Abstract
Numerous studies have investigated the potential use of TNF-related apoptosis-inducing ligand (TRAIL) as a cancer therapeutic since its discovery in 1995 – because TRAIL is a potent inducer of apoptosis in tumor cells but not in normal cells and tissues. Consequently, a great deal is known about TRAIL/TRAIL receptor expression, the molecular components of TRAIL receptor signaling, and methods of altering tumor cell sensitivity to TRAIL-induced apoptosis. Our laboratory was the first to report the possibility of TRAIL gene transfer therapy as an alternative method of using TRAIL as an antitumor therapy. As with recombinant proteins administered systemically, intratumoral TRAIL gene delivery also has limitations that can restrict its full potential. Translating the preclinical TRAIL studies into the clinic has started, with the hope that TRAIL will exhibit robust tumoricidal activity against human primary tumors in situ with minimal toxic side effects.
Keywords: TRAIL, apoptosis, tumor, adenovirus, immunotherapy
INTRODUCTION
Cell death can be generally classified into two forms based on morphological and biochemical criteria: necrosis and apoptosis [1–3]. Necrosis is the nonphysiological or passive type of cell death usually resulting from extreme cellular trauma, generally affecting groups of cells rather than individual cells, and typically evoking inflammation [4]. Cells undergoing necrosis normally have problems maintaining proper plasma membrane function, so they can no longer regulate osmotic pressure. Consequently, the cells swell and rupture, spilling their cellular contents into the surrounding tissue, resulting in the nonspecific cellular destruction that leads to an inflammatory response necessary to remove the debris and begin tissue repair.
Apoptosis is a more subtle process usually affecting scattered single cells, and is characterized histologically by the formation of small, spherical cytoplasmic fragments, some of which contain pyknotic remnants of nuclei [3]. The nuclear changes are accompanied by fragmentation of the cellular DNA into a ladder of regular subunits, the result of random double-stranded breaks in the linker regions between nucleosomes [5]. While these intracellular changes are taking place, the cell membrane becomes ruffled and blebbed in a process called zeiosis [6]. The fate of the apoptotic cell is to be phagocytosed by surrounding cells before it can rupture and release its potentially inflammatory contents. Thus, apoptotic cell death is well-suited for a role in tissue homeostasis, as it results in the deletion of cells with little tissue disruption.
The rapid phagocytosis of apoptotic cells suggests that alterations in the plasma membrane occur early in the death process, targeting these cells for removal. Phagocytic recognition of the plasma membrane alterations in dying cells can occur through several different mechanisms [7]. For example, the αvβ3 vitronectin receptor, recognition of thrombospondin, membrane carbohydrate changes, and changes in cell membrane net surface charge have all been described as recognition elements for phagocytic cells [7]. Another possible marker for apoptotic cell identification is through changes in membrane composition involving the loss of cell membrane phospholipid asymmetry [8, 9]. A normal plasma membrane exhibits a marked phospholipid asymmetry, where phosphatidylcholine and sphingomyelin are predominantly on the outer membrane leaflet and most of the phosphatidylethanolamine and phosphatidylserine (PS) are on the inner membrane leaflet [10]. This asymmetry is maintained by an ATP- and Mg2+ -dependent translocase mediating the inward transport of negatively charged phospholipids and/or association of PS with membrane skeletal proteins [11]. During apoptosis, cell membrane leaflet composition changes, with a redistribution of negatively charged PS to the outer leaflet. Surface exposure of PS occurs with chromatin condensation, precedes the increase in membrane permeability, and constitutes one of the principal targets of phagocyte recognition.
In recent years, work from Dr. Shigezaku Nagata’s laboratory has identified two molecules essential in the phagocytosis of PS-expressing apoptotic cells. One is milk fat globule epidermal growth factor (EGF) factor 8 (MFG-E8), which binds to apoptotic cells by recognizing PS and enhances the engulfment of apoptotic cells by macrophages [12, 13]. Analysis of MFG-E8-deficient mice found they contained many apoptotic lymphocytes attached to macrophages, but they were not being efficiently engulfed. In addition, MFG-E8-deficient mice were prone autoimmunity, most likely resulting from the inability to clear apoptotic cells. More recently, a report from Miyanishi and colleagues [14] identified T cell immunoglobulin- and mucin-domain-containing molecule-1 (TIM-1) and TIM-4 as PS receptors for the engulfment of apoptotic cells. Thus, the once indiscriminate process of apoptotic cell phagocytosis is now being defined at a molecular level.
With the identification of these basic characteristics, apoptotic cell death was found to be a normal physiological process associated with a variety of basic systems, such as embryonic development, tissue remodeling, immune regulation, and tumor regression, which is regulated in situ by many extracellular and intracellular signals [15, 16]. Survival signals from the environment and intracellular sensor molecules that monitor cellular integrity normally regulate a cell’s apoptotic machinery. For example, in the event a cell loses contact with its surroundings or sustains irreparable internal damage, the apoptotic death process is commonly initiated. Apoptosis also helps to balance the number of cells within a multicellular organism that arise through normal cellular proliferation, maintaining the normal homeostatic nature of the organism. Just as with apoptosis, cell proliferation is a highly regulated process with numerous regulatory proteins. A variety of growth factors and proto-oncogenes serve as positive regulators of cell cycle progression. In contrast, there are multiple tumor suppressor genes that function to oppose uncontrolled cell proliferation by inhibiting the activity of the proto-oncogenes. Thus, a balance in the cellular proliferation and death rate must be maintained for homeostasis, and alterations in proliferation or death can lead to a multitude of disease states.
Cancer is probably the most commonly studied disease associated with defects in the apoptotic process. Tumor cells from a wide array of human malignancies have a decreased ability to undergo apoptosis, and the development of therapeutic agents targeting the apoptosis pathway within tumor cells has consequently become heavily investigated – especially the induction of tumor cell apoptosis through an active, instructive process mediated by cell surface death receptors that transmit apoptotic signals when bound by their cognate death ligands. The death receptors belong to the tumor necrosis factor (TNF) receptor superfamily, and are characterized by cysteine-rich extracellular domains [17, 18]. Additionally, all death receptors contain a homologous cytoplasmic sequence called the death domain (DD) that serves as the recognition point for the apoptotic machinery [18–20]. The ligands for the death receptors belong to the TNF family of cytokines, a group of molecules that influence a variety of immunological functions. For example, TNF and Fas ligand (FasL) are two of the most studied death ligands that induce apoptosis in many physiological events, such as autoimmunity, activation-induced cell death (AICD), immune privilege, and evasion of tumors from the immune system [21–25].
TRAIL IS A POTENT INDUCER OF TUMOR CELL APOPTOSIS
TNF-related apoptosis inducing ligand (TRAIL) is another TNF family member that can induce apoptosis, and has received great attention because of its cancer therapeutic potential. While searching for molecules containing a conserved sequence present in many TNF family members, Wiley et al. identified an expressed sequence tag that was then used to clone the full length TRAIL [26] (or Apo-2 ligand [27]) cDNA. The extracellular domain of TRAIL is most homologous to Fas ligand (28% a.a. identity), but it also has significant identity to TNF (23%), lymphotoxin (LT)-α (23%), and LT-β (22%). Whereas the homology of TRAIL to other TNF family members may be considered low, examination of the crystal structure of monomeric TRAIL found it to be very similar to that of TNF and CD40 ligand [28]. TRAIL monomers contain two antiparallel β-pleated sheets that form a β sandwich core framework, and the monomers interact with other TRAIL monomers in a head-to-tail fashion, to form a bell-shaped trimer [28]. This oligomerization enhances TRAIL activity as studies with recombinant soluble TRAIL found that multimeric, or crosslinked, forms possessed the most biological activity, rather than monomeric forms of TRAIL [26].
