SUMMARY
This review summarizes the recent literature on the epidemiology of adult obstructive sleep apnea (OSA) from various population-based studies. Despite methodologic differences, comparisons have yielded similar prevalence rates of the OSA syndrome in various geographic regions and amongst a number of ethnic groups. Risk factors for OSA including obesity, aging, gender, menopause, and ethnicity are analyzed. We also provide discussion on adverse medical conditions associated with OSA including hypertension, stroke, congestive heart failure, coronary artery disease, cardiovascular mortality, insulin resistance, and neurocognitive dysfunction. Finally with the progression of the global obesity epidemic, we focus on the economic health care burden of OSA and the importance of recognizing the largely undiagnosed OSA population with emphasis on strategies to improve access to diagnostic resources.
Keywords: Epidemiology, Obstructive sleep apnea (OSA), Risk factors, Continuous positive airway pressure (CPAP), Hypertension, Insulin resistance, Cardiovascular morbidity, Economic health care costs, Prevalence, Incidence
INTRODUCTION
Several population-based studies across various geographical regions and ethnic groups have established a high prevalence of OSA. This epidemic of OSA is closely related to the obesity epidemic—an important public health related condition facing adults globally. In the United States in 2004, the estimated prevalence of adult obesity, classified as a body mass index (BMI) over 30 kg/m2, was more than 30% and the prevalence of extreme obesity (BMI > 40 kg/m2) was 2.8% in men and 6.7% in women [1]. Associated health care expenditures in obese individuals is 36% higher than normal weight persons, and it has been estimated that up to 7% of the annual health care expenditures is related to obesity [2,3]. Obesity is a well-recognized risk factor for a variety of medical conditions such as OSA, type 2 diabetes, cardiovascular diseases, hyperlipidemia, metabolic syndrome, and nonalcoholic fatty liver disease [4].
OSA, characterized by recurrent episodes of complete or partial obstruction of the upper airway during sleep, has widely gained interest since its initial description more than 40 years ago. A multitude of studies have estimated the prevalence of OSA in the general population and in the process, uncovered the despairingly large proportion of those unrecognized. In fact, up to 80% of cases of moderate or severe OSA have gone undiagnosed despite adequate access to health care [5–7]. Even more striking is the number of health related conditions implicated with OSA including diminished neurocognitive function, increased risk of motor vehicle accidents, reduced quality of life, hypertension, insulin resistance, and cardiovascular diseases [7,8].
In this article, we review the most recent literature on the epidemiology of OSA in adults with an in-depth discussion of both prevalence and incidence of disease. We also discuss the different risk factors implicated with OSA with emphasis on obesity, aging, gender, menopause, and ethnicity. We also provide an overview of the many adverse health related medical conditions associated with OSA ranging from cardiovascular diseases to insulin resistance. Other sleep disorders such as central sleep apnea and Cheyne-Stokes respirations are beyond the scope of this review article and will not be discussed. Finally, we highlight the importance of identifying the largely undiagnosed and untreated population with OSA. By understanding the epidemiology of OSA and its associated health conditions, health care providers can appreciate the public health burden of OSA and prioritize the allocation of resources to diagnose and treat this disease.
OVERVIEW OF OBSTRUCTIVE SLEEP APNEA
Definition
OSA consists of decreased airflow due to repetitive complete or partial obstruction of the upper airway associated with progressive respiratory effort to overcome the obstruction. These obstructive respiratory events are typically associated with cortical microarousals and oxygen desaturation leading to sleep fragmentation and increased sympathetic neural activity [9]. Clinical symptoms suggestive of OSA include loud snoring, witnessed breathing pauses by a bed-partner, choking or gasping during sleep, morning headaches, insomnia, and daytime sleepiness. Obstructive apneas are defined as complete or near complete cessation of airflow due to upper airway collapse lasting at least 10 seconds. Obstructive hypopneas are characterized by either a ≥ 30% decrease in airflow from baseline lasting at least 10 seconds associated with a 4% oxygen desaturation or a decrease in flow by ≥ 50% of baseline for at least 10 seconds associated with either a 3% oxygen desaturation or electroencephalographic criteria for a cortical microarousal from sleep [10]. Both obstructive apneas and hypopneas are associated with continued respiratory effort distinguishing them from central apneas [11]. Although hypopneas constitute the majority of obstructive respiratory events observed in patients with OSA, significant controversy persists around how best to define these partial upper airway obstructive events. A recent epidemiologic study demonstrated that only hypopneas that were accompanied by at least a 4% oxygen desaturation were independently associated with cardiovascular disease [12].
Diagnosis
The standard diagnostic test for OSA is an overnight polysomnogram. This study involves several measured physiologic recordings such as electroencephalogram, electrooculogram, electrocardiogram, chin and leg electromyograms, body position, finger pulse oximetry, measurements of airflow, and measurements of thoracic and abdominal respiratory effort. The gold-standard polysomnogram is attended in a laboratory by a sleep technician. However, home portable monitoring devices may eventually become an acceptable method of diagnosing OSA in individuals with a high pretest probability of at least moderate or severe OSA who do not have significant preexisting cardiopulmonary disorders [13].
The apnea-hypopnea index (AHI) is commonly used to categorize the severity of OSA and it represents the average number of apneas and/or hypopneas per hour of recorded sleep. In adults, an AHI less than 5 events per hour is considered normal. Mild OSA is defined as an AHI between 5 and 15 events per hour, moderate OSA between 15 and 30 events per hour, and severe OSA as greater than 30 events per hour. Other measures of severity used in both clinical practice and research settings include oxygen saturation nadir, percent of time with oxygen saturation below 90%, and the severity of daytime symptoms. OSA, however, can be present without any significant symptomatology. When OSA is accompanied by symptoms, most commonly excessive daytime sleepiness (EDS), it has been labeled as the OSA syndrome.
The determination of EDS varies in research settings, and usually depends on a subjective rating of factors such as daytime sleepiness, fatigue, decreased concentration, and unrefreshing sleep. Several studies use focused questionnaires that involve 3 to 5 questions, whereas others use the Epworth Sleepiness Scale—a series of 8 questions establishing daytime sleepiness. Unfortunately, no technique for assessing subjective sleepiness has been consistently validated as the reference standard [11] and the best objective evaluation of sleepiness—the Multiple Sleep Latency Test—is not routinely included in epidemiologic research settings. The Epworth Sleepiness Scale and the Multiple Sleep Latency Test are complementary tests, the former assessing self-perceived sleep propensity and the latter a marker of physiologic sleep tendency [14].
EPIDEMIOLOGIC STUDIES
Overview of Study Design
Epidemiologic studies assessing the prevalence of OSA have variable methodologic approaches, limiting accurate comparisons. These differences range from the subjects studied—clinic-based or random population samples—to methodology of the study. For example, sampling obese male subjects referred to either a general clinic or a sleep clinic markedly overestimates the prevalence of the disease and reflects selection bias because the clinic-based sample is not representative of the general population [15]. The methods by which sleep data are collected (attended laboratory polysomnography or unattended home monitoring), or criteria and techniques used to recognize obstructive respiratory events (qualitative or quantitative measurements of airflow and respiratory effort), are not routinely standardized amongst epidemiologic studies, and may lead to variable estimates of the severity of OSA [16–18]. Finally, many epidemiologic studies utilize a two staged prevalence study design, with an initial questionnaire targeted to a large screening population, followed by random selection of subjects to undergo further diagnostic testing [19]. Despite these methodologic differences, comparisons of several epidemiologic studies have yielded surprisingly similar prevalence rates of OSA and the OSA syndrome in various geographic regions and amongst a range of ethnic groups (Table 1).
TABLE 1.
