Abstract
Nuclear-pore complexes (NPCs) are large protein channels that span the nuclear envelope (NE), which is a double membrane that encloses the nuclear genome of eukaryotes. Each of the typically 2,000–4,000 pores in the NE of vertebrate cells is composed of multiple copies of 30 different proteins known as nucleoporins. The evolutionarily conserved NPC proteins have the well-characterized function of mediating the transport of molecules between the nucleoplasm and the cytoplasm. Mutations in nucleoporins are often linked to specific developmental defects and disease, and the resulting phenotypes are usually interpreted as the consequences of perturbed nuclear transport activity. However, recent evidence suggests that NPCs have additional functions in chromatin organization and gene regulation, some of which might be independent of nuclear transport. Here, we review the transport-dependent and transport-independent roles of NPCs in the regulation of nuclear function and gene expression.
Keywords: chromatin, gene regulation, nuclear-pore complex, nucleoporin, transcription
Glossary
ABL1 v-abl Abelson murine leukaemia viral oncogene homologue 1
CBP CREB-binding protein
ChIP chromatin immunoprecipitation
COPII coat protein complex II
CRM1 chromosome region maintenance 1, also known as exportin 1
Dosage compensation an epigenetic process by which gene expression from the single male X and two females Xs is equalized between the sexes; in Drosophila, this involves a twofold increase in transcription from the male X
ELYS embryonic large molecule derived from yolk sac, a nucleoporin
FG Nup nucleoporin with conserved phenylalanine glycine repeat motifs that can be of the FG, GLFG (L for leucine) or FXFG (X for any amino acid) type
Flo flotte lotte
GAL galactosidase
gp120 glycoprotein of 120 kDa, a transmembrane nucleoporin
HOX homeobox
Mex67 mRNA export
mbo members only
miRNA microRNA, a type of non-coding RNA molecule with roles in regulation of development and growth
Mlp1 myosin-like protein 1, a homologue of translocated promoter region
MNase micrococcal nuclease, an enzyme that can cleave DNA; when fused to a protein of interest, it can be used the map the binding sites of the protein using the cleavage pattern of the genome
Mtor megator
NDC1 nuclear division cycle 1, a transmembrane nucleoporin
NF-κB nuclear factor-κB
NLS nuclear-localization signal
NSD1 nuclear receptor binding SET domain protein 1
NUA nuclear pore anchor
Nuclear basket a structural element of the nuclear pore, created by the nuclear filaments attached to the core of the nuclear-pore complex and joined in a distal ring
PHD plant homeodomain
POM121 pore membrane protein of 121 kDa, a transmembrane nucleoporin
Prp20 pheromone-response pathway protein 20, also known as RanGEF or RCC1
Ran ras-related nuclear protein
SAGA Spt-Ada-Gcn5-Acetyltransferase
Sus1 suc synthase 1
THO suppressor of the transcriptional defect of hyperrecombination protein 1 by overexpression, a complex of proteins involved in messenger ribonucleoprotein biogenesis
Tpr translocated promoter region, a nucleoporin of the nuclear basket of the nuclear-pore complex
Introduction
The transport of macromolecules between the nucleus and the cytoplasm of eukaryotic cells is mediated exclusively through the nuclear-pore complexes (NPCs), which are large multiprotein channels that span the double-lipid bilayer of the nuclear envelope (NE; D'Angelo & Hetzer, 2008; Fahrenkrog et al, 2004). The selective transport of proteins, RNA and ribonucleoproteins across the NE is a highly regulated process that involves signal-dependent interactions of cargo molecules with transport receptors (Gorlich, 1997). These cargo–receptor complexes bind to proteins of the nuclear-pore channel, which, together with other crucial factors such as the Ran GTPase system, mediate active and directional transport (Weis, 2002; Terry et al, 2007). Each NPC is composed of multiple copies of approximately 30 nucleoporins (Nups; Cronshaw et al, 2002). Given their role as exclusive nucleocytoplasmic transport channels, it is not surprising that mutations in multiple Nups have been linked to cellular and developmental defects, as well as to several human diseases. The surprising fact is the observed specificity of these phenotypes, which are often manifested in specific cell types and in particular molecular pathways (Table 1; Meier & Brkljacic, 2009). These findings imply that individual pore components might act as crucial regulators of gene expression during differentiation and signal transduction.
Table 1.
Tissue-specific roles of metazoan nucleoporins
| Metazoan nucleoporins(Saccharomyces cerevisiae homologue) | Tissue-specific disorder or developmental aspect | Nature of nucleoporin mutation | References |
|---|---|---|---|
| Human Nup155 (Nup157, Nup170) | AF, which is a cardiac disorder characterized by clinical arrhythmia | Homozygous missense R391H in humans; large truncation (leaving 271/1,391 amino acids) in mouse model | Zhang et al, 2008 |
| Drosophila Nup154(Nup157, Nup170) | Male and female gametogenesis, various steps of oogenesis and spermatogenesis | P-element insertion in the 5′ region of the gene, resulting in reduced expression (hypomorph) | Gigliotti et al, 1998; Grimaldi et al, 2007 |
| Human Nup98, mouse Nup98(Nup145N, Nup116) | Acute myeloid leukaemia (and other haematological malignancies); haematopoietic stem-cell proliferation | Multiple genomic translocations, which result in fusion of Nup98 fragment, including FG repeats, to another gene | Nakamura et al, 1996; Slape & Aplan, 2004; Takeda et al, 2006 |
| Mouse Nup96(Nup145C) | Immune-system function, interferon response, B-cell and T-cell proliferation | Genetic knockout by an inserted early stop codon | Faria et al, 2006 |
| Human Nup214(Nup159) | T-cell acute lymphoblastic leukaemia | Genomic translocations, which fuse Nup214/CAN fragment to another gene | Graux et al, 2004; Saito et al, 2004 |
| Drosophila Nup88/mbo(Nup82) | Trachea, central nervous system, imaginal discs of larvae; immune response | Genetic null by removal of 5′-coding sequences | Uv et al, 2000 |
| Human Nup62(Nsp1) | Primary biliary cirrhosis (autoimmune liver degeneration) | Autoimmune antigen | Wesierska-Gadek et al, 2007 |
| Human Nup62(Nsp1) | Autosomal recessive infantile bilateral striatal necrosis (degeneration of the basal ganglia) | Homozygous missense Q391P mutation | Basel-Vanagaite et al, 2006 |
| Human Gp210(–) | Primary biliary cirrhosis (autoimmune liver degeneration) | Autoimmune antigen | Tartakovsky & Worman, 1995 |
| Human Nup358/RanBP2(–) | Familial cases of infection-triggered acute necrotizing encephalopathy | Heterozygous missense mutation T585M | Neilson et al, 2009 |
| Mouse Nup133(Nup133) | Neural stem/progenitor cell differentiation | Functional null mutation owing to truncation/stop codon | Lupu et al, 2008 |
| Human Aladin(–) | Triple A syndrome (adrenal insufficiency, absence of tear secretion and achalasia) | Various nonsense, frameshift and missense mutations | Cronshaw & Matunis, 2003 |
| Zebrafish ELYS/Flo(–) | Proliferating progenitor cells in developing retina and intestine | Truncated protein owing to early stop codon and lower expression levels | Davuluri et al, 2008; de Jong-Curtain et al, 2008 |
AF, atrial fibrillation; CAN, Nup214/CAN; ELYS, embryonic large molecule derived from yolk sac; Flo, flotte lotte; Gp210, glycoprotein of 210 kDa; mbo, members only; Nup, nucleoporin; RanBP2, Ran-binding protein 2.
