Abstract
In this study we have explored, the use of Fab-toxin proteins (immunotoxin) to target antigen-specific MHC-peptide complexes of in vitro and in vivo cancer cells. A human phage display library was used to screen for TCR-like antibodies that are highly specific for the peptide melanoma-associated antigen MART-126–35 presented by HLA-A201. We also used previously selected TCR-like antibodies specific for the peptide melanoma-associated antigen presented by HLA-A201. The gp100280–288 recombinant immunotoxin constructs were generated by fusing the targeting Fab fragment to a truncated form of Pseudomonas exotoxin (PE): PE38KDEL. These immunotoxins bound with high affinity to the EBV transformed JY cell line pulsed with the aforementioned peptides and internalized within 30 min. A significant inhibition of protein synthesis, which resulted in cell death, was detected at 24 h. MART-1 and gp-100 specific immunotoxins bound and killed HLA-A201 melanoma MART-1 and gp-100 positive cell lines that were presented at natural levels, but do not bind to HLA-A201− or to HLA-A201+ MART-1 and gp-100 negative cell lines. In SCID mice MART-1 and gp-100 immunotoxins significantly and discriminately inhibited human melanoma growth. These results show that MHC Class I/peptide complexes can serve as a specific target for passive immunotherapy of cancer.
Introduction
Observation of spontaneous antitumoral T cell response in melanoma patients led to the identification of tumor associated antigens (1, 2). Drugs that target these antigens are becoming a first line treatment for some cancer types (3). Rituximab, a monoclonal antibody that binds CD20 and promotes destruction of non Hodgkin’s lymphoma B cells via ADCC represents a success in passive immunotherapy (4). Similarly, Herceptin which targets HER-2 positive cancer cells is now standard in breast cancer therapy (5). The efficacy of such drugs has prompted efforts to develop additional antibody agents that elicit ADCC, or deliver toxin moieties to the cancerous cells. Although numerous melanoma associated antigens have been identified (6), many are intracellular rather than surface proteins, and therefore not accessible to antibodies. However, peptides derived from these intracellular antigens are presented as epitopes on major histocompatibility (MHC) Class I molecules of human cancer cells (7). These melanoma exclusive complexes represent prime targets for immunotoxins. HLA-A201-restricted CTLs derived from melanoma tumor-infiltrating lymphocytes (TIL) of patients were found to recognize epitopes from the melanocitic differentiation proteins gp100 and MART-1 (1, 2). However progression of tumors leading to patient death suggests that these T cells are ineffective in eradicating the tumor. Several mechanisms are considered in this matter to control tumor growth including the quality of the T cells i.e. low vs. high avidity (8) and the presence of local immunosuppressive processes. The specificity of the TILs remains attractive for therapeutic purposes. Recent studies show that these cells expanded in vitro and adaptively transferred back to the patient, can elicit remarkable responses particularly in the lymphoablated patients (9, 10).
Another approach is to develop Fab fragments that bind melanoma-specific peptide/MHC complexs with the specificity of the T cell receptor. Such ligands, when conjugated with therapeutic moieties, i.e. drugs, radioisotopes or tumor cell toxins constitute potential anticancer exogenous agents that may also avoid tumor regulating immunosuppressive mechanisms. By screening a large human phage display library we previously isolated high-affinity recombinant Fab antibodies Fab 2F1 and G2D12 that recognize HLA-A201 in complex with peptide gp100280–288 and gp100154–162, respectively (11). Herein, we describe the isolation of Fab antibodies (Fab CAG10 and Fab CLA12) which recognize MART-126–37 peptide in the context of HLA-A201. Fusion proteins comprised of these Fab antibodies fragment and a truncated form of Pseudomonas exotoxin (PE38KDEL) specifically kill in vitro and in vivo melanoma cells that present the corresponding peptide complexes on their surface.
Materials and Methods
Peptides and cell lines
The HLA-A201-restricted peptides used for specificity studies are gp100154–162: KTWGQYWQV; gp100209–217: IMDQVPFSV (G9–209); gp100280–288: LLLTVLTVL (G9–280); HTLV-1 TAX11–19: LLFGYPVYV (TAX); CMV P65495–503: NLVPMVATV; TARP29–37: FLRNFSLML; XAGE-1: GVFPSAPSPV; MART-126–35 EAAGIGILTV (MART-1 26–35); MART-127L ELAGIGILTV (MART-1 27L); hTERT865–873: RLVDDFLLV.
Cell lines used in this study: B cell line RMAS-HHD, which is transfected with a single-chain β2m-HLA-A201 gene, the EBV-transformed HLA-A201+ JY cells, HLA-A201+ TAP-deficient T2 cells. Melanoma cell lines: HLA-A201+/gp100+/MART-1+ :Mel624.38, Mel526, Mel501A, FM3D, Stiling. HLA-A201+/gp100−/MART-1−: Mel1938 HLA-A201−/gp100+/MART-1+: HA24, G-43; HLA-A201−/gp100−/MART-1−: PC3.
Selection and characterization of recombinant Fabs with specificity for MART-1/HLA-A201
The generation and characterization of a panel of Fabs specific for peptide/HLA-A201 were previously described in detail (12). Phage Abs were selected for binding to single-chain MHC-peptide complexes (13) using a large human Fab library containing 3.7 × 1010 different Fab clones (12). The binding specificity of the phage clones selected was tested against soluble MART-1/HLA-A201 complexes in ELISA assays. MART-1/HLA-A201-specific Fab Abs were expressed and purified as previously described (12). The eluted Fabs were dialyzed twice against PBS (overnight, 4°C) to remove residual imidazole.
