Abstract
Permethrin resistance has been reported worldwide and clinical failures to commercial pediculicides containing permethrin have likewise occurred. Permethrin resistance in head lice populations from the U.S. is widespread but is not yet uniform and the level of resistance is relatively low (~4–8 fold). Permethrin-resistant lice are cross-resistant to pyrethrins, PBO-synergized pyrethrins and to DDT. Nix®, when applied to human hair tufts following manufacture’s instructions, did not provide 100% control when assessed by the hair tuft bioassay in conjunction with the in vitro rearing system. Resistance to permethrin is due to knockdown resistance (kdr), which is the result of three point mutations within the α-subunit gene of the voltage-gated sodium channel that causes amino acid substitutions, leading to nerve insensitivity.
A three-tiered resistance monitoring system has been established based on molecular resistance detection techniques. Quantitative sequencing (QS) has been developed to predict the kdr allele frequency in head lice at a population level. The speed, simplicity and accuracy of QS made it an ideal candidate for a routine primary resistance monitoring tool to screen a large number of louse populations as an alternative to conventional bioassay. As a secondary monitoring method, real-time PASA (rtPASA) has been devised for a more precise determination of low resistance allele frequencies. To obtain more detailed information on resistance allele zygosity, as well as allele frequency, serial invasive signal amplification reaction (SISAR) has been developed as an individual genotyping method. Our approach of using three tiers of molecular resistance detection should facilitate large-scale routine resistance monitoring of permethrin resistance in head lice using field-collected samples.
Keywords: Pediculus humanus capitis, Permethrin resistance, Quantitative sequencing, real time PCR amplification of specific alleles, serial invasive signal amplification reaction
Determination of knockdown resistance
Relationship and medical importance of the human body and head louse
The human body louse, Pediculus humanus humanus (L.) and head louse, P. h. capitis, belong to the hemimetabolous order Phthiraptera (James and Harwood, 1969) and are so closely related that a long debate has occurred about whether they are subspecies or two separate species (Kitter et al., 2003;Yong et al., 2003)
Body lice pose a serious public health threat because they transmit several types of bacteria (Rickettsia prowazekii, Borrelia recurrentis and Borrelia recurrentis) that cause human diseases (epidemic typhus, louse-borne relapsing fever, five-day relapsing fever or trench fever) and these diseases have killed millions (Kelly et al., 2002). Since the advent of antibiotics, outbreaks are sporadic but in 1986, more than 50,000 people were infected with R. prowazekii in Burundi (Weiss, 1988). Thus, body lice represent a health risk during times of war, famine and social unrest (Piesman and Gage, 2000) and still serve as an important vector of re-emerging diseases in developed countries (Roux and Raoult, 1999). In fact, R. prowazekii is classified as a category B bioterrorism agent (Rotz et al., 2002).
Although not a significant disease vector, head lice represent a major economic and social concern in North America and worldwide because infestations are often associated with school-aged children, who miss substantial school days (12–24 million days) during this critical learning period (Williams et al., 2001). Income can also be lost by parents who must miss work to care for children who are sent home. Infestations often cause intense itching, which can injure skin allowing secondary infections to occur.
Pediculosis and its treatment
Pediculosis is the most prevalent parasitic infestation of humans (Raoult and Roux, 1999). Acquired immunity likely reduces louse density and impact on chronically exposed older juveniles and adults. However, louse outbreaks may constitute one of many opportunistic infestations associated with a depressed immune system (e.g., people with HIV). Infestation rates range from 6–12 million cases annually with 2.6 million households affected and 8% of all schoolchildren infested (Gratz, 1999). Most people find lice intolerable and repeatedly and prophylactically apply pediculicides (insecticides) without realizing the harm and lethality if misused or overused. Misapplications affect children in particular due to their small size and higher sensitivity to the toxic effects of pediculicides (NRC, 1993).
There are two ways to combat pediculosis: 1) proactive prevention or 2) post-infestation treatment. Although emphasis is increasingly on prevention (education) and physical removal (combing or shaving), the use of pediculicides is still the method of choice with U.S. pediculicide sales estimated at >$240 million per year with the over-the-counter (OTC) remedies accounting for > $90 million of this amount (Clark et al. 2009).
Commercial pediculicides that are currently in use consists of the over-the-counter (OTC) products and those available by prescription only (Rx). Currently, the OTC products comprise 70% of the pediculicides sold in the U.S. (Kwon et al., 2008). The OTC products consist of either pyrethroid insecticides (e.g., NIX®, 1% permethrin crème rinse, ~16% of the OTC market) or synergized pyrethrins (e.g., Rid®, Clear®, Pronto®, A-200®, ~0.3% with 3–4% piperonyl butoxide, ~7–35% of the OTC market). Because of the dominance of the pyrethrins/pyrethroids in the current pediculicide market, the remainder of this chapter will focus on these insecticides.
