Abstract
Objective:
Estradiol (E2) and its metabolites [2-hydroxyestrone (2-OHE1) and 16α-hydroxyestrone (16α-OHE1)] are believed to curtail greater oxidative stress found in the development and progression of disease conditions including atherosclerosis. We related estrogen levels to F2a-isoprostane levels, a biomarker of oxidative stress.
Design and Participants:
Data were from 1647 women, aged 47-57 years, participating in the 5th annual follow-up of the Study of Women's Health Across the Nation (SWAN), a study of the menopausal transition.
Measurements:
Serum E2 and urinary 2-OHE1 and 16α-OHE1 concentrations were assayed by ELISA while urinary F2a-isoprostanes were assayed by EIA.
Results:
F2a-isoprostane concentrations were elevated in women who smoked, a behavior associated with increased oxidative stress, but not in stages of the natural menopause. Mean F2a-isoprostane concentrations among premenopausal and postmenopausal women who smoked were 1082 and 1064 pg/mL, respectively, values double those in premenopausal (343 pg/mL) and postmenopausal (379 pg/mL) non-smoking women. 2-OHE1 and F2a-isoprostane concentrations were positively and highly related [partial correlations ρY|X = 0.44 and ρY|X = 0.43 in premenopausal and postmenopausal women, respectively]. Likewise, 16α-OHE1 concentrations were positively and highly correlated with F2a-isoprostane concentrations [ρY|X = 0.52 and ρY|X = 0.59 in premenopausal and postmenopausal women, respectively]. E2 was significantly correlated with F2a-isoprostanes only in postmenopausal women [ρY|X = 0.20]. Associations were adjusted for age, body mass index, race/ethnicity, lipids, physical activity level, and alcohol consumption.
Conclusions:
This study does not support the commonly-held hypothesis that levels of endogenous estradiol or its estrone metabolites favorably modify oxidative stress by decreasing F2a-isoprostane levels.
Keywords: isoprostanes, estrogen, oxidative stress, antioxidant, smoking
INTRODUCTION
It has been hypothesized that physiological levels of estradiol (E2) and estrogen metabolites, including 2-hydroxyestrone (2-OHE1) and 16α-hydroxyestrone (16α-OHE1), favorably modulate oxidative stress, potentially attenuating the development and progression of disease, including atherosclerosis and diabetes.1,2 While the concept of estrogens as antioxidants is intriguing and might explain differential rates or timing of cardiovascular disease between men and women, the evidence remains controversial.
High, non-physiological levels of estradiol were associated with the in vitro inhibition of low-density lipoprotein (LDL) oxidation,3,4 a prominent feature of atherosclerosis.5,6 Some,7,8 but not all studies9,10 have shown that, in postmenopausal women, estrogen replacement diminished circulating oxidized LDL levels. Studies of oxidative stress according to sex difference or menopause transition to postmenopause are too few in number to clarify the role of estrogens as antioxidants. Ide et al. reported that urinary levels of F2a-isoprostanes are increased in healthy young men compared to premenopausal women;11 however, two other studies12,13 reported F2a-isoprostane levels were about 20% lower, not higher, in men as compared to women. Helmersson et al. reported increased levels in postmenopausal women compared to premenopausal women.14
Using F2a-isoprostane production as a marker for identifying oxidative stress,15,16 we examined the potential antioxidant associations of estrogens considering stages of menopause. We hypothesized that an important antioxidant role for E2, 2-OHE1 and 16α-OHE1 would be reflected in strong, inverse correlations with F2a-isoprostanes. We postulated that the associations between estrogens and the F2a-isoprostanes would be similar in premenopausal women and postmenopausal women using hormone therapy (HT), and dissimilar to the associations in postmenopausal women without HT use. These hypotheses were tested while adjusting for lipids, body mass index, race/ethnicity, smoking, exercise, vitamin E and iron supplementation and substantial alcohol consumption.17
METHODS
Sampling and Study Population
Data are from 1647 enrollees at the 5th annual follow-up visit of the Study of Women's Health Across the Nation (SWAN), a community-based, longitudinal study of the menopausal transition.18 Eligible participants were from Boston MA, Chicago IL, the Detroit area MI, Los Angeles CA, Hudson County NJ, Oakland CA, and Pittsburgh PA. At baseline, eligibility criteria for the longitudinal cohort were: age 42 to 52 years; intact uterus and at least one ovary; no current use of estrogens or other medications known to affect ovarian function; at least one menstrual period in the 3 months before enrollment; and, self-identification as a member of one of the 5 eligible racial/ethnic groups. Sites enrolled a Caucasian and non-Caucasian sample including African-American women in Boston, Chicago, the Detroit area, and Pittsburgh, and Japanese, Chinese, and Hispanic women in Los Angeles, Oakland, and New Jersey, respectively. Two sites, Chicago and Newark, did not collect annual urine samples from enrollees so the only contribution for these sites comes from 44 women at the Chicago site who provided urine samples for a SWAN substudy. There was Institutional Review Board approval for the study protocol at each study site and all subjects gave informed consent.
