Abstract
Objective
To image the conditional activation of cardiac gene expression in vivo.
Background
The Cre–loxP system has been routinely used for conditional activation and deletion of gene expression. However, the spatiotemporal manner of these events in the heart has not yet been defined by in vivo imaging.
Methods
Adenovirus (1×109 pfu) carrying the silent positron emission tomography (PET) reporter gene, herpes simplex virus type 1 thymidine kinase (HSV1-tk), was injected into the left ventricular wall of male transgenic mice (n=15) or FVB controls (n=8). Transgenic mice expressed Cre recombinase driven by a cardiac-specific α-myosin heavy chain (α-MHC) promoter. Following injection of the 9-[4-fluoro-3-(hydroxymethyl)butyl]guanine ([18F]-FHBG; 137±25 μCi) reporter probe, micro PET imaging was used to assess the expression of HSV1-tk reporter gene in the myocardium.
Results
Two days following adenoviral injection, cardiac HSV1-tk gene activation resulted in tracer uptake of 3.20±0.51 %ID/g for α-MHC-Cre and 0.05±0.02 %ID/g for control mice (P<0.01). The in vivo results were confirmed by RT-PCR and Western blot analysis. Similar transfections were evaluated in both cardiac specific and non-cardiac specific cell lines. Enzyme activity showed a robust correlation (r2 = 0.82) between in vivo molecular imaging technique and traditional in vitro enzyme assays.
Conclusions
With further development and validation, PET imaging will likely play an important role in the non-invasive, repetitive, and quantitative measurement of conditional gene activation in the future.
Keywords: gene activation, Cre-lox recombinase, molecular imaging, PET, heart disease
INTRODUCTION
Over the past two decades, conditional gene expression and gene deletion models have been instrumental in advancing our understanding of gene function, cell development, and pathophysiology (1). These site-specific recombinase systems such as Cre-loxP, Flp-FRT, and φC31-att allow expression modulation of specific gene products in transgenic mice and various cell lines (2). In particular, the widely used Cre recombinase is a 38-kDa integrase derived from the bacteriophage P1 which catalyzes site-specific recombination between two 34-bp long loxP sequences (3). The intervening DNA between the two loxP sites positioned head-to-tail is excised. By inserting a stop cassette in the intervening sequence between the promoter and the transgene, expression of the target gene can be temporally and spatially controlled (4).
In the field of cardiovascular research on gene activation, the Cre-loxP mediated recombination heralds a new generation of mouse models of human diseases (5). Agah et al. first demonstrated that postmitotic cardiac muscle cells are amenable to Cre-mediated recombination after adenoviral gene transfer (6). Subsequently, Minamino et al. showed that the timing and tissue specificity of Cre transgene expression in the heart can be regulated by incorporating synthetic antiprogestin ligand and cardiac MHC promoter, respectively, into the system (7). Recently, Oh et al. was able to demonstrate the homing, differentiation, and fusion of cardiac progenitor cells derived from adult mouse myocardium by using Cre-loxP donor and recipient transgenic mice (α-MHC-Cre/Rosa26 reporter) (8). More critically, these studies further highlighted the utility of the site specific recombinase system for studying cardiac gene transfer, embryonic development, and stem cell biology.
While the Cre-loxP mediated recombination has become a popular system for understanding conditional gene activation, the majority of current studies involve β-galactosidase (LacZ) or green fluorescent protein (GFP) as reporter genes (8,9). The use of LacZ and GFP requires sacrifice of the animals for postmortem analysis, precluding longitudinal follow up of reporter gene expression within the same animal. Thus, the incorporation of molecular imaging technique to monitor these events can provide significant advantages. In this study, we seek to demonstrate noninvasive PET imaging of cardiac tissue-specific conditional gene activation.
MATERIALS AND METHODS
Cultivation of cell lines
H9c2 (rat embryonic cardiomyoblast) cells were grown in deficient DMEM, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/L-glutamate as described (10). The HL-1 (mouse atrial cardiomycoyte) cells were cultured in petri dishes pre-coated with 0.02% gelatin and 5 μg/ml fibronectin in complete Claycomb medium supplemented with 10% FBS, 0.1 mM norepinephrine, 0.3 mM L-ascorbic acid, 2 mM L-glutamine and antibiotics (1% penicillin/streptomycin) as described (11). The HeLa (human cervical adenocarcinoma cells) and 293-T (human embryonic kidney) cells were grown in MEM with 1% penicillin/streptomycin and 10% FBS as described (12).
Transfection with plasmid and adenovirus
The cells were grown in 10 cm dishes and were transfected with 10 μg of α-MHC-Cre plasmid using Lipofectamine 2000 (Invitrogen, CA). After three hours, the culture medium was removed and 1×109 particle forming unit (pfu) of adenovirus was added to the serum free medium for one hour, followed by addition of complete medium. The E1/E3-deleted recombinant adenovirus contains a hybrid cytomegalovirus enhancer/chicken β-actin promoter (pCAG) carrying a neomycin (Neo) selection marker and a poly adenylation (PA) as stop signal flanked by two loxP sites, followed by the HSV1-tk reporter gene (Ad-pCAG-loxP-Neo-PA-loxP-HSV1-tk-PA) (Figure 1). In this construct, the SV40 PA tail was placed downstream of Neo and the rabbit β-globin PA is placed downstream of HSV1-tk. These cells were also exposed to 1×109 pfu of adenovirus containing no transgene (Ad-null) as negative control and 1×109 pfu of adenovirus carrying constitutive CMV promoter driving herpes simplex virus type 1 thymidine kinase (Ad-HSV1-tk) as positive control. Construction, amplification, and viral titer determination of these viruses have been described previously (13).