Construction, expression, and production of melanoma specific Fab-PE38KDEL
The light chains and the heavy chain containing the variable and constant region 1 (VLCL or VHCH) of Fabs 2F1, CLA12 and H9 were cloned separately by PCR into T7-promotor based expression vector pULI9 (14). The VLCL chain was fused to a gene encoding the toxin PE38KDEL for the construction of VLCL-PE38KDEL. The VHCH of the Fabs were cloned into the same expression vector after the toxin gene was removed. These constructs were expressed separately in Escherichia coli BL21 λDE3 cells. Upon induction with IPTG, intracellular inclusion bodies that contain large amounts of the recombinant protein accumulated. Inclusion bodies of both chains were purified, solubilized, reduced, and subsequently refolded at a 1:1 ratio in a redox-shuffling buffer system containing 0.1 M Tris, 0.5 M arginine, and 0.09 mM oxidized glutathione, pH 8.0. Correctly folded Fab-PE38KDEL fusions were purified by ion-exchange chromatography on Q-Sepharose and Mono-Q (Pharmacia).
ELISA with purified Fab Abs or Fab-PE38KDEL immunotoxin
The binding specificities of individual soluble Fabs and recombinant Fab-PE38KDEL immunotoxin were determined by ELISA using biotinylated scMHC-peptide complexes. pMHC complexes were refolded using each peptide and coated via streptavidin on an ELISA plate (Falcon). After extensive washing, plates were blocked with PBS/2% skim milk and incubated with various concentrations of soluble purified Fab or Fab-PE38KDEL for 1h at room temperature. Bound clones were detected with an anti-human Fab mAb coupled to HRP or HRP-conjugated anti-PE (for Fab-immunotoxin). Detection was performed using tetramethylbenzidine reagent (Sigma-Aldrich, St. Louis, MO).
Measurement of melanoma-specific p/HLA-A201-specific Fabs or Fabs-PE38KDEL immunotoxin binding to cell surface peptide/MHC complexes
RMA-S·HHD or JY cells (106) were pulsed overnight with 50 μM peptide at 26°C or 37°C, respectively. RMAS-HHD cells were subsequently incubated at 37°C for 2–3 h to stabilize cell surface expression of MHC-peptide complexes. The cells were then washed in FACS assay medium (PBS, 2% BSA, and 0.09% sodium azide), and incubated for 1 h at 4°C with 20 μg/ml Fabs or Fab-toxin and FITC-labeled goat anti-human IgG (Fab-specific; The Jackson Laboratory, Bar Harbor, ME). Cells were washed 3 times with PBS and analyzed by FACSCalibur (BD Biosciences, Mountain View, CA). Melanoma cells were trypsinized and stained with the Fab or Fab-toxin as described above. The level of total HLA-A201 expression was detected using the mouse anti-human HLA-A201 (clone BB7.2).
Fab-toxin affinity measurement
Fab-toxin was labeled with [125I] using Bolton-Hunter reagent. 125I-labeled Fab-toxin (3–5 × 105 counts per minutes (cpm)/106 cells) was incubated with JY cells that were loaded with specific or irrelevant peptide and with increasing concentrations of unlabeled Fab-toxin for 1 h at RT. The cells were washed extensively with PBS, and the bound radioactivity was measured by a gamma counter. Nonspecific binding was determined by adding a 20- fold excess of unlabeled Fab-toxin, and was 25% of the total bound radiolabel. Values of non-specific binding were subtracted to calculate specific binding. Specifically bound 125I-labeled Fab 2F1-PE38KDEL was expressed as the percentage of the maximal bound 125I-labeled toxin, and is plotted against the concentration of the competitor. The apparent binding affinity of the recombinant immunotoxin was determined using nonlinear regression as the concentration of competitor (soluble purified Fab-toxin) required for 50% inhibition of 125I-labeled Fab-toxin binding to the cells. Data are representative of two independent experiments.
Internalization assay
JY cells were loaded with specific or control peptide, washed and incubated with 20–30 μg/ml FITC labeled Fab-toxin for 1 h on ice. Cells were then washed and resuspended in RPMI 1640 medium containing 10% FCS. Half of the cells were kept on ice while the other half was incubated at 37°C. At indicated time points, a sample was removed, washed, and fixed in Tris/glycerol/polyvinyl alcohol mounting solution. Specimens were examined with a Zeiss confocal laser fluorescence inverted microscope (LSM 410, Carl Zeiss, Oberkochen, Germany) using simultaneous lasers with excitation wavelength 488 nm.
Cytotoxicity assays on JY APCs and melanoma cell lines
JY cells were incubated overnight with 0.1 mM specific peptide or control peptides at 37°C. Peptide-loaded cells were then washed twice with medium and incubated for 24 h with increasing concentrations of recombinant Fab-PE38KDEL. For melanoma killing assay, 5 × 104 cells were plated in each well of a flat bottom 96 well plate for 36 h. Graduate amounts of Fab-toxin were then added for an additional 24 h. Protein synthesis inhibition is measured by incorporation of [3H] leucine into cell proteins. IC50 is the concentration of immunotoxin which causes 50% inhibition of protein synthesis.