By controlling the access of transcription factors or the export of specific messenger RNA (mRNA) molecules, the NPCs have a crucial transport-dependent role in gene expression. Alterations in pore function or composition can result in the subcellular mislocalization of regulatory factors and, therefore, a misregulation of cellular growth and identity (Cronshaw & Matunis, 2004). However, in addition to the well-established transport function of the NPC, the nuclear pore has been proposed to exert a more direct role in gene expression through physical interactions of Nups with the nuclear genome (Akhtar & Gasser, 2007; Brown et al, 2008). Such interactions have been proposed to contribute to chromatin organization inside the nucleus and to the transcriptional status of genes that physically associate with pore components. Therefore, to understand the tissue-specific roles of Nups and the regulatory nature of the NPC, it is important to consider both transport-dependent and transport-independent mechanisms.
The dynamic and stable structures of the NPC
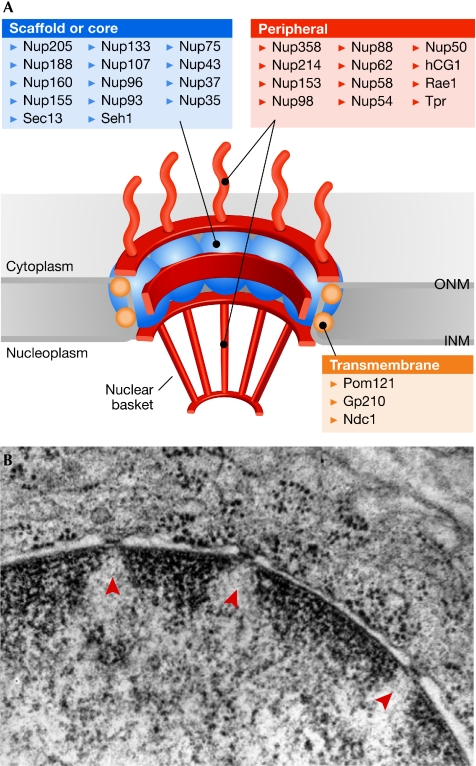
The NPC is one of the largest macromolecules in the cell; it has a molecular mass of 60–90 MDa (Rout et al, 2000) and exhibits an eightfold-symmetrical structure that forms a transport channel across the NE (D'Angelo & Hetzer, 2008; Weis, 2007; Lim & Fahrenkrog, 2006). The roughly 30 different Nups that compose the pore can be grouped into at least three categories (Fig 1A). The scaffold Nups form the core of the NPC and are assembled into two large multiprotein subcomplexes: Nup107–160 and Nup93–205 (Alber et al, 2007). Recent structural studies suggest that these scaffold Nups are similar to clathrin and COPII vesicle coats, which has led to the hypothesis that both classes of proteins share common ancestors (Alber et al, 2007; Debler et al, 2008; Devos et al, 2004). The scaffold Nups might be tethered to the NE by the transmembrane Nups POM121, NDC1 and gp210, which constitute the second class of NPC components (Hetzer et al, 2005). The third group are the peripheral Nups, which associate with the NPC scaffold, and have crucial roles in mediating the selectivity and permeability of the nuclear pore. A large proportion of the peripheral Nups is constituted by FG repeat-containing Nups. These FG-repeat motifs fill the central channel of the nuclear pore, thereby precluding the entry of molecules that are more than 40 kDa (Weis, 2007). In addition, FG Nups provide low-affinity binding sites for multiple transport receptors (Wente, 2000), thereby mediating the passage of transport receptors and their cargos, and functioning as a selective permeability barrier (for further information on the various models of NPC selectivity see D'Angelo & Hetzer, 2008; Frey & Gorlich, 2007; Rout et al, 2003; Weis, 2007). Other peripheral components of the NPC include the nuclear proteins Tpr, Nup153 and Nup50, and the predominantly cytoplasmic Nup88, Nup214 and Nup358. These distal Nups, which protrude as filaments from the NE-embedded core (Beck et al, 2007), have been implicated in the initiating steps of export and import, respectively.
Figure 1.
Nuclear pores and chromatin organization. (A) Schematic illustration of the NPC. The three different classes of Nups and their relative locations within the NPC are shown. (B) Electron micrograph image of a mammalian nucleus, illustrating the positioning of the NE against darkly stained condensed heterochromatin, and of the nuclear pores (arrowheads) against the lighter stained open euchromatin. Reproduced with permission from http://synapses.clm.utexas.edu (J. Spacek). Gp210, glycoprotein of 210 kDa; hCG1, candidate gene 1; INM, inner nuclear membrane; Ndc1, nuclear division cycle 1; NE, nuclear envelope; NPC, nuclear-pore complex; Nup, nucleoporin; ONM, outer nuclear membrane; Pom121, pore membrane protein of 121 kDa; Rae1, RNA export 1; Sec13, secretory mutant 13; Seh1, Sec13 homologue; Tpr, translocated promoter region.