Figure 1.
Schema representing conditional activation of cardiac gene expression. Cardiomyoyctes are transfected with an adenovirus carrying a cytomegalovirus enhancer/chicken β-actin promoter driving a neomycin and poly adenylation signal flanked by two loxP sites (floxed), followed by the HSV1-tk reporter gene (Ad-pCAG-loxP-Neo-PA-loxP-HSV1-tk-PA). In the absence of Cre, expression of pCAG driven Neo becomes terminated at the first PA stop cassette. In the presence of Cre, there is Cre-loxP mediated recombination event leading to expression of pCAG driven HSV1-tk. Activation of the HSV1-tk reporter gene can be detected non-invasively by PET imaging via intracellular phosphorylation and trapping of the administered [18F]-FHBG PET reporter probe.
α-MHC-Cre transgenic mice
Transgenic mice of FVB background that express Cre recombinase driven by a cardiac specific myosin heavy chain promoter were used for the in vivo experiments (6). All mice were tested and confirmed to be positive for α-MHC-Cre by PCR of genomic DNA from tail tissue. Results were obtained using sense and anti-sense primers of α-MHC-Cre recombinase (sense 5′-ATGACAGACAGATCCCTCCTATCTCC-3′ and antisense 5′-CTCATCACTCGTTGCATCGAC-3′) by setting 30 PCR cycles with denaturation at 95°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min, followed by final extension for 4 min. The expected PCR product of 250 base pairs was confirmed by running 1% agarose gel with suitable marker. Tail vein genomic DNA from Cre negative mice (FVB background) served as negative control.
Myocardial injection of adenoviruses
Male mice weighing 20–30 gm were studied under protocols approved by the Stanford Animal Research Committee. Animals received isoflurane (2%) for general anesthesia, followed by bupinorphine (2.5 mg/kg) for pain control. Anesthetized mice were intubated and mechanically ventilated (Harvard Instruments, MA) for the creation of a left thoracotomy. Using a 30-gauge needle containing 30 μl of viral volume, 1×109 pfu of Ad-pCAG-loxP-Neo-PA-loxP-HSV1-tk-PA was injected into the anterolateral wall of α-MHC-Cre transgenic mice (n=15) and Cre-negative control mice of FVB background (n=8) by a blinded surgeon (G.H.). All animals recovered uneventfully and underwent serial PET imaging. For the study group, fifteen transgenic mice underwent imaging on day 2, twelve on day 4, nine on day 7, six on day 9, and three on day 14. Three animals were sacrificed at each time point. For the control group, three Cre-negative mice underwent imaging on days 2, 4, 7, 9, and 15. We did not image all of the Cre-negative mice at each time point because based on our experience, their signals were uniformly near the background levels.
Small animal positron emission tomography imaging
Cardiac PET imaging was acquired using the R4 Concorde MicroPET system (Knoxville, TN). This scanner has a computer-controlled bed and 10.8-cm transaxial and 8-cm axial fields of view (FOVs). Animals were placed in the center of the FOV of the scanner, where the highest image resolution and sensitivity are available for the heart. The microPET studies were performed by tail-vein injection of [18F]-FHBG reporter probe (137±25 μCi) under isoflurane anesthesia. Briefly, mice were anesthetized using a mixture of 2% isoflurane and 100% oxygen and placed supine in the imager. Images from 60–75 minutes post-injection were reconstructed by filtered back projection algorithm. This particular time point was chosen based on previous data showing the maximum cardiac [18F]-FHBG activity (%ID/g) between 1–2 hour post tracer injection (14,15). No corrections for partial volume or attenuation were performed. Regions of interest (ROI) of [18F]-FHBG images were drawn by a blinded observer (F.C.) over the anterolateral wall by using vendor software (ASI Pro 5.2.4.0) on decay-corrected whole-body horizontal view. The maximum radioactivity concentration (accumulation) was obtained from mean pixel values within the multiple ROI volume, which were converted to counts/mL/min by using a conversion factor. Assuming a tissue density of 1 g/mL, the ROIs were converted to counts/g/min and then divided by the administered activity to obtain an imaging ROI–derived [18F]-FHBG percentage injected dose per gram of heart (%ID/g) as described (15,16). The effects of ROI positioning were determined by averaging at least three ROI and assessing the variability across regions. The same process was repeated on days 2, 4, 7, 9, and 15 of [18F]-FHBG injection followed by microPET imaging. To acquire whole images of the heart, animals were kept in the same position and [18F]-fluorodeoxyglucose ([18F]-FDG) (142±33 μCi) was injected intravenously approximately 2 hours after initial [18F]-FHBG injection. FDG imaging was acquired as a single 15-minute static scan starting 60 minutes post radiotracer injection. Afterwards, both the [18F]-FHBG and [18F]-FDG images were overlaid as a fusion image using the ASIPro VM software. Finally, after PET scans, mice were euthanized and the tissues were processed for RT-PCR, Western blot analysis, and HSV1-TK enzyme assay.