Antitumor activity (in vivo antitumor assay)
The antitumor activity of Fab-PE38KDEL fusions was determined in SCID mice bearing human cancer cells. Mel526 cells (10 × 106) were injected subcutaneously into irradiated NOD-SCID β2M deficient mice on day 0. When tumors developed in the animals for about 0.05 cm3 in size (by day 6–10), treatment with immunotoxin was initiated. Animals were treated with 4 intravenous injections of CLA12 Fab-PE38KDEL, 2F1 Fab-PE38KDEL or H9 Fab-PE38KDEL diluted in 0.2 ml of PBS once every other day. Fifth and sixth injections were given 4 and 8 days later. Treatment groups consisted of 4 animals. Tumors were measured with a caliper every other day, and the volume of the tumor was calculated by using the following formula: tumor volume (cm3) = length × (width)2 × 0.5.
Statistical Analysis
Tumor sizes in animal experiments are expressed as mean ± SD. For comparison between the two experimental groups, Mann-Whitney test was used. P < 0.05 is considered statistically significant.
Results
Generation of recombinant antibodies specific for HLA-A201 MART-1 peptide complexes
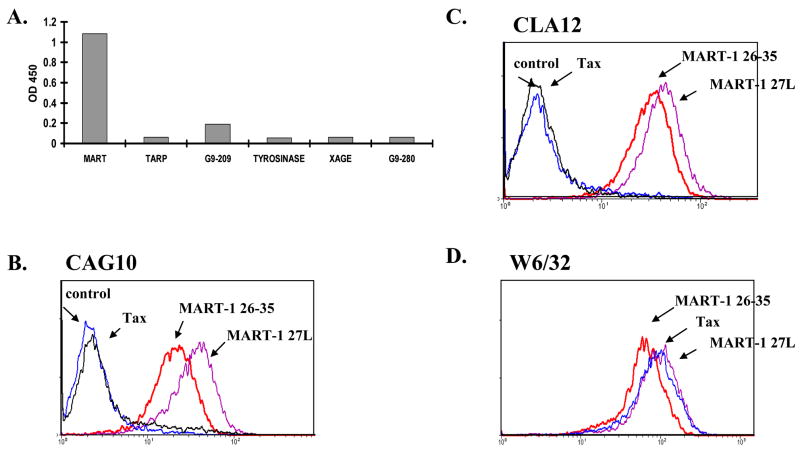
HLA-A201/MART-126–35 (AAGIGILTV) complexes were exposed to a large naive repertoire of 3.7×1010 human recombinant Fab fragments displayed on the surface of phage. A 1000- to-2500-fold enrichment in phage titer was observed after three rounds of panning. Two Fab phage clones, CAG10 and CLA12 Abs were selected and produced in a soluble form in Escherichia coli BL21 cells, then purified by IMAC as described (12). The binding specificity of these purified Fab fragments was determined by ELISA with biotinylated MHC-peptide complexes immobilized to wells through BSA-biotin-streptavidin. As shown in Fig. 1A, Fab CLA12 binds to MART-1/HLA-A201-restricted MHC-peptides but not to other HLA-A201 peptide complexes (gp100 G9–209, TARP, Tyrosinase, XAGE and gp100 G9–280). As shown in Fig. 1B and 1C, Fabs CAG10 and CLA12 respectively bound in a peptide-specific manner to HLA-A201+ RMAS-HHD cells that were loaded with the MART-1 26–35 peptide but not control HTLV-1-derived HLA-A201 restricted peptide (TAX 11–19). The Fabs also bound HLA-A201+ RMAS-HHD cells that were loaded with the anchor modified MART-1-derived peptide 27L, where the alanine in position 2 is replaced by a leucine residue, giving stronger binding to HLA-A201 (15, 16). The MART-1 26–35, 27L-35 and the TAX peptide were all presented on the surface of the pulsed RMAS-HHD as demonstrated by the binding of mAb W6/32, a conformational antibody that recognizes peptide-loaded MHC Class I (Fig. 1D).
Figure 1. Binding of Fabs CAG10 and CLA12 to peptide-loaded antigen presenting cells.
(A) Fine specificity of the purified Fab clones by ELISA. TCR-like Fabs were tested by ELISA for binding refolded peptide-MHC complexes (B–C) Detection of MART-1 peptide/HLA-A201 complexes on antigen presenting cells RMA-S-HHD. RMA-S cells were loaded with specific MART-1 (26–35 or 27L) or control TAX peptide. Complexes were probed with recombinant purified Fab CAG10 (B) and CLA12 (C) and analyzed by FACS using FITC-labeled goat anti-human Fab. (D) mAb W6/32 binding demonstrates the total peptide/HLA on the APCs cell surface.
Thus Fab CAG10 and CLA12 antibodies exhibit TCR-like fine specificity and recognize cell surface-HLA-A201 complexes bearing MART-1 26–35 peptide.
MART-1 and gp100-specific TCR-like Fab antibodies bind to HLA-A201+ melanoma cells
To test whether the melanoma-specific TCR-like Fab antibodies can bind naturally processed HLA-A201-peptide complexes on the surface of tumor cells, we performed flow cytometry studies on HLA-A201+ melanoma tumor cell lines. As shown in Fig. 2, MART-1-specific Fab antibody CLA12 reacted with the HLA-A201+/MART-1+ melanoma lines 501A (Fig. 2A), 624.38 (Fig. 2C), FM3D (Supplementary Fig. 1A), and Stilling (Supplementary Fig. 1B), but not with HLA-A201+/MART-1− melanoma 1938 (Fig. 2B) or with melanoma G-43 cells which are HLA-A201−/MART-1+ (Fig. 2D). As expected, the HLA-A201/MART-1 complexes represent a minor fraction out of the total surface HLA-A201 complexes as monitored by the binding of the HLA-A201 specific mAb BB7.2. These results indicate that the TCR-like antibody CLA12 can detect the native HLA-A201/MART-1 epitope on the surface of melanoma cells.