One unique characteristic of NPC organization, which might be crucial for understanding nuclear-pore function, was revealed by fluorescence-recovery after photobleaching (FRAP) analysis in living cells (Rabut et al, 2004). Monitoring the dissociation rates of GFP-tagged Nups in cultured cells showed that the NE localization of the scaffold nuclear-pore components remains stable over many hours, which is consistent with their role as structural nuclear-pore components. By contrast, the nuclear-pore association of many peripheral Nups—such as Nup153 and Nup98—is highly dynamic, with residence times of seconds rather than hours (Daigle et al, 2001; Rabut et al, 2004). Although these results need to be interpreted with some caution because they were obtained with tagged proteins, they raise the interesting question of whether the dynamic behaviour of peripheral Nups is crucial for transport or whether they are engaged in a different set of nuclear functions that might involve shuttling between the nuclear periphery and other intranuclear locations. The Nups that have been identified as the most dynamic in this assay—including Nup153, Nup50 and Nup98—also happen to be components or associated factors of the nuclear basket (see glossary; Stoffler et al, 2003). Interestingly, the dynamic movement of Nup98 and Nup153—which can bind to mRNA-export factors (Pritchard et al, 1999) and RNA (Ball et al, 2007), respectively—has been shown to depend on active transcription (Griffis et al, 2004). Therefore, it is plausible that the high mobility of nuclear basket Nups might reflect their involvement in transcription or in the delivery of mRNA to the nuclear pore.
In a recent study, the unique molecular organization of Nups was correlated to their expression status in dividing versus terminally differentiated cells (D'Angelo et al, 2009). Comparing expression levels revealed that both dynamic and stable Nups are transcribed in proliferating cells, whereas only the stable scaffold Nups are sharply downregulated when cells exit the cell cycle. Furthermore, the core structures of nuclear pores were shown not to turn over during the entire lifespan of non-dividing cells. The lack of a replacement mechanism for NPC scaffold components comes at a cost for long-lived post-mitotic cells such as mammalian brain neurons that can persist for the whole life of an organism. An age-dependent deterioration of the NPC was observed that resulted in a breakdown of the nuclear-permeability barrier, leading to aberrant nuclear accumulation of the cytoplasmic protein tubulin (D'Angelo et al, 2009).
The NPC in tissue-specific development and disease
Given the indispensable role of the NPC in cellular function and the highly conserved nature of its components, tissue-specific requirements for Nups might seem surprising. Nonetheless, many reports have shown the involvement of both stable and dynamic Nups in particular aspects of development and in specific human pathologies (Table 1). As a compelling example, a recent study has isolated a mutation in human NUP155—a component of the scaffold NUP93–205 complex—that underlies atrial fibrillation (AF), which is an inherited form of clinical arrhythmia that can lead to sudden cardiac death (Zhang et al, 2008). Although homozygous mice carrying the knockout Nup155 allele die around the time of mid-embryogenesis, the heterozygous animals show an AF phenotype. This suggests that a reduction in the levels of NUP155 or its mistargeting, such as that found in the naturally isolated allele of Nup155, results in a tissue-specific disorder. Interestingly, the fly homologue of NUP155, Nup154, has also been shown to have a tissue-specific role, although it seems to be important for the function of a different organ; in Drosophila, Nup154 has crucial roles in gametogenesis, affecting egg-chamber development in females and meiotic progression in both sexes (Gigliotti et al, 1998; Grimaldi et al, 2007).
Perhaps the best-studied examples of Nup alterations that underlie specific human disorders are the identified translocations of the NUP98 and NUP214 genes, which result in fusions of the Nups to transcriptional and signalling regulators, and have been characterized as mutations leading to several types of leukaemia (Nakamura et al, 1996; Saito et al, 2004; Slape & Aplan, 2004). The oncogenic fusion of NUP98 to the transcription factor HOXA9 results in acute myelogenous leukaemia and has been shown to induce the aberrant expression of HOXA9 target genes, beyond the effects of the overexpression of HOXA9 alone, and to block the differentiation of human hematopoietic cells (Ghannam et al, 2004; Takeda et al, 2006). The fusion of NUP98 to PHD-finger proteins has also been shown to produce similar effects and to lock target genes into an aberrantly active state, resulting in leukaemic transformation (Wang et al, 2009). These findings agree with the idea that the normal function of NUP98 might include the regulation of gene expression. The fusion between NUP214 and the tyrosine kinase ABL1 is present in a significant portion of acute lymphoblastic leukaemia cases. Recent data suggest that localization of NUP214/ABL1 to the NPC and its interaction with other Nups are required to induce transformation (De Keersmaecker et al, 2008). It remains to be tested whether changes in nuclear-pore composition and consequently altered function are crucial events in the pathogenesis of haematopoietic malignancies.
Interestingly, both the dynamic Nup98 and the stable Nup96—which are products of the same gene but are auto-proteolytically cleaved into two polypeptides (Fontoura et al, 1999)—also have a crucial role in the function of the immune system and are upregulated by interferons in response to viral infections (Enninga et al, 2002). A heterozygous mutation in Nup96 has been shown to affect the expression of interferon-regulated genes and to disrupt the normal function of macrophages, and B and T lymphocytes, with no detectable gross pathological abnormalities in other organs (Faria et al, 2006). Moreover, the role of Nup96 in the immune response seems to be evolutionarily conserved, as NUP96 in Arabidopsis thaliana has also been shown to be required for the basal defence and resistance responses mediated by plant immune sensors (Zhang & Li, 2005).
The cell-type specificity of the roles of Nups has similarly been observed in development and differentiation (Table 1). For example, mouse NUP133—a component of the stable NUP107–160 subcomplex—was recently shown to have a role in embryonic development of the neural lineage, such that NUP133-null neural progenitors could not efficiently produce terminally differentiated neurons (Lupu et al, 2008). Furthermore, a mutation in the zebrafish ELYS—a Nup that closely associates with the same subcomplex—was reported to inhibit normal development and proliferation of the retina and the intestine (Davuluri et al, 2008; de Jong-Curtain et al, 2008). Mutations in several plant Nups have been reported to cause diverse developmental defects, which seem to be comparatively mild and relatively specific (Meier & Brkljacic, 2009). One such example is a mutant allele of the nuclear basket Nup Tpr/NUA, which affects the flowering time, seed production and leaf morphology of A. thaliana (Xu et al, 2007).
