Abstract
A key function of memory is to use past experience to predict when something important might happen next. Indeed, cues that previously predicted arousing events (emotional harbingers) garner more attention than other cues. However, the current series of five experiments demonstrates that people have poorer memory for the context of emotional harbinger cues than of neutral harbinger cues. Participants first learned that some harbinger cues (neutral tones or faces) predicted emotionally arousing pictures and others predicted neutral pictures. Then they studied associations between the harbinger cues and new contextual details. They were worse at remembering associations with emotional harbingers than with neutral harbingers. Memory was impaired not only for the association between emotional harbingers and nearby digits but also for contextual details that overlapped with or were intrinsic to the emotional harbingers. However, new cues that were inherently emotionally arousing did not yield the same memory impairments as the emotional harbingers. Thus, emotional harbinger cues seem to suffer more from proactive interference than do neutral harbinger cues, impairing formation of new associations with cues that previously predicted something arousing.
Keywords: affective associative memory, conditioning, proactive interference
Anticipating something emotional can be as intense as—and sometimes even more intense than—the actual experience (O'Doherty et al., 2002). This anticipation is often triggered by cues that were previously associated with an emotional event (Bermpohl et al., 2006a, 2006b; Jensen et al., 2003; Kirsch et al., 2003; Knutson et al., 2005; Mackiewicz et al., 2006; McNally & Westbrook, 2006; Phelps, 2004). For instance, for most of us, the sound of a telephone ringing does not predict anything very emotional. However, for someone being hounded by creditors because of unpaid debt, every phone call in the past few days might have been from a creditor that led to either an unpleasant conversation or a nasty message. Hearing the phone ring might increase arousal because of what that sound predicts. Once associated with something arousing, even abstract neutral symbols such as a blue square can increase physiological arousal and activity in the amygdala, a key emotion-processing region of the brain (Phelps et al., 2001).
Increases in arousal often make it more likely that people will remember what was happening at the time. In general, pictures, words or events that evoke physiological arousal and amygdala activity because of their inherently emotional qualities are more likely to be remembered later than neutral stimuli (for reviews see Hamann, 2001; LaBar & Cabeza, 2006). For example, after watching a slide show, participants are more likely to remember seeing a picture of a burn victim than another picture with a neutral male face (Mather & Knight, 2005). However, emotional arousal does not necessarily lead to enhanced memory for everything that is experienced. For instance, arousing items can lead to attentional narrowing, as indicated by worse memory for peripheral details when there is a central arousing item in a scene (for reviews see Levine & Edelstein, in press; Mather, 2007; Reisberg & Heuer, 2004).
Expectations based on previous experience have a powerful influence on behavior (Lovibond & Shanks, 2002); because people tend to remember emotional events better than neutral ones, they are more likely to learn from these situations (Baumeister et al., 2007). However, despite its sway over behavior, little is known about how emotional anticipation affects explicit memory. Decades of research on conditioning have focused on the mechanisms of learning associations between aversive or pleasant events and cues that predict them (Dickinson & Mackintosh, 1978; Rescorla & Holland, 1982; Wasserman & Miller, 1997). However, previous studies have not examined how cues that predict emotional events affect explicit memory for contextual information. In the current series of experiments, we examined whether a neutral cue that previously predicted arousing outcomes (what we will call an emotional harbinger) enhances or impairs memory for information associated with that neutral cue. For instance, when hearing a phone ring, memory for the details of what else was happening at the time might be impaired or enhanced depending on whether the phone ringing predicts something aversive, neutral or pleasant.