Figure 2. Binding of Fab CLA12 to melanoma cell lines.
(A–D) Detection of MART-1 peptide/HLA-A201 complexes on HLA-A201+/MART-1+-melanoma cell lines 501A (A), 624.38 (C) and on control cell lines: the HLA-A201+/MART-1−-melanoma 1938 (B) or the HLA-A201−/MART-1+-melanoma G-43 (D) by Fab CLA12. Complexes were detected by FACS using recombinant purified Fab CLA12 and FITC-labeled goat anti-human Fab. mAb BB7.2 recognizes total HLA-A201 and was used to validate HLA-A201 expression by the indicated cell lines.
The TCR-like antibody Fab 2F1, directed against the HLA-A201/gp100-G9–280 epitope, also binds HLA-A201+/gp100+ 624.38 and 501A melanoma cells (Supplementary Fig. 2A and 2B), but does not bind to either HLA-A201+/gp100− 1938 melanoma (Supplementary Fig. 2C), or HLA-A201−/gp100− PC3 cells (Supplementary Fig. 2D).
These results show that these MART-1 and gp100 specific TCR-like antibodies bind in a peptide-dependent HLA-A201-restricted manner to target cells which express naturally processed endogenously-derived peptide-HLA-A201 complexes.
Construction and purification of TCR-like antibody-toxin fusion proteins
We generated fusion molecules in which the different TCR-like antibody Fabs are fused to truncated form of Pseudomonas exotoxin A (PE38KDEL). This truncated form of PE contains the translocation and ADP-ribosylation domains of PE but lacks the cell-binding domain, which is replaced by the Fab fragment. In addition, the 5 C-terminal amino acids REDLK of the native PE were replaced with KDEL which increases the toxin cytotoxicity (17) due to increased binding to the ER retention receptor (18). The truncated PE38KDEL gene was fused to at its N-terminus to the C-terminus of each Fab light chain (CL) as shown schematically in Fig. 3A and produced and purified as described in material and methods.
Figure 3. Analysis of recombinant purified Fab 2F1-PE38KDEL.
(A) Schematics of the Fab 2F1-PE38 fusion protein. The gene encoding PE38 that contains the translocation and ADP ribosylation domains of PE was fused to the C terminus of Fab 2F1 (Lκ) κ chain. (B) Binding of 2F1 Fab-PE38KDEL to peptide loaded APCs. JY cells were loaded with 50μM G9–280 or G9–209 peptides, incubated at 4°C in the presence of 2F1 Fab-toxin and analyzed by flow cytometry. (C) Competitive binding analysis of the ability of purified recombinant Fab 2F1-PE38KDEL to inhibit the binding of 125I-labeled Fab 2F1-PE38KDEL to JY cells loaded with 0.05 mM G9–280. Specifically bound 125I-labeled Fab 2F1-PE38KDEL is expressed as the percentage of the maximal bound 125I-labeled toxin, and is plotted versus the concentration of the competitor. Values of non-specific binding, as measured by binding of 125I-labeled toxin in the presence of excess amount of competitor were subtracted to calculate specific binding. Value of maximum binding: 2011 cpm; value of non-specific binding: 511 cpm. Data shown are representative of two independent experiments. (D) Binding of Fab-toxin - 2F1 Fab PE38KDEL to HLA-A201+gp100+ melanoma cell line Mel526. Complexes were detected by flow cytometry using FITC-labeled goat anti-human Fab.
The binding specificity of the soluble purified Fab-PE38KDEL fusion proteins was first determined by ELISA on biotinylated MHC-peptide complexes immobilized to wells through BSA-biotin-streptavidin. The binding was assessed with either anti-human Fab or anti-PE38 antibodies. Fab 2F1-PE38KDEL reacts specifically with the immobilized HLA-A201/gp100-G-280 complexes and not with control HLA-A201-peptide complexes (gp100 209, CMV p65) (Supplementary Figure 3).
The binding affinity of the soluble 2F1-PE38KDEL fusion protein was determined using a competition binding assay in which the binding of 125I-labeled Fab2F1-PE38KDEL to the specific HLA-A201/G9–280 complexes on APCs (Fig 3B) is competed with increasing concentrations of unlabeled fusion protein. Non-specific binding of the soluble 125I-labeled 2F1-PE38KDEL fusion protein was determined on APCs loaded with the specific peptide gp100-G-280 in the presence of 20 fold excess of the unlabeled 2F1-PE38KDEL fusion protein. The specific binding was calculated by subtracting the background value from the bound cpm. The percentage of the specific maximal binding was plotted against the concentration of the competitor. Relative binding affinities were inferred from the IC50 values of four-parameter curve fits. (Fig. 3C) and determined as 249 nM. The binding of the labeled Fab 2F1 and the 2F1-toxin fusion to cells was indistinguishable (not shown). Thus we have generated immunotoxin with comparable binding properties to the unconjugated Fab.