Assays
This study is limited to specimens collected during the fifth annual follow-up examination and available through the SWAN Repository. When possible, early morning blood draws occurred in the fasted state during days 2-5 of the early follicular phase of the menstrual cycle. Urine specimens for the F2a-isoprostane analyses and estrone metabolites were collected on the days of blood draw (usually on days 2-5 of the early follicular phase and, by protocol, prior to 11 AM). At follow-up visit 05, 96% of samples were acquired fasting; 97% were acquired before 11 AM; and, 53% of samples were collected in days 2-5 of the follicular phase of the menstrual cycle. Assays were completed in the CLASS laboratory, University of Michigan.
Serum estradiol (E2) concentrations were measured with a modified, off-line ACS:180 (E2-6) immunoassay (Bayer Diagnostics Corp, Norwood, MA). The assay range for E2 was calibrated to address the lower values associated with the follicular phase or with the postmenopause. Inter- and intra-assay coefficients of variation were 10.6% and 6.4%, respectively, over the assay range 20-500 pg/ml.
2-OHE1 and 16α-OHE1 were assayed by enzyme immunoassay (ESTRAMET™) in triplicate.19 The inter- and intra-assay coefficients of variation were less than 10% for each analyte. Because urinary forms of 2-OHE1 and 16α-OHE1 are found as 3-glucuronides or 3,3,16-glucuronides, it was necessary to remove these sugars to achieve recognition sites for the monoclonal antibodies. Therefore, the estrogens were deconjugated from glucuronic acid and sulfate using a mixture of ß-glucuronidase and arylsulphatase enzyme isolated from the snail Helix Pomatia. The assay range was 0.6 to 40.0 ng/mL.
Samples were purified with F2a-isoprostane affinity columns according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI). Samples were applied to the column, washed with buffer and eluted with 95% ethanol. Following evaporation of solvent, the dried samples were diluted 1:10 with 0.1 M phosphate buffer and assayed using the F2a-isoprostane enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI). The standard curve range was 3.9-500 pg/mL. Samples were read at 405 nm in a 96-well microplate reader. The post-extraction intra- and inter-assay coefficients of variation were 14.4% (51.2 pg/mL, n=83 pairs) and 17.5% (51.2 pg/mL, n=85), respectively; the intra-assay coefficient of variation was 5.8% (n=1707 pairs).
Lipids were assayed at the Medical Research Laboratories, Highland Heights, Kentucky, USA, which is certified by the Centers for Disease Control Lipid Standardization Part III program20. All lipid and lipoprotein fractions were analyzed on EDTA treated plasma. Total cholesterol and triglycerides were analyzed by enzymatic methods and HDL-C was isolated using heparin-2M manganese chloride. Participants were classified as diabetic based on a fasting glucose ≥ 126 mg/dL [measured using a hexokinase-coupled reaction on a Hitachi 747-200 (Boehringer Mannheim Diagnostics, Indianapolis, IN)] or self-reported use of insulin or oral medications for diabetes.
Physical and interview-based measures
Weight (kg) was measured using balance beam and electronic scales; height (m) was measured using stadiometers. The data were used to calculate body mass index (BMI; weight in kilograms divided by height squared in meters).
Women were designated as pre- or perimenopausal unless they had 12 months of amenorrhea and then they were designated as postmenopausal. Because of their limited number, women who had surgical menopause were excluded from these data analyses. Use of exogenous hormones (HT) was self-reported.
Data on smoke exposure were obtained from a self-administered questionnaire incorporating American Thoracic Society questions21 and validated questions on passive exposure.22 Baseline leisure physical activity was based on the frequency, intensity, and duration of the two sports or exercise activities reported by the participant as occurring in the year prior to assessment.23 Estimates of usual annual dietary data were obtained using a modified Block food frequency (FFQ) interviewer-assisted questionnaire,24 administered in four languages, English, Spanish, Chinese, or Japanese. Serving sizes were reported with the assistance of food models. Genistein and daidzein intakes25 and polyphenol levels were evaluated.26 Total alcohol consumption per day was categorized as none, less than ½ serving (< 15 gm), and at least ½ serving (≥ 15 gm). Vitamin and mineral supplement use was self-reported.
Statistical approach
Analyses were conducted using SAS Version 9.1 software. Information was excluded from the baseline enrollment of 3302 women for the following reasons: attrition by the 5th annual follow-up visit (n=696) and unavailable estrogen metabolite data because the protocol at two sites did not include annual urine collection (n=571). Of the remaining 2035 participants, 42 women had surgical menopause and 346 did not have repository specimens available for analyses. This final analytical sample consisted of 371 (23%) African-American, 837 (51%) Caucasian, 202 (12%) Chinese, and 237 (14%) Japanese women.
Median and interquartile ranges (IQR) were calculated for continuous variables, including 2-OHE1 and 16α-OHE1, serum E2, F2a-isoprostanes, lipids, BMI, and age. All continuous variables except for age were log-transformed to satisfy the normality assumption in parametric testing. Analyses of covariance and multiple variable regression analyses were the primary statistical approaches. Type III sums of squares were used to estimate the adjusted correlations. Adjusted group means were identified using analyses of covariance and comparison of the pair-wise differences. P-values were two-sided at an α < 0.05. Ninety-five percent confidence intervals (CI) were used to describe statistical probabilities of associations with ANCOVA models.