Early studies identified two unique characteristics of TRAIL. First, TRAIL-induced apoptosis occurs only in tumorigenic or transformed cells and not normal cells [26]. As with the other death-inducing members of the TNF family (i.e. FasL and TNF), cells undergoing TRAIL-induced death exhibited many of the hallmarks of apoptosis, including DNA fragmentation, expression of pro-phagocytic signals (i.e. phosphatidylserine) on the cell membrane, and cleavage of multiple intracellular proteins by caspases [26, 27, 29, 30]. Second, in contrast to other TNF family members whose expression is tightly regulated and often transiently expressed, mRNA for TRAIL is detected in a wide range of tissues, including peripheral blood lymphocytes, spleen, thymus, prostate, ovary, small intestine, colon and placenta [26]. Within the immune system, TRAIL can be expressed by human T cells after CD3 crosslinking and type I IFN stimulation - perhaps contributing to the activation-induced cell death of T cells in the natural setting [31]. In addition, human NK, B cells, monocytes, and dendritic cells express membrane-bound TRAIL following cytokine stimulation (especially type I and II IFN), transforming them into potent tumor cell killers [32–35]. Our group is also one of several to show that human polymorphonuclear neutrophils (PMN) are a rich source of TRAIL [36–40], which can be released in a functional soluble form after stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) [39, 41, 42].
TRAIL RECEPTOR SIGNAL TRANSDUCTION
Unlike FasL and TNF, which interact with a single or pair of receptors, respectively, TRAIL specifically binds to four distinct receptors: DR4 [43], DR5/TRAIL-R2 [44–46], TRID/DcR1/TRAIL-R3 [44, 45, 47], and TRAIL-R4/DcR2 [48, 49] (hereafter referred to as TRAIL-R1, -R2, -R3, and -R4, respectively). Both TRAIL-R1 and TRAIL-R2 contain a cytoplasmic DD, and crosslinking by TRAIL or receptor-specific mAb activates the apoptosis signaling pathway in sensitive cells [30, 43–46]. In contrast, neither TRAIL-R3 (which is GPI linked) nor TRAIL-R4 (which is a type I membrane protein) contains a complete cytoplasmic death domain, and neither can induce apoptosis after ligation [44, 45, 47–49]. Because TRAIL can bind to TRAIL-R3 and/or -R4 without directly signaling for cell death, it was initially proposed that these receptors inhibit TRAIL-induced apoptosis by acting either as antagonistic receptors [44, 45, 47] or via transduction of an anti-apoptotic signal [48]. Therefore, the presence or absence of TRAIL-R3 and/or -R4 was thought to determine whether a cell was resistant or sensitive, respectively, to TRAIL-induced apoptosis [44, 45, 49]. Further investigation of many tumor cell lines, however, disproved this theory as the sole mechanism regulating TRAIL-sensitivity and resistance [30, 50].
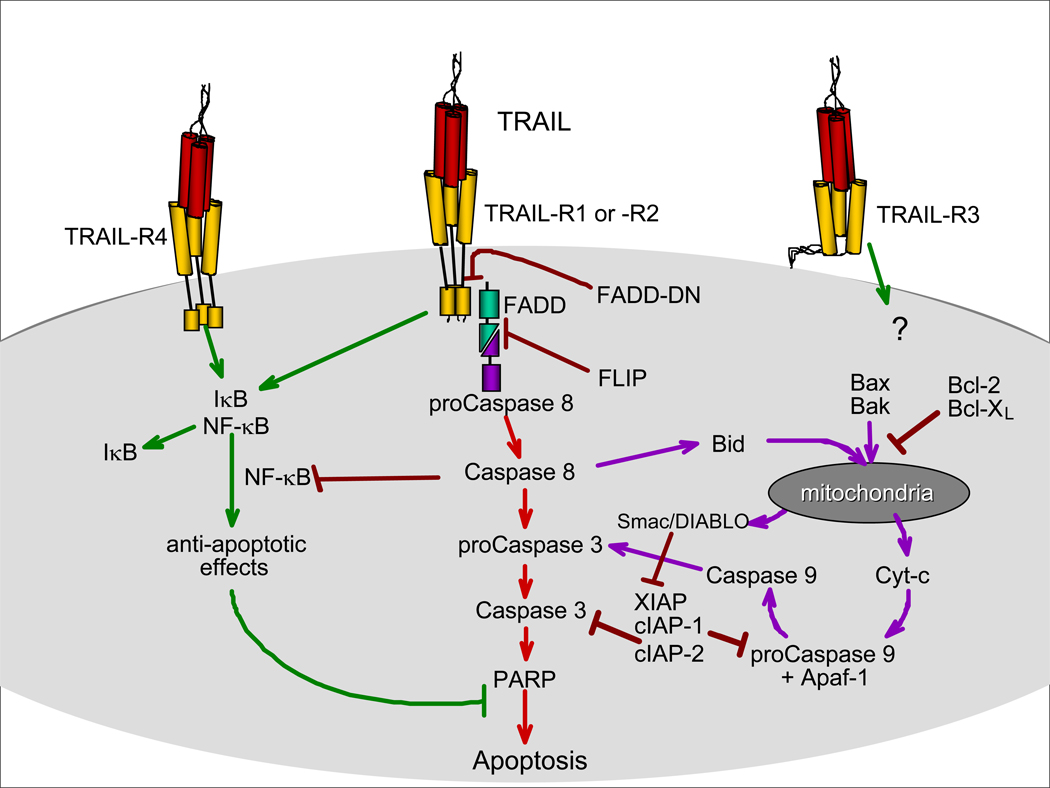
Initial evaluation of the TRAIL receptor signaling pathway proved difficult, yielding contradictory results from different laboratories. It took 5 years to fully elucidate the molecular signal transduction events leading to apoptosis induced via TRAIL/TRAIL receptor interaction [51, 52]. Death receptor, be it TRAIL-R1/R2, Fas, or TNF-R1, crosslinking leads to the formation of a multiprotein structure called the death-inducing signaling complex (DISC [53]) that includes the death receptor, the death adapter protein Fas-associated death domain protein (FADD [54]), and the proteolytic cysteine protease procaspase-8. All death receptors share a homophilic protein/protein intracellular domain, called the DD [19, 20], which serves as the recognition point for specialized apoptosis signaling molecules that often contain a DD themselves. In a homotypic interaction, the DD of FADD binds to the DD of TRAIL-R1 or –R2. The death effector domain (DED) of FADD, in turn, interacts with the DED of procaspase-8 [55]. Procaspase-8 is proteolytically cleaved and activated at the DISC, which then initiates the apoptosis executing caspase cascade. The downstream executioner caspases, caspase-3, −6, and −7, are then activated to cleave the numerous structural and regulatory proteins that maintain cellular integrity. The apoptotic signal from TRAIL-R1/-R2 can also be amplified by engaging the apoptotic mediators present in mitochondria and activation of the apoptosome [56]. Figure 1 shows a schematic representation of the principal molecules involved in the TRAIL-R1/-R2 signal transduction pathway.
Figure 1.
Schematic representation of TRAIL receptor signaling.
TRAIL AS AN ANTITUMOR THERAPEUTIC
Since the first report describing TRAIL [26], the majority of the published studies have focused on its tumoricidal activity in vitro and in vivo, with the goal of developing this molecule into an antitumor therapeutic agent. Early in vitro analyses demonstrated that recombinant, soluble TRAIL possessed the unique ability of inducing apoptosis in a broad range of tumor cell lines, while having little to no activity against normal cells [26, 27, 29]. Based on these in vitro results, it was suggested that TRAIL could be used as an anti-tumor therapy without the side effects seen with other TNF family members - namely FasL and TNF. The first reports examining the antitumor activity of TRAIL in vivo also evaluated its toxicity against normal tissues. Walczak et al. [57] compared the effects of intravenous administration of soluble TRAIL or FasL into immunocompetent mice. Injection of TRAIL (either human or murine, 200 µg) did not result in any observable toxic effects, whereas mice given human FasL (50 µg) were dead within 1 h due to massive hepatocellular degeneration, necrosis, and hemorrhage. Histologic examination of tissues obtained from mice treated for a longer period (500 µg/d for 14 days) also demonstrated no toxicity. Further investigation into the potential side effects of TRAIL was performed in nonhuman primates by Ashkenazi et al. [58]. The exposure of cynomolgus monkeys to TRAIL at 0.1–10 mg/kg/d over 7 days did not induce detectable toxicity. In contrast, TNF induced severe toxicity at 0.003 mg/kg/d. Interestingly, certain recombinant forms of soluble TRAIL have been shown to induce apoptosis in human hepatocytes in vitro [59, 60], raising some concerns about the therapeutic potential of TRAIL. The explanation for the observed hepatocyte killing may actually lie in the form of TRAIL used. A TRAIL monomer of native sequence contains a single cysteine, Cys-230, and when forming trimeric TRAIL the cysteines from three monomers are close to each other and chelate Zn2+ [28, 61]. In contrast, the poly-His tagged recombinant TRAIL version that demonstrated toxicity toward hepatocytes in vitro [59, 60] had a low Zn2+ content and displayed an aberrant structure compared to native TRAIL [62]. These results suggest that the question of liver toxicity was nothing more than an in vitro observation entirely dependent upon the form of TRAIL used, and the use of untagged TRAIL (a.k.a. Apo2L/TRAIL.0 [62]) in a therapeutic setting in humans will not display any toxicity.
