Abstract
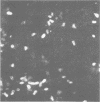
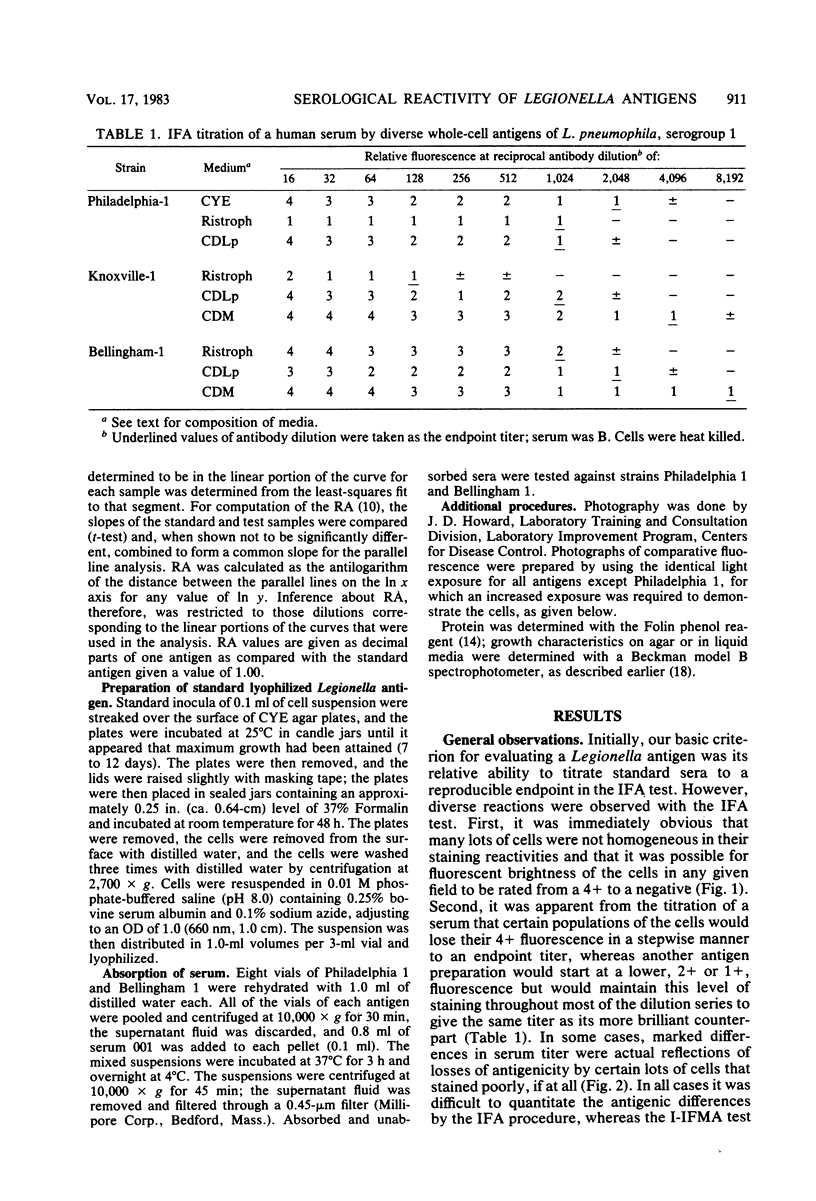
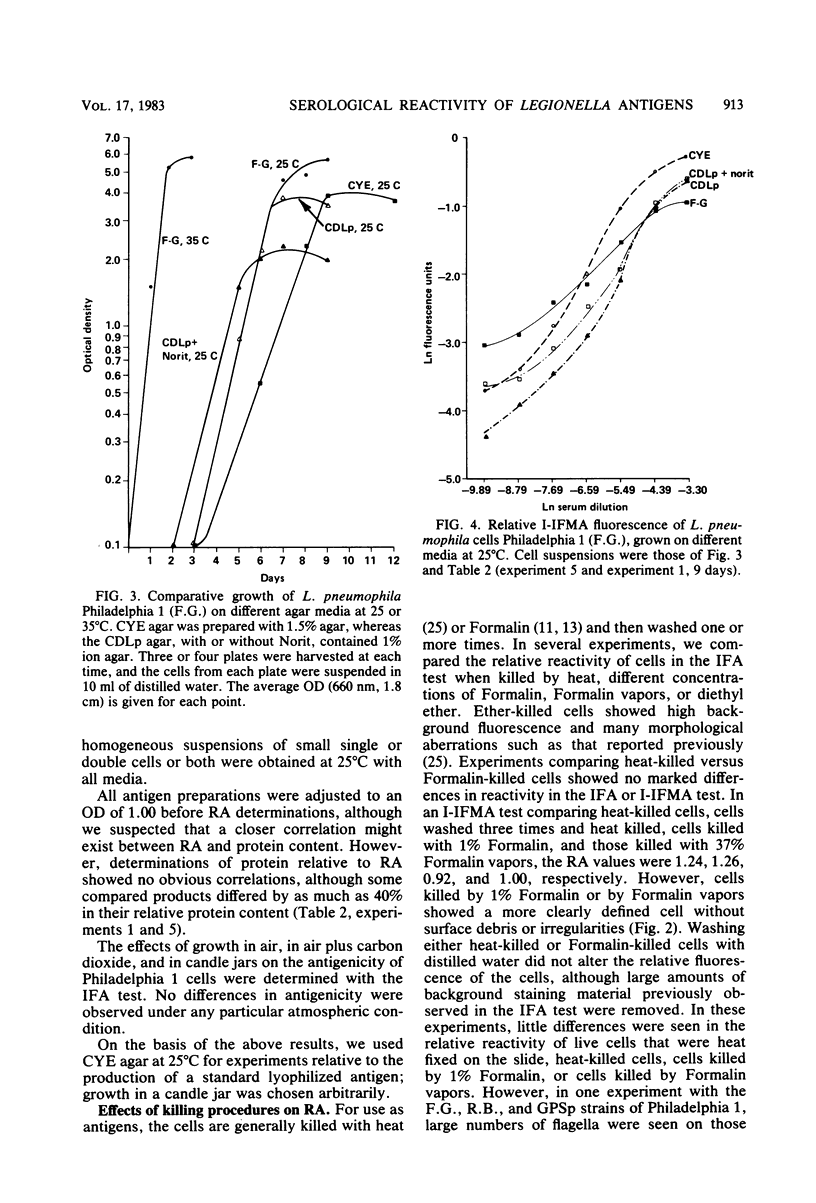
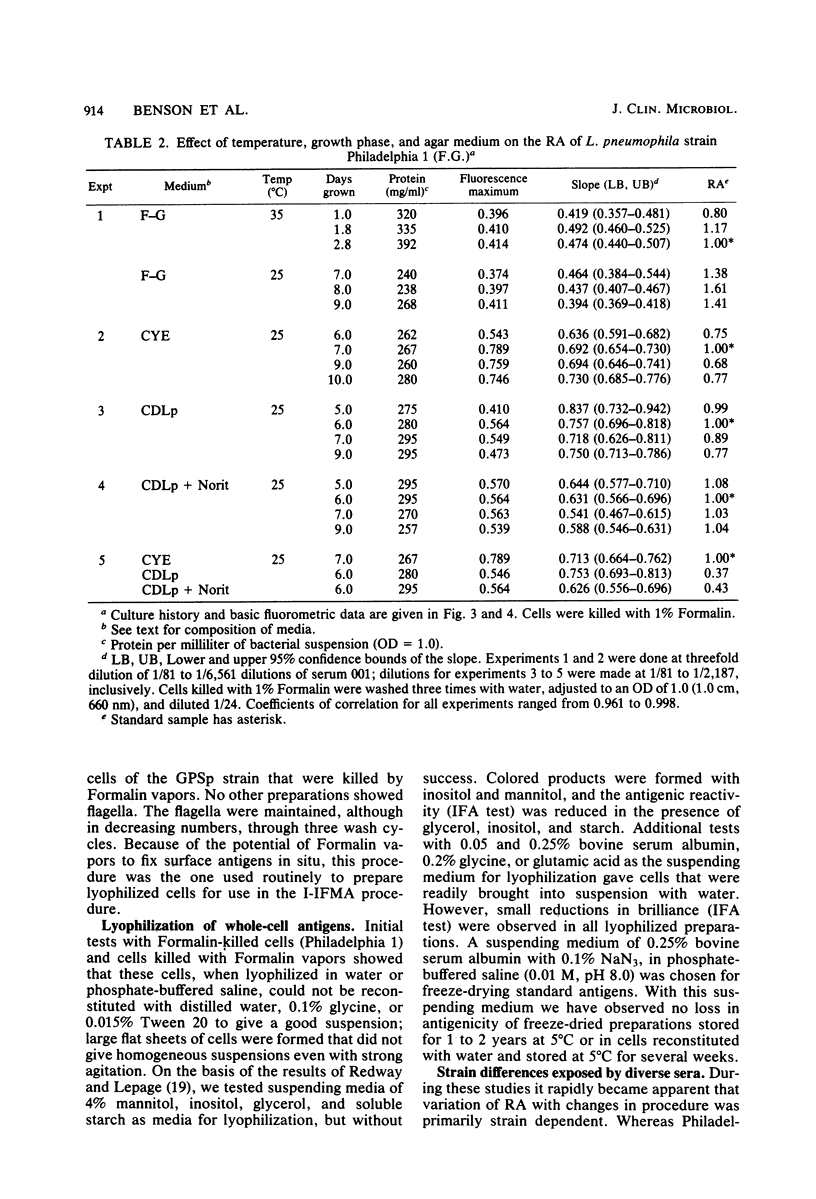
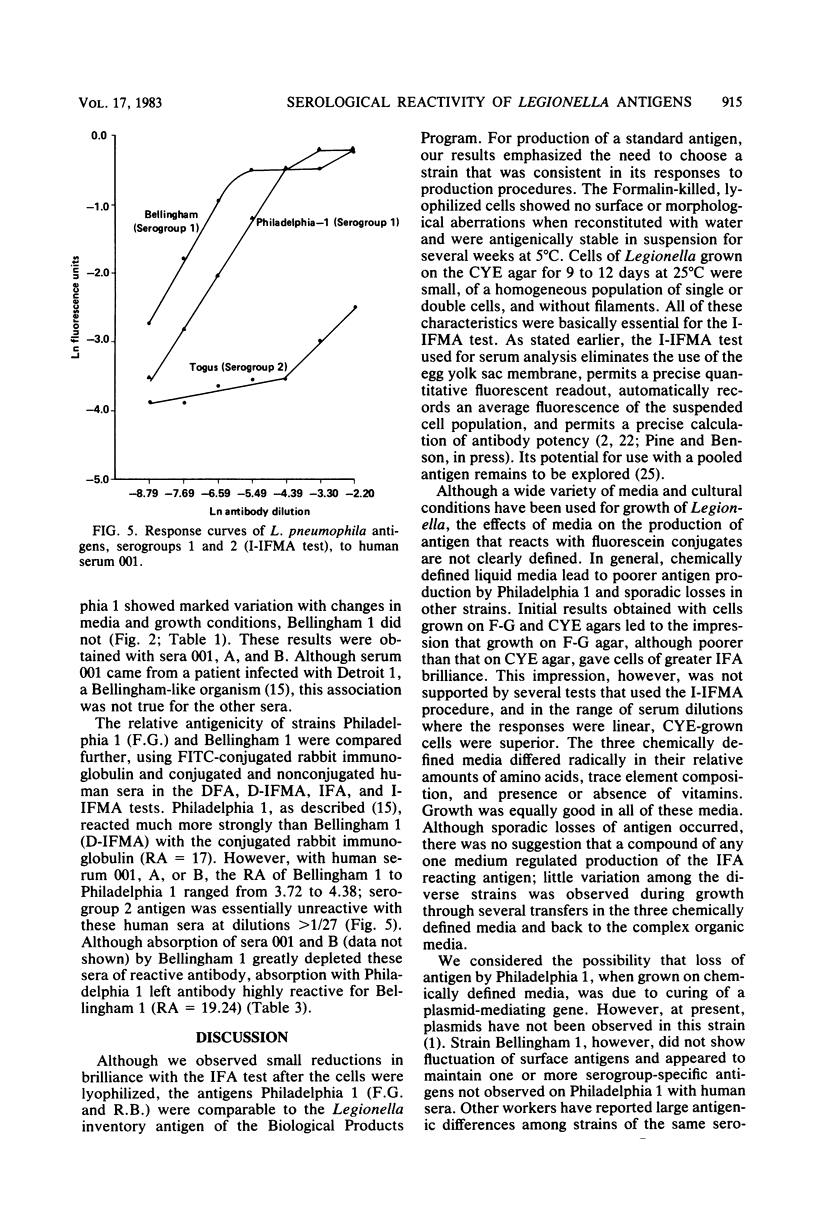
We examined several factors for their effects on the serological reactivity of Legionella antigens used for direct or indirect fluorescent-antibody tests. These factors included media, methods of killing, strain differences, and the nature of the reactivity with diverse human sera. The maximum serological reactivities were obtained with charcoal-yeast extract agar; the relative antigenicity of cells grown on a chemically defined medium could be fourfold less than those grown on the charcoal-yeast extract agar. Cells grown at 25 degrees C showed only small antigenic differences from those grown at 35 degrees C but had better morphological and staining characteristics. Cells killed by 1% Formalin or 37% Formalin vapors showed a 20% less relative antigenicity than those killed by heat, but their cell walls stained more clearly and they had fewer aberrations. As tested with several human sera, cells of Philadelphia 1 showed great variation in relative antigenicity with changes in media or methods of preparation; Bellingham 1 was quite stable under these same conditions. The data suggest that Bellingham 1 had serogroup 