Data were stratified by current smoking behavior because smoking behavior had a strong main effect and interaction with menopause status. Further, a potential statistical interaction with race could not be tested because smoking was infrequently reported by Chinese or Japanese women, making full statistical adjustment or interaction testing inappropriate. Data were adjusted for age, BMI, race, physical activity level, and lipids as continuous variables. Adjustment also included vitamin E supplement use, iron supplement use, and diabetes as categorical variables. There were no associations with intakes of dietary fat, dietary genistein, daidzein, or polyphenol consumption.
RESULTS
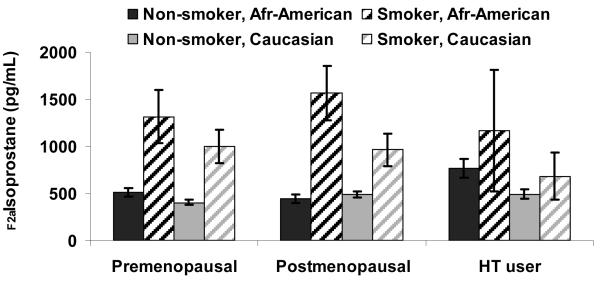
The overall median age at the 5th annual visit was 51 years, with premenopausal women slightly younger (50 years) and postmenopausal women slightly older (53 years). The median BMI was 26.5 kg/m2 (Table 1). F2a-isoprostane concentrations were significantly and markedly different in smokers vs. non-smokers before and after adjusting for covariates. As seen in Figure 1, F2a-isoprostane concentrations in smokers were almost double the mean level of non-smokers in both African-American and Caucasian women, with few smokers among Chinese and Japanese women.
Table 1.
Characteristics of 1647 participants at the 5th follow-up SWAN examination (excluding women with hysterectomy)
| Attribute | Pre- and Perimenopausal |
Postmenopausal | HT users | |||
|---|---|---|---|---|---|---|
| Median | IQR* | Median | IQR | Median | IQR | |
| Age (yr) | 50.0 | 48 - 51 | 53.0 | 51 - 54 | 52.0 | 50 - 54 |
| BMI (kg/m2) | 26.5 | 23 - 32 | 27.0 | 23 - 32 | 26.0 | 23 - 31 |
| Waist circumference (cm) | 84.2 | 74 - 97 | 86.3 | 74 - 100 | 83.3 | 74 - 96 |
| Estradiol (pg/mL) | 38.4 | 20 - 79 | 13.2 | 10 - 21 | 39.5 | 22 - 68 |
| 2-hydroxyestrone (pg/mL) | 8.7 | 5 - 15 | 5.3 | 3 - 9 | 17.3 | 8 - 41 |
| 16α-hydroxyestrone(pg/mL) | 6.1 | 4 - 10 | 4.3 | 3 - 6 | 9.0 | 5 - 18 |
| F2a-isoprostanes (pg/mL) | 433 | 235 - 785 | 413 | 231 - 747 | 495 | 262 - 872 |
| Follicle-stimulating hormone (mIU/mL) | 22.5 | 12 - 54 | 87.3 | 62 - 116 | 46.2 | 25 - 71 |
| Race | N | % | N | % | N | % |
| African-American | 205 | 22.7 | 99 | 26.2 | 66 | 18.1 |
| Caucasian | 445 | 49.3 | 168 | 44.4 | 223 | 61.1 |
| Chinese | 114 | 12.7 | 54 | 14.3 | 34 | 9.3 |
| Japanese | 138 | 15.3 | 57 | 15.1 | 42 | 11.5 |
| Smoking | ||||||
| Current | 99 | 11.0 | 50 | 13.2 | 37 | 10.1 |
| Not smoking | 803 | 89.0 | 328 | 86.8 | 328 | 89.9 |
| Exercise | ||||||
| Lowest 2 quintiles | 332 | 37.6 | 157 | 42.9 | 134 | 37.8 |
| Middle 2 quintiles | 373 | 42.2 | 136 | 37.2 | 144 | 40.7 |
| Highest quintile | 179 | 20.2 | 73 | 19.9 | 76 | 21.5 |
| Alcohol consumption | ||||||
| More than ½ serving / day | 163 | 18.8 | 70 | 19.0 | 90 | 25.1 |
| Less than ½ serving / day | 268 | 31.0 | 94 | 25.5 | 105 | 29.3 |
| None | 434 | 50.2 | 204 | 55.5 | 163 | 45.6 |
| Diabetes | ||||||
| Yes | 69 | 8.2 | 42 | 11.6 | 34 | 9.7 |
| No | 767 | 91.8 | 320 | 88.4 | 317 | 90.3 |
IQR = Interquartile range
Figure 1.
