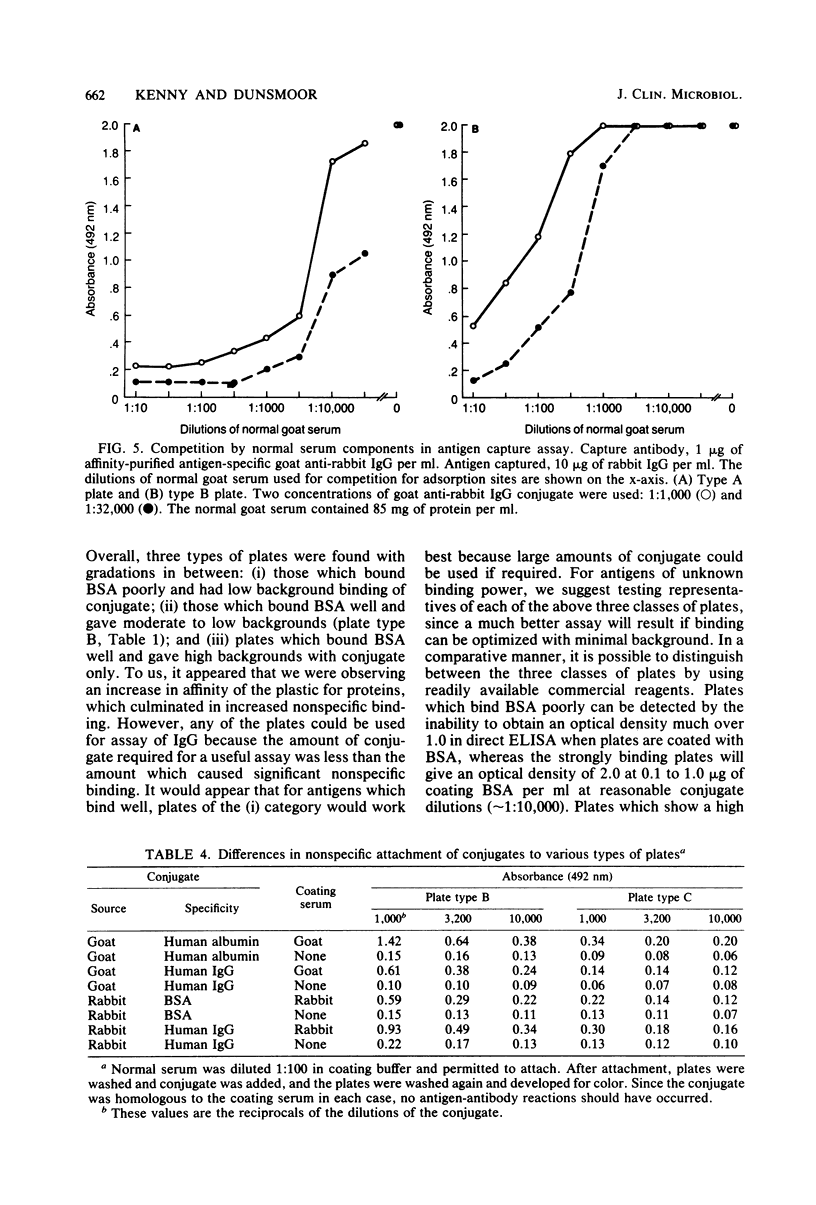
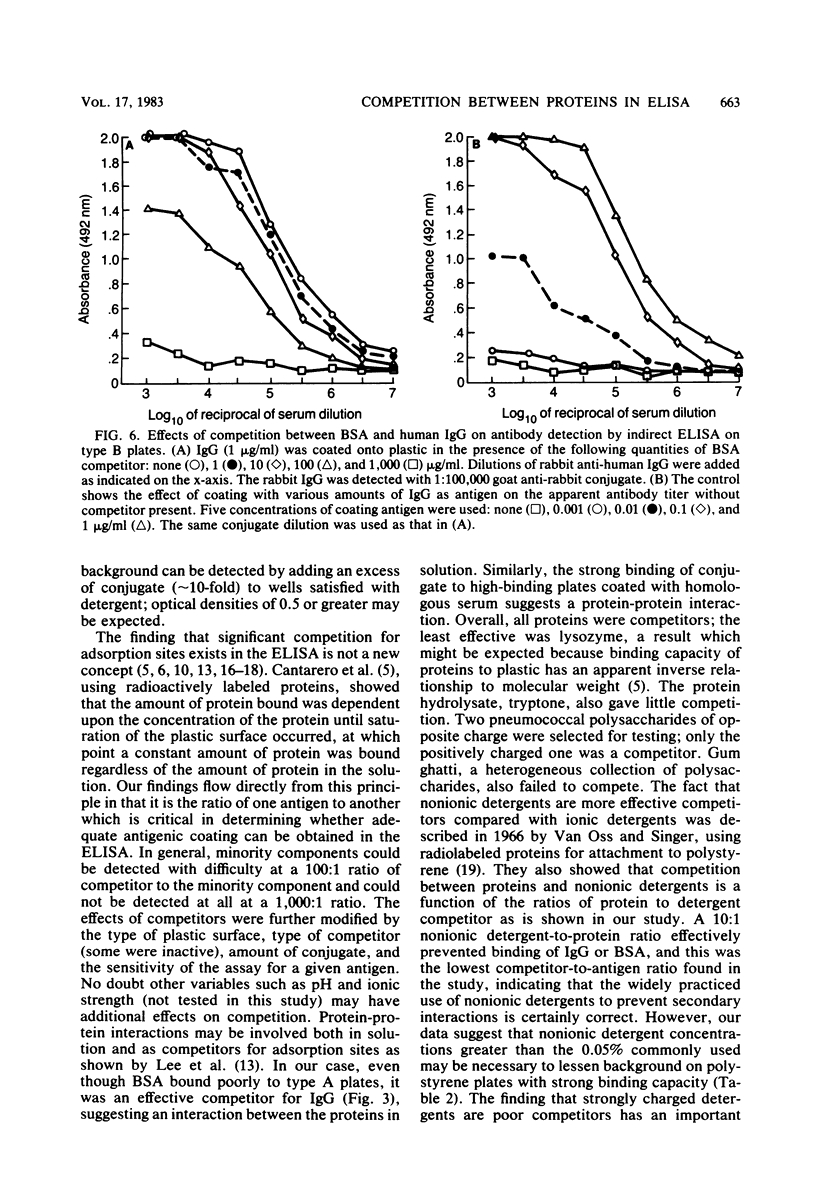
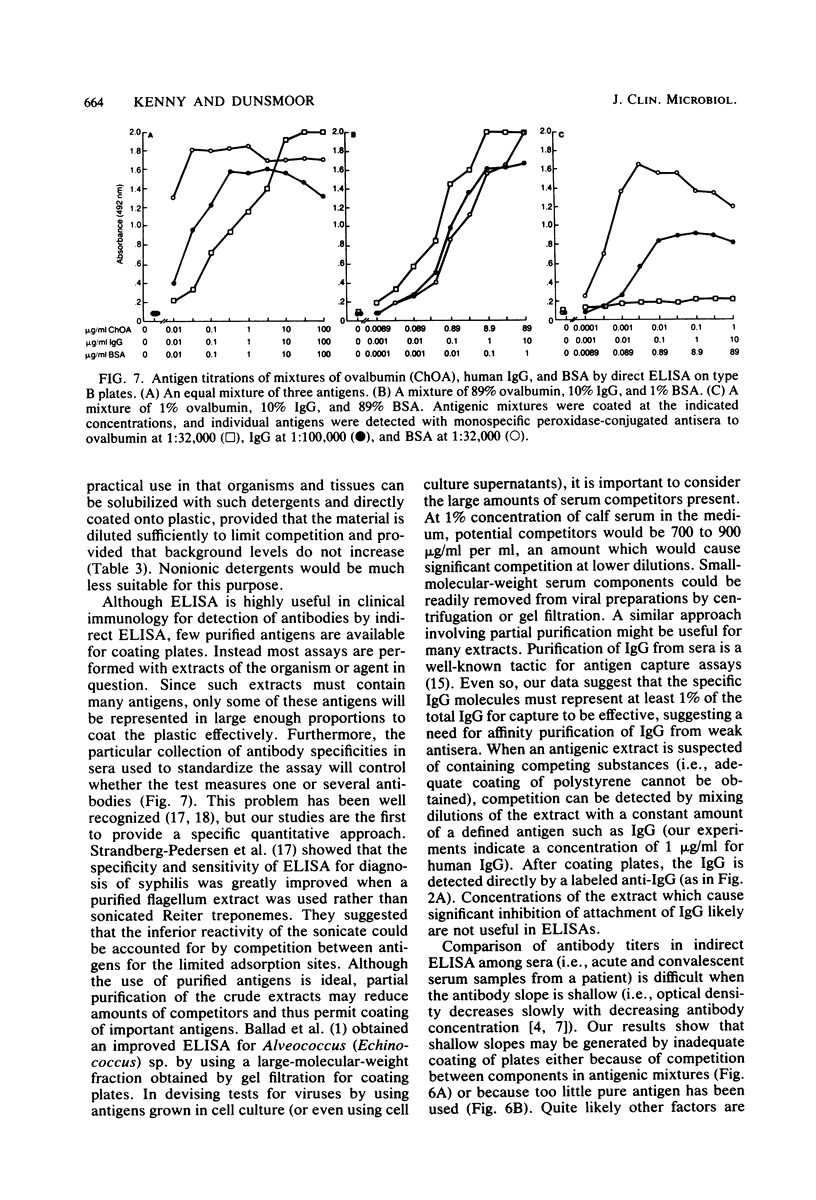
Competition between proteins and other macromolecules for adsorption sites on plastic was studied with the enzyme-linked immunosorbent assay (ELISA) to determine effects of the use of antigenic mixtures or extracts of organisms on assays of antibodies and antigens by ELISA. A comparison of a number of different polystyrene microplates with bovine albumin and human immunoglobulin G (IgG) as antigens showed two major classes of plates; those which adsorbed albumin poorly and those which adsorbed albumin well. IgG adsorbed well on all plates, but plates which adsorbed albumin best also gave significant background levels of nonspecific binding of conjugate. When mixtures of IgG and bovine serum albumin were used as coating antigens, significant competition was observed; the component present at 1% or less in the mixture was essentially undetectable unless excessive amounts of conjugate were used. The important factor was the ratio of competitor to antigen, not the absolute amount. Other proteins (ovalbumin, rabbit albumin, human albumin, and gelatin) were equally effective competitors for adsorption sites on plastic. Nonionic detergents (Tween 20, and Triton X-100) were strong competitors even at 10:1 competitor-to-antigen ratios. In antigen capture assays, normal serum components blocked attachment of antigen-specific IgG, but this competition could