The Fab-PE38KDEL fusion proteins bind to APCs and melanoma cells displaying the relevant gp100 and MART-1-derived epitopes
As found with the original MART-1 specific TCR-like Fabs CAG10 and CLA12 (Fig. 1 and 2), the derived immunotoxins CAG10-PE38KDEL and CLA12- PE38KDEL bound to T2 cells loaded with MART-1 (Supplementary Fig. 4A) but did not bind T2 cells loaded with an irrelevant peptides. As shown in Fig. 3B, Fabs 2F1-PE38KDEL, like the original gp100 G9–280 specific TCR-like Fab 2F1 (11), bound to JY cells loaded with gp100 G9–280 peptide. Thus the fusion protein could still bind to peptide/MHC complexes expressed at high density on peptide loaded APCs, leaving the question open as to whether the fusion protein would retain sufficient affinity for peptide-MHC complexes expressed at low levels as on melanoma cells. Indeed, as the original Fab (Fig. 2 and Supplementary Fig. 2), the Fab-PE38KDEL fusion proteins bound to HLA-A201+ and gp100/MART-1+ melanoma cells 526 (Fig. 3D), 501A, and 624.38 (not shown) but not to 1938 melanoma cells which are HLA-A201+ but do not express gp100 and MART-1 or to G-43 melanoma cells which are HLA-A2- but express gp100 and MART-1 (not shown).
These results demonstrate the ability of the TCR-like Fab-PE38KDEL fusion molecules to bind the authentic endogenously-derived MHC-peptide complex when at a limited density on the surface of the tumor cells.
Internalization of TCR-like antibodies
Fab 2F1-PE38KDEL fusion protein was labeled with FITC and tested for its binding and internalization on JY cells pulsed with the appropriate gp100-derived G9–280 peptide. As shown with unlabeled Fab in Fig. 3B, the FITC-labeled Fab 2F1-PE38KDEL molecule binds specifically to G9–280 peptide-pulsed JY cells but not to cells pulsed with a control peptide G9–209. Internalization was monitored by incubating cells with FITC-labeled Fab 2F1-PE38KDEL at 4°C for 1 hour and transferred to 37°C. Membranous binding was observed immediately after the cells were transferred to 37°C (time 0). No fluorescence was observed on the negative control (JY cells + peptide G9–209, not shown). After 15 minutes the majority of stain intensity was mainly on the surface of the cell (Fig. 4A) and dense areas of fluorescence were detected, which may indicate processes of micro-capping of MHC-peptide complexes. After 30 minutes, fluorescence was concentrated in small vesicles (Fig. 4B). After 1 hour, the FITC-labeled antibody was detected in larger vesicles (Fig. 4C). After 6 hours, intense staining was observed around the nucleus in the ER-Golgi compartment (Fig. 4D). Cells kept on ice for 3 h demonstrated membranous staining (not shown).
Figure 4. Cellular internalization of 2F1-PE38-FITC.
JY cells were loaded with the specific G9–280 peptide and incubated at 4°C in the presence of 2F1-PE38-FITC. Cells were then transferred to 37 °C and monitored for the immunotoxin internalization at indicated time points. 15 minutes (A); 30 minutes (B); 1 hour (C) and 6 hours (D). PI was used to detect the nucleus.
These results show that TCR-like antibodies fused to toxin rapidly internalize after binding to cell surface peptide-MHC complexes.
The TCR-like Fab-PE38KDEL immunotoxins are potent killer of APCs
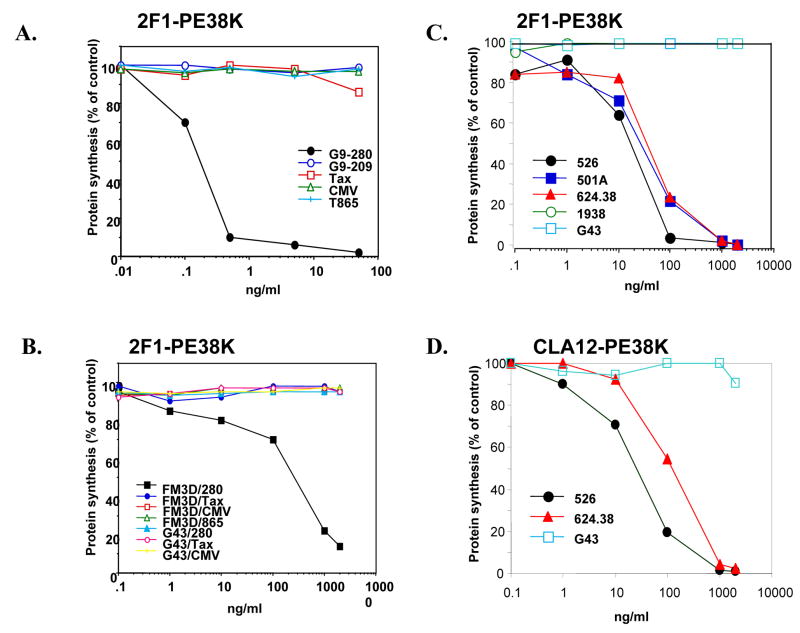
The ability of the Fab- PE38KDEL immunotoxins to inhibit protein synthesis of JY cells was used as a measure of their cytotoxic effect. As shown in Fig. 5A, cytotoxicity by 2F1 Fab-PE38KDEL was observed only when JY cells were loaded with the gp100 G9–280 peptide with an IC50 of ~0.5 ng/ml (black dots). No cytotoxic activity was observed on JY cells that were either unloaded or loaded with other HLA-A201-restricted peptides such as these from TAX or CMV. Similar results were observed with the RMAS-HHD cell line (data not shown). We then analyzed whether the toxins can also demonstrate cytotoxic activity towards melanoma cells such as FM3D, which express 20-fold fewer sites on their surface (1×104 molecules/cell) than JY cells (1.5–2×105 molecules/cell; (19)). As shown in Fig. 5B, 2F1 Fab-PE38KDEL induced killing in FM3D cells pulsed with the gp100 G9–280 peptide, but not with other HLA-A201-restricted control peptides. Furthermore, it did not kill the HLA-A201 negative melanoma G-43 cells pulsed with G9–280 or control peptides. The IC50 for G9–280-pulsed FM3D was ~200 ng/ml possibly reflecting the lower number of HLA-A201/G9–280 sites compared to G9–280-pulsed JY cells. Similar results were observed with the MART-1-specific CLA12 Fab-PE38KDEL fusion proteins (Supplementary Fig. 4B).