F2a-isoprostanes concentrations among premenopausal women, postmenopausal women, and hormone therapy users, by race and smoking status at the 5th follow-up SWAN examination
Non-smokers
There were no statistically significant differences observed in mean F2a-isoprostane concentrations in premenopausal vs. postmenopausal stages (343 vs. 379 pg/mL, P=0.09) among women who did not smoke (Table 2). The mean F2a-isoprostane concentration in women using HT (425 pg/mL) was 24% higher than the mean for premenopausal women (P=0.0002).
Table 2.
Mean (SEM) of estrogen levels (pg/mL) by smoking and menopausal status at the 5th follow-up SWAN examination, adjusted for covariates*
| Non-Smokers | Smokers | |||||
|---|---|---|---|---|---|---|
| Premenopausal N=803 |
Postmenopausal N=328 |
Using HT N=328 |
Premenopausal N=99 |
Postmenopausal N=50 |
Using HT N=37 |
|
| Estradiol (pg/mL)* | 38.9 (2.4) | 14.7 (0.9) | 36.0 (1.5) | 35.7 (4.5) | 19.4 (3.2) | 46.1 (8.4) |
| 2-hydroxyestrone (pg/mL)* | 7.4 (0.3) | 4.9 (0.3) | 15.3 (0.9) | 12.5 (1.6) | 7.3 (1.2) | 29.8 (5.5) |
| 16α-hydroxyestrone (pg/mL)* | 5.4 (0.2) | 4.1 (0.02) | 9.5 (0.05) | 8.8 (1.1) | 6.5 (1.0) | 11.3 (2.0) |
| F2a-isoprostanes (pg/mL)* | 343 (12.4) | 379 (19.5) | 425 (21.9) | 1082 (139.6) | 1064 (174.1) | 871 (159.6) |
Adjusted for age, BMI, race, lipids, menopausal status, exercise, alcohol consumption, and diabetes
In non-smokers, the mean E2 concentrations in premenopausal women and HT users were similar (P=0.26) but were markedly lower (−62%, P<0.0001) in postmenopausal women as compared to premenopausal women, seen in Table 2.
The mean 2-OHE1 concentration in postmenopausal women was 34% lower (P<0.0001) than premenopausal women. Women using HT had a mean 2-OHE1 concentration 107% (P<0.0001) higher than premenopausal women.
Compared to premenopausal women, the mean 16α-OHE1 concentration was 24% (P<0.0001) lower in postmenopausal women and 76% (P<0.0001) higher in women using HT.
Smokers
Among smokers, mean F2a-isoprostane concentrations for women who were premenopausal, postmenopausal, or using HT were 1082, 1064, and 871 pg/mL, respectively. These values were not statistically different from each other.
Among smokers, the mean E2 concentration in premenopausal women was greater than postmenopausal women (35.7 vs. 19.1 pg/mL, P<0.004). E2 concentrations in women using HT were somewhat higher than premenopausal women (46.1 pg/mL, P=0.24).
Among smokers, mean 2-OHE1 concentrations for women who were premenopausal, postmenopausal, or using HT were 12.5, 7.3 and 29.8 pg/ml, respectively. These values were significantly different from each other. Among smokers, mean 16α-OHE1 levels for women who were premenopausal, postmenopausal, or using HT were 8.8 pg/mL, 6.5 pg/mL, and 11.3 pg/mL, respectively, values that were not statistically significantly different from each other (see Table 2).
Associations of estrogens with isoprostanes
As seen in Table 3, among non-smoking women, E2 concentrations were not significantly associated with F2a-isoprostanes in premenopausal women or those that used HT. In postmenopausal women, the partial correlation was ρY|X=0.20 (95% CI 0.08, 0.31). This and all subsequent partial correlations include adjustment for age, BMI, race, lipids, menopausal status, exercise, alcohol consumption, and diabetes.
Table 3.