Pyrethrins are natural apolar esters that are solvent-extracted from the flower heads of Chrysanthemum cineraiaefolium. They are excellent insecticides with low toxicity to mammals due to their poor absorption and rapid metabolism to non-toxic metabolites. Unfortunately, they are also rapidly broken down in the environment by photolysis and hydrolysis, making them unsuitable as field-applied insecticides. Permethrin, a synthetic pyrethrin called a pyrethroid, was designed as a field-stable insecticide by removing sites of photolytic degradation, hydrolysis and xenobiotic metabolic attack. Both, along with DDT, share a common mode of action as agonists at voltage-gated sodium channels (VGSC) in the nervous systems of insects. Because of this, they both also share a common resistance mechanism with DDT, knockdown resistance (kdr), which is due to selective point mutations in the α-subunit gene of the VGSC that results in nerve insensitivity to these channel agonists.
To date, NIX® has failed clinically (Meinking et al. 2002) and 78–82% of patients treated with synergized pyrethrins (Rid® 36%, Pronto® 7% of the OTC market) or permethrin remained infested with 63% treating themselves more often or at higher doses than mandated by the manufacturer (Pray, 2003).
Insecticide resistance threatens the success of all vector control programs but is particularly problematic in the control of human lice for several reasons. First, they are obligate human blood feeders that are exposed to pediculicides at all stages because most infestations are treated (few if any refugia). Second, they have low mobility, short generational time, and high fecundity. Third, there are a small and dwindling number of effective commercial pediculicidal products, the majority of which share common chemistry and elicit cross-resistance. These aspects combine and produce a worst-case scenario for the evolution of resistance in human lice.
Resistance to pyrethrins/pyrethroids
Pyrethrum (the natural pyrethrin extract) has been used to control human ectoparasites, including lice, since ancient times. Pyrethroids, including d-phenothrin and permethrin, have been registered as pediculicides since the 1970s and have been widely available as OTC products since the 1980s. Despite these introductions of effective pediculicides, the number of cases of louse infestation has increased worldwide since the mid-1960s (Gratz, 1997) and dramatically since the mid-1990s (Meinking et al., 2002). Currently, resistance to the pyrethroid pediculicides has been reported in France (1994), followed rapidly by additional reports from Europe (Czech Republic/1995, UK/1999, and Denmark/2005), the Middle East (Israel/1995), North (United States/1999) and South (Argentina/1998) America, Asia (Japan/2003) and Australia/2003 (Clark et al., 2008).
Unfortunately, much of the published work on the level of insecticide resistance in head lice and on the possible mechanisms involved is largely anecdotal and unreplicated. This problem stemmed from the lack of laboratory colonies of insecticide-susceptible and -resistant head lice necessary to standardize bioassay protocols. Over the past 5 years, a series of papers from the Clark research group describes the development of an in vitro rearing system for human head lice that has resolved this dilemma (Takano-Lee et al., 2003; Takano-Lee et al., 2003; Yoon et al., 2006).
Permethrin resistance is widespread in the U.S. (MA, FL, TX, and CA) at a level of ~ 4–8 fold more than in the insecticide-susceptible EC-HL strain (Yoon et al., 2003). Additionally, the FL-HL and CA-HL strains show cross-resistance to pyrethrins and to PBO-synergized pyrethrins (~3 fold), and to DDT (~3 fold) (Yoon et al., 2004). These findings indicate that oxidative metabolism is not yet involved in pyrethrin/permethrin resistance in U.S. populations but target site insensitivity is likely.
More recently, the efficacies of three commercial pediculicidal products were assessed using the hair tuft bioassay in conjunction with the in vitro rearing system (Yoon et al., 2006). Treatments of 1% permethrin in acetone, Nix®, Rid®, or Pronto Plus® to hair tufts following manufactures’ instructions were highly efficacious (100% mortality) against the susceptible EC-HL strain but differentially efficacious (62–84% mortality) against the permethrin-resistant strain from south Florida (SF-HL) when examined eight days post-treatment. SF-HL that survived the first treatment received an identical second treatment eight days following the first treatment. Survivors (13–30%) developed into adults, mated, and females laid fertile eggs that hatched into first instars. These results confirm resistance to permethrin- and pyrethrin-based pediculicidal formulations and validate resistance previously determined using filter-paper contact bioassays with unformulated neat insecticides.
Mechanisms of kdr
Resistance mechanisms to permethrin
Lee et al., (2000) first hypothesized that permethrin resistance in head lice was most likely due to kdr based on the fact that permethrin-resistant head lice were cross-resistant to DDT, only showed low levels of metabolic synergism, and were tolerant to knockdown in behavioral bioassays.
Molecular analysis of kdr-type resistance in permethrin-resistant head lice
Using a homology probing strategy, cDNA fragments that spanned the IIS4~IIS6 region of para-orthologous head louse sodium channel α-subunit gene, where most of the mutations associated with kdr are located, were PCR amplified (Lee et al., 2000). Through molecular cloning and sequencing, two point mutations, T929I and L932F (T917I and L920F in the numbering of the head louse amino acid sequence), located in the IIS5 transmembrane segment of cDNAs from only the permethrin-resistant FL-HL and BR-HL populations, were identified.
