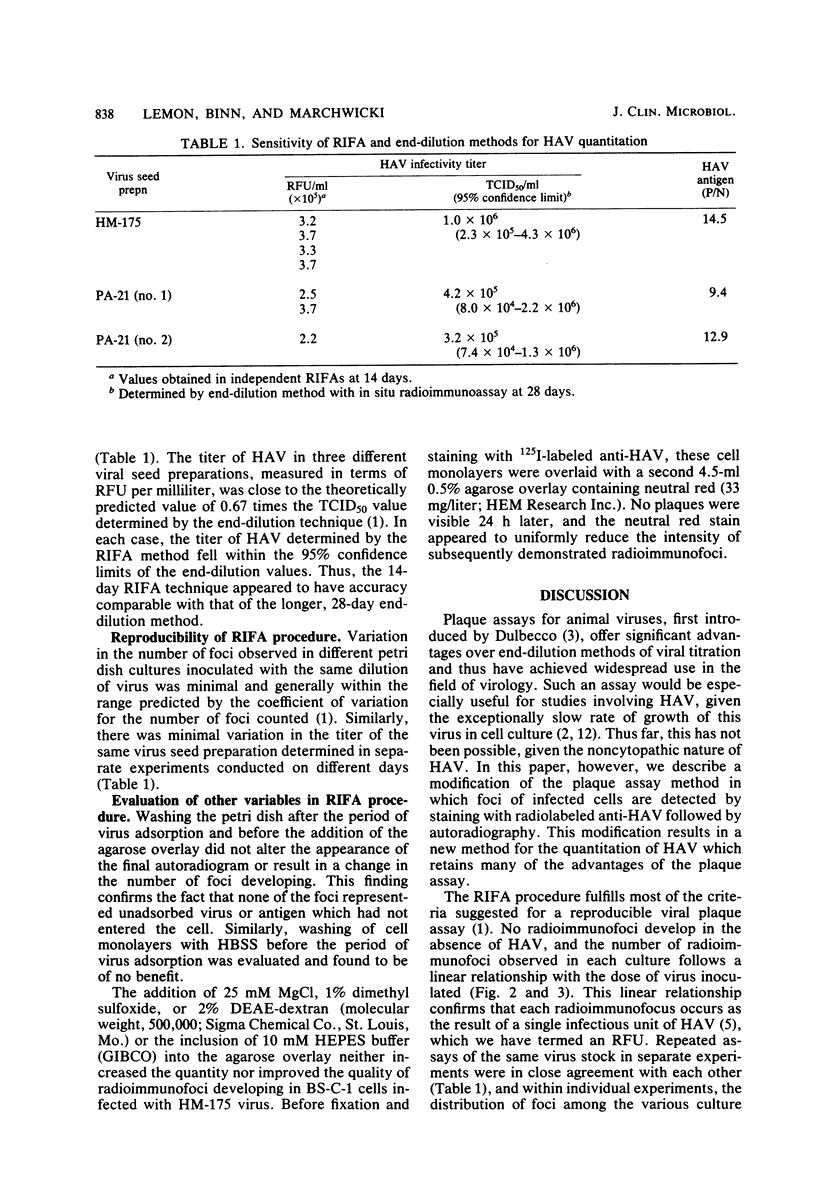
Abstract
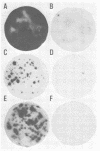
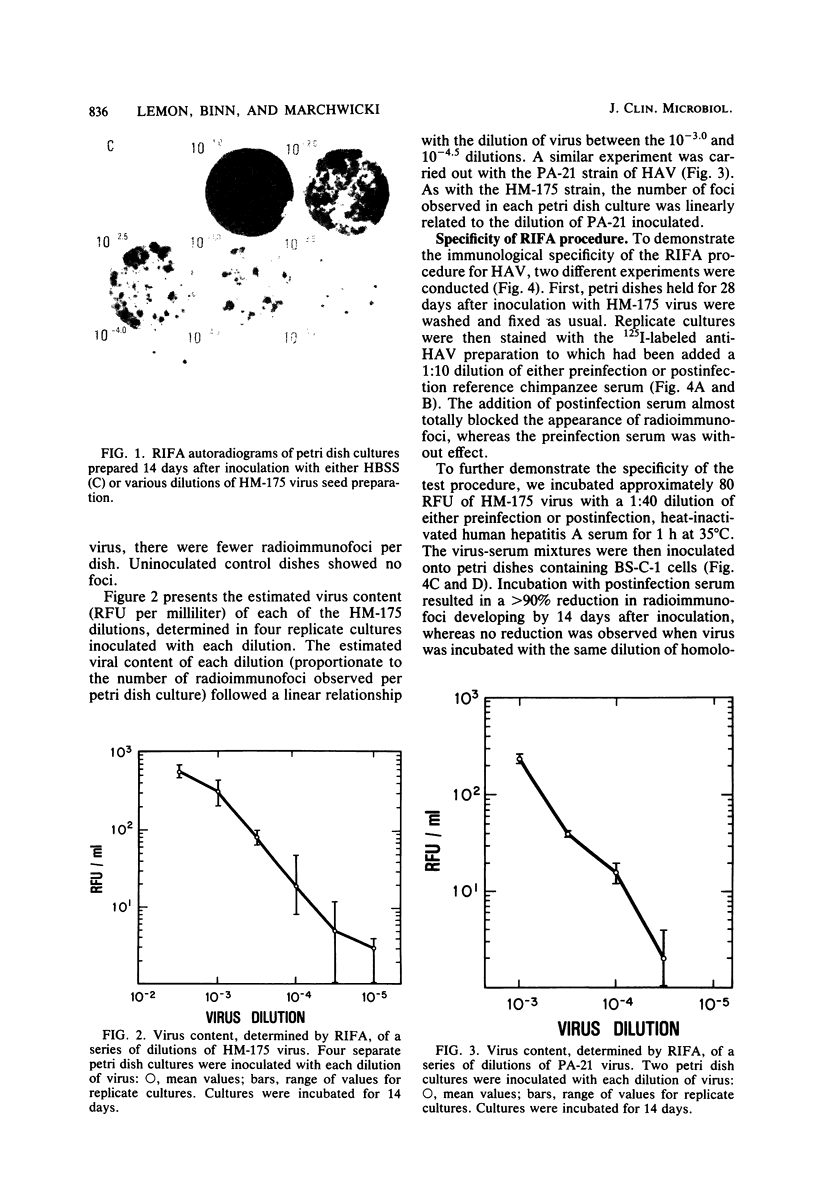
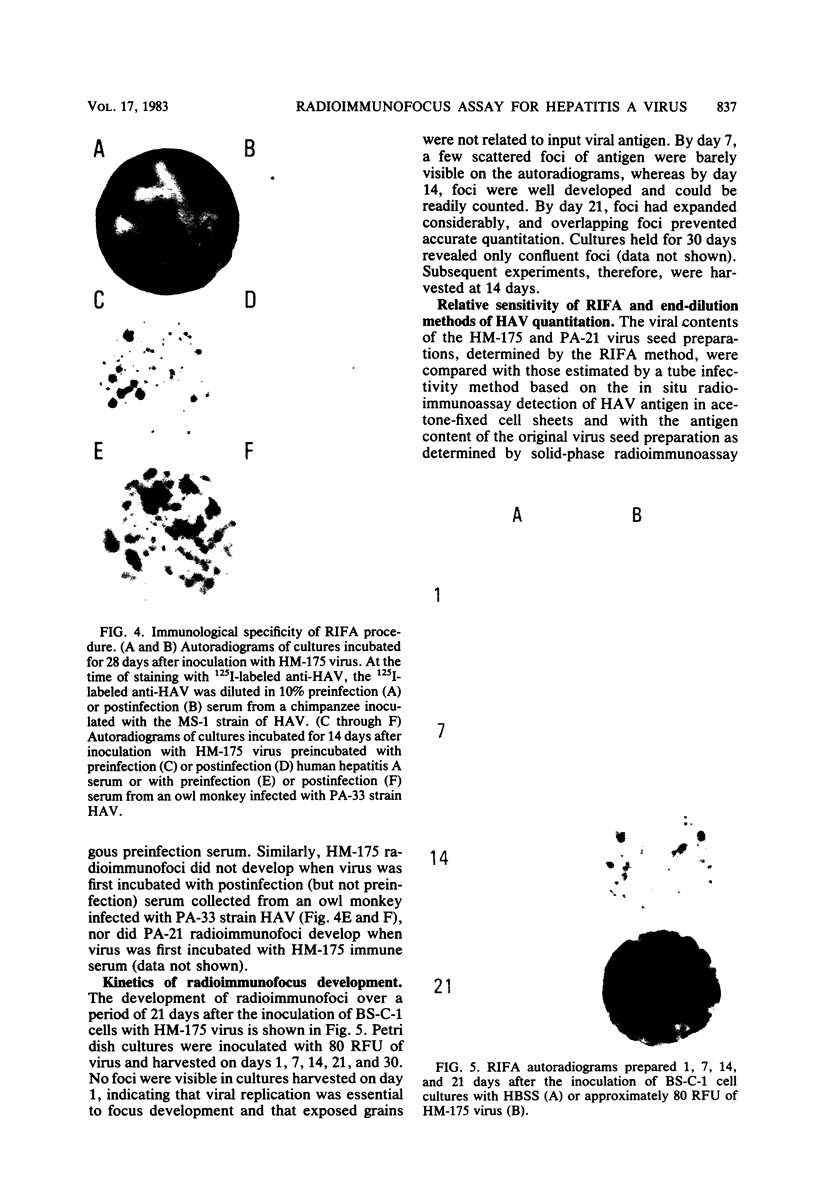
A new method is described for the quantitation of hepatitis A virus in cell cultures, based on the immune autoradiographic detection of foci of infected cells (radioimmunofoci) developing beneath an agarose overlay 14 days after the inoculation of petri dish cultures of continuous African green monkey kidney cells (BS-C-1). The number of foci developing in each culture was linearly related to the dose of hepatitis A virus (either HM-175 or PA-21 strain) inoculated. Focus development was prevented by prior incubation of virus with specific antisera, and the specificity of the radiolabeled antibody reaction was confirmed in competitive blocking experiments. This new assay method retains many of the advantages of conventional plaque assays for virus. Compared with existing end-dilution methods for the quantitation of hepatitis A virus, the radioimmunofocus