Reverse transcriptase polymerase chain reaction
Total RNA was prepared from heart, liver, spleen, and kidney tissues using the TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). The second strand synthesis by PCR with gene specific primer of α-MHC-Cre and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was carried out as follows: α-MHC-Cre forward primer 5′-ATGACAGACAGA TCCCTCCTATCTCC-3′; α-MHC-Cre reverse primer 5′–CTCATCACTCGTTGCATCGAC-3′; GAPDH forward primer 5′-GGCATGGACTGTGGTCATGA-3′; GAPDH reverse primer 5′-TTCACCACCATGGAGAAGGC -3′. The reaction was cycled at 94°C for 30 sec, 56°C for 30 sec, 72°C for 45 sec for 30 times and a final extension step at 72°C for 5 min in a DNA Engine Thermal Cycler (MJ Research, Waltham, MA). All amplification products were subjected to 1% agarose gel electrophoresis in TBE buffer. The resulting bands were assessed by using Labworks 4.6 Image Acquisition and analysis software (UVP Bio-imaging systems, Upland, CA).
Western blot analysis
The heart, liver, spleen, and kidney tissues were dissected from the euthanized animals after PET imaging. Electrophoresis and immunodetection of α-MHC-Cre were carried out according to methods as previously described (7), using polyclonal rabbit anti-Cre antibody (Novagen, 1:1000) for primary detection and HRP-conjugated goat anti-rabbit IgG (Promega, 1:4000) for secondary detection. Protein expression was visualized using substrates from enhanced chemiluminescence kit (Amersham) by exposing the Kodak X-ray film for 10 seconds. The membrane was immediately washed with TSBT over night at 4°C in order to use again for α-tubulin detection as an internal loading control.
Thymidine kinase enzyme assays
Cultured cells (H9c2, HL-1, HeLa, 293-T) or tissue samples were homogenized in HSV1-TK buffer and the enzyme activity determined as described previously (15,16). Rat glioma (C6) and C6 stably transfected HSV1-sr39tk (C6-stb-sr39tk+) cells served as negative and positive controls, respectively. The radioisotope 8-[3H]penciclovir (8-[3H]PCV) was obtained from Moravek Biochemicals (La Brea, CA). Results are expressed as percent conversion of 8-[3H]PCV in (dpm/μg protein/min of cell or tissue extract)/(dpm of control sample) × 100.
Statistical analysis
Data are presented as mean ± S.E.M. ANOVA and repeated measures ANOVA were applied to determine significant differences between cell lines and PET values for the animal cohort, respectively. A two-tailed Student’s T-test was used where appropriate. Standard linear regression analysis was used to determine correlation between in vivo imaging and in vitro assays. Significance was defined at P<0.05.
RESULTS
Conditional activation of HSV1-tk gene in different tissue specific cell lines
To assess both tissue specificity and conditional activation of gene expression in vitro, the H9c2 (rat embryonic cardiomyoblasts), HL-1 (mouse atrial cardiomyocyte), HeLa (human cervical adenocarcinoma), and 293-T (human embryonic kidney) cells were transfected with the following conditions: (1) Ad-null as negative control, (2) Ad-loxP-Neo-PA-loxP-HSV1-tk-PA alone, (3) α-MHC-Cre plasmid alone, (4) Ad-HSV1-tk as positive control, or (5) α-MHC-Cre plasmid and Ad-loxP-Neo-PA-loxP-HSV1-tk-PA (Figure 2). Transfection with adenovirus containing no transgene (Ad-null) yielded background thymidine kinase activity (<0.2 dpm/μg protein/total count) in all four cell lines. Likewise, single transfection with silenced HSV1-tk (Ad-loxP-neo-PA-loxP-HSV1-tk) or cardiac specific plasmid (α-MHC-Cre) alone led to background thymidine kinase activity similar to Ad-null. In contrast, transfection with the constitutively active wild-type Ad-HSV1-tk induced a 35–100 fold higher levels of thymidine kinase activity in all cell types (P<0.01 vs. Ad-null). Double transfection with Ad-loxP-Neo-PA-loxP-HSV1-tk and α-MHC-Cre resulted in significant activation of thymidine kinase activity in H9c2 and HL-1 cell lines (P<0.01) but not in HeLa and 293-T cell lines. Thus, the expression of Cre enzyme in the presence of cardiac-specific cell lines (H9c2 and HL-1) led to Cre-mediated excision of loxP-Neo-PA-loxP sequence, allowing for the expression of the downstream HSV1-tk gene. In contrast, Cre enzyme was not expressed in human cervical or kidney tissues, and thus the HSV1-tk remained silenced by the upstream poly adenylation (PA) stop cassette (Figure 1). Finally, the similar levels of thymidine kinase activities between the single transfection (wild-type Ad-HSV1-tk) and the conditional activation (Ad-loxP-Neo-PA-loxP-HSV1-tk-PA and α-MHC-Cre) within cardiac-specific cell lines attest to the robustness of Cre-loxP mediated recombination events.