1-specific antigens, reactive with human sera, which were not present in Philadelphia 1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black C. M., Pine L., Reimer C. B., Benson R. F., Wells T. W. Characterization of antibody responses in legionellosis with an immunofluorometric assay. J Clin Microbiol. 1982 Jun;15(6):1077–1084. doi: 10.1128/jcm.15.6.1077-1084.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome C. V., Goings S. A., Thacker S. B., Vogt R. L., Beaty H. N., Fraser D. W. The Vermont epidemic of Legionnaires' disease. Ann Intern Med. 1979 Apr;90(4):573–577. doi: 10.7326/0003-4819-90-4-573. [DOI] [PubMed] [Google Scholar]
- Cherry W. B., Pittman B., Harris P. P., Hebert G. A., Thomason B. M., Thacker L., Weaver R. E. Detection of Legionnaires disease bacteria by direct immunofluorescent staining. J Clin Microbiol. 1978 Sep;8(3):329–338. doi: 10.1128/jcm.8.3.329-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Pasiecznik K. A., Yasui V. K., Meyer R. D. Susceptibility of Legionella spp. to mycinamicin I and II and other macrolide antibiotics: effects of media composition and origin of organisms. Antimicrob Agents Chemother. 1982 Jul;22(1):90–93. doi: 10.1128/aac.22.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. A., Johnson W., Helms C. M. Ultrastructural localization and protective activity of a high-molecular-weight antigen isolated from Legionella pneumophila. Infect Immun. 