These results clearly demonstrated the safety of large, systemic doses of TRAIL, leading to further studies that evaluated the antitumor activity in vivo. In these experiments, immunocompromised mice were subcutaneously injected with human tumor cells, after which intraperitoneal or intravenous injections of soluble TRAIL were started after tumor implantation [57, 58]. Multiple doses of TRAIL beginning the day after tumor implantation suppressed tumor outgrowth, with many animals becoming tumor-free. Further studies have shown that the in vivo antitumor activity of TRAIL is enhanced when combined with chemotherapeutics and ionizing radiation [49, 58, 63]. The antitumor activity of systemically administered TRAIL in these experiments demonstrated that TRAIL could interact with the primary tumor and, potentially, any metastases that would normally be difficult to detect and/or treat. One major drawback to these findings, however, was that large amounts of TRAIL (up to 500 µg/d) were required to inhibit tumor formation, since most of the protein was cleared within 5 hours [57]. Furthermore, the systemic administration of TRAIL appeared to be most successful when administered shortly after tumor implantation [49, 57, 58, 63]. Although these previous results show promise, successful treatment relied on repeated, systemic administration of large amounts of soluble TRAIL to have an effect. Yet, the administration of equivalent doses of recombinant TRAIL protein into humans may be problematic. Despite these possible concerns, recombinant soluble TRAIL protein and several TRAIL-R1 and –R2-specific agonistic mAb have been tested clinically [64]. The generation of large amounts of clinical-grade recombinant TRAIL protein or agonistic mAb can be accomplished with current technologies, and because these are human proteins there is little/no immunological response induced that could potentially neutralize their cytotoxic effects. That said, the systemic administration of these molecules requires repeated injections to maintain high enough concentrations to have a potential therapeutic response. Thus, the development of an alternative means of delivery may increase the relative activity of TRAIL such that larger, more established tumors may be eradicated as efficiently as smaller tumors.
An alternative approach, which was first reported from our laboratory, is the delivery of the TRAIL cDNA using a nonreplicative adenoviral vector [65]. Since that report, there have been a number of recombinant viral vectors containing the TRAIL cDNA described in the literature [66–78]. Localized therapy of solid tumors has been successful in a number of settings. For example, the treatment of prostate cancer with local (intraprostatic) regimens is also common practice, with cryotherapy, brachytherapy, and several experimental viral-based studies serving as current treatment options [79–81]. Of the viral-based therapies, published data indicate minimal toxicity for adenovirus injection into the prostate up to doses of 1011 pfu [82]. As demonstrated in the studies performed to date, transfer of the TRAIL gene by Ad5-TRAIL into human prostate tumor cells in vitro and in vivo led to the rapid transcription and translation of the transferred TRAIL gene into functional TRAIL protein that, when expressed on the cell surface, induced apoptotic death in TRAIL-sensitive tumor cell targets but not normal cells [65, 69]. In addition, we observed sustained TRAIL expression (up to 7 d) after a single intratumoral injection of Ad5-TRAIL [69] – something that would not be possible with the administration of recombinant TRAIL protein.
Adenoviral-mediated gene transfer has been well-studied over the past decade, and one major criticism of this approach is the fact that adenovirus is highly immunogenic and most people have been exposed to adenovirus at some point in their life [83, 84]. This fact is one of the reasons for the limited clinical success of adenoviral-mediated gene transfer protocols. In their study of agents to enhance the delivery of recombinant viral vectors, Siemens et al. observed that the coinjection of adenovirus in a collagen-based matrix led to enhanced adenoviral transgene expression, and stimulated the generation of transgene-specific CTL responses – even in the presence of preexisting anti-adenoviral immunity [85]. Thus, in addition to generating “less immunogenic” adenoviral vectors through genetic modifications, there are ways to minimize the immunological response that could restrict the therapeutic potential of adenoviral vectors.
We recently completed a Phase I clinical trial with Ad5-TRAIL in men with locally-confined prostate cancer, where the primary objectives were to determine the toxicity profile and maximal tolerated dose. The ability of the vector to induce tumor cell death was also evaluated. Patients with histologically confirmed adenocarcinoma of the prostate [clinical stage (as per AJCC 2002, 6th edition) -T1c, T2a, T2b all of whom are clinically N0, M0] and scheduled to undergo radical prostatectomy within 10 days following study entry were eligible for the study. The vector was administered intraprostatically in a collagen matrix (Gelfoam, 30 mg/ml) to 12 patients using the following dose escalation: Level 1 – 1.3 × 108 pfu (4.2 × 109 particles); Level 2 – 4.2 × 108 pfu (1.3 × 1010 particles); Level 3 – 1.3 × 109 pfu (4.2 × 1010 particles); Level 4 – 4.2 × 109 pfu (1.3 × 1011 particles). Each group received injections of Ad5-TRAIL in Gelfoam divided equally into both lobes of the prostate, further divided into 2 injection points in each lobe. No adverse reactions were observed in any of the patients treated, and all patients tolerated the injection. Further, there were no complications with the surgical removal of the prostate after Ad5-TRAIL injection.
The clinical trial protocol specified that 3 subjects be enrolled at each of 4 dose levels with a 4 week break between dose levels to allow observation for side effects at each level. The Gelfoam could be detected by histological assessment in the injected prostate after prostatectomy. Inflammatory cells were evident in the areas where Gelfoam was present, as well as TUNEL-positive staining – indicating DNA fragmentation that is indicative of apoptotic death. Serum samples from each patient were also assayed for caspase 3 activity, another marker of apoptotic death. Increases in active caspase 3 were detected in every patient. These are the first pieces of data, to our knowledge, evaluating TRAIL gene transfer therapy in humans. Studies are planned to further test the therapeutic potential of Ad5-TRAIL in patients with metastatic disease.
While one of the intended uses of Ad5-TRAIL is to kill tumor cells present at the site of injection, we also envision that intratumoral injection of Ad5-TRAIL will generate sufficient apoptotic tumor cell debris that a systemic tumor-specific immune response will develop. We recently published a report that offered a glimpse of the ability of localized, intratumoral delivery of Ad5-TRAIL to induce systemic antitumor immunity [86]. It was our hypothesis that increasing the amount of antigen for presentation would elicit a stronger antitumor T cell response. When Ad5-TRAIL was given intratumorally to mice bearing experimental renal cell carcinoma (Renca) tumors, the mice displayed only a minimal increase in survival and a low level of CTL activity. To enhance DC efficiency, an immunostimulatory CpG oligodeoxynucleotide (CpG ODN) was co-administered intratumorally with the Ad5-TRAIL. This combination therapy augmented in vivo antigen-specific T cell proliferation and CTL activity, as well as significantly prolonging the survival of Renca tumor-bearing mice. Interestingly, depletion of CD4+ or CD25+ cells prior to therapy further enhanced survival and in vivo CTL activity In addition, tumor-free mice depleted of CD4+ cells were also able to reject a subsequent challenge of Renca cells, but not MHC-matched RM-11 prostate tumor cells, demonstrating the existence of immunologic memory. In recent years, the pivotal role that CD4+ T cells play in the induction of CD8+ T cell responses has been highlighted [32, 87–89], where most CD8+ T cell-mediated responses depend on concomitant CD4+ T cell priming to be effective. In addition, CD8+ T cell priming in the absence of CD4+ T cell help leads to their deletion from the periphery, an effect that can be overcome by supplying help during the initial priming phase [90]. Thus, we were surprised by the observation that CD4+ cell depletion prior to Ad5-mTRAIL/CpG ODN treatment augmented the CD8+ T cell response and significantly enhanced animal survival. CD40-CD154 interactions between APC and CD4+ T cells are important in facilitating CD8+ CTL cross-priming events [32, 87], and CD40 ligation on the APC increases CD80/CD86 expression [91], which is essential for proper T cell activation. In this regard, Prilliman et al. [92] demonstrated that CD8+ CTL cross-priming could still occur in the absence of CD4+ T cell help provided there was sufficient CD28 signaling with an agonistic mAb. Because of its profound immunostimulatory nature, we hypothesize that the CpG ODN used in our system substituted for the required CD4+ T cell help. The report by Cho et al. [93] supports this concept, where they were able to induce CD8+ CTL activity in CD4 or MHC class II-deficient animals. These results importantly demonstrated that local treatment with Ad5-TRAIL and CpG ODN (in this experimental setting) can augment tumor antigen cross presentation resulting in T cell proliferation, enhanced CTL activity, and increased animal survival. Current studies are assessing the Ad5-TRAIL/CpG ODN-induced immune response in an experimental model of metastatic cancer.