One of the most interesting questions regarding the regulatory functions of the NPC is whether various cell types have different nuclear-pore compositions. Cell type-specific expression of Nups has been documented for the dynamic nuclear-pore proteins NUP50 and GP210 in mice (Olsson et al, 2004; Smitherman et al, 2000). Similarly, the expression of Nup88/mbo, as well as its mutant phenotype, seems to be tissue specific in Drosophila (Uv et al, 2000). Gamete formation and spermatogenesis, in particular, seem to rely on transport-mediated developmental regulation (Hogarth et al, 2005). In addition to the fly Nup154, mammalian NUP BS-63, which is homologous to Nup358, is expressed specifically in spermatids and human sperm (Cai et al, 2002). Although the molecular basis of most of these disparate cases of tissue-specific roles of Nups is not yet characterized, the potential mechanisms can be grouped into transport-dependent and transport-independent gene regulation, as discussed below.
Transport-dependent gene regulation by Nups
There are several mechanisms by which a tissue-specific transport function of the NPC can be achieved. It can occur through the selective transport of a specific protein such as the import or export of a particular transcription factor, or through the regulated export of a crucial RNA (Fig 2). These mechanisms can be achieved either by expressing tissue-specific transport receptors or by changing the relative composition of NPCs. In support of the former idea, transport receptors seem to have differential affinities for different FG domains, suggesting that a particular FG Nup will probably interact with only a subset of transport receptors (Damelin & Silver, 2000; Pyhtila & Rexach, 2003; Strawn et al, 2004). An example of the latter is the nuclear accumulation of Dorsal (a transcription factor with roles in development and immune response), which has been shown to depend specifically on Nup88/mbo (Uv et al, 2000). Although mbo mutants do not show defects in general NLS-mediated protein import or mRNA export, they fail to accumulate Dorsal in the nuclei and, consequently, to activate the immune response properly. Interestingly, Nup88 was proposed to regulate the location of the nuclear export receptor CRM1, which becomes mislocalized to the nucleus in mbo mutants and seems to drive the aberrant export of Dorsal (Roth et al, 2003). In addition, the same group proposed that variations in levels of the Nup214–Nup88 complex at the pore change the amount of NPC-bound CRM1, thereby influencing the relative strength and duration of NF-κB-signalling responses (Xylourgidids et al, 2006).
Figure 2.
Models of transport-dependent and transport-independent mechanisms of gene regulation by the nuclear-pore complex. The NPC consists of stable core components (dark blue) and peripheral Nups (red), which are often dynamic. (A) Transport-dependent regulation might involve interactions between cell type-specific TFs (green) and a specific NPC component, between an IMP (gray) that carries a TF (orange) and a specific FG Nup, or between a given mRNA and a particular Nup. (B) Transport-independent regulation might involve chromatin tethering to the NPC (nucleosomes are shown as gray discs), or the recruitment of active genes (Pol II is shown in green) to the NPC through interactions with SAGA/Sus1 (gray), mRNA or the associated mRNA export and processing machinery (light blue). Alternatively, dynamic Nups (red) could shuttle away from the nuclear pore and interact with active genes, mRNA or mRNA export/processing machinery inside the nucleus. IMP, import receptor; mRNA, messenger RNA; NPC, nuclear-pore complex; Nup, nucleoporin; Pol II, RNA polymerase II; SAGA, Spt-Ada-Gcn5-Acetyltransferase; Sus1, suc synthase 1; TF, transcription factor.
A role in the export of particular mRNAs has been reported for NUP96 in mammalian macrophages (Faria et al, 2006). The lower levels of NUP96 in Nup96+/− mice seem to disrupt the nuclear export of a subclass of immune-related transcripts, probably contributing to the reduced levels of some of the proteins that they encode. Interestingly, the same Nup96+/− condition is reported to affect the cell cycle-dependent nucleocytoplasmic distribution of several mRNAs in T cells (Chakraborty et al, 2008). In contrast to the nuclear accumulation observed in macrophages, most of the tested mRNAs were seen to accumulate aberrantly in the cytoplasm of Nup96+/− T cells, suggesting that NUP96 can facilitate or inhibit specific mRNA export, perhaps depending on the cell type, cell-cycle stage or type of mRNA.
Several mutants of plant Nups show an accumulation of mRNA in the nucleus (Meier & Brkljacic, 2009), although none has been linked to a particular mRNA subset that would help to explain the relatively specific developmental problems that they induce. Intriguingly, the levels of three specific miRNAs are significantly reduced in a Tpr/NUA mutant of Arabidopsis (Jacob et al, 2007). As miRNAs are known to be involved in many aspects of development, the link between Nups and miRNA metabolism could be crucial to understanding the tissue-specific roles of nuclear-pore components.
Despite the examples described above, it is often difficult to envision how transport specificity can be achieved through different nuclear-pore compositions, as each pore must establish interactions with various molecules simultaneously. Although the involvement of FG Nups in specific transport events can be based on their proposed affinities for particular transport receptors (Weis, 2007), it is more challenging to explain these types of tissue-specific behaviour for components of the NPC core. One possibility is that an altered nuclear-pore number or altered transport rate can have distinct effects on different tissues. Early studies reported markedly altered transport rates and nuclear-pore permeability in cells of varying cell division and differentiation states (Feldherr & Akin, 1990). A recent study on the role of zebrafish ELYS might provide an example of the developmental consequences of the reduction of nuclear-pore number (de Jong-Curtain et al, 2008). Flo—a mutant of ELYS, which is thought to be required for nuclear-pore assembly—caused tissue-specific defects, most markedly during the development of the intestinal epithelium. It could be that the number of nuclear pores is considerably reduced in the highly proliferating intestine of the Flo mutant, as without correct nuclear-pore insertion, every cell division dilutes the total number further. In more slowly proliferating tissues, the nuclear-pore number is less severely affected and cells develop normally. Although this maybe true, the cell-proliferation rate does not seem to be a consistent distinguishing factor for the tissues that Nup mutations have been described to alter. The phenotypes of Nup alleles suggest that alternative roles of the NPC might have to be considered to explain the full range of the regulatory functions of the nuclear pore.
Transport-independent gene regulation by Nups
The proposed involvement of the nuclear pore in chromatin organization and transcriptional regulation might also explain the tissue-specific roles of nuclear-pore components. High-resolution images of mammalian nuclei distinctly show the non-random association of decondensed chromatin with nuclear pores (Fig 1B), suggesting a relationship between NCPs and active genes. One can envision that it would be advantageous for a cell to co-regulate the two endpoints of nuclear gene expression—that is, the production of mRNA and its eventual exit into the cytoplasm. According to this idea—known as the gene-gating hypothesis—nuclear pores are able to specifically interact with, and perhaps regulate, active sites in the genome (Blobel, 1985). Several lines of experimental evidence, mainly obtained in yeast, have provided support for the gene-gating hypothesis, showing a connection between the NPC and transcriptional activation.