Figure 2.
Comparison of constitutive versus conditional gene expression in different cellular systems. (A) HL-1 mouse atrial cardiomyocyte, (B) H9c2 rat embryonic cardiomyoblast, (C) HeLa human cervical adenocarcinoma cells, and (D) 293-T human embryonic kidney cells were investigated under various conditions. These include transfection with E1/E3-deleted recombinant adenovirus carrying no transgene (Ad-null), adenovirus carrying pCAG-loxP-Neo-PA-loxP-HSV1-tk-PA (Ad-loxP-TK), plasmid with cardiac tissue specific promoter driving Cre (MHC-Cre), adenovirus with constitutive CMV promoter driving HSV1-tk (Ad-TK), and double transfection with MHC-Cre and conditional Ad-loxP-TK. In HL-1 and H9c2cell lines, both constitutive expression and conditional activation of HSV1-tk led to comparable thymidine kinase enzyme activities (dpm/μg protein/total count) that are significantly higher compared to Ad-null, Ad-loxP-TK, or MHC-Cre single transfection (*P<0.01). In contrast, there was no conditional activation of HSV1-tk expression by the cardiac specific MHC-Cre plasmid in the HeLa cervical cell line and 293-T kidney cell line.
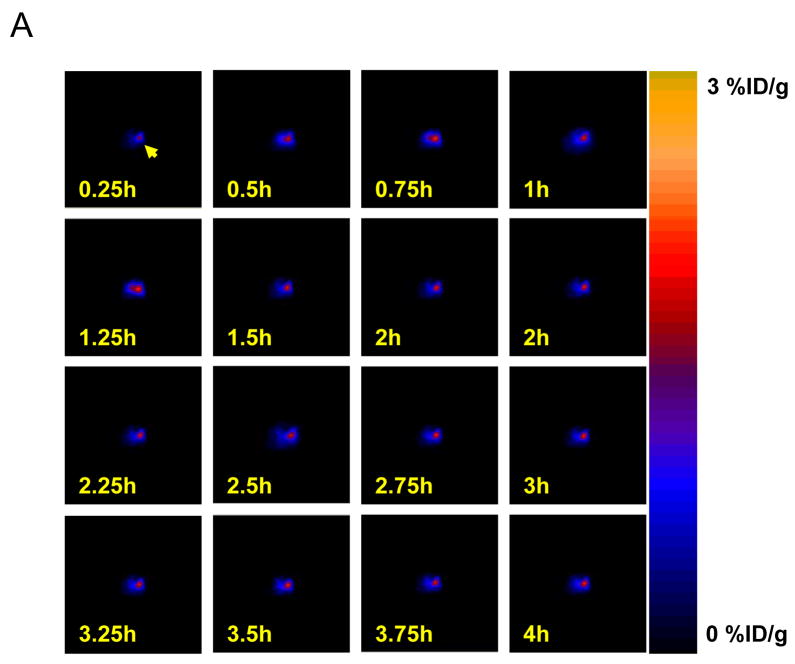
Pharmacokinetics of [18F]-FHBG tracer uptake and tracer retention in mouse heart
In order to determine the time-activity curve of [18F]-FHBG uptake in the heart, both α-MHC-Cre transgenic mice and Cre-negative control mice were injected with Ad-loxP-Neo-PA-loxP-HSV1-tk-PA. Serial [18F]-FHBG scans were performed at 15-min time intervals for 4 hours. Representative coronal images at different time points after administration of the tracer are shown in Figure 3A. The cardiac uptake of [18F]-FHBG in α-MHC-Cre mice was 2.32±0.26, 3.22±0.44, 3.21±0.36, 3.19±0.35, and 2.06±0.07 %ID/g at 15 min, 1 hour, 2 hour, 3 hour, and 4 hour after injection, respectively (P<0.01 vs. control). In contrast, control animals uniformly have background activity levels of around 0.05±0.02 %ID/g (Figure 3B). Peak activity was observed during the 1- to 2-hour period post tracer injection with a signal-to-background ratio (heart-to-lung ratio) ratio of 6.70±0.14 at 60 min after injection. This pattern of increased %ID/g at later time points is also consistent with findings by other investigators (14,17).
Figure 3.
Kinetics of [18F]-FHBG myocardial tracer uptake. (A) Micro PET horizontal slices of representative α-MHC-Cre transgenic mouse at 0 to 4 hours after intravenous injection of PET reporter probe. (B) Time-activity curves of [18F]-FHBG tracer uptake in both α-MHC-Cre mice and control mice 2 days after intramyocardial injection of Ad-loxP-Neo-PA-loxP-HSV1-tk-PA.