be lessened to a degree by the use of strongly binding polystyrene plates. In indirect ELISA for measurement of serum antibody, the use of antigenic mixtures gave significantly lower antibody titers when the desired antigen was less than 1% of the total protein coated. Therefore, the use of mixed or crude antigens in ELISA presents significant problems concerning the sensitivity and specificity of tests.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballad N. E., Leikina E. S., Egorov A. M., Gavrilova E. M., Zorikhina V. I. Effektivnost' razlichnykh modifikatsii immunofermentnogo metoda s ochishchennymi antigenami al'vokokka v diagnostike al'veokokkoza cheloveka. Soobshchenie I. Mikrometod v probikakh. Med Parazitol (Mosk) 1982 Jan-Feb;51(1):66–70. [PubMed] [Google Scholar]
- Berntsson E., Broholm K. A., Kaijser B. Serological diagnosis of pneumococcal disease with enzyme-linked immunosorbent assay (ELISA). Scand J Infect Dis. 1978;10(3):177–181. doi: 10.3109/inf.1978.10.issue-3.04. [DOI] [PubMed] [Google Scholar]
- Bullock S. L., Walls K. W. Evaluation of some of the parameters of the enzyme-linked immunospecific assay. J Infect Dis. 1977 Oct;136 (Suppl):S279–S285. doi: 10.1093/infdis/136.supplement_2.s279. [DOI] [PubMed] [Google Scholar]
- Butler J. E. The amplified ELISA: principles of and applications for the comparative quantitation of class and subclass antibodies and the distribution of antibodies and antigens in biochemical separates. Methods Enzymol. 1981;73(Pt B):482–523. doi: 10.1016/0076-6879(81)73087-8. [DOI] [PubMed] [Google Scholar]