The reported interaction between the yeast nuclear basket component Nup2, which is a homologue of Nup153, and the promoters of active genes—known as the Nup-PI phenomenon—indicates that nuclear pores can interact with active genes during the initial steps of transcription (Schmid et al, 2006). In this regard, enhancing the association of an inducible gene with the NPC was shown to boost its transcript levels (Taddei et al, 2006). Furthermore, a genome-wide ChIP analysis in Saccharomyces cerevisiae showed that a subset of Nups, including the nuclear basket components Mlp1 (which is a homologue of Tpr), Nup2 and Nup60, often occupy regions of highly transcribed genes (Casolari et al, 2004). A recent study of Drosophila dosage compensation (see glossary) showed that Nup153 and Mtor/Tpr are necessary for the twofold hypertranscription of the male X (Mendjan et al, 2006), implicating NPC components in the establishment of active chromatin and suggesting that metazoans could similarly use the NPC for the epigenetic regulation of their gene-expression programmes. Furthermore, mammalian NUP98 has been reported to interact physically with histone acetyltransferase CBP/p300 (Kasper et al, 1999). This interaction seems to be highly relevant to the cancer-associated roles of NUP98, as an oncogenic fusion of NUP98 to NSD1 histone methyltransferase was shown to produce aberrant activation, hyperacetylation and recruitment of p300 to Hox-A genes in leukaemogenesis (Wang et al, 2007).
What mediates the interaction between active genes and the NPC? Sus1—a member of the mRNA-export complex that interacts physically with the NPC component Nup1—was shown to associate with the histone acetyltransferase complex SAGA, which is involved directly in transcriptional activation (Rodriguez-Navarro et al, 2004). In addition, the recruitment of active GAL genes to the nuclear periphery was shown to depend on Sus1 and other SAGA components (Cabal et al, 2006; Luthra et al, 2007), which is perhaps the strongest evidence in support of the gene-gating hypothesis. The mRNA-export receptor Mex67 was shown to contribute to the association of active genes with the nuclear periphery (Dieppois et al, 2006). The mRNA molecule could itself be another ‘physical glue' for the proposed Nup-active gene association. However, although Nup153—which is the homologue of Nup1, Nup2 and Nup60—can physically bind to RNA (Ball et al, 2007), and the chromatin association of Mlp1 was found to be RNAse sensitive (Casolari et al, 2005), no specific RNA-based interactions have been identified so far. Two new interesting candidates have recently been implicated in the association between transcribing genes and NPCs: some components of the exosome seem to mediate the post-transcriptional tethering of GAL genes to the NE (Vodala et al, 2008), and the THO complex seems to link 3′-end RNA processing to nuclear-pore association (Rougemaille et al, 2008).
Interestingly, the sites of NPC interactions with chromatin, which were identified by the ChIP approach in yeast, were preferentially located in the 3′ ends of active genes (Casolari et al, 2004). This is in contrast to the observed Nup-PI phenomenon, which was discovered by mapping the MNase–Nup2 fusion, and was found to involve Nup binding to the promoter and 5′ regions (Schmid et al, 2006). One explanation for this apparent contradiction would be the presence of both gene ends at the NPC through interactions with different Nups, resulting in DNA looping. It has been suggested that such chromatin looping at the NPC could establish active chromatin domains, into which protein factors involved in activation are clustered (Akhtar & Gasser, 2007; Menon et al, 2005).
By contrast, the high-resolution images of the nucleus (Fig 1B) suggest another potential function of the NPC. Heterochromatin seems to be interspersed by pores in a highly periodic manner, and it seems plausible that the NPC would have a role in setting up this organization by acting as a boundary between condensed and decondensed regions. Boundary elements are generally defined by their ability to prevent communication between active and silent chromatin, which they are thought to do by physically partitioning the chromatin fibre (Burgess-Beusse et al, 2002; Gerasimova et al, 1995). Examples of this type of involvement have been reported for the NPC: yeast Nup2, Nup60 and Prp20/RanGEF were identified in a screen for chromatin-boundary activities that can prevent the spreading of repressing factors into neighbouring genes (Ishii et al, 2002). In addition, Prp20-bound histones show a pattern of histone modifications typical for both heterochromatin and euchromatin, implying that Prp20, which can interact with Nup2, could establish a chromatin boundary at the NPC (Dilworth et al, 2005). Interestingly, the yeast genome ChIP studies discussed above correlated the binding of Prp20 to non-transcribing genes (Casolari et al, 2004), supporting the idea that the Nup-associated boundary activity could be an additional function of the NPC, separable from its involvement in transcriptional activation. Intriguingly, the only characterization of a genome-wide binding pattern of a metazoan Nup performed so far showed an enrichment of repressive heterochromatic histone marks (Brown et al, 2008). ChIP analysis of Nup93 showed that its binding sites correlate with regions of high trimethylation of H3K9, H4K20 and H3K27, although the results were obtained from a different cell type than that used for the analysis of genome-wide histone modifications, and therefore should be interpreted with caution. Nonetheless, they support the involvement of nuclear pores in tethering non-active chromatin regions for the purposes of genome organization.
During mitosis in higher eukaryotes, the NE and the nuclear pores break down and reform around newly segregated chromatin (D'Angelo & Hetzer, 2008). Mitotic propagation of cell-specific chromatin architecture is intimately linked to this reassembly; yet, how post-mitotic nuclear reformation around chromatin translates into interphase chromatin organization is unknown. Studies in Xenopus cell-free systems showed that the Nup107–160 complex, ELYS, Nup153 and Nup358 are recruited to chromatin early in the post-mitotic reassembly independently of nuclear membranes (Franz et al, 2007; Rasala et al, 2006; Walther et al, 2003). The association of Nups with chromatin represents one of the initial events in nuclear reorganization, suggesting that they could be involved in the propagation of chromatin organization through mitosis and, therefore, in epigenetic memory. An interesting role in epigenetic memory has been proposed for the yeast NPC, in which a long-term association of an inducible gene with the nuclear pore was reported to occur for several cell generations after the initial activation in yeast (Brickner et al, 2007). Although yeasts undergo closed mitosis and do not need to reform their NE, a role of the NPC in positional or epigenetic memory remains an intriguing possibility.