1981 Feb;31(2):822–824. doi: 10.1128/iai.31.2.822-824.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. A., Johnson W. Immunological and biochemical relationships among flagella isolated from Legionella pneumophila serogroups 1, 2, and 3. Infect Immun. 1981 Aug;33(2):602–610. doi: 10.1128/iai.33.2.602-610.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms C. M., Renner E. D., Viner J. P., Hierholzer W. J., Jr, Wintermeyer L. A., Johnson W. Indirect immunofluorescence antibodies to Legionella pneumophila: frequency in a rural community. J Clin Microbiol. 1980 Sep;12(3):326–328. doi: 10.1128/jcm.12.3.326-328.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W., Pesanti E., Elliott J. Serospecificity and opsonic activity of antisera to Legionella pneumophila. Infect Immun. 1979 Nov;26(2):698–704. doi: 10.1128/iai.26.2.698-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lattimer G. L., Cepil B. A. Legionnaires' disease serology. Effect of antigen preparation on specificity and sensitivity of the indirect fluorescent antibody test. J Clin Pathol. 1980 Jun;33(6):585–590. doi: 10.1136/jcp.33.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney R. M., Thacker L., Harris P. P., Lewallen K. R., Hebert G. A., Edelstein P. H., Thomason B. M. Four serogroups of Legionnaires' disease bacteria defined by direct immunofluorescence. Ann Intern Med. 1979 Apr;90(4):621–624. doi: 10.7326/0003-4819-90-4-621. [DOI] [PubMed] [Google Scholar]