- Cantarero L. A., Butler J. E., Osborne J. W. The adsorptive characteristics of proteins for polystyrene and their significance in solid-phase imunoassays. Anal Biochem. 1980 Jul 1;105(2):375–382. doi: 10.1016/0003-2697(80)90473-x. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Tregear G. W., Burger H. G., Skermer C. Antibody-coated tube method for radioimmunoassay of human growth hormone. Clin Chim Acta. 1970 Feb;27(2):267–279. doi: 10.1016/0009-8981(70)90344-x. [DOI] [PubMed] [Google Scholar]
- Cremer N. E., Cossen C. K., Hanson C. V., Shell G. R. Evaluation and reporting of enzyme immunoassay determinations of antibody to herpes simplex virus in sera and cerebrospinal fluid. J Clin Microbiol. 1982 May;15(5):815–823. doi: 10.1128/jcm.15.5.815-823.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Jonsson K., Perlmann P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta. 1971 Dec 28;251(3):427–434. doi: 10.1016/0005-2795(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Kenny G. E., Wentworth B. B., Beasley R. P., Foy H. M. Correlation of circulating capsular polysaccharide with bacteremia in pneumococcal pneumonia. Infect Immun. 1972 Oct;6(4):431–437. doi: 10.1128/iai.6.4.431-437.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochwa S., Brownell M., Rosenfield R. E., Wasserman L. R. Adsorption of proteins by polystyrene particles. I. Molecular unfolding and acquired immunogenicity of IgG. J Immunol. 1967 Nov;99(5):981–986. [PubMed] [Google Scholar]