Predictions based on previous research
Neutral cues that predict arousing events increase anxiety and arousal (e.g., Grillon & Davis, 1997; Phelps et al., 2001). According to an influential model of anxiety (Gray, 1982), a behavioral inhibition system responds to stimuli associated with punishment or reward by inhibiting current behavior but increasing arousal and attention to the external environment. Consistent with this model, stimuli previously associated with aversive stimuli attract more attention than stimuli not previously associated with aversive stimuli (Armony & Dolan, 2002; Beaver et al., 2005; Dawson et al., 1982; Kelly & Forsyth, 2007; Koster et al., 2004; Richards & Blanchette, 2004; Smith et al., 2006; Van Damme et al., 2004). Thus, one potential prediction for our experiments is that this increased attention will enhance the encoding of events appearing at the same time as the emotional harbinger. In addition, this increased attention could improve people's likelihood of making lasting associations between the emotional harbinger and its contextual details. Indeed, a growing number of studies indicate that people show enhanced memory binding for features of inherently arousing stimuli (e.g., D'Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; Kensinger & Corkin, 2003; MacKay & Ahmetzanov, 2005; MacKay et al., 2004). For instance, they are more likely to remember the association between an emotional picture and its location (Mather et al., under review; Mather & Nesmith, 2008; Nashiro & Mather, under review), or the specific visual details associated with an emotional object (e.g., Kensinger et al., 2007a, 2007b; Kensinger & Schacter, 2007), than they are for neutral items.
In addition, the effects of emotional harbingers on memory may vary depending on the length of time since encoding. One study testing paired-associate learning found that memory for which digit was paired with words that elicited a larger arousal response increased with time, whereas memory for which digit was paired with less arousing words decreased with time (Kleinsmith & Kaplan, 1963). Although these striking findings have been difficult to replicate, probably because of a confound in the experimental design (for more details see Mather, 2007), other studies have also found that increasing the test delay increases the memory advantage that arousing stimuli have over neutral stimuli (LaBar & Phelps, 1998; Sharot & Phelps, 2004; Sharot & Yonelinas, 2008; but see Mather et al., under review; Mather & Knight, 2005; Payne et al., 2006). Thus, as time passes, people might be less likely to forget contextual details of emotional harbingers than contextual details of neutral cues.
The current study
We began by testing whether memory was enhanced or impaired for digits presented at the same time as neutral auditory tones that previously predicted arousing pictures. This first experiment revealed that, contrary to our initial prediction, participants were worse at remembering which numbers were associated with emotional harbingers (neutral tones that previously predicted negative pictures) than which numbers were associated with neutral harbingers (neutral tones that previously predicted neutral pictures). Experiment 2 revealed that cues that previously predicted positive events also yielded impaired memory for associated digits, indicating the emotional harbinger effect is due to emotional arousal rather than negative valence. Our next question was whether the memory impairment for information associated with the emotional harbingers would be seen for intrinsic features of the harbinger cue as well as for distinct items. To test this, Experiments 3 and 4 used visual cues, instead of auditory cues. Memory was impaired even for contextual details that were part of the same object as the emotional harbinger cue, in contrast with the arousal-enhanced memory binding seen in previous studies for arousing pictures and words (for a review, see Mather, 2007). Therefore, to test whether we could see these contrasting effects in the same experiment, in the final experiment, we compared context memory for faces that previously predicted emotional pictures to context memory for inherently emotional faces. Emotional harbinger faces had the opposite effect on location memory as inherently arousing faces.
Experiment 1
This experiment addresses the question of how having neutral cue repeatedly predict arousing events affects people's ability to learn new associations to that cue later. As shown in Figure 1A, in the cue-learning phase of Experiment 1, several specific neutral tones always preceded negative pictures whereas others always preceded neutral pictures. Immediately after the cue-learning phase, participants completed a study phase in which they heard each tone while seeing a digit and were asked to learn the tone-digit associations (Figure 1B). No inherently emotional stimuli were involved in this study phase. Either immediately or 24 hours later, we tested memory for the tone-digit pairings by playing the tone and asking participants to select which of two digits had been seen with the tone during the study phase. Of interest was whether tone-digit association memory would be better for tones that previously predicted arousing pictures or for tones that previously predicted neutral pictures.
Figure 1.