Population-based studies reporting the prevalence of OSA and OSA syndrome
| Study | Number of subjects | AHI ≥ 5 | AHI ≥ 15 | OSA syndrome | Methodology | Hypopnea Definition† |
|---|---|---|---|---|---|---|
| Wisconsin, U.S.A. ‡ 1993 [20] | Men: 352 Women: 250 (age 30–60) |
Men: 24% Women: 9% |
Men: 9% Women: 4% |
Men: 4% Women:2% |
Attended PSG (oronasal airflow and respiratory inductance plethysmography) | Discernable reduction in airflow plus ≥ 4% oxygen desaturation † |
| Pennsylvania, U.S.A.¶ 1998, 2001 [22,23] | Men: 741 Women: 1000 (age 20–100) |
Men: 17% Women: 5% |
Men: 7% Women: 2% |
Men: 3.3% Women:1.2% |
Attended PSG (oronasal thermocouple) | Discernable reduction in airflow and ≥ 4% oxygen desaturation † |
| Spain ¶ 2001 [21] | Men: 325 Women: 235 (age 30–70) |
Men: 26% Women:28% |
Men: 14% Women:7% |
Men: 3.4% Women:3% |
Attended PSG (oronasal thermister) | 50% airflow reduction Accompanied by either ≥ 4% oxygen desaturation or an EEG arousal |
| Australia ‡ 1995 [24] | 294 men (age 40–65) | Men: 25.9% | Men: 10% (AHI ≥ 10) | Men: 3.1% Women: n/a |
MESAM IV portable monitoring (snoring and oximetry) | ≥ 3% oxygen desaturation along with increased heart rate of 10 beats/minute or burst of snoring † |
| Hong Kong, China ‡ 2001, 2004 [25,26] | Men: 153 Women:106 (age 30–60) |
Men: 8.8% Women: 3.7% |
Men:5.3% Women: 1.2% |
Men: 4.1% Women:2.1% |
Attended PSG (oronasal thermister, thoracic and abdominal impedance belts) | Discernable reduction in airflow and ≥ 4% oxygen desaturation † |
| Korea ‡ 2004 [27] | Men: 309 Women: 148 (age 40–69) |
Men: 27% Women: 16% |
Men: 10.1% Women: 4.7% |
Men: 4.5% Women:3.2% |
In laboratory or home PSG (oronasal thermister) | Discernable reduction in airflow and ≥ oxygen 4% desaturation † |
| India ‡ 2004 [28] | 250 men (age 35–65) | Men: 19.5% | Men: 8.4% | Men: 7.5% Women: n/a |
Home PSG (oronasal thermister) | Discernable 50% reduction in airflow and ≥ 4% oxygen desaturation † |
| India ‡ 2006 [29] | Men: 88 Women: 63 (age 30–60) |
Men: 19.7% Women: 7.4% |
n/a | Men: 4.9% Women:2.1% |
Attended in laboratory PSG | Discernable 50% reduction in airflow and ≥ 4% oxygen desaturation † |
Studies that did not use EEG microarousal as part of the definition of hypopnea
OSA Syndrome defined as: AHI ≥ 5 + EDS
OSA Syndrome defined as: AHI ≥ 10 + EDS
MESAM IV: Madaus Medizin-Elektronik – Freiburg, Germany; PSG: Polysomnography
Population-based Prevalence Studies
The most rigorous population-based study determining epidemiologic features of OSA is the Wisconsin Sleep Cohort Study [20]. Using in-laboratory polysomnography, the prevalence of OSA (AHI ≥ 5) in 602 middle-aged adults between 30 and 60 years of age was 9% for women and 24% for men. The OSA syndrome, characterized by both an AHI ≥ 5 along with daytime sleepiness (assessed by 3 subjective questions), was present in 2% of women and 4% of men. Other similarly designed studies have yielded comparable prevalence rates. The higher prevalence of OSA in a Spanish cohort between 30 and 70 years of age—28% in women and 26.2% in men—could be explained by including arousals to score hypopneas [21]. However, the prevalence of the OSA syndrome (AHI ≥ 10 and EDS) in this Spanish population was similar to the Wisconsin Sleep Cohort Study. Likewise, in 1,741 patients from the Pennsylvania cohort, the prevalence of the OSA syndrome (defined as AHI ≥ 10 and EDS) was 3.9% in men, and 1.2% in women [22,23]. Finally, Bearpark and colleagues evaluated 294 men between 40 and 65 years of age from Australia using portable monitoring and identified the OSA syndrome (AHI ≥ 5 and EDS) in 3.1% of the population [24]. Altogether, these large studies of predominately white populations estimate the prevalence of OSA syndrome at approximately 3–4% in men and 2% in women. Interestingly, despite the overall lower BMI in the studies from Asia, the prevalence of the OSA syndrome is similar in Asian countries when compared to their Western counterparts. The similar prevalence amongst these two distinct populations highlights the importance of non-obesity related risk factors [25–29] (Table 1).
Taken together, the results of several population-based studies across various geographical regions and ethnic groups demonstrate a similar prevalence rate of OSA syndrome despite differences in study designs and technical aspects for identifying respiratory events.
Population-based Incidence Studies
The incidence of OSA in adults can be estimated from 3 population-based longitudinal studies (Table 2). Using home polysomnography, the Cleveland Family Study investigators identified 286 subjects without evidence of OSA (AHI < 5) at baseline and followed the progression of OSA five years later [30]. The majority of subjects, 63%, did not develop OSA, yet the overall 5 year incidence of OSA was 7.5% for those developing at least moderate OSA or worse (AHI > 15), and 16% for those developing an AHI > 10. Predictors of an increase in AHI included age (up to 60 years), male gender, higher BMI, increases in the waist-to-hip ratio, and increases in serum cholesterol. In the Wisconsin Sleep Cohort, 690 subjects had baseline attended polysomnography with follow up studies 4 years later. The AHI was below 5 in 554 of these subjects (80%) and over 4 years, 10.6% developed an AHI ≥ 5 [31]. Finally in the Sleep Heart Health Study, the incidence rate over 5 years for those with an AHI < 5 developing an AHI ≥ 15 was 11.1% in men and 4.9% in women [32].
TABLE 2.
Incidence of OSA from population-based longitudinal studies
RISK FACTORS FOR OSA
Several risk factors have been identified in the development of OSA but undoubtedly, the strongest risk factor is obesity reflected by several markers including BMI, neck circumference, and waist-to-hip ratio [30,33]. Other risk factors include aging (up to age 65), male gender, menopause, craniofacial abnormalities, upper airway anatomy, smoking, alcohol, and genetic predisposition [7]. We review major risk factors including obesity, aging, menopause, and ethnicity. Other important known risk factors such as alcohol, craniofacial abnormalities, and genetic predisposition are not discussed in this review. Table 3 summarizes both modifiable and non-modifiable risk factors associated with OSA.
TABLE 3.
Risk factors for developing OSA
| Modifiable Risk Factors for OSA |
| Obesity |
| Drugs – opiates, benzodiazepines, and alcohol intake |
| Smoking (active and passive) |
| Supine body position during sleep |
| * Nasal congestion or obstruction |
| Non-modifiable Risk Factors for OSA |
| Gender: men more than women |
| Genetic predisposition |
| Race |
| Aging |
| Menopause |
| * Craniofacial anatomy (retrognathia and micrognathia) |
potentially corrected by surgery
Obesity
Obesity has been well established as a risk factor for OSA in multiple population-based studies and in fact there is direct relationship between the OSA epidemic and the obesity epidemic [20,30,32,34]. Several studies have demonstrated an increase in the prevalence of OSA with any increase in measures of body habitus (BMI, waist-to-hip ratio, or neck girth) (Table 4). Moreover, more than half of the prevalence of OSA is attributable to excess body weight [20,34]. In fact, per each unit increase in BMI the adjusted odds ratio for developing OSA is 1.14 (95% CI 1.10–1.19). However, the impact of BMI on OSA becomes less significant after age 60 [30].
TABLE 4.
Prevalence of OSA by body mass index in population-based studies utilizing polysomnography. Reprinted with permission from Young et al. [34]
| Study | Number of subjects | Age group (years) | Definition of OSA | BMI (kg/m2) | Prevalence of OSA (percentage) |
|---|---|---|---|---|---|
| Pennsylvania, U.S.A. Bixler et al. [22,23] | 1,741 | 20–100 | AHI ≥ 15 | Women < 32.3 ≥ 32.3 Men < 32.3 ≥ 32.3 |
Women 1.1 % 7.2% Men 2.0% 13.8 % |
| Sleep Heart Health, U.S.A. Young et al. [33] | 5,615 | 40–98 | AHI ≥ 15 | Quartiles 16–24 24–28 28–32 32–59 |
10 % 13 % 17 % 32 % |
| Hong Kong, China Ip et al. [25,26] | 259 | 30–60 | AHI ≥ 5 | Women < 23 ≥ 23 Men < 23 ≥ 23 |
Women 0.9 % 14.5 % Men 0.9 % 8.8 % |
OSA: obstructive sleep apnea; BMI: body mass index
Weight changes have also been associated with the progression and regression of OSA [32]. In subjects with no OSA or mild OSA at baseline (AHI < 15), a 10% weight gain increases the odds of developing moderate or worse OSA (AHI ≥ 15) by 6-fold (95% CI, 2–17) [31]. The impact of weight loss on the severity of OSA is supported by studies evaluating bariatric surgery outcomes [35]. The significant weight loss seen in these patients leads to dramatic improvements in self-reporting of frequent apneas, snoring, and EDS [36].