There might be two distinct types of mechanism by which the NPC regulates gene expression in a transport-independent manner (Fig 2). One is the physical association of the stable NE-embedded nuclear-pore structure with the chromatin fibre, which could have a role in tethering a chromatin boundary or in the general establishment of the three-dimensional organization of chromatin inside the nucleus. As the core of the NPC does not turn over during the entire lifespan of a cell (D'Angelo et al, 2009), this extreme stability makes the core components of the NPC ideal candidates for establishing long-term transcription programmes. We hypothesize that the highly stable central core of the nuclear pore can be viewed as a scaffold for nuclear architecture and could be used as a basis for epigenetic memory in differentiated cells. By contrast, a subset of NPC components seems to have a role in transcriptional activation or in coordinating the processes of transcription and mRNA processing and export. In this sense, the mechanism would not be entirely transport independent, as the overall purpose of this interaction might be to achieve the efficient or regulated transport of mRNA. The NE-embedded NPC could accommodate the recruitment of crucial machinery or the DNA looping necessary to establish an active chromatin domain. Interestingly, the Nups that have been implicated in transcriptional activation, including Nup153/Nup1/Nup2/Nup60 and Nup98, are also highly dynamic (Rabut et al, 2004). An intriguing possibility is that the dynamic behaviour of these Nups underlies their role in transcriptional activation. Dynamic Nups could move away from the nuclear pore to interact with transcribing genes or with mRNA and associated machinery inside the nucleus—that is, not at NE contact sites; this idea is supported by the reported transcription-dependent mobility of Nup153 and Nup98 (Griffis et al, 2004). The classification of these dynamic proteins as Nups might not fully reflect their functional diversity as, in addition to transport, they could also have roles in transcriptional gene regulation. The nuclear pore might sequester and/or use these proteins until signals alter their distribution to fulfil their additional roles away from the nuclear pore. This view of the NPC as a chromatin-interacting regulatory entity could provide an explanation of how tissue-specific functions of NPC components are achieved.
Conclusions
Our knowledge of the nuclear pore has expanded significantly for more than 50 years, since its initial identification as the exclusive transport channel between the nucleoplasm and the cytoplasm. The cell and tissue-specific transport events and regulatory roles of the NPC, in the context of normal and abnormal cell physiology, now await elucidation (Sidebar A). Evidence is emerging from studies in yeast, plants and animals that NPC composition varies among cell types, stages of the cell cycle and developmental stages. Furthermore, a new picture is emerging in which the NPC is active in chromatin organization and gene regulation in a transport-independent manner. On the one hand, the immobile and stable nature of the NPC core could provide a scaffold for tethering and partitioning the chromatin template; on the other hand, highly dynamic nuclear-pore components can move to non-peripheral locations inside the nucleus, where they might be similarly involved in transcriptional regulation. It is likely that at least some of the uncovered tissue-specific roles of Nups originate from these genome-organizing functions of the NPC, which is a idea that awaits further exploration.
Sidebar A | In need of answers.
Do nuclear pores have different nucleoporin (Nup) composition in various cell types? Which Nups are expressed in particular tissues and/or at specific developmental times?
Is the deterioration of the permeability function of the nuclear-pore complexes a general mechanism for ageing in long-lived post-mitotic cells?
Are transcribing loci recruited to the nuclear pores in metazoan cells, similar to that observed in yeast? Do certain metazoan Nups interact preferentially with transcribing genes versus heterochromatic loci?
What is the function of the dynamic behaviour of some peripheral Nups? Does it have a role in transport or in a pore-independent transcription-dependent nuclear process?
What role do alterations in nuclear-pore composition and Nup expression have in leukaemogenesis, and do other cancers involve perturbations of nuclear pore function?

Maya Capelson

Martin W. Hetzer
Acknowledgments
We thank members of the Hetzer laboratory for discussions of the presented ideas, critical evaluation and help with manuscript preparation. M.C. is supported by a Damon Runyon fellowship; M.W.H. by NIH RO1 GM57438.
References
- Akhtar A, Gasser SM (2007) The nuclear envelope and transcriptional control. Nat Rev Genet 8: 507–517 [DOI] [PubMed] [Google Scholar]
- Alber F et al. (2007) The molecular architecture of the nuclear pore complex. Nature 450: 695–701 [DOI] [PubMed] [Google Scholar]
- Ball JR, Dimaano C, Bilak A, Kurchan E, Zundel MT, Ullman KS (2007) Sequence preference in RNA recognition by the nucleoporin Nup153. J Biol Chem 282: 8734–8740 [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L et al. (2006) Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol 60: 214–222 [DOI] [PubMed] [Google Scholar]
- Beck M, Lucic V, Forster F, Baumeister W, Medalia O (2007) Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature 449: 611–615 [DOI] [PubMed] [Google Scholar]
- Blobel G (1985) Gene gating: a hypothesis. Proc Natl Acad Sci USA 82: 8527–8529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner DG, Cajigas I, Fondufe-Mittendorf Y, Ahmed S, Lee PC, Widom J, Brickner JH (2007) H2A.Z-mediated localization of genes at the nuclear periphery confers epigenetic memory of previous transcriptional state. PLoS Biol 5: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA (2008) Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev 22: 627–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess-Beusse B, Farrell C, Gaszner M, Litt M, Mutskov V, Recillas-Targa F, Simpson M, West A, Felsenfeld G (2002) The insulation of genes from external enhancers and silencing chromatin. Proc Natl Acad Sci USA 99 (Suppl 4): 16433–16437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal GG et al. (2006) SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441: 770–773 [DOI] [PubMed] [Google Scholar]