Though our results showed Ad5-TRAIL was effective in suppressing tumor outgrowth in vivo, additional debulking of the tumor by increasing the tumoricidal activity of Ad5-TRAIL should only make the task of removing any residual tumor cells by the immune system easier. Methods to enhance the distribution and expression of recombinant genes are currently being investigated. For entry into a cell by receptor-mediated endocytosis, Group C adenovirus (e.g. adenovirus type 5) requires interaction between the viral fiber capsid protein with the coxsackie and adenovirus receptor (CAR) and the viral penton base binding to αv integrins [94–96]. With the principal viral vector for cancer gene therapy being adenovirus, the success of any adenoviral-based gene transfer therapy is dictated by (CAR) recognition [95, 97]. While CAR is ubiquitously expressed in most benign epithelial tissues, there can be marked variations in amount of CAR expressed by different tumor cell lines of the same tissue origin [98]. For example, Okegawa et al. examined three prostate cancer cell lines and found that CAR is down-regulated in the tumorigenic cell line PC-3 compared to DU-145 and LNCaP [99]. Despite these variations, adenoviral infection of human prostate tumor cells appears to be very efficient, and numerous reports have evaluated the use of recombinant adenoviral vectors in the treatment of prostate cancer [100–107]. Furthermore, the utility of replication-deficient adenoviral vectors for cancer gene therapy is also restricted by their inability of infect every cell within a solid tumor mass [108]. If CAR expression on target cells could be increased, one would predict that the clinical efficacy of adenoviral-based gene transfer therapy for cancer would also be increased. Consequently, numerous substances have been tested as vehicles to enhance gene delivery and sustain expression to both cancerous and normal tissues [109–111].
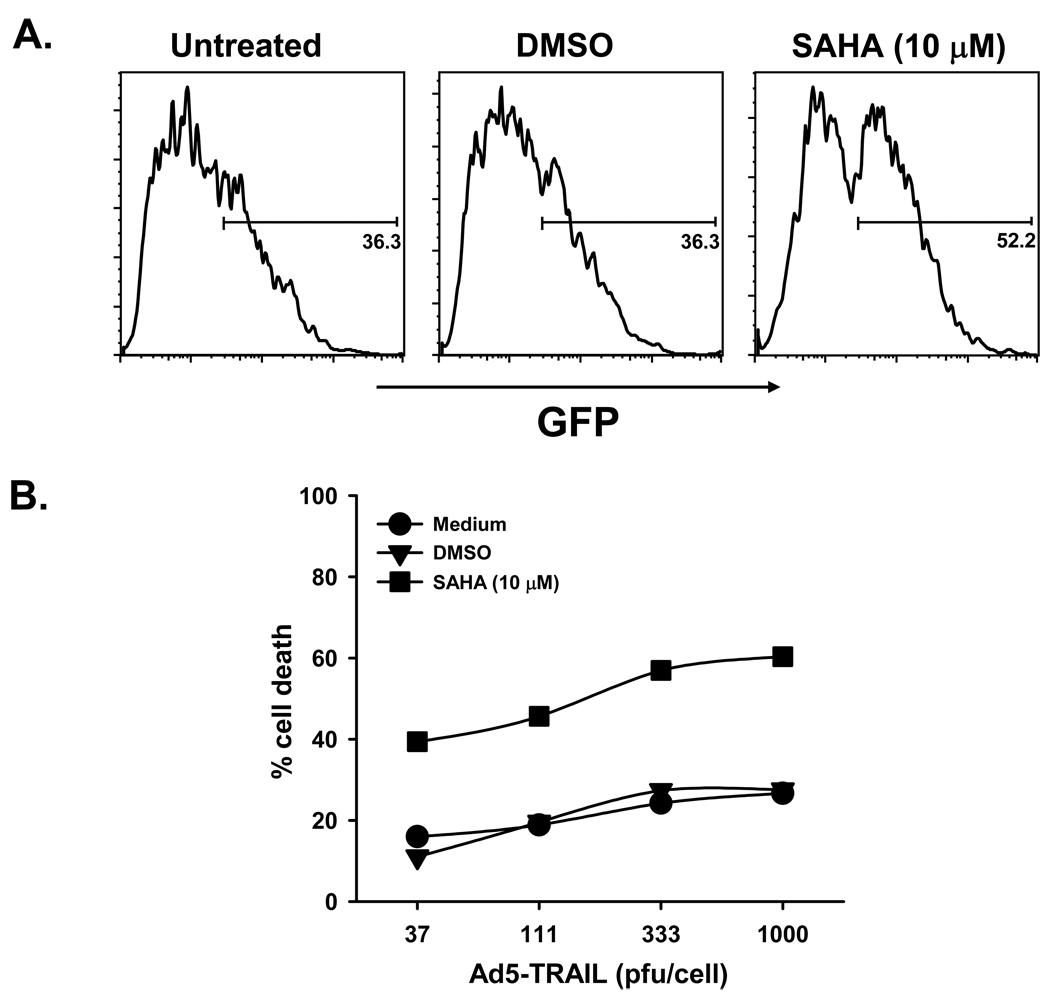
Histone deacetylases (HDAC) catalyze the removal of acetyl groups from the ɛ-amino group on lysine residues of proteins, including the core nucleosomal histones H2A, H2B, H3, and H4, and together with histone acetyltransferases regulate histone acetylation levels. The balance of nucleosomal histone acetylation plays an important regulatory role in chromatin structure and transcription of many genes [112–115]. HDAC inhibition leads to the accumulation of marked amounts of acetylated histone species that result in profound effects on tumor cells, such as inhibiting cell cycle, or inducing differentiation or apoptosis [113, 116, 117]. Currently, there are several HDAC inhibitors (HDACi) undergoing Phase I and II testing as single agent therapy as a treatment for cancer. We became interested in combining Ad5-TRAIL with HDACi because they were also shown to increase adenoviral infection of tumor cells and enhance adenoviral transgene expression [98, 118, 119]. We have performed studies with a number of histone deacetylase HDACi, including depsipeptide, MS-275, oxamflatin, suberoylanilide hydroxamic acid (SAHA), sodium butyrate, trichostatin A, and valproic acid. These HDAC inhibitors display differential abilities to increase the susceptibility of tumor cells to adenoviral infection, augment the level of adenoviral transgene expression in vitro and in vivo, and increase the tumoricidal activity of Ad5-TRAIL [120–124]. For example, human prostate tumor cells became more susceptible to adenoviral infection and Ad5-TRAIL-induced killing after SAHA pretreatment compared to untreated or vehicle-treated cells (Figure 2). Not only do HDACi increase tumor cell susceptibility to adenoviral infection, but adenoviral transgene expression is also enhanced in HDACi-treated cells [124]. In addition, our studies have identified several mechanisms by which tumor cell susceptibility to Ad5-TRAIL-induced death occurs, including facilitating TRAIL-R1/-R2 redistribution to lipid rafts, overall increased TRAIL-R1/-R2 expression, increased caspase-2 activity, and accelerated DISC formation [121–124]. Reports describing the efficacy of TRAIL-based therapies combined with HDACi are beginning to appear in the literature [125–129], which will open new avenues of cancer therapy.