Longitudinal imaging of Cre-loxP mediated gene expression in the heart
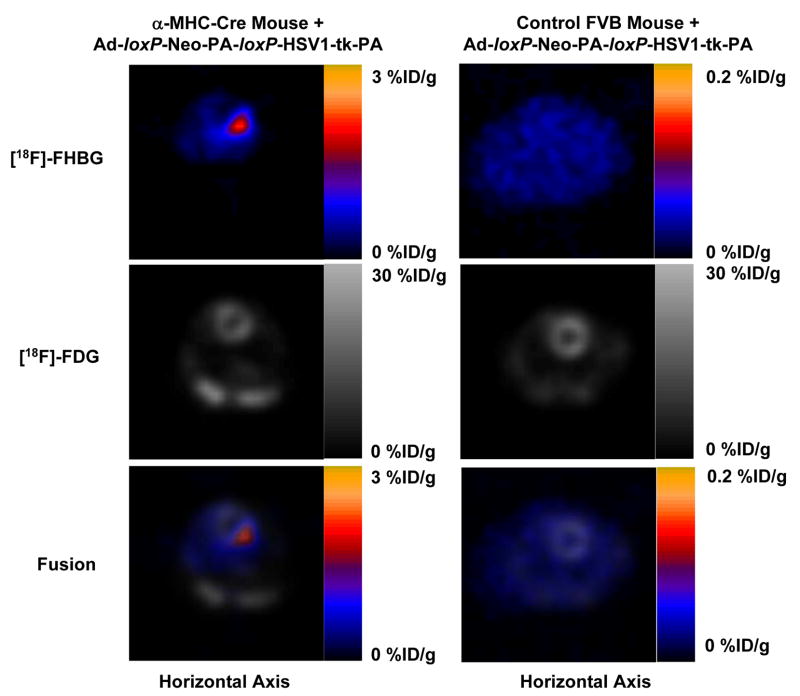
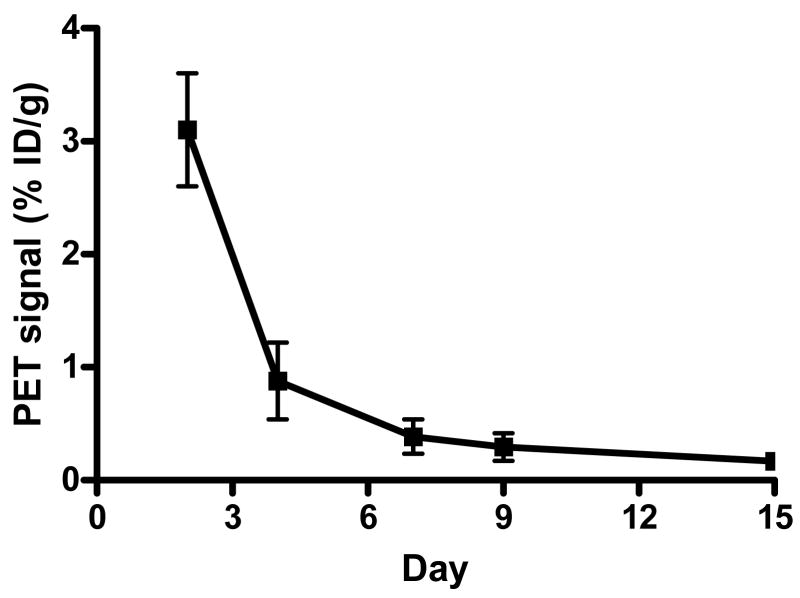
In order to demonstrate the magnitude and duration of Cre-loxP mediated gene expression in the heart, microPET imaging of [18F]-FHBG uptake was performed on days 2, 4, 7, 9, and 15 in both transgenic mice and control mice. Figure 4A shows that two days after adenoviral injection, the %ID/g in the heart of MHC-Cre mice was 3.20±0.51 compared to background activity of 0.05±0.02 in control mice (P<0.01). For [18F]-FDG imaging, the %ID/g was typically 20–30 %ID/g for both groups of mice, depending on the anesthetic and fasting state (18–20). Fusion of the [18F]-FHBG and [18F]-FDG images show the activity of HSV1-tk expression at the anterolateral wall. Thus, in the control mice, there was no excision of the loxP-Neo-PA-loxP sequence to activate HSV1-tk gene expression. In the transgenic mice, the Cre-loxP mediated activation of HSV1-tk gene expression can be monitored noninvasively over an extended period of time by repeated PET imaging. However, the HSV1-tk activity decreases progressively from day 4 (0.88±0.20) to day 7 (0.39±0.09) to day 9 (0.29±0.07) and to day 14 (0.17±0.03 %ID/g) (Figure 4B).
Figure 4.
Micro PET image of Cre-loxP mediated HSV1-tk expression in the heart. (A) In the α-MHC-Cre transgenic mouse, intramyocardial injection of adenovirus carrying pCAG-loxP-Neo-PA-loxP-HSV1-tk-PA led to significant cardiac expression of HSV1-tk reporter gene. The activity can be expressed as %ID/g of [18F]-FHBG reporter probe (top row), overlaid with the whole heart [18F]-FDG image (middle row), which provide a detailed anatomic location of the cardiac gene expression at the anterolateral wall (bottom row). Injection of the same adenovirus into control mouse heart did not conditionally activate HSV1-tk gene expression, resulting in background [18F]-FHBG signals. (B) Conditional activation of HSV1-tk gene expression could be imaged repetitively for >2 weeks. However, there was significant drop off of signal activity from day 2 to day 15.