- Cai Y, Gao Y, Sheng Q, Miao S, Cui X, Wang L, Zong S, Koide SS (2002) Characterization and potential function of a novel testis-specific nucleoporin BS-63. Mol Reprod Dev 61: 126–134 [DOI] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA (2004) Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117: 427–439 [DOI] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Drubin DA, Rando OJ, Silver PA (2005) Developmentally induced changes in transcriptional program alter spatial organization across chromosomes. Genes Dev 19: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P et al. (2008) Nucleoporin levels regulate cell cycle progression and phase-specific gene expression. Dev Cell 15: 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw JM, Matunis MJ (2003) The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci USA 100: 5823–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw JM, Matunis MJ (2004) The nuclear pore complex: disease associations and functional correlations. Trends Endocrinol Metab 15: 34–39 [DOI] [PubMed] [Google Scholar]
- Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ (2002) Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 158: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo MA, Hetzer MW (2008) Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol 18: 456–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo MA, Raices M, Panowski SH, Hetzer MW (2009) Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136: 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle N, Beaudouin J, Hartnell L, Imreh G, Hallberg E, Lippincott-Schwartz J, Ellenberg J (2001) Nuclear pore complexes form immobile networks and have a very low turnover in live mammalian cells. J Cell Biol 154: 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damelin M, Silver PA (2000) Mapping interactions between nuclear transport factors in living cells reveals pathways through the nuclear pore complex. Mol Cell 5: 133–140 [DOI] [PubMed] [Google Scholar]
- Davuluri G, Gong W, Yusuff S, Lorent K, Muthumani M, Dolan AC, Pack M (2008) Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress. PLoS Genet 4: e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong-Curtain TA et al. (2008) Abnormal nuclear pore formation triggers apoptosis in the intestinal epithelium of elys-deficient zebra fish. Gastroenterology 136: 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keersmaecker K et al. (2008) Kinase activation and transformation by NUP214-ABL1 is dependent on the context of the nuclear pore. Mol Cell 31: 134–142 [DOI] [PubMed] [Google Scholar]
- Debler EW, Ma Y, Seo HS, Hsia KC, Noriega TR, Blobel G, Hoelz A (2008) A fence-like coat for the nuclear pore membrane. Mol Cell 32: 815–826 [DOI] [PubMed] [Google Scholar]
- Devos D, Dokudovskaya S, Alber F, Williams R, Chait BT, Sali A, Rout MP (2004) Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol 2: e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieppois G, Iglesias N, Stutz F (2006) Cotranscriptional recruitment to the mRNA export receptor Mex67p contributes to nuclear pore anchoring of activated genes. Mol Cell Biol 26: 7858–7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth DJ, Tackett AJ, Rogers RS, Yi EC, Christmas RH, Smith JJ, Siegel AF, Chait BT, Wozniak RW, Aitchison JD (2005) The mobile nucleoporin Nup2p and chromatin-bound Prp20p function in endogenous NPC-mediated transcriptional control. J Cell Biol 171: 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J, Levy DE, Blobel G, Fontoura BM (2002) Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 295: 1523–1525 [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Koser J, Aebi U (2004) The nuclear pore complex: a jack of all trades? Trends Biochem Sci 29: 175–182 [DOI] [PubMed] [Google Scholar]
- Faria AM, Levay A, Wang Y, Kamphorst AO, Rosa ML, Nussenzveig DR, Balkan W, Chook YM, Levy DE, Fontoura BM (2006) The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity 24: 295–304 [DOI] [PubMed] [Google Scholar]
- Feldherr CM, Akin D (1990) The permeability of the nuclear envelope in dividing and nondividing cell cultures. J Cell Biol 111: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura BM, Blobel G, Matunis MJ (1999) A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J Cell Biol 144: 1097–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, Antonin W (2007) MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep 8: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Gorlich D (2007) A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130: 512–523 [DOI] [PubMed] [Google Scholar]
- Gerasimova TI, Gdula DA, Gerasimov DV, Simonova O, Corces VG (1995) A Drosophila protein that imparts directionality on a chromatin insulator is an enhancer of position-effect variegation. Cell 82: 587–597 [DOI] [PubMed] [Google Scholar]
- Ghannam G, Takeda A, Camarata T, Moore MA, Viale A, Yaseen NR (2004) The oncogene Nup98-HOXA9 induces gene transcription in myeloid cells. J Biol Chem 279: 866–875 [DOI] [PubMed] [Google Scholar]
- Gigliotti S, Callaini G, Andone S, Riparbelli MG, Pernas-Alonso R, Hoffmann G, Graziani F, Malva C (1998) Nup154, a new Drosophila gene essential for male and female gametogenesis is related to the nup155 vertebrate nucleoporin gene. J Cell Biol 142: 1195–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich D (1997) Nuclear protein import. Curr Opin Cell Biol 9: 412–419 [DOI] [PubMed] [Google Scholar]
- Graux C et al. (2004) Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nat Genet 36: 1084–1089 [DOI] [PubMed] [Google Scholar]
- Griffis ER, Craige B, Dimaano C, Ullman KS, Powers MA (2004) Distinct functional domains within nucleoporins Nup153 and Nup98 mediate transcription-dependent mobility. Mol Biol Cell 15: 1991–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi MR, Cozzolino L, Malva C, Graziani F, Gigliotti S (2007) nup154 genetically interacts with cup and plays a cell-type-specific function during Drosophila melanogaster egg-chamber development. Genetics 175: 1751–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer M, Walther TC, Mattaj IW (2005) Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu Rev Cell Dev Biol 21: 347–380 [DOI] [PubMed] [Google Scholar]
- Hogarth C, Itman C, Jans DA, Loveland KL (2005) Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays 27: 1011–1025 [DOI] [PubMed] [Google Scholar]
- Ishii K, Arib G, Lin C, Van Houwe G, Laemmli UK (2002) Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109: 551–562 [DOI] [PubMed] [Google Scholar]
- Jacob Y, Mongkolsiriwatana C, Veley KM, Kim SY, Michaels SD (2007) The nuclear pore protein AtTPR is required for RNA homeostasis, flowering time, and auxin signaling. Plant Physiol 144: 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, van Deursen JM (1999) CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol Cell Biol 19: 764–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RY & Fahrenkrog B (2006) The nuclear pore complex up close. Curr Opin Cell Biol 18: 342–347 [DOI] [PubMed] [Google Scholar]
- Lupu F, Alves A, Anderson K, Doye V, Lacy E (2008) Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev Cell 14: 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra R, Kerr SC, Harreman MT, Apponi LH, Fasken MB, Ramineni S, Chaurasia S, Valentini SR, Corbett AH (2007) Actively transcribed GAL genes can be physically linked to the nuclear pore by the SAGA chromatin modifying complex. J Biol Chem 282: 3042–3049 [DOI] [PubMed] [Google Scholar]