From 1986 to 2000 the prevalence of extreme obesity defined as a BMI ≥ 40 kg/m2 has quadrupled and that of BMI ≥ 50 kg/m2 has increased by 5-fold in the United States. Such degree of obesity can lead to severe OSA and in some cases can lead to daytime and nocturnal hypoventilation, a condition known as the Pickwickian syndrome or obesity hypoventilation syndrome [37]. The prevalence of obesity hypoventilation syndrome has been estimated to be as high as 25% in patients with OSA who are extremely obese [38].
Aging
OSA remains highly prevalent in the elderly and the gender differences diminish significantly after menopause. Various studies using different methodologies have reported that among the elderly, the prevalence of at least moderate OSA (AHI ≥ 15) varies widely from as low as 7% to as high as 44% [21–23,39]. It remains unclear, however, as to why the steady increase in the prevalence of OSA during the middle-aged years plateaus around age 65. There are several possibilities to explain this phenomenon. It has been shown that the BMI has a smaller influence on the AHI in the elderly when compared to middle-aged individuals [22,23,30,32] and that changes in the BMI, particularly related to weight gain, are less significant in the elderly. Other possible explanations include either increase mortality in patients with OSA or disease remission in the elderly. However, there is no evidence to support disease remission with aging and there is growing evidence that untreated OSA can increase cardiovascular morbidity and mortality [40–42].
Sleep-disordered breathing may be phenotypically different in the elderly. It has been long recognized that most patients with OSA developperiods of stable breathing, particularly during non-REM slow wave sleep [43]. Normal aging is associated with a significant decline in slow wave sleep, particularly in the older men [44,45]. An increase in respiratory events and periodic breathing in the elderly may be in part related to the significant reduction in slow wave sleep and an increase in sleep state instability or to an increase in upper airway resistance [46,47]. Although OSA is highly prevalent in the elderly population, its long term impact remains less well understood. Many of the elderly with OSA do not develop symptoms; thus the higher prevalence of OSA compared to their younger counterparts does not necessarily lead to an increase in the prevalence of OSA syndrome (AHI ≥ 5 plus symptoms) [23]. However, classic OSA syndrome is frequently observed in the elderly with a wide range of clinical sequelae. With careful follow-up and adequate adherence with therapy, the elderly experience similar degrees of improvement in daytime alertness and in other symptoms associated with OSA when compared to middle-aged adults [48].
Gender
Although clinic-based studies had previously reported a significant gender gap in the prevalence of OSA, more recent large population-based studies have demonstrated that the prevalence of OSA is only 1.5–3 times higher in men than women and this gender gap narrows even further after menopause [20–27,29]. This discrepancy between clinic and population-based prevalence suggests a strong referral bias for evaluating OSA favoring men and has been hypothesized to be partly attributed to different clinical presentations of OSA in women. Women may not present with the “classic” symptoms of OSA (loud snoring, EDS, nocturnal choking episodes, or witnessed apneas), and therefore may less likely be referred for a formal evaluation [49,50]. In two clinic-based studies comparing men and women with similar degrees of obesity and OSA, women were more likely to present with “atypical complaints” such as insomnia or depression, and less likely to have observed apneas [51,52]. However, two population-based studies have reported similar symptoms of OSA in men and women [26,53]. As a result, there may be other reasons for the gender disparity in diagnosing OSA such as the possibility that health care providers disregard typical symptoms in women or alternatively, women are less likely to seek medical attention because of loud snoring. The lack of recognition of OSA in women may impact mortality, as one study found that women with OSA had a higher 5 year mortality rate when compared to men with OSA [54]. Taken together, the evidence suggests that women are underdiagnosed and consequently undertreated for OSA compared to men.
Menopause
In contrast to men, women with similar degree of obesity have a less collapsible upper airway [55]. This pathophysiologic difference in the upper airway anatomy could explain the overall lower prevalence of OSA in premenopausal women in whom the obstructive respiratory events commonly tend to cluster during REM sleep [56]. The increased prevalence of OSA in menopausal women—independent of age, body habitus and other factors such as alcohol use and health status—draws attention to hormonal differences in the pathogenesis of OSA [22,57]. Even more intriguing is the observation of reduced OSA prevalence in women taking hormone replacement therapy [22,58] suggesting a protective role of progesterone and/or estrogen due to its impact on the upper airway dilator muscle activity [59]. It is also not entirely clear if hormone replacement therapy is the only reason for the lower prevalence of OSA or if other unidentified variables are responsible for the modest decrease in the risk of OSA with hormone replacement therapy. For example, postmenopausal women who opt to take hormone replacement therapy may be healthier at baseline, more health conscious, less obese, and in earlier stages of menopause [60]. Hormone replacement therapy has not been shown to be an effective treatment of OSA in post-menopausal women; therefore, the optimal treatment for OSA in postmenopausal women remains continuous positive airway pressure (CPAP) therapy [61].
Ethnicity
Although the Sleep Heart Health Study reported a similar prevalence of OSA in African-Americans compared to other ethnic groups [33], other investigators have reported a disproportionately higher severity of OSA in young and elderly African Americans compared to White subjects after controlling for confounders [62,63].
There is limited data on the prevalence of OSA in Hispanics. More recently however, the Sleep Heart Health Study reported a substantially higher odds ratio of self-reported snoring and apneas in both Hispanic men and women compared with Whites and African Americans [64].
Despite the overall lower average BMI in the Asian population when compared to Western societies, the prevalence of OSA syndrome is similar and indicates that other non obesity related risk factors contribute to the pathogenesis of the disease. The prevalence of OSA in the Chinese population-based studies was similar to the White population despite a significantly lower BMI, only 5% of men had a BMI above 30 kg/m2 [25]. The similar prevalence of OSA in these two populations could be attributed to cephalometric differences between Asian and White patients such as an inferiorly positioned hyoid bone, enlarged soft palate, and reduced upper airway width at the soft palate [65–67].
In summary, there are ethnic differences in the prevalence and severity of OSA. However, in some of these studies the ethnicity is self reported, which may be inaccurate and may not incorporate the genetic heterogeneity amongst populations [19].
MEDICAL DISORDERS WITH HIGH PREVALENCE OF OSA
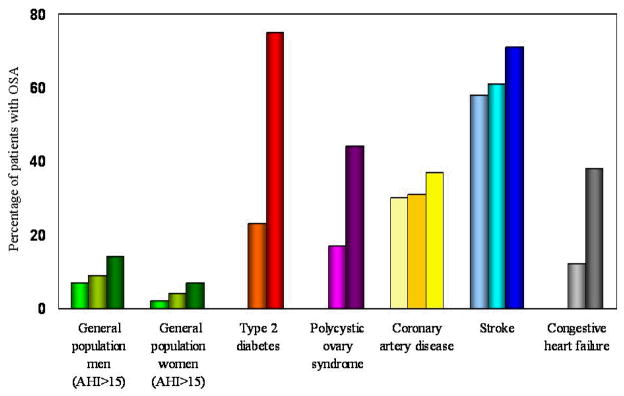
In certain medical disorders, the prevalence of OSA exceeds that seen in the general population (Figure 1). These medical conditions include, but are not limited to, type 2 diabetes [68,69], polycystic ovary syndrome [70–72], refractory hypertension [73], coronary artery disease [74–76], congestive heart failure with systolic dysfunction [77–79], and stroke [80–82]. Therefore, we believe it is prudent for clinicians to routinely screen patients with the above-mentioned medical conditions for OSA.
Figure 1.
The prevalence of OSA (defined as either AHI ≥ 10 or 15) in various medical disorders. Compared to three population-based prevalence studies [20–23], patients with Type 2 diabetes [68,69], polycystic ovary syndrome [70,72], coronary artery disease [74–76], congestive heart failure [77–79], and stroke [80–82] have a much higher prevalence of OSA. Each bar in the figure represents an individual study.