Sequence comparisons of the complete open reading frame identified one additional novel mutation (M815I), which was located in the IIS1–2 extracellular loop of αsubunits from only permethrin-resistant head louse populations (Lee et al., 2003). Sequence analyses of cloned cDNA fragments and genomic DNA fragments from individual louse samples, both containing the three mutation sites, confirmed that all the mutations exist en bloc as a resistant haplotype. Northern blot analysis identified a single 7.2 kb transcript.
Functional significance of louse VGSC mutations
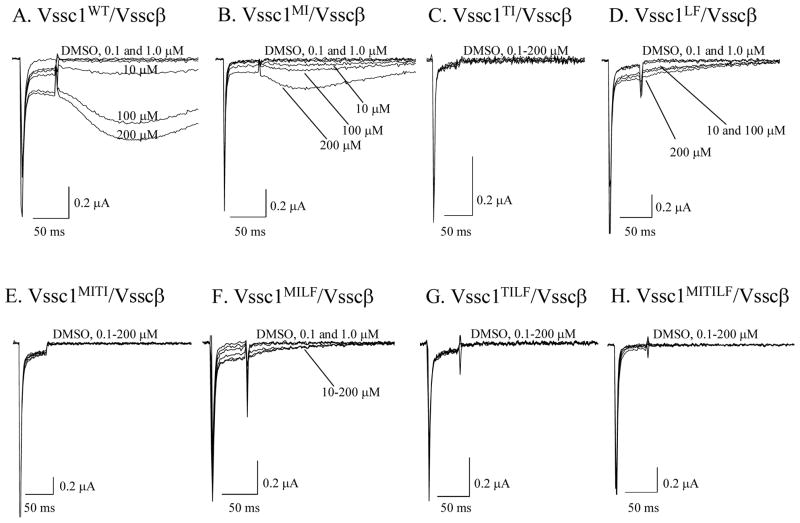
The three louse mutations were inserted in all combinations using site-directed mutagenesis at the corresponding amino acid sequence positions (M827I, T929I and L932F) of the house fly para-orthologous VGSC α-subunit (Vssc1WT) gene and heterologously co-expressed with the sodium channel auxiliary subunit of house fly (Vsscβ) in Xenopus oocytes (Yoon et al., 2008). The M827I and L932F mutations reduced permethrin sensitivity when expressed alone but the T929I mutation, either alone or in combination, virtually abolished permethrin sensitivity (Fig. 1). Thus, the T929I mutation is the principal cause of permethrin resistance in head lice via a kdr-type nerve insensitivity mechanism.
Fig. 1.
Comparative sodium current traces from Xenopus oocytes obtained before and after exposure to increasing concentrations of permethrin. Reproduced with permission from Yoon et al, 2008. Copyright 2008 Elsevier.
Use of kdr mutations in resistance management
The pyrethrins and pyrethroids are the major commercially available pediculicides in the current market. Extensive use of the pyrethrins/pyrethroids as OTC pediculicides has, however, been rapidly followed by resistance. Pyrethroid resistance in head louse populations appears to be widespread in both the United States and other countries but varies in intensity and is not yet uniform (Gao et al., 2003). Loss of these valuable pediculicides due to resistance would cause a serious problem in the control of pediculosis. Thus, establishment of proactive resistance management system is essential to maximize the life span of these major pediculicides.
Detection of the early phase of resistance is crucial to the long-term, efficient resistance management that can delay and reverse resistance development. Early resistance detection is often difficult, however, using conventional bioassay-based monitoring methods, particularly when resistance is recessive. In addition, collecting large numbers of live specimens, particularly in case of lice, is impractical and always difficult. To circumvent these limitations, various individual genotyping techniques for the detection of resistance allele frequencies using genomic DNA extracted from target insects have been employed as alternative resistance monitoring tool (Clark et al., 2001; Kim et al., 2005). For the efficient monitoring of head lice resistance in the field based on the kdr genotype, we have developed a sequence of molecular tools, including quantitative sequencing (QS), real-time PCR amplification of specific allele (rtPASA) and serial invasive signal amplification reaction (SISAR).
Molecular detection of kdr mutations for resistance monitoring
QS
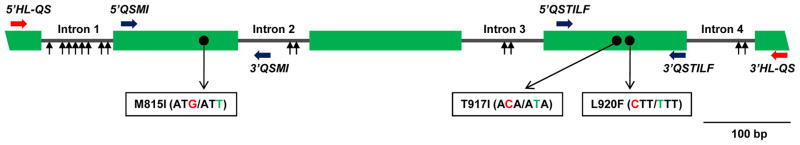
A QS protocol has been developed as a population genotyping method for the prediction of the VGSC mutation frequencies in head louse populations (Kwon et al., 2008). Briefly, a 908-bp genomic DNA fragment of the VGSC α-subunit gene, encompassing the three mutation sites (M815I, T917I and L920F), was PCR-amplified from individual genomic DNAs (Fig. 2). Once the genotypes and the intron sequences were confirmed, the PCR products with or without mutations were mixed to generate the standard DNA mixture templates in following molar ratios: 0:10, 1:9, 3:7, 5:5, 7:3, 9:1 and 10:0 (resistant allele : susceptible allele at each mutation site). Standard DNA template mixtures (10 ng) were sequenced using two sets of sequencing primers for the sense- and antisense-directional sequencing, respectively. The nucleotide signal intensities of the resistant and susceptible alleles at each mutation site were determined and signal ratios calculated using Equation 1.