Validation of in vivo activation of HSV1-tk with traditional in vitro assays
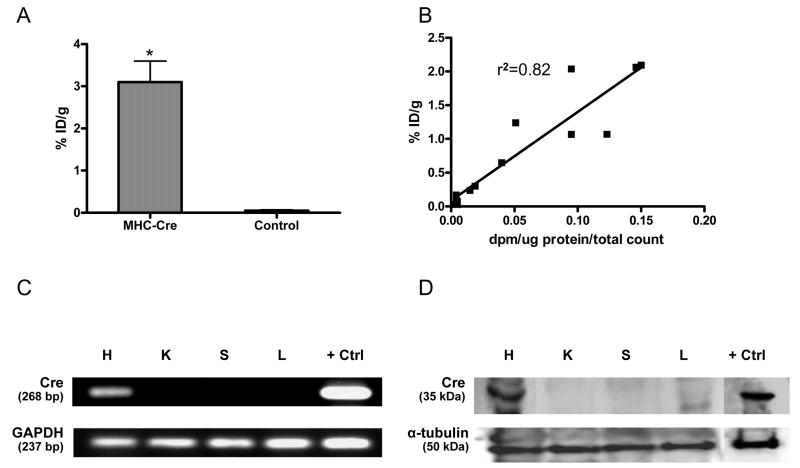
In order to substantiate our cardiac molecular imaging results, we sacrificed the animals at different time points following PET imaging. At day 2, the in vitro HSV1-TK enzyme activity was significantly higher in the myocardium of α-MHC-Cre transgenic compared to Cre-negative control mice, which is consistent with our imaging data (P<0.01) (Figure 5A). Likewise, there was a robust correlation (r2=0.82) between in vitro enzyme activity (dpm/μg protein/total count) and in vivo image (%ID/g) at all time points of sacrifice (Figure 5B). This suggests that in vivo cardiac PET gene imaging can be used in lieu of or in parallel with traditional in vitro assays. The expression of Cre was also examined by RT-PCR in heart, kidney, spleen, and liver samples of α-MHC-Cre transgenic and control mice. As expected, Cre messenger RNA was present only within the myocardium of α-MHC-Cre transgenic mice but not in other tissues (Figure 5C). Finally, Western blot analysis confirmed the presence of cardiac Cre proteins in α-MHC-Cre transgenic but not in other tissues (Figure 5D).
Figure 5.
Validation of in vivo imaging with traditional in vitro assays. (A) Enzyme assay shows significantly higher levels of HSV1-TK activity in explanted hearts from α-MHC-Cre mice compared to control mice at day 2 (*P<0.01 vs. control). (B) A robust correlation also exists between imaging signal (%ID/g) and enzyme assays (dpm/μg protein/total count) at all time points. (C) Representative RT-PCR shows the presence of Cre mRNA in the heart tissues of α-MHC-Cre mice but not in kidney, spleen, or liver tissues. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, serving as an internal control, was detected in all tissue samples. (D) Representative Western blot shows the presence of Cre protein in the heart of α-MHC-Cre mouse but not in kidney, spleen, or liver tissues. Detection of α-tubulin protein served as a loading control in the Western blot assays. Cre-positive control samples for both RT-PCR and Western blot were obtained from H9c2 cells transfected with α-MHC-Cre plasmid using lipofectamine.
DISCUSSION
Over the past decade, remarkable progress in recombinant DNA technology has enabled the development of molecular and cellular treatments for coronary artery disease. The field of cardiac gene therapy in particular has evolved from in vitro studies to pre-clinical testing to multi-center trials (21). One of the key issues in successfully implementing gene therapies in the clinical setting is to be able to regulate gene expression very tightly and consistently as and when it is needed (22). The ability to switch transgenes on and off would be of paramount importance not only when the therapy is no longer needed, but also in the case of the development of adverse side effects to the therapy. Co-development of noninvasive techniques for monitoring these events in vivo could provide significant values. In this context, we have demonstrated for the first time that a silenced PET reporter gene (HSV1-tk) carried in a replication-deficient adenovirus can be conditionally activated in cardiac tissue using the Cre-loxP system in living animals. The in vivo imaging results were further corroborated by traditional in vitro assays using RT-PCR, Western blots, and enzyme assays. The duration of gene expression was ~2 weeks due to host cellular immune response against the adenoviral vector, consistent with previous studies (17,23,24). Our results are also consistent with two separate imaging studies involving conditional gene activation in the liver (13) and tumors (25) of transgenic mice. Taken together, these proof-of-principle experiments illustrate the potential of molecular imaging for monitoring conditional gene activation and gene deletion in the cardiovascular system.
The ideal type of inducible gene activation or deletion systems should have the following characteristics: (i) no leaky transgene activity when the system is off; (ii) high levels of transcriptional activity when the system is activated; (iii) no impact on normal biological processes of the animal; (iv) specific induction of targeted cells (no mosaic expression); (v) sustained induction over long periods; (vi) induction agents that are readily available, inexpensive, and easy to administer; and (vii) the ability to reverse the process (1). One of the most well developed biological tools that best meet these criteria is the Cre-loxP recombination system. The Cre-loxP is a useful method for conditional gene expression which allows spatial and temporal control via tissue specific promoters or ligand-inducible promoters (7,26–28). The selective alteration of the genome using Cre recombinase to target the rearrangement of genes flanked by loxP recognition sequences has required the use of two separate genetic constructs, one containing Cre and the other containing the gene of interest flanked by loxP sites. Recently, the development of inducible, tissue-specific activation (or inactivation) of gene expression in a single vector has been reported (29). This approach may be applicable for targeted gene therapy in the future. However, despite their obvious advantages, there are no examples of regulatable gene expression systems in human clinical trial so far. This in part is due to the continuing challenges of adenoviral vector toxicity and host immunogenicity that remain to be resolved in the field of gene therapy (21).