ADVERSE CONSEQUENCES ASSOCIATED WITH OSA
Hypertension
Population-based cross-sectional and prospective longitudinal studies have shown a strong and independent association of various measures of OSA severity (AHI and measures of oxygenation) with hypertension. In two large population-based cross-sectional studies, subjects with moderate or worse OSA (AHI ≥ 15) had a 1.42 to 1.72 increased odds ratio for prevalent hypertension when compared to subjects with no OSA (AHI < 1.5) after adjusting for BMI and other known confounders [83,84]. A longitudinal prospective population-based study with four year follow-up assessed the impact of OSA on hypertension. After controlling for various known confounders (age, body habitus, baseline hypertension, alcohol and smoking), compared to subjects with no OSA, the adjusted odds ratio for prevalent hypertension was 2.03 (95% CI 1.29–3.17) for mild OSA and 2.89 (95% CI 1.46–5.64) for moderate or worse OSA [85]. In contrast to middle-aged individuals in whom there is a significant relationship between OSA and prevalent hypertension, the impact of OSA on systemic blood pressure is insignificant in the elderly [83,86]. The lack of significant association between OSA and hypertension in the elderly may be due to the fact that those affected did not survive or that alternatively, significant cardiovascular disease has already occurred by that age and the contribution of OSA on disease progression becomes less important.
Studies investigating the effect of CPAP treatment can provide further insights into the cause and effect relationship between OSA and hypertension. However, results from randomized controlled trials have been inconsistent [87–92]. The variable results of the randomized controlled trials are related to enrollment of patients who are not hypertensive at baseline, suboptimal CPAP adherence, and methodological differences between the studies. Despite these limitations, a recent meta-analysis of several randomized, placebo-controlled trials assessing the impact of CPAP therapy on blood pressure in patients with OSA demonstrated a small but significant decrease in mean 24-hr blood pressure (1.69 mmHg; 95% CI, −2.69 to −0.69 mm Hg) when compared to placebo. However, the reduction in ambulatory blood pressure was greater in patients with more severe OSA and sleep fragmentation at baseline and with increasing adherence to nightly CPAP therapy [93].
Stroke
Several large population-based epidemiologic studies have found an independent association between snoring [94] or OSA and stroke [95,96]. After adjusting for known confounders, data from the Sleep Heart Health Study demonstrated an odds ratio for self-reported stroke of 1.58 (95% CI 1.02–2.46) for AHI > 11 compared to subjects with no OSA [96]. Similarly, the Wisconsin Sleep Cohort data revealed an adjusted odds ratio for stroke of 4.33 (95% CI 1.32–14.24) for an AHI ≥ 20 vs. an AHI < 5. In addition, on four-year longitudinal follow-up of this cohort, there was a trend towards increased risk of incident stroke [95]. Further evidence to support the impact of OSA on incident stroke came from a large observational clinic-based cohort study in which the adjusted hazards ratio for incident stroke or death from any cause was 1.97 (95% CI, 1.12–3.48) in patients with mild or worse OSA (AHI ≥ 5) when compared to patients with no OSA [42]. Studies are needed to assess the impact of OSA therapy on primary or secondary prevention of stroke given its associated high burden of morbidity and mortality.
Coronary Artery Disease
Coronary artery disease (CAD), defined as angina pectoris and/or myocardial infarction, has been independently associated with OSA in both population-based [96] and clinic-based studies [97]. The Sleep Heart Health Study, a large population-based study, documented a modest association between OSA and self-reported (CAD). In subjects with the highest quartile of AHI (AHI > 11), an adjusted odds ratio of 1.27 was observed relative to subjects with no OSA [96]. Similarly, in a clinic-based study, OSA was a significant and independent predictor of incident CAD (relative risk 4.60) [97].
In patients with documented CAD, the prevalence of OSA has ranged from 30% to 57% [74–76,98–100]. Moreover, in a prospective observational study with a follow-up period of 7 years, patients with OSA had a significantly elevated incidence of CAD (16.2%) compared to snorers without OSA (5.4%) [97]. Several prospective studies have reported an increased rate of adverse secondary cardiovascular events in patients with CAD and concomitant OSA [99,101,102]. There are data demonstrating that effective treatment of OSA can serve as both primary prevention [97] and secondary prevention [103] of adverse cardiovascular outcomes.
Congestive Heart Failure and Arrhythmias
Limited data from population-based studies have demonstrated an independent association between OSA and congestive heart failure. In the Sleep Heart Health Study subjects in the highest quartile of AHI (> 11) had an adjusted odds ratio of 2.38 for self-reported diagnosis of congestive heart failure compared to subjects in the lowest AHI quartile [96]. Several clinic-based cohort studies have established a high prevalence of sleep-disordered breathing (OSA and/or central sleep apnea) in patients with congestive heart failure and reduced systolic function [77–79]. Although epidemiologic studies have established an association between OSA and CHF, the direction of causality has not been established. However, a few short-term, randomized controlled trials have been able to demonstrate that effective treatment of OSA with CPAP therapy in patients with systolic congestive heart failure can lead to significant improvements in the ejection fraction, measures of daytime sleepiness, and quality of life [104,105].
Cardiac arrhythmias have also been associated with OSA in both clinic-based and population-based studies. Investigators from the Sleep Heart Health Study reported that subjects with severe OSA had two-to-fourfold higher odds of complex arrhythmias than those without OSA even after adjustment for potential confounders. In particular, the adjusted odds ratio for co-existing atrial fibrillation was 4.02 (95% CI, 1.03 15.74) [106]. In a retrospective population-based study, nocturnal hypoxemia due to OSA was an independent predictor of incident atrial fibrillation in patients less than 65 years of age [107].
Cardiovascular Mortality Attributed to OSA
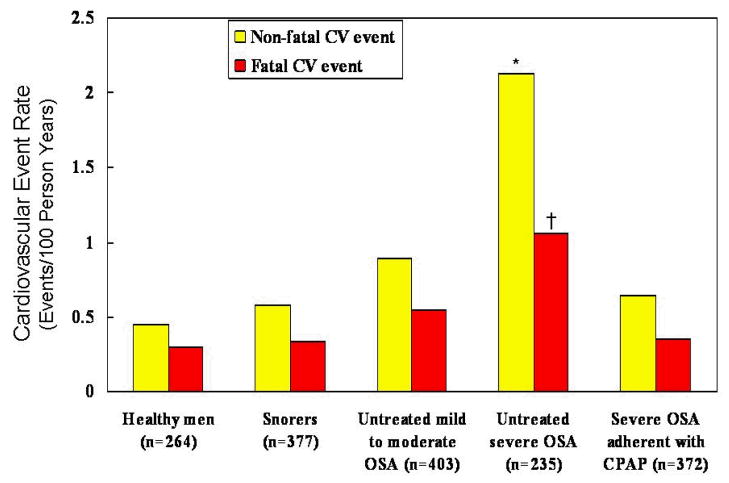
As to date, there are no long-term, large, randomized controlled trials assessing the impact of CPAP therapy vs. placebo on cardiovascular mortality. However, there are a number of studies supporting the adverse impact of untreated OSA on cardiovascular outcomes. Several retrospective studies examined the association of OSA and mortality. In one study, patients with severe untreated OSA had a higher mortality than patients with similar degree of OSA severity who were either treated by tracheostomy or CPAP therapy [108]. In another retrospective study individuals with OSA had a peakin sudden death from cardiac causes during the sleeping hours, in contrast to the general population [109]. Furthermore, three recent large observational studies have confirmed the adverse consequences of untreated OSA [40,41,110]. All together, a total of 2,396 patients with various degrees of severity of OSA were evaluated and followed in these three studies. The findings of these studies suggest that patients with untreated severe OSA have increased fatal and nonfatal cardiovascular outcomes compared to those who were treated with CPAP therapy (Figures 2 and 3). Of interest, patients who adhered to the prescribed CPAP therapy had more comorbidities, higher AHI and BMI compared to those who did not adhere with CPAP therapy. The main limitation of these observational studies includes the possibility that patients who were nonadherent with CPAP therapy may have also been less adherent with other therapies recommended by their physicians such as prescribed medications for hypertension, dyslipidemia, and type 2 diabetes. Conversely, one could argue that the nonadherent patients in these observational studies typify the usual patient seen by physicians in clinical practice.
Figure 2.
Incidence of non-fatal and fatal cardiovascular events in 10 years (events/100 person years) in controls, snorers, untreated mild to moderate OSA, untreated severe OSA, and OSA treated with CPAP in an observational study. All participants in this study were men. The healthy men were age and BMI matched to patients with OSA (healthy men had a mean age of 49.6 years and a mean BMI of 29.8 kg/m2 vs. men with untreated severe OSA who had a mean age of 49.9 years and a mean of BMI 30 kg/m2). Adherence with CPAP treatment was assessed with the timer built into each CPAP device. Patients with severe OSA had a mean CPAP use of ≥ 4 h/day. Data adapted from Marin et al. [40].