Figure 5. Cytotoxic activity of different immunotoxins toward peptide loaded APCs and melanoma cell lines.
(A) Cytotoxic activity of recombinant Fab 2F1-PE38KDEL towards JY cells loaded with G9–280 peptide or with other control HLA-A201 restricted peptides. Cells were incubated for 24 hr with recombinant Fab 2F1-PE38KDEL. [3H] leucine incorporation into cellular protein was measured. The results are expressed as a percentage of control where no immunotoxin was added. (B) Same assay as in A was done on HLA-A201+ FM3D cells and HLA-A201− G43 cells loaded with the specific G9–280, or control HLA-A201 restricted peptides. (C–D) Cytotoxic activity of 2F1-PE38KDEL (C), and CLA12-PE38KDEL (D) toward melanoma cell lines Mel526 (-●-), Mel624.38 (-▲-), Mel501A (-■-), G43 (-□-), 1938 (-○-) after 24 h of incubation.
[3H] leucine incorporation into cellular protein was measured. The results are expressed as a percentage of control where no immunotoxin was added.
These results demonstrate that Fab-PE38KDEL fusion proteins kill cells bearing specific peptide/MHC complexes.
Cytotoxic activity of TCR-like Fab-PE38KDEL immunotoxins toward melanoma cells expressing endogenous gp100 and MART-1
Naturally expressed target antigens, HLA-A201+ melanoma cells that express gp100 and MART-1 were used to evaluate Fab-PE38KDEL fusion molecules’ activity. The gp100 G9–280 specific 2F1 Fab-PE38KDEL fusion protein exhibited cytotoxic activity on gp100+, HLA-A201+ melanoma cells Mel526, 501A, and 624.38. However it does not show cytotoxic activity on HLA-A201−, −/gp100+ G-43 cells or on HLA-A201+, gp100− 1938 cells (Fig. 5C). Similarly, MART-1 specific CLA12 Fab-PE38KEDL fusion protein exhibited cytotoxic activity toward MART-1+, +/HLA-A201+ melanoma cells Mel526 and Mel624.38 but it does not show cytotoxic activity on HLA-A201−, gp100+ G-43 cells (Fig 5D). The IC50 of the immunotoxin molecules 2F1 Fab-PE38KDEL and CLA12 Fab-PE38KDEL to antigen+ and HLA-A201+ cells was ~ 20–100 ng/ml, depending on the cell type and the target antigen. (Fig. 5C and 5D). Thus, these results indicate that TCR-like antibodies fused to PE38KDEL display specific cytotoxic activity on melanoma cells that express natural endogenous differentiation antigens gp100 and MART-1.
In vivo anti-tumor activity of TCR-like Fab-PE38KDEL fusion proteins
Based on the in vitro cytotoxic activity of 2F1 Fab-PE38KEDL and CLA12 Fab-PE38KDEL, we analyzed their ability to alter the development of human melanoma in irradiated NOD SCID β2M-deficient mice. Mice, inoculated with 107 Mel-526 tumor cells were randomly assigned to treatment groups, with 4 mice in each group. Treatment began on day 10 post inoculation when the tumor size reached approximately 55 mm2 and four IV doses of CLA12-PE38KDEL at either 0.05 mg/kg, or 0.125 mg/kg were administered at 48 h intervals. Injection of PBS was used as a control. A fifth and sixth injections were given on day 20 and 24 respectively. Tumor volumes were recorded for 34 days. By day 34, tumors in mice receiving PBS diluent grew to a size averaging 530 ± 127 mm3. Treatment with 0.05 mg/kg CLA12-PE38KDEL delayed tumor development compared with control and by day 34 tumor size reached 259 ± 92 mm3 (P=0.0006) (Fig. 6A). The effect on tumor was dose-dependent; since treatment with 0.125 mg/kg CLA12-PE38KDEL delayed tumor development to a greater extent, reaching ~ 25% (135 ± 56 mm3) of the size of the tumors in the control group on day 34 (Fig. 6B P=0.0002). To further assess the specificity of 2F1 Fab-PE38KEDL and CLA12 Fab-PE38KDEL, a non relevant immunotoxin recognizing the complex of CMV peptide p65 bound to HLA-A201 (H9-PE38KDEL) was used as a control (Fig. 6C and 6D). Mice bearing a 55 mm2 melanoma tumor were injected using the same protocol with 0.125 mg/kg of the gp100-specific or MART-1-specific immunotoxins 2F1-PE38KDEL or CLA12-PE38KDEL, respectively. PBS or CMV-specific immunotoxin H9-PE38KDEL were used as a control. By day 35, tumor size reached 791±161 mm3. Treatment with control immunotoxin (H9 Fab-PE38KDEL) had no significant effect on tumor growth that had reached 721±120 mm3 by day 35. Treatment with either 2F1 Fab-PE38KEDL or CLA12 Fab-PE38KDEL, however, considerably and consistently delayed tumor growth. By day 35 the tumor size reached 164.5±22 mm3 and 162.5±27 mm3 respectively.