Fig. 2.
Exon-intron structure of the 908-bp head louse VSSC genomic region that contains the M815I, T917I and L920F mutations. Shaded boxes and solid lines indicate exons and introns, respectively. Locations of three resistance mutations are marked with black circles. Vertical arrows indicate the approximate locations of intron polymorphisms. Horizontal arrows indicate the locations of the QS primers.
| Equation 1 |
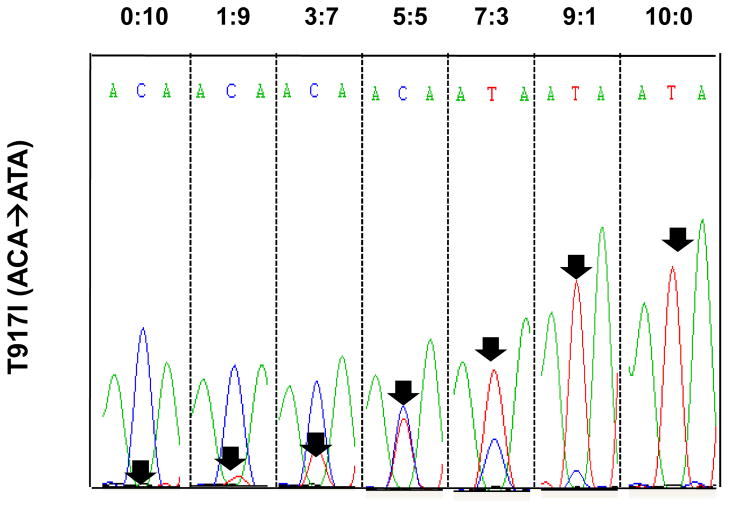
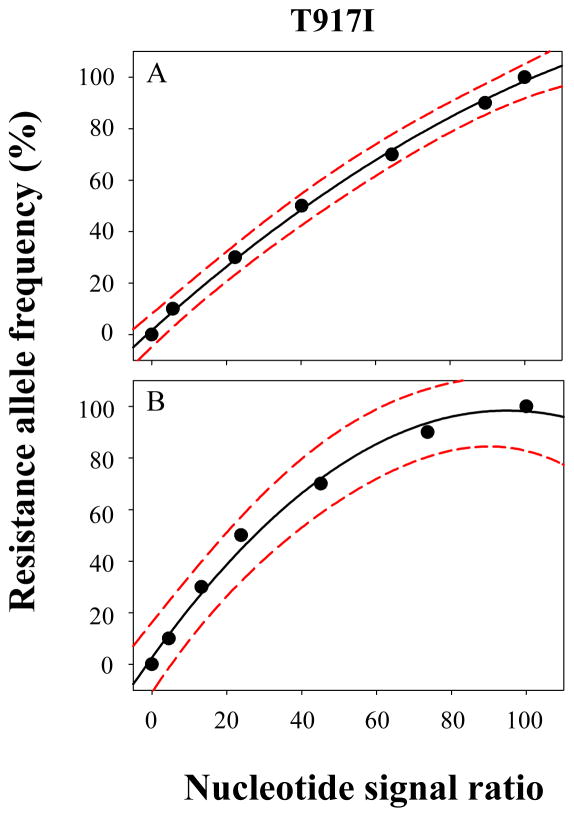
The signal ratios of template DNA mixtures were normalized by multiplying them with the normalization factor (signal ratio of the heterozygous DNA template/signal ratio of the 5:5 standard DNA template). A series of normalized signal ratios were plotted against the corresponding resistance allele frequencies and standard regression equations together with lower and upper prediction equations were generated for the estimation of resistance allele frequencies of unknown samples. As seen in Fig. 3, the signal intensity of the T917I mutation increased as the resistance allele frequency increased. When nucleotide signal ratios at the T917I mutation locus were plotted against the corresponding resistance allele frequencies and fitted to quadratic equations (Fig. 4), the resulting regression curve showed high correlation coefficients, demonstrating that the nucleotide signal ratio is highly proportional to the resistance allele frequencies (Table 1). Similar results were also obtained with other two mutations (data not shown) (Kwon et al. 2008)
Fig. 3.
Sequencing chromatograms of the standard template DNA mixtures with different ratios of resistant and susceptible alleles at the T917I mutation site. The numbers on the top of each column indicate the resistance allele ratios at each mutation site. The relative intensities of the resistance allele signals are indicated with arrows.
Fig. 4.
Resistance sequence signal ratios obtained from the sense (A)- and antisense (B)-directional sequencing were plotted with corresponding resistance allele frequencies at the T917F mutation site. Quadratic regression lines are indicated by solid black lines with the upper and lower 95% prediction lines indicated by dotted red lines. Nucleotide signal ratio (x-axis) was calculated as [resistant nucleotide signal/(resistant nucleotide signal + susceptible nucleotide signal)].
Table 1.