Figure 2.
HDACi treatment increases prostate tumor cell sensitivity to adenovirus infection and Ad5-TRAIL-induced cell death. DU-145 cells were treated for 16 h with vehicle or suberoylanilide hydroxamic acid (SAHA) at the indicated concentrations. A The cells were then cultured with (A) Ad5-eGFP (100 pfu/cell) or (B) Ad5-TRAIL (at the indicated doses) for 24 h. Cell infection was determined by quantitiating the percentage of GFP+ cells by flow cytometry. Cell death was determined by crystal violet staining.
MECHANISMS REGULATING SENSITIVITY/ RESISTANCE TO TRAIL-INDUCED APOPTOSIS
Despite TRAIL’s ability to induce apoptosis in a wide variety and number of human tumor cell lines, there are many reports describing the existence of TRAIL-resistant tumor cells. Consequently, investigation into the mechanism for tumor cell resistance to TRAIL-induced apoptosis, and ways to overcome the resistance, has become an intense area of research. It is also believed that studies examining TRAIL-resistant tumor cells may reveal clues to explain the profound level of resistance shown by normal cells and tissues to TRAIL-induced apoptosis. TRAIL mRNA is constitutively expressed in a wide variety of tissue and cell types [26], suggesting that the restricted expression of the different TRAIL receptors regulates the induction of TRAIL-mediated apoptosis. However, as previously discussed, mRNA for the four TRAIL receptors has been detected in a wide range of normal cells and tissues, and each of the four TRAIL receptors is capable of binding TRAIL with comparable affinity [47, 48]. Therefore, the initial hypothesis to explain a cell’s ability to respond to TRAIL was that the non-death-inducing TRAIL receptors (TRAIL-R3 and –R4) were acting as “decoys”, and were the chief molecules determining whether a cell was resistant or sensitive to TRAIL-induced death [44, 45, 49]. This hypothesis seemed logical and drew support from experiments utilizing TRAIL-sensitive cells overexpressing either TRAIL-R3 or -R4, resulting in an inhibition of TRAIL-induced apoptotic cell death [44, 45, 49]. Rather surprisingly, though, immunohistochemical analysis of several human tissues (brain, colon, heart, liver, lung, kidney, and testis) found TRAIL-R3 expression only within the brain, heart, liver, and testis [130]. Additionally, TRAIL-R4 may be more effective than TRAIL-R3 in protecting target cells from TRAIL-induced death [48], perhaps because TRAIL-R4 ligation activates NF-κB, a known inhibitor of death-ligand-induced apoptosis [131, 132]. NF-κB activation can prevent cells from undergoing TNF-induced cell death, probably by up-regulating expression of a gene or group of genes whose products are anti-apoptotic [131, 132]. Support for the protective role of TRAIL-R3 and/or –R4 against TRAIL-induced tumor cell death was recently provided by Sanlioglu et al, who reported that TRAIL-R4 expression was well correlated with TRAIL resistance in human breast tumor cell lines [133]. Additional studies from this group elegantly showed that modulation of TRAIL receptor composition, especially TRAIL-R4, on human lung and prostate tumor cells using an siRNA approach improved the tumoricidal activity of adenoviral-mediated delivery of the TRAIL cDNA into the cells [134, 135]. Thus, the premise of tumor cell sensitivity/resistance being controlled by TRAIL-R3/-R4 expression remains a plausible theory. That said, TRAIL-R1 and -R2 ligation also activates NF-κB [136, 137], though still resulting in apoptotic cell death. Thus, such explanations cannot fully account for resistance to TRAIL-induced apoptosis. While TRAIL-R3 and/or -R4 expression may indeed be a means of regulating TRAIL-mediated apoptosis, analysis of TRAIL receptor mRNA and protein expression in a panel of human tumor cell lines by RT-PCR, immunoblotting, and flow cytometry have indicated no correlation between TRAIL resistance and TRAIL-R3/-R4 mRNA expression.
A more defendable hypothesis involves the differential expression of any of a number of pro- and anti-apoptotic proteins within the tumor cell that collectively help to regulate the signals generated from trimerized TRAIL-R1 and/or –R2. Molecules such as FLIP, Bcl-2 family members, inhibitors of apoptosis (IAP) proteins, the ERK survival pathway, Akt, and Toso, have all been implicated in regulating the TRAIL receptor signal transduction pathway [29, 138–147]. Furthermore, identification of numerous chemotherapeutic drugs that specifically regulate the levels of these proteins, as well as others important in the TRAIL receptor signaling pathway, has been shown to alter tumor cell sensitivity to TRAIL. It is likely that therapies that combine TRAIL with agents that target apoptosis-regulatory proteins will be necessary to make TRAIL a feasible alternative approach for treating cancer. Recently, it was found that expression of the peptidyl O-glycosyltransferase GALNT14 correlated with TRAIL-sensitivity in a number of human tumor cell lines [61]. O-glycosylation of TRAIL-R1 and –R2 promoted ligand-stimulated clustering of the receptor, which mediated the recruitment and activation of caspase-8. Further evidence demonstrating the ability of GALNT14 to sensitize tumor cells to TRAIL was obtained by RNAi-mediated knock-down, which reduced cellular sensitivity to TRAIL-mediated killing.
A third option for explaining the differences in tumor cell sensitivity to TRAIL-induced apoptosis focuses on cell cycle progression. Apoptosis of dividing cells occurs at various stages of the cell cycle depending on the cell type and/or death-inducing stimulus. For example, TNF- and Fas ligand-induced apoptosis is influenced by cell cycle stage. Moreover, inappropriate regulation of the cell cycle machinery can also result in induction of apoptosis. A study by Jin et al. [148] determined, by using SW480 colon cancer and H460 lung cancer cell lines, that arresting these cells at the G0/G1 phase resulted in increased sensitivity to TRAIL-induced apoptosis compared to the same cells arrested at other cell cycle phases. The mechanism by which G1-arrested cells display increased sensitivity to TRAIL remains to be determined. One possible explanation may lie in the levels of anti-apoptotic proteins during the different cell cycle phases. Though using a different system, Algeciras-Schimnich et al. [149] found that activated T cells arrested in G1 phase contained high levels of FLIP protein, which correlated with an increase in TCR-induced apoptosis observed in other cell cycle phases. It is possible that FLIP and/or other pro- or anti-apoptotic proteins fluctuate in a similar fashion in tumor cells.
Tumor cells are commonly found to contain genetic abnormalities that account for the production of mutated proteins. Thus, it is possible that some tumors possess mutations that inhibit TRAIL-induced apoptosis and the blockade of TRAIL-induced apoptosis facilitates the survival of the tumor cells. The TRAIL-R2 gene has been mapped to human chromosome 8p21 [150], a region that is frequently the site of losses of heterozygosity in many types of cancer [151]. Mutations of the TRAIL-R2 gene have been identified in head and neck cancer, non-small cell lung cancer, breast cancer, non-Hodgkin’s lymphoma, colorectal cancer, gastric cancer, and hepatocellular carcinoma [152–157]. In each case, the mutations have been located within the DD, which is important for connecting the receptor to the apoptotic signal transduction pathway. The genes for the four TRAIL receptors are tightly clustered on human chromosome 8p21–22 [46–48], suggesting they evolved relatively recently via gene duplication. It remains to be determined if the other TRAIL receptor genes are also mutated in some cancers that alter tumor cell sensitivity to TRAIL-induced apoptosis.