Figure 6. Anti-tumor activity of different immunotoxins on subcutanous human melanoma tumor in SCID mice.
Groups of animals were injected with 10×106 Mel526 melanoma cells on day 0. When tumor reached 55 mm3, animals were administrated in 48 h intervals with four i.v. injections of 0.05mg/kg (A) and 0.125 mg/kg (B) CLA12 Fab-PE38KDEL in PBS. A fifth and sixth injections were given 4 and 8 days later. Control group received diluent alone. (C–D) animals were administrated in 48 h intervals with four i.v. injections of 0.125 mg/kg CLA12 Fab-PE38KDEL (C) or 2F1 Fab-PE38KDEL (D) in PBS. A fifth and sixth injections were given 4 and 8 days later. Control groups received diluent alone or non binding immunotoxin CMV specific H9 Fab-PE38KDEL. No cytotoxicity was observed at these doses. Comparison of tumor size between treated and control groups gave P < 0.0006. Data are expressed as the mean ± SD (n=4).
We therefore conclude that 2F1-PE38KDEL and CLA12-PE38KDEL are specific and effective in slowing the growth of HLA-A201+gp100+MART-1+ tumors at non-toxic doses.
Discussion
Here we report the isolation of two novel human recombinant antibody fragments Fab-CAG10 and Fab-CLA12 that recognize HLA-A201/MART-126–37 complex. They possess a similar affinity as previously reported for Fab 2F1 and Fab G2D12 that bind to HLA-A201/gp100280–288, and HLA-A201/gp100154–162 complexes, respectively (11). Differences in binding of these antibody fragments to melanoma cell lines, revealed the differential expression of the various specific peptide-HLA-A201 complexes. MART-1 specific and gp100 G9–280 specific HLA-A201 complexes express higher levels in melanoma cell lines compared to G9–154 epitopes of the gp100 protein (not shown).
Our extended panel of melanoma-specific antibodies with T cell receptor-like specificity was used for targeting toxin to cells that express specific peptide/MHC complex in vitro and in vivo. We constructed different human antibody-toxin hybrid molecules (immunotoxins), in which a human Fab antibody is fused to the Pseudomonas exotoxin derivative, PE38KDEL.
All the recombinant immunotoxins we have analyzed show high binding specificity to APCs and melanoma cells expressing the specific peptide/HLA-A201 complex. The immunotoxin 2F1-PE38KEDL has a high rate of internalization (within 30 min) and accumulation in the cytosol of the cell (at 6 h) where it exerts its function. When tested in vitro, Fab-PE38KDEL immunotoxins kill APCs and melanoma cells in a peptide-dependent MHC-restricted manner. Immunotoxin G2D12-PE38KDEL was not as potent in killing melanoma cell lines compared to 2F1-PE38KDEL, CAG10-PE38KDEL and CLA12-PE38KDEL (not shown), and this order correlates with lower expression of p154/HLA-A201 complexes on the surface of the melanoma cell line. Furthermore, CLA12-PE38KDEL and 2F1-PE38KDEL have specific antitumor activity in a mouse xenograft model for human melanoma. It is therefore able to penetrate solid tumor and substantially delay tumor growth in mice at doses that do not produce animal toxicity.
Having constructed immunotoxin against several melanoma epitopes, we aimed at increasing the probability of eradicating tumor cell variants. As tumor cells tend to mutate, they may lack the target antigen entirely or express it at levels too low for effective immunotoxin-mediated killing. Such mutant cells could be eradicated with cocktails of two or more immunotoxins recognizing different target antigens (20). In our in vitro study we did not observe a significantly higher cytotoxic effect when using more than one immunotoxin. This may be due to relatively homogenous expression of the target antigen in melanoma cell lines. However, in patients carrying a large tumor mass, mutations are abundant and using a cocktail of two or more immunotoxins may show a beneficial effect.
The only immunotoxin that is directed to tumor cells and currently being evaluated for treatment metastatic melanoma is comprised of ricin A chain conjugated to a murine monoclonal antibody directed against high molecular weight melanoma antigens. In this case, the use of murine antibody may induce the development of anti-immunotoxin antibodies which will result in decreased efficacy and limit repetitive dosing with an immunotoxin (21–23) Thus we propose the evaluation of a different class of toxin target for melanoma, the use of a human antibody fragment which is less immunogenic than a mouse antibody and of smaller size, which should allow a better tumor penetration and repeated administrations. In addition, strategies to reduce the toxicity and the immunogenicity of the toxin, such as a chemical modification with polyethylene glycol (24), will be further required in order to maximize the toxin therapeutic potency in human.
The data presented here are a proof of principle that specific MHC complexes can be used in humans as target therapy for melanoma. Moreover, the delivery of TCR-like-antibodies-PE38KDEL could serve as a first line therapy, to debulk tumor mass and prevent further rigorous growth and may be a general approach that can be readily extended to known immunodominant peptides for other HLA (25, 26) and different cancer types.