Pearson (ρ, 95% confidence intervals) and partial Pearson correlations (ρY|X, 95% confidence intervals) between F2a-isoprostane concentrations and estrogen measures, by menopause and smoking status
| Premenopausal | Postmenopausal | Using hormones | ||||
|---|---|---|---|---|---|---|
| Nonsmokers | N=778 | N=323 | N=325 | |||
| Unadjusted | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI |
| Estradiol (pg/mL) | 0.13 | 0.06, 0.20 | 0.31 | 0.21, 0.41 | 0.04 | −0.07, 0.15 |
| 2-hydroxyestrone (pg/mL) | 0.43 | 0.37, 0.49 | 0.43 | 0.33, 0.51 | 0.34 | 0.24, 0.44 |
| 16α-hydroxyestrone (pg/mL) | 0.57 | 0.52, 0.62 | 0.60 | 0.53, 0.67 | 0.44 | 0.35, 0.52 |
| Adjusted* | ρY|X | 95% CI | ρY|X | 95% CI | ρY|X | 95% CI |
| Estradiol (pg/mL) | 0.08 | 0.00, 0.15 | 0.20 | 0.08, 0.31 | 0.02 | −0.09, 0.14 |
| 2-hydroxyestrone (pg/mL) | 0.44 | 0.38, 0.50 | 0.43 | 0.33, 0.52 | 0.39 | 0.29, 0.49 |
| 16α-hydroxyestrone (pg/mL) | 0.52 | 0.47, 0.57 | 0.59 | 0.51, 0.66 | 0.46 | 0.36, 0.54 |
| Smokers | N=87 | N=44 | N=33 | |||
| Unadjusted | ρ | 95% CI | ρ | 95% CI | ρ | 95% CI |
| Estradiol (pg/mL) | −0.007 | −0.22, 0.21 | 0.28 | −0.03, 0.55 | 0.04 | −0.31, 0.38 |
| 2-hydroxyestrone (pg/mL) | 0.22 | 0.01, 0.41 | 0.45 | 0.18, 0.66 | 0.49 | 0.18, 0.71 |
| 16α-hydroxyestrone (pg/mL) | 0.36 | 0.16, 0.53 | 0.62 | 0.40, 0.78 | 0.43 | 0.10, 0.67 |
| Adjusted* | ρY|X | 95% CI | ρY|X | 95% CI | ρY|X | 95% CI |
| Estradiol (pg/mL) | 0.10 | −0.15, 0.34 | 0.38 | −0.01, 0.68 | 0.10 | −0.43, 0.45 |
| 2-hydroxyestrone (pg/mL) | 0.28 | 0.04, 0.49 | 0.39 | 0.03, 0.66 | 0.55 | 0.16, 0.79 |
| 16α-hydroxyestrone (pg/mL) | 0.39 | 0.16, 0.58 | 0.64 | 0.35. 0.81 | 0.58 | 0.19, 0.81 |
Adjusted for age, BMI, race group, lipids, physical activity level, alcohol, and having diabetes.
In women who did not smoke, consistent, substantial and positive associations were observed between 2-OHE1 and F2a-isoprostane concentrations in women using hormones (ρY|X = 0.39), postmenopausal women (ρY|X = 0.43) and in premenopausal women (ρY|X = 0.44). 16α-OHE1 concentrations were highly and positively associated with F2a-isoprostane concentrations in premenopausal women (ρY|X=0.52), postmenopausal women (ρY|X = 0.59), and women using HT (ρY|X = 0.46), see Table 3.
Among women who smoked, F2a-isoprostane levels were not significantly associated with E2 concentrations. F2a-isoprostane levels were positively associated with 2-OHE1 concentrations in premenopausal women and in women who reported HT use [ρY|X=0.28 and 0.55, respectively], as well as in postmenopausal women [ρY|X=0.39] after adjustment for covariates. F2a-isoprostane levels were positively associated with 16α-OHE1 concentrations in premenopausal women (ρY|X= 0.39), postmenopausal women (ρY|X = 0.64), and women using HT (ρY|X = 0.58).
The patterns between estrogens and F2a-isoprostanes were similar even when data were stratified by race groups or BMI categories of obesity (data not shown).
DISCUSSION
It has been argued9 that estrogens could exert antioxidant actions in modifying F2a-isoprostane production due to the presence of an aromatic hydroxyphenol structure.27 However, these data do not support that hypothesis; indeed, the opposite may be true. While there was a statistically significant correlation between endogenous E2 and F2a-isoprostanes in postmenopausal women, the direction of the correlation was positive, not negative, and circulating levels of E2 were low. The same direction was observed with exogenous hormone use. The correlations of the estrone metabolites with F2a-isoprostanes were positive, not negative, and quite strong. Further, the strengths of these associations were not diminished following adjustment for race, menopause status, BMI, lipids, smoking behavior, vitamin E and iron supplement use, and level of alcohol consumption. This evidence led us to conclude that higher levels of these estrone metabolites were associated with greater oxidative stress and did not support our a priori hypothesis. However, these three aspects of estrogen status do not reflect all estrogen metabolites and it is possible that a non-measured analyte may have a relationship different than the very striking relationships we observed. For example, Tang et al. reported that 4-hydroyxestrone was a potent inhibitor of lipid peroxidation in a cell culture system28 and Seeger et al. reported that 2-methoxyestrone and 2-hydroxyestrone inhibited lipid peroxidation better than estradiol and 16a-hydroxyestrone in pooled samples from healthy individuals.29 Evaluation of measures of the range of estrogen metabolites in association with health-related states has been limited by the availability of reproducible assays that target specific metabolites and that can implemented in substantially-sizes populations.
Our conclusion that E2 and estrone metabolites were not acting like potent antioxidants is contingent on the integrity of the F2a-isoprostanes to serve as informative markers of oxidative stress. F2a-isoprostanes, compounds produced by the free radical-induced peroxidation of arachidonic acid,30,31 have been characterized as superior markers of the in vivo oxidative stress response.15,32-34 Further, elevated levels of F2a-isoprostanes are associated with behaviors or conditions thought to include oxidative stress such as smoking,35,36 alcohol consumption,17 hypercholesterolemia,37,38 diabetes,39 liver disease,40 breast cancer,41 and atherosclerotic lesions.42 Indeed, we showed that F2a-isoprostanes values were more than doubled in women who smoked. Additionally, F2a-isoprostanes levels were also correlated with HDL-c in pre- and post-menopausal women who did not smoke and with LDL in post-menopausal women who smoked (data not shown).