CONCLUDING THOUGHTS
The development of TRAIL as a potential anti-cancer therapeutic arose from its ability to potently induce apoptosis in tumor cells, while having little or no detectable cytotoxic effects on normal cells and tissues. The identification of four distinct receptors that can bind TRAIL has significantly increased the potential complexity of this receptor/ligand system in terms of understanding normal physiological functions and as a therapeutic molecule. Not only will it be essential to optimize the form of TRAIL (protein or gene) used in therapy, as well as the mode of delivery, it may be even more important to further understand the mechanism(s) by which tumor cells regulate their sensitivity to TRAIL-induced apoptosis. At the same time, elucidating the molecular basis that protects normal cells and tissues from TRAIL will also be essential in formulating TRAIL-based therapies for cancer that will not be toxic to normal cells within the body. Since its identification over 10 years ago, there has been a great deal learned regarding how TRAIL functions within the body in tumor and non-tumor scenarios. Future studies will continue to develop TRAIL into the powerful anticancer therapeutic many believe it can become.
ACKNOWLEDGEMENTS
The authors wish to thank Badri Konety, M.D., Fadi Joudi, M.D., Richard Williams, M.D., Timothy Ratliff, Ph.D., Tammy Madsen, Carlene Etscheidt, and Barbara Zeigler for their essential contributions to the Ad5-TRAIL clinical trial. This work was supported by the following funding agencies: Alliance for Cancer Gene Therapy, Department of Defense Prostate Cancer Research Program, and the National Cancer Institute (CA109446).
REFERENCES
- 1.Nadler R, Luo Y, Zhao W, et al. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guerin (BCG) mediated antitumour activity. Clin Exp Immunol. 2003;131:206–216. doi: 10.1046/j.1365-2249.2003.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 3.Vaux DL, Strasser a. The molecular biology of apoptosis. Proc Natl Acad Sci U S A T. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 5.Cao XX, Mohuiddin I, Ece F, McConkey DJ, Smythel WR. Histone deacetylase inhibitor down regulation of bcl-xl gene expression leads to apoptotic cell death in mesothelioma. Am J Respir Cell Mol Biol. 2001;25:562–568. doi: 10.1165/ajrcmb.25.5.4539. [DOI] [PubMed] [Google Scholar]
- 6.Godman GC, Miranda AF, Deitch AD, Tanenbaum SW. Action of cytochalasin D on cells of established lines. III. Zeiosis and movements at the cell surface. J Cell Biol. 1975;64:644–667. doi: 10.1083/jcb.64.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadok VA, Savill JS, Haslett C, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 8.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 9.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993;5:661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 11.Bevers EM, Tilly RH, Senden JM, Comfurius P, Zwaal RF. Exposure of endogenous phosphatidylserine at the outer surface of stimulated platelets is reversed by restoration of aminophospholipid translocase activity. Biochemistry. 1989;28:2382–2387. doi: 10.1021/bi00432a007. [DOI] [PubMed] [Google Scholar]
- 12.Hanayama R, Tanaka M, Miyasaka K, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka K, Hanayama R, Tanaka M, Nagata S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur J Immunol. 2004;34:1414–1422. doi: 10.1002/eji.200424930. [DOI] [PubMed] [Google Scholar]
- 14.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 15.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 17.Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6:407–413. doi: 10.1016/0952-7915(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 18.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 19.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNFreceptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 20.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 21.Cerami A, Beutler B. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol Today. 1988;9:28–31. doi: 10.1016/0167-5699(88)91353-9. [DOI] [PubMed] [Google Scholar]
- 22.Alderson MR, Tough TW, Davis-Smith T, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 24.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 25.Hahne M, Rimoldi D, Schroter M, et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 26.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 27.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 28.Cha SS, Kim MS, Choi YH, et al. 2.8 A resolution crystal structure of human TRAIL, a cytokine with selective antitumor activity. Immunity. 1999;11:253–261. doi: 10.1016/s1074-7613(00)80100-4. [DOI] [PubMed] [Google Scholar]
- 29.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 30.Griffith TS, Rauch CT, Smolak PJ, et al. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol. 1999;162:2597–2605. [PubMed] [Google Scholar]
- 31.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: A novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;88:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemp TJ, Moore JM, Griffith TS. Human B Cells Express Functional TRAIL/Apo-2 Ligand after CpG-Containing Oligodeoxynucleotide Stimulation. J Immunol. 2004;173:892–899. doi: 10.4049/jimmunol.173.2.892. [DOI] [PubMed] [Google Scholar]
- 36.Tecchio C, Huber V, Scapini P, et al. IFNalpha-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood. 2004;103:3837–3844. doi: 10.1182/blood-2003-08-2806. [DOI] [PubMed] [Google Scholar]
- 37.Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64:1037–1043. doi: 10.1158/0008-5472.can-03-1808. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig AT, Moore JM, Luo Y, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 39.Kemp TJ, Ludwig AT, Earel JK, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–3482. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassatella MA, Huber V, Calzetti F, et al. Interferon-activated neutrophils store a TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) intracellular pool that is readily mobilizable following exposure to proinflammatory mediators. J Leukoc Biol. 2006;79:123–132. doi: 10.1189/jlb.0805431. [DOI] [PubMed] [Google Scholar]
- 41.Simons MP, Leidal KG, Nauseef WM, Griffith TS. TNF-related apoptosis-inducing ligand (TRAIL) is expressed throughout myeloid development, resulting in a broad distribution among neutrophil granules. J Leukoc Biol. 2008;83:621–629. doi: 10.1189/jlb.0707452. [DOI] [PubMed] [Google Scholar]
- 42.Simons MP, Moore JM, Kemp TJ, Griffith TS. Identification of the mycobacterial subcomponents involved in the release of tumor necrosis factor-related apoptosis-inducing ligand from human neutrophils. Infect Immun. 2007;75:1265–1271. doi: 10.1128/IAI.00938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 44.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 45.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 46.Walczak H, Degli-Esposti MA, Johnson RS, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degli-Esposti MA, Smolak PJ, Walczak H, et al. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 49.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 50.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 51.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 52.Sprick MR, Weigand MA, Rieser E, et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 53.Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 55.Medema JP, Scaffidi C, Kischkel FC, et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 57.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 58.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brandau S, Suttmann H, Riemensberger J, et al. Perforin-mediated lysis of tumor cells by Mycobacterium bovis Bacillus Calmette-Guerin-activated killer cells. Clin Cancer Res. 2000;6:3729–3738. [PubMed] [Google Scholar]
- 60.Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 61.Wagner KW, Punnoose EA, Januario T, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 62.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 63.Chinnaiyan AM, Prasad U, Shankar S, et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ashkenazi A, Herbst RS. To kill a tumor cell: The potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffith TS, Anderson RD, Davidson BL, Williams RD, Ratliff TL. Adenoviral-mediated transfer of the TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces tumor cell apoptosis. J Immunol. 2000;165:2886–2894. doi: 10.4049/jimmunol.165.5.2886. [DOI] [PubMed] [Google Scholar]