assay offers greatly improved accuracy and comparable sensitivity, yet is relatively rapid and highly conservative of reagents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOPER P. D. The plaque assay of animal viruses. Adv Virus Res. 1961;8:319–378. doi: 10.1016/s0065-3527(08)60689-2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemer R. J., Feinstone S. M., Gust I. D., Purcell R. H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981 Apr;32(1):388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Production of Plaques in Monolayer Tissue Cultures by Single Particles of an Animal Virus. Proc Natl Acad Sci U S A. 1952 Aug;38(8):747–752. doi: 10.1073/pnas.38.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frösner G. G., Deinhardt F., Scheid R., Gauss-Müller V., Holmes N., Messelberger V., Siegl G., Alexander J. J. Propagation of human hepatitis A virus in a hepatoma cell line. Infection. 1979;7(6):303–305. doi: 10.1007/BF01642154. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Kojima H., Shibayama T., Sato A., Suzuki S., Ichida F., Hamada C. Propagation of human hepatitis A virus in conventional cell lines. J Med Virol. 1981;7(4):273–286. doi: 10.1002/jmv.1890070404. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Brown C. D., Brooks D. S., Simms T. E., Bancroft W. H. Specific immunoglobulin M response to hepatitis A virus determined by solid-phase radioimmunoassay. Infect Immun. 1980 Jun;28(3):927–936. doi: 10.1128/iai.28.3.927-936.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., LeDuc J. W., Binn L. N., Escajadillo A., Ishak K. G. Transmission of hepatitis A virus among recently captured Panamanian owl monkeys. J Med Virol. 1982;10(1):25–36. doi: 10.1002/jmv.1890100105. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Banker F. S., Giesa P. A., McAleer W. J., Buynak E. B., Hilleman M. R. Progress toward a live, attenuated human hepatitis A vaccine. Proc Soc Exp Biol Med. 1982 May;170(1):8–14. doi: 10.3181/00379727-170-41387. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]