Quadratic regression and prediction equations of the resistant nucleotide signal ratio versus corresponding resistant allele frequency at the M815I, T917I and L920F mutation sites.
| Mutation | Sequencing direction | Regression equationa | Regression coefficient (r2) | 95% prediction equation |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| T917I | Sense | y =1.7+130.2x − 33.5x2 | 0.9985 | y =−4.6+131.8x − 35.3x2 | y =8.0+128.6x − 31.7x2 |
| Antisense | y =2.7+201.6x − 106.3x2 | 0.9928 | y =−10.5+205.1x − 111.8x2 | y =15.9+198.2x − 100.8x2 | |
In the equation, y and x represents the % resistance allele frequency and nucleotide signal ratio [resistant nucleotide signal/(resistant nucleotide signal + susceptible nucleotide signal)], respectively.
Using the lower and upper 95% prediction equations, the lower detection limits for the three resistance mutations were 4.4~9.7% (7.4 ± 2.7%), suggesting that QS can be employed as a preliminary resistance monitoring tool for the detection of resistance allele frequencies higher than ca. 7.4% at the 95% confidence level (Kwon et al., 2008).
Resistance allele frequencies in several head louse populations were predicted accurately by QS compared with those determined by individual sequencing and the resistance allele frequency in a population mostly composed of heterozygous individuals was likewise precisely estimated by QS (data not shown) (Kwon et al., 2008). Presence of phenotypically susceptible heterozygous lice in a field population would not be detected by a traditional bioassay due to the recessive trait of kdr (Yoon et al. 2003), emphasizing the importance of DNA-based detection of resistance allele frequency and zygosity in resistance monitoring.
Furthermore, there were no significant differences in predicted allele frequencies between the ‘pooled DNA’ (DNA extracted from individual louse specimens, then combined and prepared) and ‘pooled specimen DNA’ (DNA extracted and prepared from multiple louse specimens all at once) samples, as long as the size (developmental stage) and quality (collected and stored in the same way) of individual lice are identical (Kwon et al., 2008). This finding validates our approach of using the ‘pooled specimen DNA’ as the DNA template for QS-based population genotyping. The use of DNA extracted from combined multiple lice for QS greatly reduces the overall cost and effort as repetitive DNA extraction from individual lice is labor-intensive and costly.
rtPASA
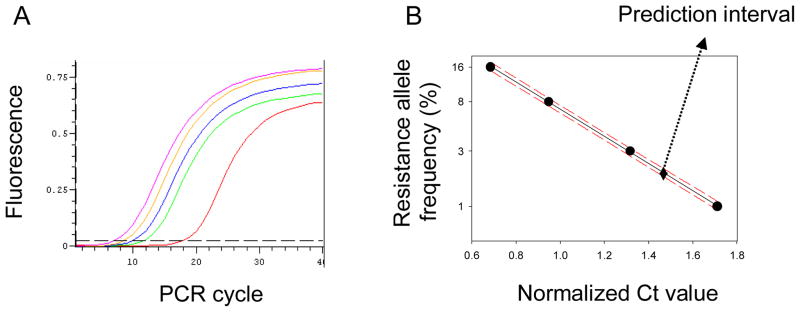
A rtPASA protocol was developed to detect the resistance allele frequencies below the lower detection limit of QS (ca. 7.4%). The same 908-bp genomic DNA fragment that was used as the template for QS, was PCR-amplified and used for rtPASA. The standard DNA mixture templates for rtPASA were prepared by combining the PCR-amplified fragments with and without the M815I, T917I and L920F mutations in following ratios: 0:100, 1:99, 3:97, 8:92, 16:84 (resistant allele : susceptible allele at each mutation site). Resistant allele-specific primers were designed to match the I917 and F920 alleles simultaneously (Fig. 5). Real-time PCR (rtPCR) with allele-specific primers (each 5 pM) and standard DNA mixture template (1 ng) was conducted using the Chromo 4™ real-time thermal cycler (Bio-Rad, Hercules, CA). Following rtPCR, threshold cycles (Ct) were determined from each amplification curve, and plotted against respective resistance allele frequencies. Standard linear regression lines were generated by plotting the log of the resistance allele frequency versus normalized Ct value using the SIGMA plot version 10.0 (Systat Software Inc.). The PCR amplification efficiency (E) was calculated from the slope of the standard curve using the following equation: E=10−1/slope.
Fig. 5.
Diagram of the exon 3 fragment in the VSSC genomic DNA region that encompasses the T917I and L920F mutation sites. Locations of the two mutations are marked with black circles. Horizontal arrows indicate the locations of the rtPASA primers.
The optimum conditions for the rtPASA were determined to be 64°C annealing temperature, 5 pM each primer, 1 ng DNA template (Fig. 6A). Based on the relationship between resistance allele frequencies (0~16%) and corresponding normalized Ct values, a linear regression line (y=−3.24x + 10.32, r2=0.999) was generated by converting the frequency to log values (Fig. 6B). The high regression coefficient demonstrated a very strong correlation between resistance allele frequency and Ct value. The resistance allele frequency of 1% was clearly distinguishable from 0% and considering the prediction interval, the lower detection limit was determined as 1.13% at the 95% confidence level.
Fig. 6.