- 66.Armeanu S, Lauer UM, Smirnow I, et al. Adenoviral gene transfer of tumor necrosis factor-related apoptosis-inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Res. 2003;63:2369–2372. [PubMed] [Google Scholar]
- 67.Carlo-Stella C, Lavazza C, Di Nicola M, et al. Antitumor activity of human CD34+ cells expressing membrane-bound tumor necrosis factor-related apoptosis-inducing ligand. Hum Gene Ther. 2006;17:1225–1240. doi: 10.1089/hum.2006.17.1225. [DOI] [PubMed] [Google Scholar]
- 68.Dong F, Wang L, Davis JJ, et al. Eliminating established tumor in nu/nu nude mice by a tumor necrosis factor-alpha-related apoptosis-inducing ligand-armed oncolytic adenovirus. Clin Cancer Res. 2006;12:5224–5230. doi: 10.1158/1078-0432.CCR-06-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Griffith TS, Broghammer EL. Suppression of tumor growth following intralesional therapy with TRAIL recombinant adenovirus. Mol Ther. 2001;4:257–266. doi: 10.1006/mthe.2001.0439. [DOI] [PubMed] [Google Scholar]
- 70.Kagawa S, He C, Gu J, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- 71.Kock N, Kasmieh R, Weissleder R, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9:435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee J, Hampl M, Albert P, Fine HA. Antitumor activity and prolonged expression from a TRAIL-expressing adenoviral vector. Neoplasia. 2002;4:312–323. doi: 10.1038/sj.neo.7900245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lillehammer T, Engesaeter BO, Prasmickaite L, Maelandsmo GM, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J Gene Med. 2007;9:440–451. doi: 10.1002/jgm.1036. [DOI] [PubMed] [Google Scholar]
- 74.Seol JY, Park KH, Hwang CI, et al. Adenovirus-TRAIL can overcome TRAIL resistance and induce a bystander effect. Cancer Gene Ther. 2003;10:540–548. doi: 10.1038/sj.cgt.7700597. [DOI] [PubMed] [Google Scholar]
- 75.Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002;9:164–172. doi: 10.1038/sj.cgt.7700420. [DOI] [PubMed] [Google Scholar]
- 76.Wenger T, Mattern J, Haas TL, et al. Apoptosis mediated by lentiviral TRAIL transfer involves transduction-dependent and -independent effects. Cancer Gene Ther. 2007;14:316–326. doi: 10.1038/sj.cgt.7701016. [DOI] [PubMed] [Google Scholar]
- 77.Yang F, Shi P, Xi X, et al. Recombinant adenoviruses expressing TRAIL demonstrate antitumor effects on non-small cell lung cancer (NSCLC) Med Oncol. 2006;23:191–204. doi: 10.1385/MO:23:2:191. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Ma H, Zhang J, Liu S, Liu Y, Zheng D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life Sci. 2008;82:1154–1161. doi: 10.1016/j.lfs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 79.Denis LJ. The role of active treatment in early prostate cancer. Radiother Oncol. 2000;57:251–258. doi: 10.1016/s0167-8140(00)00284-x. [DOI] [PubMed] [Google Scholar]
- 80.Stone NN, Stock RG. Prostate brachytherapy in patients with prostate volumes >/= 50 cm(3): dosimetic analysis of implant quality. Int J Radiat Oncol Biol Phys. 2000;46:1199–1204. doi: 10.1016/s0360-3016(99)00516-7. [DOI] [PubMed] [Google Scholar]
- 81.Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 82.Herman JR, Adler HL, Aguilar-Cordova E, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther. 1999;10:1239–1249. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 83.Collins SA, Guinn BA, Harrison PT, Scallan MF, O'Sullivan GC, Tangney M. Viral vectors in cancer immunotherapy: which vector for which strategy? Curr Gene Ther. 2008;8:66–78. doi: 10.2174/156652308784049345. [DOI] [PubMed] [Google Scholar]
- 84.Kaplan JM. Adenovirus-based cancer gene therapy. Curr Gene Ther. 2005;5:595–605. doi: 10.2174/156652305774964677. [DOI] [PubMed] [Google Scholar]
- 85.Siemens DR, Elzey BD, Lubaroff DM, et al. Cutting edge: restoration of the ability to generate CTL in mice immune to adenovirus by delivery of virus in a collagen-based matrix. J Immunol. 2001;166:731–735. doi: 10.4049/jimmunol.166.2.731. [DOI] [PubMed] [Google Scholar]
- 86.VanOosten RL, Griffith TS. Activation of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG oligodeoxynucleotide combination therapy. Cancer Res. 2007;67:11980–11990. doi: 10.1158/0008-5472.CAN-07-1526. [DOI] [PubMed] [Google Scholar]
- 87.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 88.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 89.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 90.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 92.Datta SK, Redecke V, Prilliman KR, et al. A Subset of Toll-Like Receptor Ligands Induces Cross-presentation by Bone Marrow-Derived Dendritic Cells. J Immunol. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- 93.Cho HJ, Takabayashi K, Cheng PM, et al. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat Biotechnol. 2000;18:509–514. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 94.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 95.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 96.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia W, Zhou Q. Viral vectors for cancer gene therapy: viral dissemination and tumor targeting. Curr Gene Ther. 2005;5:133–142. doi: 10.2174/1566523052997460. [DOI] [PubMed] [Google Scholar]
- 98.Hemminki A, Kanerva A, Liu B, et al. Modulation of coxsackie-adenovirus receptor expression for increased adenoviral transgene expression. Cancer Res. 2003;63:847–853. [PubMed] [Google Scholar]
- 99.Okegawa T, Li Y, Pong RC, Bergelson JM, Zhou J, Hsieh JT. The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 2000;60:5031–5036. [PubMed] [Google Scholar]
- 100.Eastham JA, Hall SJ, Sehgal I, et al. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995;55:5151–5155. [PubMed] [Google Scholar]
- 101.Hall SJ, Mutchnik SE, Yang G, et al. Cooperative therapeutic effects of androgen ablation and adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy in experimental prostate cancer. Cancer Gene Ther. 1999;6:54–63. doi: 10.1038/sj.cgt.7700004. [DOI] [PubMed] [Google Scholar]
- 102.Nasu Y, Bangma CH, Hull GW, et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6:338–349. doi: 10.1038/sj.gt.3300834. [DOI] [PubMed] [Google Scholar]
- 103.Anello R, Cohen S, Atkinson G, Hall SJ. Adenovirus mediated cytosine deaminase gene transduction and 5-fluorocytosine therapy sensitizes mouse prostate cancer cells to irradiation. J Urol. 2000;164:2173–2177. [PubMed] [Google Scholar]
- 104.Shariat SF, Desai S, Song W, et al. Adenovirus-mediated transfer of inducible caspases: a novel “death switch” gene therapeutic approach to prostate cancer. Cancer Res. 2001;61:2562–2571. [PubMed] [Google Scholar]
- 105.Cao G, Su J, Lu W, et al. Adenovirus-mediated interferon-beta gene therapy suppresses growth and metastasis of human prostate cancer in nude mice. Cancer Gene Ther. 2001;8:497–505. doi: 10.1038/sj.cgt.7700333. [DOI] [PubMed] [Google Scholar]
- 106.Katner AL, Hoang QB, Gootam P, et al. Induction of cell cycle arrest and apoptosis in human prostate carcinoma cells by a recombinant adenovirus expressing p27(Kip1) Prostate. 2002;53:77–87. doi: 10.1002/pros.10124. [DOI] [PubMed] [Google Scholar]
- 107.Flynn V, Jr, Ramanitharan A, Moparty K, et al. Adenovirus-mediated inhibition of NF-kappaB confers chemo-sensitization and apoptosis in prostate cancer cells. Int J Oncol. 2003;23:317–323. [PubMed] [Google Scholar]
- 108.Gomez-Navarro J, Curiel DT, Douglas JT. Gene therapy for cancer. Eur J Cancer. 1999;35:867–885. doi: 10.1016/s0959-8049(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 109.Lee YC, Simamora P, Yalkowsky SH. Systemic delivery of insulin via an enhancer-free ocular device. J Pharm Sci. 1997;86:1361–1364. doi: 10.1021/js970191c. [DOI] [PubMed] [Google Scholar]
- 110.Machan L, Burt HM, Hunter WL. Local delivery of chemotherapy: a supplement to existing cancer treatments. A case for surgical pastes and coated stents. Adv Drug Deliv Rev. 1997;26:199–207. doi: 10.1016/s0169-409x(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 111.Siemens DR, Austin JC, Hedican SP, Tartaglia J, Ratliff TL. Viral vector delivery in solid-state vehicles: gene expression in a murine prostate cancer model. J Natl Cancer Inst. 2000;92:403–412. doi: 10.1093/jnci/92.5.403. [DOI] [PubMed] [Google Scholar]