- Meier I, Brkljacic J (2009) The nuclear pore and plant development. Curr Opin Plant Biol 12: 87–95 [DOI] [PubMed] [Google Scholar]
- Mendjan S et al. (2006) Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol Cell 21: 811–823 [DOI] [PubMed] [Google Scholar]
- Menon BB, Sarma NJ, Pasula S, Deminoff SJ, Willis KA, Barbara KE, Andrews B, Santangelo GM (2005) Reverse recruitment: the Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc Natl Acad Sci USA 102: 5749–5754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T et al. (1996) Fusion of the nucleoporin gene NUP98 to HOXA9 by the chromosome translocation t(7;11)(p15;p15) in human myeloid leukaemia. Nat Genet 12: 154–158 [DOI] [PubMed] [Google Scholar]
- Neilson DE et al. (2009) Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 84: 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Scheele S, Ekblom P (2004) Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp Cell Res 292: 359–370 [DOI] [PubMed] [Google Scholar]
- Pritchard CE, Fornerod M, Kasper LH, van Deursen JM (1999) RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains. J Cell Biol 145: 237–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyhtila B, Rexach M (2003) A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J Biol Chem 278: 42699–42709 [DOI] [PubMed] [Google Scholar]
- Rabut G, Doye V, Ellenberg J (2004) Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol 6: 1114–1121 [DOI] [PubMed] [Google Scholar]
- Rasala BA, Orjalo AV, Shen Z, Briggs S, Forbes DJ (2006) ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc Natl Acad Sci USA 103: 17801–17806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro S, Fischer T, Luo MJ, Antunez O, Brettschneider S, Lechner J, Perez-Ortin JE, Reed R, Hurt E (2004) Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 116: 75–86 [DOI] [PubMed] [Google Scholar]
- Roth P, Xylourgidis N, Sabri N, Uv A, Fornerod M, Samakovlis C (2003) The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol 163: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemaille M et al. (2008) THO/Sub2p functions to coordinate 3′-end processing with gene-nuclear pore association. Cell 135: 308–321 [DOI] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT (2000) The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol 148: 635–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Magnasco MO, Chait BT (2003) Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 13: 622–628 [DOI] [PubMed] [Google Scholar]
- Saito S, Miyaji-Yamaguchi M, Nagata K (2004) Aberrant intracellular localization of SET-CAN fusion protein, associated with a leukemia, disorganizes nuclear export. Int J Cancer 111: 501–507 [DOI] [PubMed] [Google Scholar]
- Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, Laemmli UK (2006) Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol Cell 21: 379–391 [DOI] [PubMed] [Google Scholar]
- Slape C, Aplan PD (2004) The role of NUP98 gene fusions in hematologic malignancy. Leuk Lymphoma 45: 1341–1350 [DOI] [PubMed] [Google Scholar]
- Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE (2000) Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol Cell Biol 20: 5631–5642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffler D, Feja B, Fahrenkrog B, Walz J, Typke D, Aebi U (2003) Cryo-electron tomography provides novel insights into nuclear pore architecture: implications for nucleocytoplasmic transport. J Mol Biol 328: 119–130 [DOI] [PubMed] [Google Scholar]
- Strawn LA, Shen T, Shulga N, Goldfarb DS, Wente SR (2004) Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat Cell Biol 6: 197–206 [DOI] [PubMed] [Google Scholar]
- Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM (2006) Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441: 774–778 [DOI] [PubMed] [Google Scholar]
- Takeda A, Goolsby C, Yaseen NR (2006) NUP98-HOXA9 induces long-term proliferation and blocks differentiation of primary human CD34+ hematopoietic cells. Cancer Res 66: 6628–6637 [DOI] [PubMed] [Google Scholar]
- Tartakovsky F, Worman HJ (1995) Detection of Gp210 autoantibodies in primary biliary cirrhosis using a recombinant protein containing the predominant autoepitope. Hepatology 21: 495–500 [PubMed] [Google Scholar]
- Terry LJ, Shows EB, Wente SR (2007) Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 318: 1412–1416 [DOI] [PubMed] [Google Scholar]
- Uv AE, Roth P, Xylourgidis N, Wickberg A, Cantera R, Samakovlis C (2000) members only encodes a Drosophila nucleoporin required for rel protein import and immune response activation. Genes Dev 14: 1945–1957 [PMC free article] [PubMed] [Google Scholar]
- Vodala S, Abruzzi KC, Rosbash M (2008) The nuclear exosome and adenylation regulate posttranscriptional tethering of yeast GAL genes to the nuclear periphery. Mol Cell 31: 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC et al. (2003) The conserved Nup107–160 complex is critical for nuclear pore complex assembly. Cell 113: 195–206 [DOI] [PubMed] [Google Scholar]
- Wang GG, Cai L, Pasillas MP, Kamps MP (2007) NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol 9: 804–812 [DOI] [PubMed] [Google Scholar]
- Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, Luo JL, Patel DJ, Allis CD (2009) Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature 459: 847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis K (2002) Nucleocytoplasmic transport: cargo trafficking across the border. Curr Opin Cell Biol 14: 328–335 [DOI] [PubMed] [Google Scholar]
- Weis K (2007) The nuclear pore complex: oily spaghetti or gummy bear? Cell 130: 405–407 [DOI] [PubMed] [Google Scholar]
- Wente SR (2000) Gatekeepers of the nucleus. Science 288: 1374–1377 [DOI] [PubMed] [Google Scholar]
- Wesierska-Gadek J, Klima A, Komina O, Ranftler C, Invernizzi P, Penner E (2007) Characterization of autoantibodies against components of the nuclear pore complexes: high frequency of anti-p62 nucleoporin antibodies. Ann NY Acad Sci 1109: 519–530 [DOI] [PubMed] [Google Scholar]
- Xu XM, Rose A, Muthuswamy S, Jeong SY, Venkatakrishnan S, Zhao Q, Meier I (2007) NUCLEAR PORE ANCHOR, the Arabidopsis homolog of Tpr/Mlp1/Mlp2/megator, is involved in mRNA export and SUMO homeostasis and affects diverse aspects of plant development. Plant Cell 19: 1537–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xylourgidis N, Roth P, Sabri N, Tsarouhas V, Samakovlis C (2006) The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFκB activation in Drosophila. J Cell Sci 119: 4409–4419 [DOI] [PubMed] [Google Scholar]
- Zhang X et al. (2008) Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell 135: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X (2005) A putative nucleoporin 96 Is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1,constitutive 1. Plant Cell 17: 1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]