Typical amplification patterns of rtPASA using the DNA templates containing 0~16% resistant alleles (A) and the regression line generated from the plot of normalized Ct value versus the log of resistance allele frequency (B). The regression line is indicated by a solid line with the upper and lower 95% prediction lines indicated by dashed lines in Panel B.
Using genomic DNA samples extracted from head louse populations collected from Ecuador, Thailand and Korea, the resistance allele frequencies determined by rtPASA were 8.0%, 0%, and 16.2%, respectively, which corroborated well with those determined by QS (5.2%, 1.5% and 11.7%) (data not shown) (Kwon et al., 2008).
SISAR
The SISAR technology is based on a fluorescence resonance energy transfer (FRET) detection format and uses structure-specific endonucleolytic cleavage by eubacterial DNA polymerases to detect single nucleotide polymorphisms (SNPs) (Hall et al., 2000). This high throughput genotyping method detects VGSC mutations associated with permethrin resistance in individual head lice and detailed protocols are described elsewhere (Kim et al., 2004).
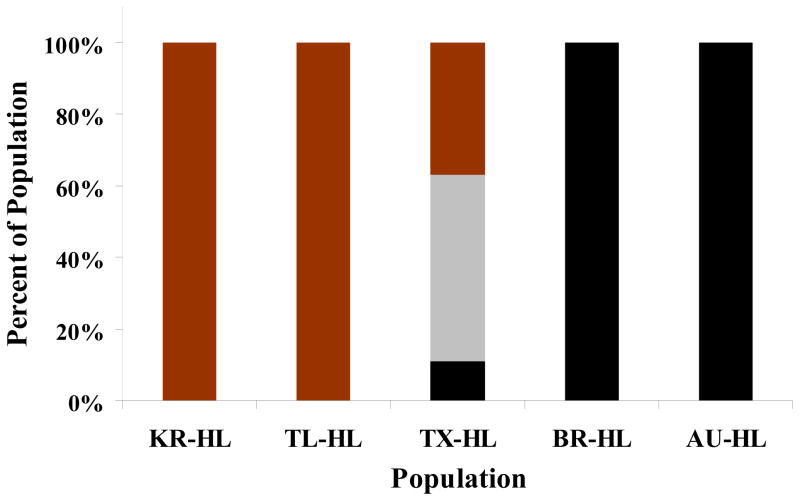
The genotypes of 27 individual head lice, collected from Mathis, TX (TX-HL), US, were analyzed by SISAR and compared with those determined by direct DNA sequencing. All SISAR kits for each mutation discriminated the genotypes with 100% accuracy (data not shown) (Kim et al., 2004). The frequencies of the resistant homozygous, heterozygous and susceptible homozygous alleles from the TX-HL population were determined by SISAR to be 11.1%, 51.9%, and 37.0%, respectively, resulting in the total resistance allele frequency of 37.1%. Head louse populations from the U.K (BR-HL) and Australia (AU-HL) were similarly determined to be 100% homozygous resistant and populations from Korea (KR-HL) and Thailand (TL-HL) were 100% homozygous susceptible (Fig. 7), indicating again that kdr is widespread but not uniform worldwide.
Fig. 7.
Comparisons of the genotypic make up of head louse populations by SISAR. Red bars are homozygotes susceptible (SS), grey bars are heterozygotes (RS) and black bars are homozygote resistant (RR).
The individual genotyping based on SISAR is very accurate and efficient in obtaining detailed information on resistance allele frequency and allelic zygosity. Nevertheless, SISAR requires a larger sample size (50–100) to ensure accurate estimation of resistance allele frequency and therefore is more suitable for the secondary or tertiary resistance monitoring step.
Head louse resistance management
The three molecular techniques used to detect kdr in head lice for head lice were compared (Table 2). QS-based population genotyping can process a large number of louse populations simultaneously for the evaluation of resistance allele frequencies. It is expected that QS of 90 different population samples can be completed within 2 days in moderately equipped laboratories (1 day for genomic DNA extraction, 3 hrs for PCR to generate DNA templates for QS, and 5 hrs for sequencing). The technique dependency of QS is also relatively low compared to other population genotyping techniques such as rtPASA-TaqMan (Livak, 1999) and rtPASA. Thus, the speed, simplicity and moderate sensitivity of QS make it an ideal candidate for a routine primary resistance monitoring tool to screen a large number of louse populations as an alternative to conventional bioassay. Since the sensitivity of QS is ca. 7.4%, a small to medium-size sampling (7–14 lice) per louse population should be sufficient, making this method practical considering the difficulty of collecting a large number of lice samples.
Table 2.
Comparison of molecular techniques for resistance detection
| QS | rtPASA | SISAR | |
|---|---|---|---|
| Technical dependency | Low | Moderate | Low |
| Reliability | High | High | Very high |
| Sensitivity (Detection limit for resistance allele frequency) | Moderate (7.4%) | High (1.12%) | Can detect actual frequency |
| Zygosity detection | No | No | Yes |
| Cost per samplea | $2.0 | $1.5 | $1.5 |
| Time per 96 samplesb | 2 days | 2 days | 2 days |
| High throughput analysis | Yes | Yes | Yes |
| Suggested usage in resistance monitoring | Primary | Secondary | Secondary or tertiary |
| Suggested no. of lice per analysis for resistance monitoring | 7~14 | 50~100 | 50~100 |
Costs include DNA extraction and PCR. The cost for QS and rtPASA is for analyzing a single population as a unit whereas the cost of SISAR for analyzing a single individual as a unit.