To better understand the relationship of estrogen and F2a-isoprostanes, we adjusted for or stratified by factors that had previously been identified as generating variation in F2a-isoprostane concentrations. In some7,8 but not all9,10 studies of postmenopausal women, estrogen therapy acted like an antioxidant by diminishing the level of circulating oxidized LDL. However, responses to HT may have been inconsistently observed because preparations included progestin products as well as estrogens. It has been reported that progesterone opposes the antioxidant actions of estrogen on plasma LDL oxidation in primates.33
Health-related and lifestyle behaviors may influence oxidative capacity34,43. We observed little modifying effect by diet of the association of estrogens with F2a-isoprostanes, based on intakes of genistein, daidzein, or total dietary fat. This is consistent with the findings of Block12 who reported a very modest association with fruits, and none with vegetables, dairy foods, grains, meats, or total fat intake. In contrast to diet, we identified a very strong association of oxidative stress with smoking behavior. A greater proportion of African-American and Caucasian women smoked, compared to the low proportion of smokers in Chinese and Japanese women, leading to greatly exaggerated mean F2a-isoprostane values for African-American and Caucasian women. Though we evaluated the role of passive smoking, we found only an association with personal smoking behavior. Women who reported more physical activity had lower F2a-isoprostane concentrations. Others have reported that endurance exercise is associated with increased plasma F2a-isoprostanes in dogs,44 horses,45 and humans.46
This study had a number of strengths and limitations. A substantial number of women were evaluated with representation from four racial/ethnic groups. While this was a cross-sectional study, it was nested in a longitudinal study of the menopausal transition, so that menopausal status and HT use were definable within the context of those longitudinal observations. In spite of these strengths, the cross-sectional design precluded our ability to establish temporal sequence between single-time measures of estrogens and F2a-isoprostanes index of oxidative stress. Study protocol dictated that serum and urine be collected in the early follicular phase of the menstrual cycle rather than in the later follicular phase or luteal phase when estrogen levels will be higher. As a result, we are describing that low estrogen levels did not suppress oxidative stress; however, this does not necessarily preclude the possibility that higher estrogen levels as seen later in the menstrual cycle might suppress oxidative stress. Initial criticism of the F2a-isoprostane measurement by EIA has been largely overcome by the use of affinity columns to prepare the samples for analyses as well as by the improvement in the assays, a procedure we applied in our assay procedures.47 EIA from eluted samples correlated very well with other methods, including gas chromatography-mass spectrometry.48,49 A major advantage of the urinary measurement of F2a-isoprostanes was that they are not formed by ex vivo autooxidation in urine and levels were stable even when urine was maintained at room temperature for as much as 10 days.50,51 Further, the concentration of F2a-isoprostanes was unchanged with storage at −20 degrees C.52,53 Values from repeated days of urinary excretion tended to be the same with low intra-subject coefficients of variation in persons whether they were healthy or diseased.11,32,36,51 Little diurnal variation is observed, and therefore, measurement of F2a-isoprostanes in a single early morning sample has been described as adequate to represent the daily isoprostane excretion in humans.52
In summary, these data indicate major differences in F2a-isoprostanes between smoking and non-smoking women across the menopause transition. However, there was no evidence that the serum measure of early follicular phase E2 or urinary estrone metabolite measures were acting like antioxidants; indeed, the strong adjusted and unadjusted positive correlations between the estrone metabolites and F2a-isoprostanes suggest an active metabolic environment associated with generation of free radicals. This study refutes the commonly-held hypothesis that levels of endogenous estradiol or its estrone metabolites favorably modify oxidative stress through a decrease in F2a-isoprostanes.
ACKNOWLEDGEMENTS
The Study of Women's Health Across the Nation (SWAN) and the SWAN Repository have grant support from the National Institutes of Health (NIH), Department of Health and Human Services (DHHS), through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants AG17104, AG017719, NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, AG17104). The estrogen metabolites and isoprostane data were the result of an estrogen metabolism grant for assay of urine samples from the SWAN Repository (AG17104, AG017719).
This report is based on samples from the SWAN Core Repository. If scientists are interested in developing studies based on this resource, a description of the SWAN Core and DNA Repositories and how to obtain access to the resources can be found at www.swanrepository.org.