- Lee R. G., Adamson C., Kim S. W. Competitive adsorption of plasma proteins onto polymer surfaces. Thromb Res. 1974 Mar;4(3):485–490. doi: 10.1016/0049-3848(74)90083-8. [DOI] [PubMed] [Google Scholar]
- Lehtonen O. P., Viljanen M. K. Antigen attachment in ELISA. J Immunol Methods. 1980;34(1):61–70. doi: 10.1016/0022-1759(80)90225-2. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Petersen C. S., Vejtorp M., Axelsen N. H. Serodiagnosis of syphilis by an enzyme-linked immunosorbent assay for IgG antibodies against the Reiter treponeme flagellum. Scand J Immunol. 1982 Apr;15(4):341–348. doi: 10.1111/j.1365-3083.1982.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Pesce A. J., Ford D. J., Gaizutis M., Pollak V. E. Binding of protein to polystyrene in solid-phase immunoassays. Biochim Biophys Acta. 1977 Jun 24;492(2):399–407. doi: 10.1016/0005-2795(77)90091-5. [DOI] [PubMed] [Google Scholar]
- Tolo K., Schenck K., Brandtzaeg P. Enzyme-linked immunosorbent assay for human IgG, IgA, and IgM antibodies to antigens from anaerobic cultures of seven oral bacteria. J Immunol Methods. 1981;45(1):27–40. doi: 10.1016/0022-1759(81)90091-0. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Singer J. M. The binding of immune globulins and other proteins by polystyrene latex particles. J Reticuloendothel Soc. 1966 May;3(1):29–40. [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]