Supplementary Material
Acknowledgments
We thank Dr. Malka Epel, Dr. Oryan Makler, Dr. Roy Noy and Kfir Oved for help. We thank Dina Segal at the Technion and Amanda Cobb and Dr. Yanying Cao at BIIR for technical support. We thank Dr. Gerard Zurawski and Dr. Jonathan Sohnis for critical reading and discussion.
This work was supported by grants from the Israel Science Foundation (ISF grant No 229/05 to YR), the National Institutes of Health (RO-1 CA115550 to YR), Baylor Health Care Systems Foundation, Falk Foundation, the National Institutes of Health (RO-1 CA78846, RO-1 CA85540, PO-1 CA84512, U-19 AI-57234 to JB) JB holds the W.W. Caruth, Jr. Chair for Transplantation Immunology Research. AKP holds the Ramsay Chair for Cancer Immunology.
References
- 1.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 3.von Mehren M, Adams GP, Weiner LM. Monoclonal antibody therapy for cancer. Annu Rev Med. 2003;54:343–69. doi: 10.1146/annurev.med.54.101601.152442. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DR, Grillo-Lopez A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin’s B-cell lymphoma. Biochem Soc Trans. 1997;25:705–8. doi: 10.1042/bst0250705. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–31. [PubMed] [Google Scholar]
- 6.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yewdell JW. Plumbing the sources of endogenous MHC class I peptide ligands. Curr Opin Immunol. 2007;19:79–86. doi: 10.1016/j.coi.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denkberg G, Cohen CJ, Lev A, Chames P, Hoogenboom HR, Reiter Y. Direct visualization of distinct T cell epitopes derived from a melanoma tumor-associated antigen by using human recombinant antibodies with MHC- restricted T cell receptor-like specificity. Proc Natl Acad Sci U S A. 2002;99:9421–6. doi: 10.1073/pnas.132285699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lev A, Denkberg G, Cohen CJ, et al. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–94. [PubMed] [Google Scholar]
- 13.Denkberg G, Cohen CJ, Segal D, Kirkin AF, Reiter Y. Recombinant human single-chain MHC-peptide complexes made from E. coli By in vitro refolding: functional single-chain MHC-peptide complexes and tetramers with tumor associated antigens. Eur J Immunol. 2000;30:3522–32. doi: 10.1002/1521-4141(2000012)30:12<3522::AID-IMMU3522>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann U, Lee BK, Pastan I. Recombinant immunotoxins containing the VH or VL domain of monoclonal antibody B3 fused to Pseudomonas exotoxin. J Immunol. 1993;150:2774–82. [PubMed] [Google Scholar]
- 15.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750. [PubMed] [Google Scholar]
- 16.Rivoltini L, Squarcina P, Loftus DJ, et al. A superagonist variant of peptide MART1/Melan A27–35 elicits anti-melanoma CD8+ T cells with enhanced functional characteristics: implication for more effective immunotherapy. Cancer Res. 1999;59:301–6. [PubMed] [Google Scholar]
- 17.Seetharam S, Chaudhary VK, FitzGerald D, Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biol Chem. 1991;266:17376–81. [PubMed] [Google Scholar]
- 18.Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem J. 1995;307:29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen CJ, Sarig O, Yamano Y, Tomaru U, Jacobson S, Reiter Y. Direct phenotypic analysis of human MHC class I antigen presentation: visualization, quantitation, and in situ detection of human viral epitopes using peptide-specific, MHC-restricted human recombinant antibodies. J Immunol. 2003;170:4349–61. doi: 10.4049/jimmunol.170.8.4349. [DOI] [PubMed] [Google Scholar]
- 20.Ghetie MA, Tucker K, Richardson J, Uhr JW, Vitetta ES. The antitumor activity of an anti-CD22 immunotoxin in SCID mice with disseminated Daudi lymphoma is enhanced by either an anti-CD19 antibody or an anti-CD19 immunotoxin. Blood. 1992;80:2315–20. [PubMed] [Google Scholar]
- 21.Oratz R, Speyer JL, Wernz JC, et al. Antimelanoma monoclonal antibody-ricin A chain immunoconjugate (XMMME-001-RTA) plus cyclophosphamide in the treatment of metastatic malignant melanoma: results of a phase II trial. J Biol Response Mod. 1990;9:345–54. [PubMed] [Google Scholar]
- 22.Gonzalez R, Salem P, Bunn PA, Jr, et al. Single-dose murine monoclonal antibody ricin A chain immunotoxin in the treatment of metastatic melanoma: a phase I trial. Mol Biother. 1991;3:192–6. [PubMed] [Google Scholar]
- 23.Selvaggi K, Saria EA, Schwartz R, et al. Phase I/II study of murine monoclonal antibody-ricin A chain (XOMAZYME-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother. 1993;13:201–7. doi: 10.1097/00002371-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci U S A. 2000;97:8548–53. doi: 10.1073/pnas.140210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chames P, Hufton SE, Coulie PG, Uchanska-Ziegler B, Hoogenboom HR. Direct selection of a human antibody fragment directed against the tumor T-cell epitope HLA-A1-MAGE-A1 from a nonimmunized phage-Fab library. Proc Natl Acad Sci U S A. 2000;97:7969–74. doi: 10.1073/pnas.97.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krogsgaard M, Wucherpfennig KW, Cannella B, et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85–99 complex. J Exp Med. 2000;191:1395–412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.