- 112.Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184:1–16. doi: 10.1002/(SICI)1097-4652(200007)184:1<1::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 113.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 114.Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13:477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 115.Marks PA, Rifkind RA, Richon VM, Breslow R. Inhibitors of histone deacetylase are potentially effective anticancer agents. Clin Cancer Res. 2001;7:759–760. [PubMed] [Google Scholar]
- 116.Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp Cell Res. 1998;241:126–133. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- 117.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 118.Yamano T, Ura K, Morishita R, Nakajima H, Monden M, Kaneda Y. Amplification of transgene expression in vitro and in vivo using a novel inhibitor of histone deacetylase. Mol Ther. 2000;1:574–580. doi: 10.1006/mthe.2000.0074. [DOI] [PubMed] [Google Scholar]
- 119.Kitazono M, Goldsmith ME, Aikou T, Bates S, Fojo T. Enhanced adenovirus transgene expression in malignant cells treated with the histone deacetylase inhibitor FR901228. Cancer Res. 2001;61:6328–6330. [PubMed] [Google Scholar]
- 120.Earel JK, Jr, VanOosten RL, Griffith TS. Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res. 2006;66:499–507. doi: 10.1158/0008-5472.CAN-05-3017. [DOI] [PubMed] [Google Scholar]
- 121.VanOosten RL, Earel JK, Griffith TS. Enhancement of Ad5-TRAIL cytotoxicity against renal cell carcinoma with histone deacetylase inhibitors. Cancer Gene Ther. 2006 doi: 10.1038/sj.cgt.7700939. [DOI] [PubMed] [Google Scholar]
- 122.VanOosten RL, Earel JK, Jr, Griffith TS. Histone deacetylase inhibitors enhance Ad5-TRAIL killing of TRAIL-resistant prostate tumor cells through increased caspase-2 activity. Apoptosis. 2007;12:561–571. doi: 10.1007/s10495-006-0009-9. [DOI] [PubMed] [Google Scholar]
- 123.VanOosten RL, Moore JM, Karacay B, Griffith TS. Histone deacetylase inhibitors modulate renal cell carcinoma sensitivity to TRAIL/Apo-2L–induced apoptosis by enhancing TRAIL-R2 expression. Cancer Biol Ther. 2005;4:1104–1112. doi: 10.4161/cbt.4.10.2022. [DOI] [PubMed] [Google Scholar]
- 124.VanOosten RL, Moore JM, Ludwig AT, Griffith TS. Depsipeptide ( FR901228) enhances the cytotoxic activity of TRAIL by redistributing TRAIL receptor to membrane lipid rafts. Mol Ther. 2005;11:542–552. doi: 10.1016/j.ymthe.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Inoue H, Shiraki K, Ohmori S, et al. Histone deacetylase inhibitors sensitize human colonic adenocarcinoma cell lines to TNF-related apoptosis inducing ligand-mediated apoptosis. Int J Mol Med. 2002;9:521–525. [PubMed] [Google Scholar]
- 126.Zhang XD, Gillespie SK, Borrow JM, Hersey P. The histone deacetylase inhibitor suberic bishydroxamate: a potential sensitizer of melanoma to TNF-related apoptosis-inducing ligand (TRAIL) induced apoptosis. Biochem Pharmacol. 2003;66:1537–1545. doi: 10.1016/s0006-2952(03)00509-4. [DOI] [PubMed] [Google Scholar]
- 127.Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003;2:1273–1284. [PubMed] [Google Scholar]
- 128.Gray SG, Qian CN, Furge K, Guo X, Teh BT. Microarray profiling of the effects of histone deacetylase inhibitors on gene expression in cancer cell lines. Int J Oncol. 2004;24:773–795. doi: 10.3892/ijo.24.4.773. [DOI] [PubMed] [Google Scholar]
- 129.Kim YH, Park JW, Lee JY, Kwon TK. Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells. Carcinogenesis. 2004 doi: 10.1093/carcin/bgh188. [DOI] [PubMed] [Google Scholar]
- 130.Spierings DC, de Vries EG, Vellenga E, et al. Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem. 2004;52:821–831. doi: 10.1369/jhc.3A6112.2004. [DOI] [PubMed] [Google Scholar]
- 131.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 132.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 133.Sanlioglu AD, Dirice E, Aydin C, Erin N, Koksoy S, Sanlioglu S. Surface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cells. BMC Cancer. 2005;5:54. doi: 10.1186/1471-2407-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aydin C, Sanlioglu AD, Karacay B, et al. Decoy receptor-2 small interfering RNA (siRNA) strategy employing three different siRNA constructs in combination defeats adenovirus-transferred tumor necrosis factor-related apoptosis-inducing ligand resistance in lung cancer cells. Hum Gene Ther. 2007;18:39–50. doi: 10.1089/hum.2006.111. [DOI] [PubMed] [Google Scholar]
- 135.Sanlioglu AD, Karacay B, Koksal IT, Griffith TS, Sanlioglu S. DcR2 (TRAIL-R4) siRNA and adenovirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells. Cancer Gene Ther. 2007;14:976–984. doi: 10.1038/sj.cgt.7701087. [DOI] [PubMed] [Google Scholar]
- 136.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 137.Schneider P, Thome M, Burns K, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 138.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 139.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Griffith TS, Fialkov JM, Scott DL, et al. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093–3099. [PubMed] [Google Scholar]
- 141.Ng CP, Bonavida B. X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO) Mol Cancer Ther. 2002;1:1051–1058. [PubMed] [Google Scholar]
- 142.Shiiki K, Yoshikawa H, Kinoshita H, et al. Potential mechanisms of resistance to TRAIL/Apo2L–induced apoptosis in human promyelocytic leukemia HL-60 cells during granulocytic differentiation. Cell Death Differ. 2000;7:939–946. doi: 10.1038/sj.cdd.4400727. [DOI] [PubMed] [Google Scholar]
- 143.Goke R, Goke A, Goke B, El-Deiry WS, Chen Y. Pioglitazone inhibits growth of carcinoid cells and promotes TRAIL-induced apoptosis by induction of p21waf1/cip1. Digestion. 2001;64:75–80. doi: 10.1159/000048843. [DOI] [PubMed] [Google Scholar]
- 144.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 145.Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol. 2008;75:1946–1958. doi: 10.1016/j.bcp.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee TJ, Lee JT, Park JW, Kwon TK. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem Biophys Res Commun. 2006;351:1024–1030. doi: 10.1016/j.bbrc.2006.10.163. [DOI] [PubMed] [Google Scholar]
- 147.Vaculova A, Hofmanova J, Soucek K, Kozubik A. Different modulation of TRAIL-induced apoptosis by inhibition of pro-survival pathways in TRAIL-sensitive and TRAIL-resistant colon cancer cells. FEBS Lett. 2006;580:6565–6569. doi: 10.1016/j.febslet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 148.Jin Z, Dicker DT, El-Deiry WS. Enhanced sensitivity of G1 arrested human cancer cells suggests a novel therapeutic strategy using a combination of simvastatin and TRAIL. Cell Cycle. 2002;1:82–89. [PubMed] [Google Scholar]
- 149.Algeciras-Schimnich A, Griffith TS, Lynch DH, Paya CV. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J Immunol. 1999;162:5205–5211. [PubMed] [Google Scholar]
- 150.MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–25420. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- 151.Pineau P, Nagai H, Prigent S, et al. Identification of three distinct regions of allelic deletions on the short arm of chromosome 8 in hepatocellular carcinoma. Oncogene. 1999;18:3127–3134. doi: 10.1038/sj.onc.1202648. [DOI] [PubMed] [Google Scholar]
- 152.Martinez-Lorenzo MJ, Alava MA, Gamen S, et al. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur J Immunol. 1998;28:2714–2725. doi: 10.1002/(SICI)1521-4141(199809)28:09<2714::AID-IMMU2714>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 153.Carnaud C, Lee D, Donnars O, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 154.Shin MS, Kim HS, Lee SH, et al. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001;61:4942–4946. [PubMed] [Google Scholar]
- 155.Arai T, Akiyama Y, Okabe S, Saito K, Iwai T, Yuasa Y. Genomic organization and mutation analyses of the DR5/TRAIL receptor 2 gene in colorectal carcinomas. Cancer Lett. 1998;133:197–204. doi: 10.1016/s0304-3835(98)00230-4. [DOI] [PubMed] [Google Scholar]
- 156.Park WS, Lee JH, Shin MS, et al. Inactivating mutations of KILLER/DR5 gene in gastric cancers. Gastroenterology. 2001;121:1219–1225. doi: 10.1053/gast.2001.28663. [DOI] [PubMed] [Google Scholar]
- 157.Jeng YM, Hsu HC. Mutation of the DR5/TRAIL receptor 2 gene is infrequent in hepatocellular carcinoma. Cancer Lett. 2002;181:205–208. doi: 10.1016/s0304-3835(02)00051-4. [DOI] [PubMed] [Google Scholar]