*p < 0.0001 versus healthy men
†p < 0.0012 versus healthy men
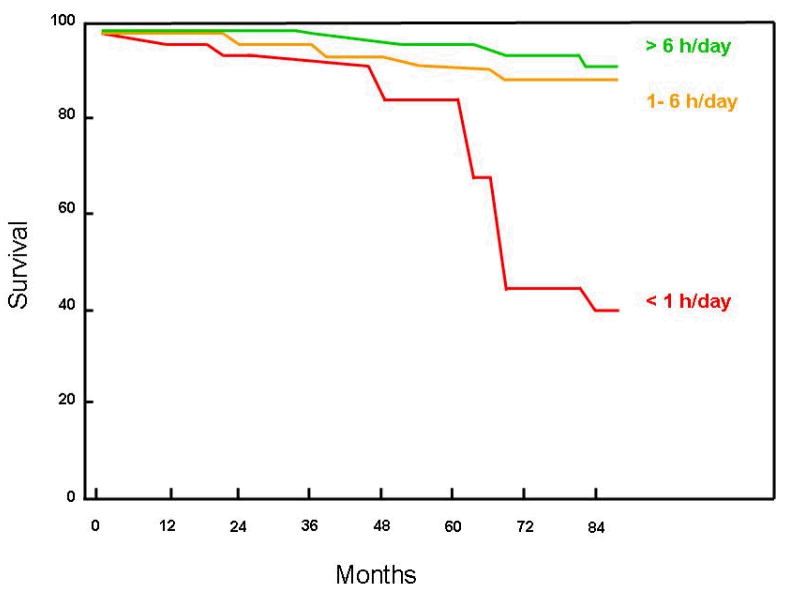
Figure 3.
Kaplan-Meier cumulative survival curve according to long-term CPAP adherence in patients with OSA emphasizing the relationship between average daily adherence with CPAP therapy and mortality. In this study, a historical cohort of 871 patients with moderate to severe OSA (mean age 55.4 ± 10.6 years, 81% men, mean AHI and CPAP use were 55.1 ± 28.7 and 10.1 ± 2.1 cm H2O respectively) were followed for an average of 49 ± 23 months. There were 322 patients in the group with CPAP adherence > 6 h/day (mean adherence 7.6 ± 1.2 hours), 342 patients in the group with 1–6 hour/day (mean adherence 3.9 ± 1.4 hours) and 85 patients in the group with < 1 h/day (mean adherence 0.3 ± 0.2 hours). Variables that independently correlated with mortality in themultivariate analysis were CPAP adherence, hypertension, and forced expiratory volume in one second (FEV1) percentpredicted. Approximately 50% of the deaths were cardiovascular in origin. Cumulative survival rates in the CPAP > 6-h group and in the CPAP 1–6 h group were significantly higher than the CPAP < 1 h group, p< 0.00005 and p= 0.01, respectively. Adapted from Campos-Rodriguez et al. [41].
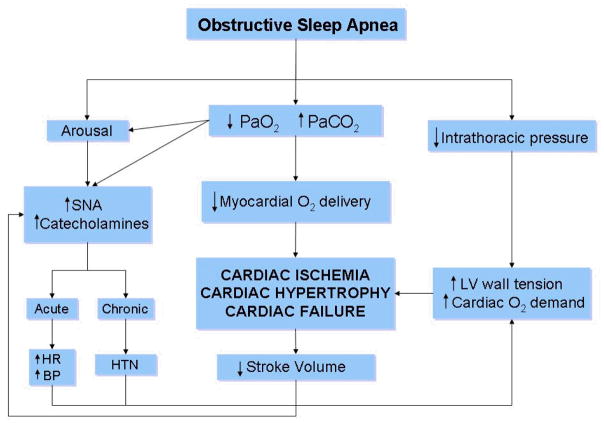
In summary, mounting evidence from clinic-based and epidemiologic population-based studies have established a strong and independent association between OSA and various cardiovascular conditions (Table 5). Moreover, uncontrolled large observational studies have shown that if left untreated, patients with severe OSA have an increased cardiovascular morbidity and mortality. The strongest evidence for adverse cardiovascular events associated with OSA seems to be linked to those with severe disease, whereas the data for the link in mild-moderate patients is less clear. Figure 4 summarizes the potential mechanisms by which OSA adversely impacts the cardiovascular system [111]. Although more data is needed in this area, it is challenging to perform randomized controlled trials to assess the impact of CPAP therapy on cardiovascular outcomes in patients with severe OSA due to ethical concerns.
TABLE 5.
Association of OSA with cardiovascular disease from population-based studies
| Condition | Number | Age | BMI | Measure of Risk | AHI ≥ 11 vs. < 5 |
|---|---|---|---|---|---|
| Hypertension¶ Peppard et al. [85] |
709 | 46 | 29 | Adjusted odds ratio† | 2.89 (95% CI, 1.46–5.64) |
| Hypertension¶ Nieto et al. [84] |
6,132 | Age ≥ 65 in 46.7% of subjects | 28.5 | Adjusted odds ratio†† | 1.37 (95% CI, 1.03–1.83) |
| Congestive heart failure Shahar et al. [96] |
6,424 | 66 (OSA) 61 (control) |
30.9 25.9 |
Adjusted odds ratio* | 2.20 (95% CI, 1.11–4.37) |
| Stroke¶ Arzt et al. [95] |
1,475 | 47 | 30 | Adjusted odds ratio§ | 4.33 (95% CI, 1.32–14.24) |
| Stroke Shahar et al [96] |
6424 | 66 (OSA) 61 (control) |
30.9 25.9 |
Adjusted odds ratio* | 1.58 (95% CI, 1.02–2.46) |
| Coronary artery disease Shahar et al. [96] |
6,424 | 66 (OSA) 61 (control) |
30.9 25.9 |
Adjusted odds ratio* | 1.27 (95% CI, 0.99–1.62) |
| Atrial fibrillation Mehra et al. [106] |
566 | 70.6 (OSA) 68.6 (control) |
30.1 28.5 |
Adjusted odds ratio‡ | 4.02 (95% CI, 1.03–15.74) |
BMI: body mass index (kg/m2); AHI: apnea-hypopnea index, OSA: Obstructive sleep apnea; CI: confidence interval; NR: not reported for the entire cohort.
Studies in which the BMI and/or age are the mean of the entire population.
Adjusted for:
Age, sex, BMI, hypertension, neck and waist circumference, alcohol and smoking
Age, sex, ethnicity, BMI, neck circumference, waist-to-hip ratio, alcohol and smoking
Age, sex, race, self-reported diabetes and hypertension, cholesterol and smoking
Age, sex, BMI, alcohol and smoking
Age, sex, BMI, coronary heart disease
Figure 4.
The pathophysiological mechanisms by which OSA can lead to adverse cardiovascular outcomes. SNA: sympathetic neural activity, HR: heart rate, BP: blood pressure, HTN: hypertension, LV: left ventricle, O2: oxygen, PaO2: partial pressure of oxygen, PaCO2: partial pressure of carbon dioxide. Reprinted with permission from Leung RS et al. [111].
Type 2 Diabetes and Insulin Resistance/Glucose Intolerance
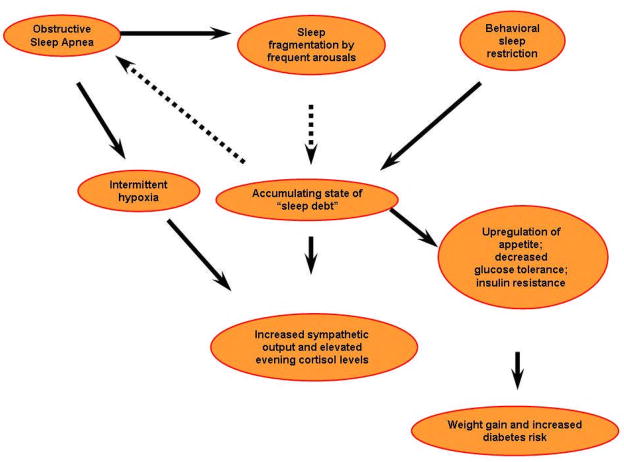
Several population-based studies from various geographical regions and involving a variety of ethnic groups have reported an independent association between self-reported snoring [112–118] or various measures of severity of OSA quantified by polysomnography (AHI or oxygen saturation) to altered glucose metabolism, insulin resistance, metabolic syndrome, and type 2 diabetes [115,119–124]. These associations have been established independent of the degree of obesity and adiposity which are major determinants of glucose metabolism dysregulation. The vast majority of the epidemiologic data has been provided by cross-sectional studies as opposed to longitudinal studies. Definitive evidence supporting the direction of causality is still needed from both population-based longitudinal studies and large-scale studies with carefully selected patient populations with OSA, adequately controlling for potential confounders, in particular visceral adiposity [125,126]. In addition, OSA is highly prevalent amongst patients with type 2 diabetes [68,69]. These remarkable associations raise the possibility that OSA may be a novel risk factor for type 2 diabetes or alternatively, chronic hyperglycemia may promote OSA. Figure 5 describes the putative mechanisms by which OSA can lead to insulin resistance, weight gain and increased risk of type 2 diabetes [127]. Therefore, we believe that clinicians would be well advised to systematically evaluate the risk of OSA in patients with type 2 diabetes, and conversely to rule out type 2 diabetes/glucose intolerance in patients with known OSA.