In patients with ischemic heart disease, several clinical trials have relied on the injection of vascular endothelial growth factor (VEGF) gene (30) or fibroblast growth factor (FGF) gene (31) that are driven by a constitutive CMV promoter. Because the gene expression is always active, investigators are unable to control the gene expression once or if therapeutic effects have been achieved. Indeed, unregulated and continuous expression of VEGF has been shown to be associated with a high rate of death and formation of endothelial cell-derived intramural vascular tumors in animal models (32). Thus, we and others propose that a safer version of gene therapy vector may involve the therapeutic gene flanked by loxP sites that can be inactivated if necessary (33,34). This molecular event can then be monitored in vivo using the HSV1-tk reporter gene placed downstream of the loxP sites.
To date, most of the cardiac Cre-loxP conditional recombination studies have relied on the use of conventional reporter genes such as lacZ, GFP, or luciferase for static postmortem analysis (6–8,35–38). For example, Iwatate el al. injected adenovirus carrying Cre recombinase gene into these reporter mice and found robust expression of lacZ in myocardial tissues (35). In contrast, PET imaging offers distinct advantages for characterizing, visualizing, and quantifying biological processes at the cellular and molecular levels within living subjects (17,39–41). PET imaging is highly sensitive because it can detect picomolar to nanomolar (10−12 – 10−9 mol/L) concentrations of radiolabeled reporter probes. Since the absolute levels of radioisotopes can be measured with tracer kinetic modeling, PET can be used to quantify the underlying biochemical processes (39). PET imaging is tomographic so relatively precise location of gene expression can be identified within the myocardium. Because of these favorable characteristics, molecular imaging with PET has already been advanced into monitoring and management of cancer patients with glioblastomas (42) and hepatic carcinomas (43). Although similar clinical studies involving cardiac imaging have yet to be conducted, the feasibility of tracking the location, magnitude, and duration of cardiac transgene expression in large animal pig models was recently demonstrated by Bengel and colleagues using clinical PET scanners (17). This raises the possibility that PET imaging may one day be used to monitor patients receiving cardiac gene therapy. In the meantime, further investigation and validation will be needed to evaluate the optimal gene delivery vector, PET reporter gene, PET reporter probe, and patient profiles (33).
In conclusion, the Cre-loxP recombinase expression system has been widely used for creation of transgenic mice, gene targeting, and gene rescue in diverse biological systems (5). Besides basic research application, the same setup may be an invaluable asset for silencing therapeutic gene expression (by placing it in between floxed sites) after a certain clinical endpoint has been achieved in gene therapy trials (21,34). With further development and validation, we believe PET imaging for examining gene expression, signal transduction, and cellular metabolism may translate to the clinical realm in the future.
Acknowledgments
This work was supported in part by grants from NHLBI, AHA, and ACCF/GE (JCW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–55. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 2.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- 3.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci U S A. 1988;85:5166–70. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Y, Sauer B. Conditional gene knockout using Cre recombinase. Mol Biotechnol. 2001;17:269–75. doi: 10.1385/MB:17:3:269. [DOI] [PubMed] [Google Scholar]
- 5.Chien KR. To Cre or not to Cre: the next generation of mouse models of human cardiac diseases. Circ Res. 2001;88:546–9. doi: 10.1161/01.res.88.6.546. [DOI] [PubMed] [Google Scholar]
- 6.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–79. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minamino T, Gaussin V, DeMayo FJ, Schneider MD. Inducible gene targeting in postnatal myocardium by cardiac-specific expression of a hormone-activated Cre fusion protein. Circ Res. 2001;88:587–92. doi: 10.1161/01.res.88.6.587. [DOI] [PubMed] [Google Scholar]
- 8.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamoto S, Niwa H, Tashiro F, et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470:263–8. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- 10.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–5. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claycomb WC, Lanson NA, Jr, Stallworth BS, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan MG, Ooi LL, Aw SE, Hui KM. Cloning and identification of hepatocellular carcinoma down-regulated mitochondrial carrier protein, a novel liver-specific uncoupling protein. J Biol Chem. 2004;279:45235–44. doi: 10.1074/jbc.M403683200. [DOI] [PubMed] [Google Scholar]
- 13.Sundaresan G, Paulmurugan R, Berger F, et al. MicroPET imaging of Cre-loxP-mediated conditional activation of a herpes simplex virus type 1 thymidine kinase reporter gene. Gene Ther. 2004;11:609–18. doi: 10.1038/sj.gt.3302194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyagawa M, Anton M, Haubner R, et al. PET of cardiac transgene expression: comparison of 2 approaches based on herpesviral thymidine kinase reporter gene. J Nucl Med. 2004;45:1917–23. [PubMed] [Google Scholar]