- Ormsbee R. A., Peacock M. G., Lattimer G. L., Page L. A., Fiset P. Legionnaires' disease: antigenic peculiarities, strain differences, and antibiotic sensitivities of the agent. J Infect Dis. 1978 Aug;138(2):260–264. doi: 10.1093/infdis/138.2.260. [DOI] [PubMed] [Google Scholar]
- Pasculle A. W., Feeley J. C., Gibson R. J., Cordes L. G., Myerowitz R. L., Patton C. M., Gorman G. W., Carmack C. L., Ezzell J. W., Dowling J. N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980 Jun;141(6):727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- Pine L., George J. R., Reeves M. W., Harrell W. K. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1979 May;9(5):615–626. doi: 10.1128/jcm.9.5.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redway K. F., Lapage S. P. Effect of carbohydrates and related compounds on the long-term preservation of freeze-dried bacteria. Cryobiology. 1974 Feb;11(1):73–79. doi: 10.1016/0011-2240(74)90040-6. [DOI] [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Hutner S. H., George J. R., Harrell W. K. Metal requirements of Legionella pneumophila. J Clin Microbiol. 1981 Apr;13(4):688–695. doi: 10.1128/jcm.13.4.688-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Gowda S. Chemically defined medium for Legionella pneumophila growth. J Clin Microbiol. 1981 Jan;13(1):115–119. doi: 10.1128/jcm.13.1.115-119.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. S., Al-Hakiem M. H., Landon J. A review of fluoroimmunoassay and immunofluorometric assay. Ann Clin Biochem. 1981 Sep;18(Pt 5):253–274. doi: 10.1177/000456328101800501. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. W., Brake B. J. Formalin-killed versus heat-killed Legionella pneumophila serogroup 1 antigen in the indirect immunofluorescence assay for legionellosis. J Clin Microbiol. 1982 Nov;16(5):979–981. doi: 10.1128/jcm.16.5.979-981.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Cruce D. D., Broome C. V. Validation of Legionella pneumophila indirect immunofluorescence assay with epidemic sera. J Clin Microbiol. 1981 Jan;13(1):139–146. doi: 10.1128/jcm.13.1.139-146.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Fikes B. J., Cruce D. D. Indirect immunofluorescence test for serodiagnosis of Legionnaires disease: evidence for serogroup diversity of Legionnaires disease bacterial antigens and for multiple specificity of human antibodies. J Clin Microbiol. 1979 Mar;9(3):379–383. doi: 10.1128/jcm.9.3.379-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]