REFERENCES
- 1.Praticò D, Rockach J, Lawson J, FitzGerald GA. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chemistry and Physics of Lipids. 2004;128:165–171. doi: 10.1016/j.chemphyslip.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems, resolutions, and preliminary results from nutritional supplementation studies. Free Radical Research. 1998;29:469–486. doi: 10.1080/10715769800300531. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga E, Rojas A, Villaseca P, Bianchi M, Arteaga A, Durán D. In vitro effect of estradiol, progesterone, testosterone, and of combined estradiol/progestins on low density lipoprotein (LDL) oxidation in postmenopausal women. Menopause. 1998;5:16–23. [PubMed] [Google Scholar]
- 4.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proceedings of the National Academy of Sciences USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.aWitztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. Journal of Clinical Investigation. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cracowski JL, Ormezzano O. Isoprostanes, emerging biomarkers and potential mediators in cardiovascular diseases. European Heart Journal. 2004;25:1675–1678. doi: 10.1016/j.ehj.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Sack MN, Rader DJ, Cannon RO., III Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women. Lancet. 1994;343:269–270. doi: 10.1016/s0140-6736(94)91117-7. [DOI] [PubMed] [Google Scholar]
- 8.Wakatsuki A, Ikenoue N, Sagara Y. Effects of estrogen on susceptibility to oxidation of low-density and high-density lipoprotein in postmenopausal women. Maturitas. 1998;28:229–234. doi: 10.1016/s0378-5122(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 9.Hermenegildo C, García-Martínez MC, Tarín JJ, Cano A. Inhibition of low-density lipoprotein oxidation by the pure antiestrogens ICI 182780 and EM-652 (SCH 57068) Menopause. 2002;9:430–435. doi: 10.1097/00042192-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Santanam N, Shern-Brewer, R, McClatchey R, Castellano PZ, Murphy AA, Voelkel S, Parthasarathy S. Estradiol as an antioxidant: incompatible with its physiological concentrations and function. Journal of Lipid Research. 1998;39:2111–2118. [PubMed] [Google Scholar]
- 11.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 12.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. American Journal of Epidemiology. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 13.Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 14.Helmersson J, Mattsson P, Basu S. Prostaglandin F2α metabolite and F2-isoprostane excretion rates in migraine. Clinical Science. 2002;102:39–43. [PubMed] [Google Scholar]
- 15.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 16.Roberts LJ, II, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radical Biology & Medicine. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 17.Hartman TJ, Baer DJ, Graham LB, Stone WL, Gunter EW, Parker CE, Albert PS, Dorgan JF, Clevidence BA, Campbell WS, Tomer KB, Judd JT, Taylor PR. Moderate alcohol consumption and levels of antioxidant vitamins and isoprostanes in postmenopausal women. European Journal of Clinical Nutrition. 2005;59:161–168. doi: 10.1038/sj.ejcn.1602051. [DOI] [PubMed] [Google Scholar]
- 18.Sowers MF, Crawford S, Sternfeld B, Morgenstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. Design, survey sampling and recruitment methods of SWAN: A multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; San Diego: 2000. pp. 175–188. [Google Scholar]
- 19.Klug TL, Bradlow HL, Sepkovic DW. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16α-hydroxyestrone in urine. Steroids. 1994;59:648–655. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 20.Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control–National Heart, Lung, and Blood Institute Lipid Standardization Program: an approach to accurate and precise lipid measurements. Clinical Laboratory Medicine. 1989;9:105–135. [PubMed] [Google Scholar]
- 21.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) American Review of Respiratory Disease. 1978;118:1–20. [PubMed] [Google Scholar]
- 22.Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. American Journal of Epidemiology. 1989;130:696–704. doi: 10.1093/oxfordjournals.aje.a115391. [DOI] [PubMed] [Google Scholar]
- 23.Sternfeld B, Ainsworth BE, Quesenbury CP., Jr. Physical activity patterns in a diverse population of women. Preventive Medicine. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 24.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. American Journal of Epidemiology. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 25.Reinli K, Block G. Phytoestrogen content of foods – a compendium of literature values. Nutrition and Cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 26.Sowers MR, Crawford S, McConnell DS, Randolph JF, Jr., Gold EB, Wilkin MK, Lasley B. Selected diet and lifestyle factors are associated with estrogen metabolites in a multi-racial/ethnic population of women. Journal of Nutrition. 2006;136:1588–1595. doi: 10.1093/jn/136.6.1588. [DOI] [PubMed] [Google Scholar]
- 27.Nathan L, Chaudhuri G. Antioxidant and prooxidant actions of estrogens: potential physiological and clinical implications. Seminars in Reproductive Endocrinology. 1998;16:309–314. doi: 10.1055/s-2007-1016289. [DOI] [PubMed] [Google Scholar]
- 28.Tang M, Abplanalp W, Ayres S, Subbiah MT. Superior and distinct antioxidant effects of selected estrogen metabolites on lipid peroxidation. Metabolism: Clinical & Experimental. 1996;45:411–414. doi: 10.1016/s0026-0495(96)90212-7. [DOI] [PubMed] [Google Scholar]