- 15.Wu JC, Inubushi M, Sundaresan G, Schelbert HR, Gambhir SS. Positron emission tomography imaging of cardiac reporter gene expression in living rats. Circulation. 2002;106:180–3. doi: 10.1161/01.cir.0000023620.59633.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambhir SS, Bauer E, Black ME, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci U S A. 2000;97:2785–90. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengel FM, Anton M, Richter T, et al. Noninvasive imaging of transgene expression by use of positron emission tomography in a pig model of myocardial gene transfer. Circulation. 2003;108:2127–33. doi: 10.1161/01.CIR.0000091401.26280.A0. [DOI] [PubMed] [Google Scholar]
- 18.Fueger BJ, Czernin J, Hildebrandt I, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47:999–1006. [PubMed] [Google Scholar]
- 19.Toyama H, Ichise M, Liow JS, et al. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol. 2004;31:251–6. doi: 10.1016/S0969-8051(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 20.Verberne HJ, Sloof GW, Beets AL, Murphy AM, van Eck-Smit BL, Knapp FF. 125I-BMIPP and 18F-FDG uptake in a transgenic mouse model of stunned myocardium. Eur J Nucl Med Mol Imaging. 2003;30:431–9. doi: 10.1007/s00259-002-0999-7. [DOI] [PubMed] [Google Scholar]
- 21.Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 22.Goverdhana S, Puntel M, Xiong W, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JC, Chen IY, Wang Y, et al. Molecular imaging of the kinetics of vascular endothelial growth factor gene expression in ischemic myocardium. Circulation. 2004;110:685–91. doi: 10.1161/01.CIR.000013815302213.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–15. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons SK, Meuwissen R, Krimpenfort P, Berns A. The generation of a conditional reporter that enables bioluminescence imaging of Cre/loxP-dependent tumorigenesis in mice. Cancer Res. 2003;63:7042–6. [PubMed] [Google Scholar]
- 26.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–6. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–9. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 28.Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–5. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaczmarczyk SJ, Green JE. A single vector containing modified cre recombinase and LOX recombination sequences for inducible tissue-specific amplification of gene expression. Nucleic Acids Res. 2001;29:E56–6. doi: 10.1093/nar/29.12.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grines CL, Watkins MW, Helmer G, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 31.Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–83. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 32.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 33.Wu JC, Yla-Herttuala S. Human gene therapy and imaging: cardiology. Eur J Nucl Med Mol Imaging. 2005;32 (Suppl 2):S346–57. doi: 10.1007/s00259-005-1897-6. [DOI] [PubMed] [Google Scholar]
- 34.Heine HL, Leong HS, Rossi FM, McManus BM, Podor TJ. Strategies of conditional gene expression in myocardium: an overview. Methods Mol Med. 2005;112:109–54. doi: 10.1007/978-1-59259-879-3_8. [DOI] [PubMed] [Google Scholar]
- 35.Iwatate M, Gu Y, Dieterle T, et al. In vivo high-efficiency transcoronary gene delivery and Cre-LoxP gene switching in the adult mouse heart. Gene Ther. 2003;10:1814–20. doi: 10.1038/sj.gt.3302077. [DOI] [PubMed] [Google Scholar]
- 36.Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ Res. 2004;94:e56–60. doi: 10.1161/01.RES.0000125294.04612.81. [DOI] [PubMed] [Google Scholar]
- 37.Schindehutte J, Fukumitsu H, Collombat P, et al. In vivo and in vitro tissue-specific expression of green fluorescent protein using the cre-lox system in mouse embryonic stem cells. Stem Cells. 2005;23:10–5. doi: 10.1634/stemcells.2004-0163. [DOI] [PubMed] [Google Scholar]
- 38.Sohal DS, Nghiem M, Crackower MA, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–5. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 39.Phelps ME. Inaugural article: positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci U S A. 2000;97:9226–33. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyagawa M, Anton M, Wagner B, et al. Non-invasive imaging of cardiac transgene expression with PET: comparison of the human sodium/iodide symporter gene and HSV1-tk as the reporter gene. Eur J Nucl Med Mol Imaging. 2005;32:1108–14. doi: 10.1007/s00259-005-1854-4. [DOI] [PubMed] [Google Scholar]
- 41.Bengel FM, Anton M, Avril N, et al. Uptake of radiolabeled 2′-fluoro-2′-deoxy-5-iodo-1-beta-D-arabinofuranosyluracil in cardiac cells after adenoviral transfer of the herpesvirus thymidine kinase gene: the cellular basis for cardiac gene imaging. Circulation. 2000;102:948–50. doi: 10.1161/01.cir.102.9.948. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs A, Voges J, Reszka R, et al. Positron-emission tomography of vector-mediated gene expression in gene therapy for gliomas. Lancet. 2001;358:727–9. doi: 10.1016/s0140-6736(01)05904-9. [DOI] [PubMed] [Google Scholar]
- 43.Penuelas I, Mazzolini G, Boan JF, et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology. 2005;128:1787–95. doi: 10.1053/j.gastro.2005.03.024. [DOI] [PubMed] [Google Scholar]