Figure 5.
Putative mechanisms linking OSA and sleep fragmentation/loss with insulin resistance and increased risk of Type 2 diabetes. Reprinted with permission from Ip M et al. [127].
Neurocognitive Function, Depression, and Motor Vehicle Accidents
OSA can cause daytime sleepiness and adversely impact daytime functioning such as work performance, motor function, and neurocognitive function [128–130]. Although the degree of OSA defined by the AHI is usually assumed to be predictive of cognitive impairment [131], another important factor is the duration and severity of hypoxemia which has been shown independently to have an adverse impact on cognitive tasks [132]. To further support the cause and effect relationship between OSA and neurocognitive dysfunction, investigators have been able to demonstrate a linear relationship between adherence with CPAP therapy for OSA and the percentage of patients achieving normal daytime function those with longer duration of therapy (over 7 hours) experiencing the most clinical benefits [133,134].
Several clinic-based studies have established an association between OSA and depression [135]. In a longitudinal population-based study, the Sleep Heart Health investigators were able to demonstrate a causal link between OSA severity and depression. In this study, subjects with moderate or worse OSA (AHI ≥ 15) had a 2.6 fold increase odds of developing depression after adjusting for confounders [136]. Studies demonstrating improvement in depression after successful treatment of OSA with CPAP therapy provide further evidence for a cause and effect relationship between OSA and depression [137].
The motor and neurocognitive dysfunction induced by OSA has been implicated as a risk factor for motor vehicle accidents [8,131,138,139]. Not only does untreated OSA increase the rate of motor vehicle accidents when compared with age and gender matched controls, but the severity of the accidents can lead to more personal injury [140]. Effective therapy for OSA can improve motor and neurocognitive function, resulting in enhanced driving performance and reduction in motor vehicle accidents to the rate seen in the general population [139,141].
IMPACT OF UNDIAGNOSED AND UNTREATED OSA
The Impact of OSA on Healthcare Utilization
Several studies have shown that patients with the OSA syndrome utilize healthcare resources almost 2 fold higher—with heavier use of resources seen in women—compared with control patients matched for age, gender, and area of residency [142–145]. Most of these costs were attributed to more days spent in the hospital, higher physician fees including more specialist consultations, and increase in prescribed medications. Furthermore, effective treatment of OSA with CPAP improved associated healthcare costs. When comparing healthcare utilization in men and women with OSA from 5 years before establishing the diagnosis to 5 years after the diagnosis, patients treated effectively with CPAP had healthcare costs that gradually decreased whereas matched controls continued to have an increase in healthcare utilization costs [146,147].
Unfortunately, the shortcomings of these studies was the inability to match control subjects for BMI; therefore the health care cost difference could be attributable to obesity—known to be associated with increased health care costs [148]. Also, the majority of these studies target patients with severe OSA; therefore, generalizing the decreases in health care costs to those with milder forms of OSA may not be appropriate [149]. Despite these methodologic limitations, the above studies collectively draw attention to the profound economic impact of OSA, especially if left untreated, and the beneficial effects of appropriate therapy. Future studies matching control patients by degree of obesity can clarify the impact that OSA has on healthcare economic costs.
The Burden of Undiagnosed OSA
Evidence suggests that a large proportion of individuals with OSA remain undiagnosed. Analysis of two large population-based studies—Wisconsin Sleep Cohort and the Sleep Heart Health Study—estimated that up to 80% of individuals with moderate or severe OSA enrolled in these studies more than a decade ago had remained undiagnosed by their physicians despite adequate access to health care [5,6]. With the ongoing obesity epidemic, there is no compelling reason to believe that the proportion of undiagnosed OSA in the population has decreased over the years despite increased overall awareness of OSA amongst the medical community and the lay population. In fact, the 2005 Sleep in America Poll identified 25% of the respondents as being high risk for OSA further supporting the high burden of this condition in the Western society [150].
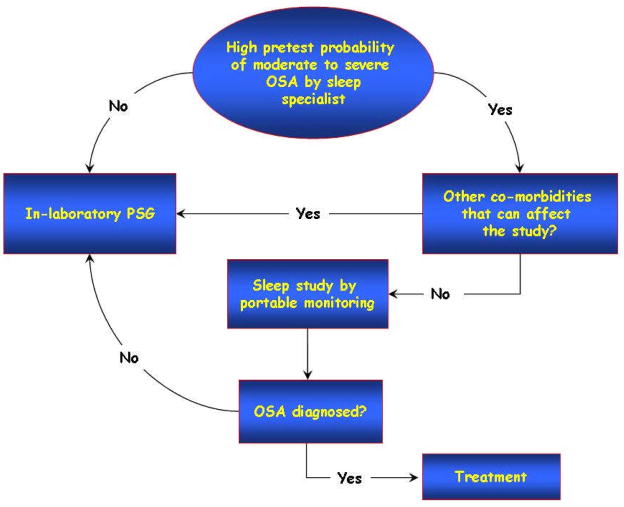
Now more than ever, there is a need to improve access to diagnostic and effective treatment strategies for patients with OSA [151]. Although an overnight polysomnogram is considered the gold standard diagnostic study, it has several limitations including a prolonged wait time depending on available local resources, inconvenience of an overnight sleep study and expense, especially for lower socioeconomic areas. Split night studies, which combine a diagnostic polysomnogram and therapeutic positive pressure titration in one night, are useful in limiting the amount of time spent in the sleep laboratory. Split night studies are cost effective, more convenient for patients, and lead to improved access to care. Although most useful for patient’s with severe OSA, the disadvantages include decreased time devoted to CPAP titration [152]. Of particular interest is the utility of portable monitoring and empiric auto-CPAP treatment in patients with a high pretest clinical probability for at least moderate or severe OSA who do not have co-existing cardiopulmonary conditions complicating OSA [153]. Although portable monitoring may emerge as a new diagnostic tool, clinical guideless to address careful interpretation is imperative to continue to advance the field. Figure 6 describes a useful approach to effective use of home monitoring as proposed by the American Academy of Sleep Medicine [13].
Figure 6.
Algorithmic approach to using portable monitoring to diagnose OSA as proposed by the American Academy of Sleep Medicine [13].
EXPERT COMMENTARY
In the last decade, extensive well-designed epidemiologic research has clarified the incidence and risk factors for developing OSA as well as its global prevalence. There is a growing body of literature linking OSA with cardiovascular morbidity and mortality, metabolic dysfunction, and neurocognitive impairment. Current evidence suggests that effective treatment of OSA can improve neurocognitive function and reduce the risk for adverse cardiovascular events.
FIVE YEAR VIEW
During the next 5 years, the health care community will continue to face a variety of medical conditions associated with obesity particularly an increasing global prevalence of OSA. The gradual recognition of this disease by both the lay and medical community will lead to improved identification of patients with OSA. With such a high prevalence of OSA in the general population, further research is needed to clarify the role of various diagnostic strategies such as in-laboratory polysomnography and portable home monitoring. In addition, the beneficial impact of CPAP therapy in mild to moderate OSA, particularly in the “non-sleepy” patients warrants further investigation. Finally, additional research is needed to evaluate the impact of OSA therapy on cardiovascular and metabolic outcomes. The field of sleep medicine is still in its infancy with a plethora of data waiting to be uncovered in the near future.
KEY ISSUES
Obstructive sleep apnea, characterized by repeated episodes of complete or partial obstruction of the upper airway during sleep, has gained significant interest due to the global obesity epidemic.