- 29.Seeger H, Mueck AO, Lippert TH. Effect of estradiol metabolites on the susceptibility of low density lipoprotein to oxidation. Life Sciences. 1997;61:865–868. doi: 10.1016/s0024-3205(97)00588-2. [DOI] [PubMed] [Google Scholar]
- 30.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., II A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Zwart, LL, Meerman JH, Commandeur JN, Vermeulen NP. Biomarkers of free radical damage applications in experimental animals and humans. Free Radical Biology & Medicine. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 32.Cracowski JL, Cracowski C, Bessard G, Pepin JL, Bessard J, Schwebel C, Stanke-Labesque, F, Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2001;164:1038–1042. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- 33.McKinney KA, Duell PB, Wheaton DL, Hess DL, Patton PE, Spies HG, Burry KA. Differential effects of subcutaneous estrogen and progesterone on low-density lipoprotein size and susceptibility to oxidation in postmenopausal rhesus monkeys. Fertility and Sterility. 1997;68:525–530. doi: 10.1016/s0015-0282(97)00237-9. [DOI] [PubMed] [Google Scholar]
- 34.Richelle M, Turini ME, Guidoux R, Tavazzi I, Métairon S, Fay LB. Urinary isoprostane excretion is not confounded by the lipid content of the diet. FEBS Letters. 1999;459:259–262. doi: 10.1016/s0014-5793(99)01259-4. [DOI] [PubMed] [Google Scholar]
- 35.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., II Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. New England Journal of Medicine. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 36.Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 37.Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni, G, Patrono C. In vivo formation of 8-epi-prostaglandin F2α is increased in hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:3230–3235. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 38.Reilly MP, Praticò D, Delanty N, DiMinno G, Tremoli E, Rader D, Kapoor S, Rokach J, Lawson J, FitzGerald GA. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–2828. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- 39.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. In vivo formation of 8-iso-prostaglandin F2α and platelet activation in diabetes mellitus: Effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 40.Pratico D, Iuliano L, Basili S, Ferro D, Camastra C, Cordova C, FitzGerald GA, Violi F. Enhanced lipid peroxidation in hepatic cirrhosis. Journal of Medical Investigation. 1998;46:51–57. [PubMed] [Google Scholar]
- 41.Kumar K, Thangaraju M, Sachdanandam P. Changes observed in antioxidant system in the blood of postmenopausal women with breast cancer. Biochemistry International. 1991;25:371–380. [PubMed] [Google Scholar]
- 42.Oguogho A, Kritz H, Wagner O, Sinzinger H. 6-oxo-PGF1α and 8-epi-PGF2α in the arterial wall layers of various species: a comparison between intact and atherosclerotic areas. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2001;64:167–171. doi: 10.1054/plef.2001.0257. [DOI] [PubMed] [Google Scholar]
- 43.Gopaul NK, Zacharowski K, Halliwell B, Änggard EE. Evaluation of the postprandial effects of a fast-food meal on human plasma F2-isoprostane levels. Free Radical Biology & Medicine. 2000;28:806–814. doi: 10.1016/s0891-5849(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 44.Hinchcliff KW, Reinhart GA, DiSilvestro R, Reynolds A, Blostein-Fujii, A, Swenson RA. Oxidant stress in sled dogs subjected to repetitive endurance exercise. American Journal of Veterinary Research. 2000;61:512–517. doi: 10.2460/ajvr.2000.61.512. [DOI] [PubMed] [Google Scholar]
- 45.Kirschvink N, Smith N, Fievez L, Bougnet V, Art T, Degand G, Marlin D, Roberts C, Genicot B, Lindsey P, Lekeux P. Effect of chronic airway inflammation and exercise on pulmonary and systematic antioxidant status of healthy and heaves-affected horses. Equine Veterinary Journal. 2002;34:563–571. doi: 10.2746/042516402776180223. [DOI] [PubMed] [Google Scholar]
- 46.Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radical Biology & Medicine. 2001;31:911–922. doi: 10.1016/s0891-5849(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 47.Proudfoot J, Barden A, Mori TA, Burke V, Croft KD, Beilin LJ, Puddey IB. Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation – A comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Analytical Biochemistry. 1999;272:209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- 48.Devaraj S, Hirany SV, Burk RF, Jialal I. Divergence between LDL oxidative susceptibility and urinary F2-isoprostanes as measures of oxidative stress in Type 2 diabetes. Clinical Chemistry. 2001;47:1974–1979. [PubMed] [Google Scholar]
- 49.Walsh SW, Vaughan JE, Wang Y, Roberts LJ., II Placenta isoprostane is significantly increased in preeclampsia. The FASEB Journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 50.Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin E on lipid peroxidation in healthy persons. JAMA: Journal of the American Medical Association. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 51.Praticò D, Barry OP, Lawson JA, Adiyaman M, Hwang SW, Khanapure SP, Iuliano L, Rokach J, FitzGerald GA. IPF2α -I: An index of lipid peroxidation in humans. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Ciabattoni G, Créminon C, Lawson J, FitzGerald GA, Patrono C, Maclouf J. Immunological characterization of urinary 8-epi-prostaglandin F2α excretion in man. Journal of Pharmacology and Experimental Therapeutics. 1995;275:94–100. [PubMed] [Google Scholar]
- 53.Ohashi N, Yoshikawa M. Rapid and sensitive quantification of 8-isoprostaglandin F2α in human plasma and urine by liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography. B, Biomedical Sciences and Applications. 2000;746:17–24. doi: 10.1016/s0378-4347(00)00201-2. [DOI] [PubMed] [Google Scholar]