The time includes DNA extraction and PCR. The time for QS and rtPASA is for analyzing 90 populations plus 6 standard DNA templates whereas the time for SISAR for 96 individuals. Reproduced with permission from Lee et al. 2009. Copyright 2009 ACS Books (in press).
If a more precise determination of resistance allele frequency is required on a population basis, rtPASA can be employed as a supporting monitoring step. This method enabled the detection of the kdr allele frequency in the head lice at the level as low as 1.13%. To detect the resistance allele frequencies lower than ca. 1%, however, a large-size sampling (50~100 lice per population) would be required. In addition, the technical dependency of rtPASA is relatively high, requiring a well-optimized protocol and experimental system to ensure an accurate prediction.
Although both QS and rtPASA enable the prediction of resistance allele frequencies on a population basis, thereby allowing rapid screening of resistant populations, they do not provide information on allele zygosity. If the information on resistance allele zygosity as well as allele frequency in a population is required, individual genotyping methods such as SISAR (Kim et al., 2004) can be conducted on a much reduced number of preselected populations as the secondary or tertiary resistance monitoring step. Information on allele zygosity would be particularly useful for understanding the resistance population dynamics at the early phase of resistance. SISAR requires, however, a large number of analyses (50~100 analyses of individual lice per population) to ensure accurate estimation of resistance allele frequency, which limits its applicability for a primary routine resistance monitoring tool.
In addition to detecting permethrin resistance mediated by kdr trait, accumulation of yearly and regional database on resistance allele frequencies will greatly facilitate the monitoring and understanding of resistance evolution patterns in different geographical regions over time. Based on the resistance allele frequencies estimated by these molecular techniques, differential actions for resistance management can be implemented. In regions where resistance allele frequency is saturated or near saturation, pyrethroids use should be curtailed and alternative pediculicides with different mode of actions used instead. In regions where the resistance allele frequencies are low or near zero, pyrethroids should be used cautiously and in conjunction with resistance monitoring program. This approach will extend the effective life span for this valuable group of pediculicides.
Acknowledgments
This work was supported by the NIH/NIAID (R01 AI045062-04A3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clark JM, Lee SH, Kim HJ, Yoon KS, Zhang A. DNA-based genotyping techniques for the detection of point mutations associated with insecticide resistance in Colorado potato beetle. Pest Management Sci. 2001;57:1–7. doi: 10.1002/ps.369. [DOI] [PubMed] [Google Scholar]
- Clark JM, Lee SH, Yoon KS, Strycharz JP, Kwon DH. Human head lice: Status, control and resistance. In: Clark JM, Bloomquist JR, Kawada H, editors. Advances in Human Vector Control; ACS Symposium Series XXX; Washington D.C: ACS Books; 2009. in press. [Google Scholar]
- Gao JR, Yoon KS, Lee SH, Takano-Lee M, Edman JE, Meinking T, Taplin D, Clark JM. Increased frequency of the T929I and L932F mutations associated with knockdown resistance in permethrin-resistant populations of the human head louse, Pediculus capitis, from California, Florida and Texas. Pest Biochem Physiol. 2003;77:115–124. [Google Scholar]
- Gratz NG. WHO/CTD/WHOPES/97.8. World Health Org; Switzerland: 1997. Human lice: Their prevalence, control and resistance to insecticides; p. 61. [Google Scholar]
- Gratz NG. Emerging and resurging vector-borne diseases, Ann. Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- Hall JG, Eis PS, Law SM, Reynaldo LP, Prudent JR, Marshall DJ, Allawi HT, Mast AL, Dahlberg JE, Kwiatkowski RW, de Arruda M, Neri BP, Lyamichev VI. Sensitive detection of DNA polymorphisms by the serial invasive signal amplification reaction, Proc. Natl Acad Sci U S A. 2000;97:8272–8277. doi: 10.1073/pnas.140225597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MT, Harwood RF. Herm’s Medical Entomology. 6. MacMillian Publ. Co. Inc; New York: 1969. The Lice; pp. 135–146. [Google Scholar]
- Kelly DJ, Richards AL, Temenak J, Strickman D, Dasch GA. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34 (Suppl 4):S245–S169. doi: 10.1086/339908. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Symington SB, Lee SH, Clark JM. Serial invasive signal amplification reaction for genotyping permethrin-resistant (kdr-like) human head lice, Pediculus capitis. Pestic Biochem Physiol. 2004;80:173–182. [Google Scholar]