Several population-based studies across various geographical regions and ethnic groups demonstrate a surprisingly similar prevalence of OSA syndrome, approximately 4 % in men and 2% in women.
Risk factors for developing OSA include obesity, aging (up to age 65), male gender, menopause, craniofacial abnormalities, upper airway anatomy, smoking and alcohol intake, and genetic predisposition. Obesity is the most important modifiable risk factor for OSA but the impact of obesity is diminished in the elderly.
OSA is an independent risk factor for hypertension; however, the treatment of OSA in hypertensive patients has generally resulted in mixed results.
OSA has been linked to adverse cardiovascular outcomes including coronary artery disease, stroke, congestive heart failure, and atrial fibrillation. Evidence from large observational studies demonstrates that treatment can improve cardiovascular morbidity and mortality.
Patients with the OSA syndrome utilize more healthcare resources compared with matched control patients.
Footnotes
Disclosure of conflict of interest: The authors do not have any financial or other potential conflicts of interest to declare.
Contributor Information
Won Lee, Fellow, Sleep Medicine, Section of Pulmonary and Critical Care Medicine, The University of Chicago Pritzker School of Medicine, 5841 S. Maryland Ave, Sleep Disorders Center W 4, Chicago, Illinois 60637, won.lee@uchospitals.edu
Swamy Nagubadi, Fellow, Sleep Medicine, Section of Pulmonary and Critical Care Medicine, The University of Chicago Pritzker School of Medicine, 5841 S. Maryland Ave, Sleep Disorders Center W 4, Chicago, Illinois 60637, swamy.nagubadi@uchospitals.edu
Meir H. Kryger, Director of Research and Education, Gaylord Hospital Sleep Medicine, Gaylord Farm Road, Wallingford, CT, 06492, mkryger@gaylord.org
Babak Mokhlesi, Assistant Professor of Medicine, Section of Pulmonary and Critical Care Medicine, Director, Sleep Disorders Center and Sleep Medicine Fellowship Program, The University of Chicago Pritzker School of Medicine, Chicago, Illinois 60637, bmokhles@medicine.bsd.uchicago.edu
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Ruhm CJ, Kosa KM. Economic causes and consequences of obesity. Annu Rev Public Health. 2005;26:239–257. doi: 10.1146/annurev.publhealth.26.021304.144628. [DOI] [PubMed] [Google Scholar]
- 3.Hensrud DD, Klein S. Extreme obesity: a new medical crisis in the United States. Mayo Clin Proc. 2006;81:S5–10. doi: 10.1016/s0025-6196(11)61175-0. [DOI] [PubMed] [Google Scholar]
- 4.Haslam DW, James WP. Obesity Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 5.Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 8.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–956. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 9.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–197. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 12.Punjabi NM, Newman A, Young T, Resnick HE, Sanders M. Sleep-disordered Breathing and Cardiovascular Disease: An Outcome-based Definition of Hypopneas. Am J Respir Crit Care Med. 2008 Feb 14; doi: 10.1164/rccm.200712-1884OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.*.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. This article provides the most recent statement from the American Academy of Sleep Medicine’s position on portable monitoring for OSA. [PMC free article] [PubMed] [Google Scholar]
- 14.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test . Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 15.**.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. Comprehensive review providing in depth insight into OSA epidemiology. [DOI] [PubMed] [Google Scholar]
- 16.Manser RL, Rochford P, Pierce RJ, Byrnes GB, Campbell DA. Impact of different criteria for defining hypopneas in the apnea-hypopnea index . Chest. 2001;120:909–914. doi: 10.1378/chest.120.3.909. [DOI] [PubMed] [Google Scholar]
- 17.Redline S, Kapur VK, Sanders MH, et al. Effects of varying approaches for identifying respiratory disturbances on sleep apnea assessment . Am J Respir Crit Care Med. 2000;161:369–374. doi: 10.1164/ajrccm.161.2.9904031. [DOI] [PubMed] [Google Scholar]
- 18.Tsai WH, Flemons WW, Whitelaw WA, Remmers JE. A comparison of apnea-hypopnea indices derived from different definitions of hypopnea . Am J Respir Crit Care Med. 1999;159:43–48. doi: 10.1164/ajrccm.159.1.9709017. [DOI] [PubMed] [Google Scholar]
- 19.*.Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9:419–436. doi: 10.1016/j.smrv.2005.04.005. This article reviews different ethnic features contributing to OSA in the non-white population. [DOI] [PubMed] [Google Scholar]
- 20.**.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. The most rigorous and carefully performed population study evaluating the prevalence of OSA in adults. [DOI] [PubMed] [Google Scholar]
- 21.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 22.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender . Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 23.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 24.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea A population study in Australian men . Am J Respir Crit Care Med. 1995;151:1459–1465. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 25.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong . Chest. 2001;119:62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences . Chest. 2004;125:127–134. doi: 10.1378/chest.125.1.127. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women . Am J Respir Crit Care Med. 2004;170:1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 28.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men . Am J Respir Crit Care Med. 2004;169:168–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 29.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–156. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 30.*.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–2237. doi: 10.1001/jama.289.17.2230. Longitudinal study clarifying the incidence of OSA over 5 years. [DOI] [PubMed] [Google Scholar]
- 31.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 33.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study . Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 34.*.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. This article reviews the impact of obesity on OSA epidemiology. [DOI] [PubMed] [Google Scholar]
- 35.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis . JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 36.Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjostrom L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30:703–710. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome . Proc Am Thorac Soc. 2008;5:218–225. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea . Sleep Breath. 2007;11:117–124. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 39.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.*.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. This large observational study demonstrated that treatment of severe OSA with CPAP can decrease cardiovascular morbidity and mortality. [DOI] [PubMed] [Google Scholar]
- 41.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure . Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 42.*.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. This large prospective clinic-based study provided compelling evidence on the impact of OSA on stroke and death. [DOI] [PubMed] [Google Scholar]
- 43.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep . Respir Physiol. 1991;83:189–200. doi: 10.1016/0034-5687(91)90028-h. [DOI] [PubMed] [Google Scholar]
- 44.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 45.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 46.Hudgel DW, Devadatta P, Hamilton H. Pattern of breathing and upper airway mechanics during wakefulness and sleep in healthy elderly humans. J Appl Physiol. 1993;74:2198–2204. doi: 10.1152/jappl.1993.74.5.2198. [DOI] [PubMed] [Google Scholar]
- 47.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep . Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults . Sleep medicine reviews. 2007;11:99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 50.Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing . Clin Chest Med. 2004;25:257–268. doi: 10.1016/j.ccm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Quintana-Gallego E, Carmona-Bernal C, Capote F, et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients . Respir Med. 2004;98:984–989. doi: 10.1016/j.rmed.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome . Sleep. 2005;28:309–314. [PubMed] [Google Scholar]
- 53.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? . Arch Intern Med. 1996;156:2445–2451. [PubMed] [Google Scholar]
- 54.Young T, Finn L. Epidemiological insights into the public health burden of sleep disordered breathing: sex differences in survival among sleep clinic patients. Thorax. 1998;53 (Suppl 3):S16–19. doi: 10.1136/thx.53.2008.s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea . J Appl Physiol. 2005;99:2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea . Am J Respir Crit Care Med. 2000;161:1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 57.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 58.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing . Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 59.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status . J Appl Physiol. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 60.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 61.White DP. The hormone replacement dilemma for the pulmonologist . Am J Respir Crit Care Med. 2003;167:1165–1166. doi: 10.1164/rccm.2302007. [DOI] [PubMed] [Google Scholar]
- 62.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 63.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–1949. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 64.O’Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study . Sleep. 2003;26:74–79. [PubMed] [Google Scholar]
- 65.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 66.Sakakibara H, Tong M, Matsushita K, Hirata M, Konishi Y, Suetsugu S. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–410. doi: 10.1183/09031936.99.13240399. [DOI] [PubMed] [Google Scholar]
- 67.Yu X, Fujimoto K, Urushibata K, Matsuzawa Y, Kubo K. Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest. 2003;124:212–218. doi: 10.1378/chest.124.1.212. [DOI] [PubMed] [Google Scholar]
- 68.Foster G, Kuna ST, Sanders M, et al. Sleep apnea in obese adults with type 2 diabetes: baseline results from the Sleep AHEAD study . Sleep. 2005;28:A606. [Google Scholar]
- 69.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes . Thorax. 2006;61:945–950. doi: 10.1136/thx.2005.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome . J Clin Endocrinol Metab. 2001;86:1175–1180. doi: 10.1210/jcem.86.3.7316. [DOI] [PubMed] [Google Scholar]
- 71.Tasali E, Van Cauter E, Ehrmann DA. Relationships between sleep disordered breathing and glucose metabolism in polycystic ovary syndrome . J Clin Endocrinol Metab. 2006;91:36–42. doi: 10.1210/jc.2005-1084. [DOI] [PubMed] [Google Scholar]
- 72.Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance . J Clin Endocrinol Metab. 2001;86:517–520. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]
- 73.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension . J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 74.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 75.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–256. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 76.Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 77.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report . Int J Cardiol. 2006;106:21–28. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 78.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure . Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 80.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack . Am J Respir Crit Care Med. 2000;161:375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 81.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 82.Hui DS, Choy DK, Wong LK, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: results in chinese patients with first-ever ischemic stroke . Chest. 2002;122:852–860. doi: 10.1378/chest.122.3.852. [DOI] [PubMed] [Google Scholar]
- 83.Bixler EO, Vgontzas AN, Lin HM, et al. Association of hypertension and sleep-disordered breathing . Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 84.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study Sleep Heart Health Study . JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 85.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 86.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study . Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 87.Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness . Thorax. 2006;61:1083–1090. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial . Chest. 2006;129:1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 89.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure . Hypertension. 2006;47:840–845. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 90.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients . Eur Respir J. 2006;27:1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 91.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea . Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 92.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome . Am J Respir Crit Care Med. 2001;163:344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 93.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials . Arch Intern Med. 2007;167:757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 94.Hu FB, Willett WC, Manson JE, et al. Snoring and risk of cardiovascular disease in women . J Am Coll Cardiol. 2000;35:308–313. doi: 10.1016/s0735-1097(99)00540-9. [DOI] [PubMed] [Google Scholar]
- 95.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke . Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study . Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 97.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 98.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men . Lancet. 1990;336:261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 99.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 100.Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92:79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 101.Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–86. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 102.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 103.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study . Eur Heart J. 2004;25:728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea . N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 105.Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure . Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 106.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study . Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation . J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 108.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients . Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 109.*.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. This study uncovered the alarming finding of increased sudden death at night in individuals with OSA in contrast to the general population. [DOI] [PubMed] [Google Scholar]
- 110.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk . Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 111.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease . Am J Respir Crit Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 112.Joo S, Lee S, Choi HA, et al. Habitual snoring is associated with elevated hemoglobin A1c levels in non-obese middle-aged adults . J Sleep Res. 2006;15:437–444. doi: 10.1111/j.1365-2869.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- 113.Renko AK, Hiltunen L, Laakso M, Rajala U, Keinanen-Kiukaanniemi S. The relationship of glucose tolerance to sleep disorders and daytime sleepiness. Diabetes Res Clin Pract. 2005;67:84–91. doi: 10.1016/j.diabres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 114.Shin C, Kim J, Kim J, et al. Association of habitual snoring with glucose and insulin metabolism in nonobese Korean adult men . Am J Respir Crit Care Med. 2005;171:287–291. doi: 10.1164/rccm.200407-906OC. [DOI] [PubMed] [Google Scholar]
- 115.Elmasry A, Lindberg E, Berne C, et al. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population-based study . J Intern Med. 2001;249:153–161. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 116.Grunstein RR, Stenlof K, Hedner J, Sjostrom L. Impact of obstructive sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. Int J Obes Relat Metab Disord. 1995;19:410–418. [PubMed] [Google Scholar]
- 117.Jennum P, Schultz-Larsen K, Christensen N. Snoring, sympathetic activity and cardiovascular risk factors in a 70 year old population. Eur J Epidemiol. 1993;9:477–482. doi: 10.1007/BF00209524. [DOI] [PubMed] [Google Scholar]
- 118.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study . Am J Epidemiol. 2002;155:387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 119.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study . Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 120.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance . Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 122.Okada M, Takamizawa A, Tsushima K, Urushihata K, Fujimoto K, Kubo K. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med. 2006;45:891–896. doi: 10.2169/internalmedicine.45.1592. [DOI] [PubMed] [Google Scholar]
- 123.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29:777–783. doi: 10.1093/sleep/29.6.777. [DOI] [PubMed] [Google Scholar]
- 124.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men . Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 125.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation . Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 126.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 127.Ip M, Mokhlesi B. Sleep and glucose intolerance/diabetes mellitus. Sleep Med Clin. 2007;2:19–29. doi: 10.1016/j.jsmc.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Engleman HM, Douglas NJ. Sleep 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome . Thorax. 2004;59:618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation . Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 130.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea . Clin Chest Med. 2003;24:249–259. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 131.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20:608–613. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 132.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study . Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 133.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning . Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis . Arch Intern Med. 2003;163:565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 135.Saunamaki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2007;116:277–288. doi: 10.1111/j.1600-0404.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 136.*.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–1715. doi: 10.1001/archinte.166.16.1709. This study further clarified the significant association between OSA and depression. [DOI] [PubMed] [Google Scholar]
- 137.Schwartz DJ, Karatinos G. For individuals with obstructive sleep apnea, institution of CPAP therapy is associated with an amelioration of symptoms of depression which is sustained long term. J Clin Sleep Med. 2007;3:631–635. [PMC free article] [PubMed] [Google Scholar]
- 138.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander . N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 139.**.Ellen RL, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. Comprehensive review of literature on the relationship between OSA and motor vehicle accidents. [PubMed] [Google Scholar]
- 140.Mulgrew A, Nasvadi G, Butt A, et al. Risk and Severity of Motor Vehicle Crashes in Patients with Obstructive Sleep Apnea Hypopnea. Thorax. 2008 Jan 30; doi: 10.1136/thx.2007.085464. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 141.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP . Thorax. 2001;56:508–512. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? . Chest. 2002;121:164–172. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 143.Tarasiuk A, Greenberg-Dotan S, Brin YS, Simon T, Tal A, Reuveni H. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005;128:1310–1314. doi: 10.1378/chest.128.3.1310. [DOI] [PubMed] [Google Scholar]
- 144.Greenberg-Dotan S, Reuveni H, Simon-Tuval T, Oksenberg A, Tarasiuk A. Gender differences in morbidity and health care utilization among adult obstructive sleep apnea patients. Sleep. 2007;30:1173–1180. doi: 10.1093/sleep/30.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, Oksenberg A, Reuveni H. The effect of obstructive sleep apnea on morbidity and health care utilization of middle-aged and older adults. J Am Geriatr Soc. 2008;56:247–254. doi: 10.1111/j.1532-5415.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 146.Albarrak M, Banno K, Sabbagh AA, et al. Utilization of healthcare resources in obstructive sleep apnea syndrome: a 5-year follow-up study in men using CPAP . Sleep. 2005;28:1306–1311. doi: 10.1093/sleep/28.10.1306. [DOI] [PubMed] [Google Scholar]
- 147.Banno K, Manfreda J, Walld R, Delaive K, Kryger MH. Healthcare utilization in women with obstructive sleep apnea syndrome 2 years after diagnosis and treatment . Sleep. 2006;29:13071311. doi: 10.1093/sleep/29.10.1307. [DOI] [PubMed] [Google Scholar]
- 148.Wittmann V, Rodenstein DO. Health care costs and the sleep apnea syndrome . Sleep medicine reviews. 2004;8:269–279. doi: 10.1016/j.smrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 149.Ayas NT, Marra C. Continuous positive airway pressure therapy for obstructive sleep apnea syndrome: do the dollars make sense? . Sleep. 2005;28:1211–1213. doi: 10.1093/sleep/28.10.1211. [DOI] [PubMed] [Google Scholar]
- 150.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130:780–786. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 151.Phillips B. Improving access to diagnosis and treatment of sleep-disordered breathing. Chest. 2007;132:1418–1420. doi: 10.1378/chest.07-1793. [DOI] [PubMed] [Google Scholar]
- 152.Patel NP, Ahmed M, Rosen I. Split-night polysomnography. Chest. 2007;132:1664–1671. doi: 10.1378/chest.06-1801. [DOI] [PubMed] [Google Scholar]
- 153.*.Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146:157–166. doi: 10.7326/0003-4819-146-3-200702060-00004. Randomized study supporting portable monitoring and auto-CPAP for high risk patients with OSA. [DOI] [PubMed] [Google Scholar]