- Kim HJ, Hawthorne DJ, Peters T, Dively GP, Clark JM. Application of DNA-based genotyping techniques for the detection of kdr-like pyrethroid resistance in field populations of Colorado potato beetle. Pestic Biochem Physiol. 2005;81:85–96. [Google Scholar]
- Kittler RM, Kayser M, Stoneking M. Molecular evolution of Pediculus humanus and the origins of clothing. Curr Biol. 2003;13:1414–1417. doi: 10.1016/s0960-9822(03)00507-4. Erratum (2004) 14:2309. [DOI] [PubMed] [Google Scholar]
- Kwon DH, Yoon KS, Strycharz JP, Clark JM, Lee SH. Determination of permethrin resistance allele frequency of human head louse populations by quantitative sequencing. J Med Entomol. 2008;45:912–920. doi: 10.1603/0022-2585(2008)45[912:dopraf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lee SH, Yoon KS, Williamson MS, Goodson SJ, Takano-Lee M, Edman JD, Devonshire AL, Clark JM. Molecular analysis of kdr-type resistance in permethrin-resistant strains of head lice, Pediculus capitis. Pestic Biochem Physiol. 2000;66:130–143. [Google Scholar]
- Lee SH, Gao JR, Yoon KS, Mumcuoglu KY, Taplin D, Edman JD, Takano-Lee M, Clark JM. Sodium channel mutations associated with knockdown resistance in the human head louse, Pediculus capitis, (De Geer) Pestic Biochem Physiol. 2003;75:79–91. [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Meinking TLC, CM, Chen C, Kolber MA, Tipping RW, Furtek CI, Villar ME, Guzzo CA. An observer-blinded study of 1% permethrin crème rinse with and without adjunctive combing in patients with head lice. J Pediatr. 2002;141:665–670. doi: 10.1067/mpd.2002.129031. [DOI] [PubMed] [Google Scholar]
- National Research Council. Pesticides in the diet of infants and children. The National Academies Press; Washington, DC: 1993. [Google Scholar]
- Piesman P, Gage L. Bacterial and rickettsial diseases. In: Eldridge BF, Edman JD, editors. Medical Entomology. Kluwer Academic Publ; Boston, MA: 2000. pp. 337–413. [Google Scholar]
- Pray WS. Pediculicide resistance in head lice: A survey. Hosp Pharm. 2003;38:241–246. [Google Scholar]
- Raoult D, Roux V. The body louse as a vector of reemerging human disease. Clin Infect Dis. 1999;29:888–911. doi: 10.1086/520454. [DOI] [PubMed] [Google Scholar]
- Roux V, Raoult D. Body lice as tools for diagnosis and surveillance of reemerging diseases. J Clin Microbiol. 1999;54:596–599. doi: 10.1128/jcm.37.3.596-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano-Lee M, Yoon KS, Edman JD, Mullens BA, Clark JM. In vivo and in vitro rearing of Pediculus humanus capitis (Anoplura:Pediculidae) J Med Entomol. 2003;40:628–635. doi: 10.1603/0022-2585-40.5.628. [DOI] [PubMed] [Google Scholar]
- Takano-Lee M, Velten RK, Edman JD, Mullens BA, Clark JM. An automated feeding apparatus for in vitro maintenance of the human head louse, Pediculus humanus capitis, (Anoplura:Pediculidae) J Med Entomol. 2003;40:795–799. doi: 10.1603/0022-2585-40.6.795. [DOI] [PubMed] [Google Scholar]
- Weiss K. The role of rickettsioses in history. In: Walker DH, editor. Biology of Rickettsial Diseases. Vol. 1. CRC; Boca Raton, FL: 1988. pp. 1–14. [Google Scholar]
- Williams LK, Reichart A, MacKenzie WR, Hightower AW, Blake PA. Lice, nits, and school policy. Pediatrics. 2001;107:1011–1015. doi: 10.1542/peds.107.5.1011. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Gao JR, Lee SH, Clark JM, Brown L, Taplin D. Permethrin-resistant human head lice. Pediculus capitis, and their treatment. Arch of Dermatol. 2003;139:994–1000. doi: 10.1001/archderm.139.8.994. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Gao JR, Lee SH, Coles GG, Meinking TL, Taplin D, Edman JD, Takano-Lee M, Clark JM. Resistance and cross-resistance to insecticides in human head lice from Florida and California. Pestic Biochem Physiol. 2004;80:192–201. [Google Scholar]
- Yoon KS, Strycharz JP, Gao JR, Takano-Lee M, Edman JD, Clark JM. An improved in vitro rearing system for the human head louse allows the determination of resistance to formulated pediculicides. Pestic Biochem Physiol. 2006;86:195–202. [Google Scholar]
- Yoon KS, Symington SB, Lee SH, Soderlund DM, Clark JM. Three mutations identified in the voltage-sensitive sodium channel α-subunit gene of permethrin-resistant human head lice abolish permethrin sensitivity of the house fly Vssc1 expressed in Xenopus oocytes. Insect Biochem & Mol Biol. 2008;38:296–306. doi: 10.1016/j.ibmb.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Z, Fournier PE, Rydkina E, Raoult D. The geographical segregation of human lice preceded that of Pediculus humanus capitis and Pediculus humanus humanus. CR Biol. 2003;326:565–574. doi: 10.1016/s1631-0691(03)00153-7. [DOI] [PubMed] [Google Scholar]