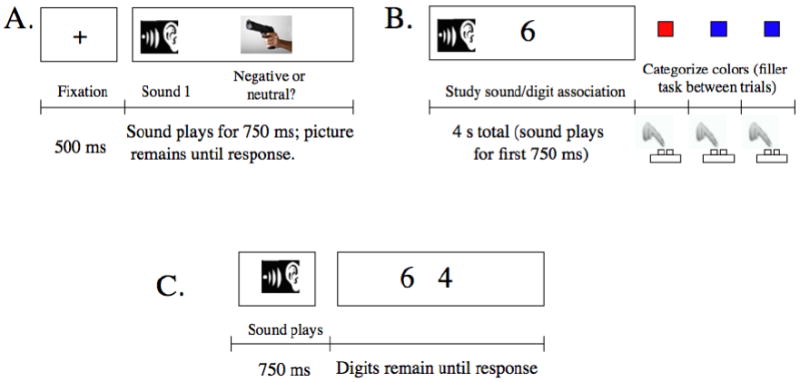
In Experiments 1 and 2, participants first completed a cue-learning phase (A) in which some tones always preceded negative pictures and others always preceded neutral pictures (the tones started playing 250 ms before the picture appeared) and then completed a study phase in which they heard the each tone again while seeing a digit (B). In Experiment 2, positive arousing pictures were introduced as an additional category. Memory for the digit-tone associations was tested using forced choice between two digits after hearing the sound (C).
Method
Participants
Forty-eight undergraduates participated for course credit (M age = 22.35, SD = 8.01, 18 males and 30 females) and were randomly assigned to condition (10 males and 15 females in the no delay condition and 8 males and 15 females in the 24-hour delay condition).
Materials
Four computer-generated tone sequences always predicted arousing pictures and four always predicted neutral pictures (which sounds preceded arousing or neutral pictures was counterbalanced across participants). We used 64 high arousal negative pictures (M arousal = 5.70, SD = .62; M valence = 2.55, SD = .56) and 64 low arousal neutral pictures (M arousal = 2.91, SD = .42; M valence = 5.08, SD = .38) from the International Affective Picture System (Lang et al., 1999), which includes standardized ratings of the valence of each picture from 1 (most unpleasant) to 9 (most pleasant) and for arousal level from 1 (least arousing) to 9 (most arousing).
Procedure
The procedure had three phases: 1) cue learning, 2) association study, and 3) association memory test. In the cue-learning phase (Figure 1A), each trial consisted of a fixation cross (500 ms) immediately followed by one of the tones (750 ms). Shortly (250 ms) after the tone started playing, a picture appeared along with the prompts “NEGATIVE” and “NEUTRAL” in the left and right lower corners of the screen. Participants were instructed to categorize each picture as quickly and as accurately as they could (no mention was made of the predictive nature of the tones). The picture remained on the screen until they pressed a key, at which point the fixation appeared for the next trial. Each of the eight tones was presented 16 times with different pictures from the same emotional category. Presentation order was random.
On each trial of the association study phase, one of the eight sounds played for 750 ms while a digit from 2 to 9 appeared for 4 seconds (the sound ended while the digit was still displayed). The participants' main task was to try to remember each tone-digit pairing for a later memory test. To prevent carry-over effects from the arousal of one trial to the next, they next saw a series of three red or blue squares and had to indicate the color of each one. To avoid a floor effect, there were four cycles through each of the eight tone-digit pairs.
Participants completed a forced-choice memory test either immediately after the study phase or 24 hours later. During each trial of the memory test, participants first heard one of the tones for 750 ms and then saw two digits on the screen and were asked to indicate which digit had previously been paired with the sound. For two of the negative sounds, the foils were digits previously paired with other negative sounds and for the other two negative sounds, the foils were digits previously paired with neutral sounds.
Results and Discussion
We analyzed the proportion of correct responses on the tone-digit conjunction memory test using a 2 (Neutral/Emotional Harbinger Tone) × 2 (Delay) ANOVA. Memory for sound-digit pairings was worse for sounds that previously predicted negative stimuli (M = .60, SE = .04) than for sounds that previously predicted neutral stimuli (M = .71, SE = .04), F(1,46) = 6.33, p<.05, ηp2=.12 (see left side of Figure 2). Also, participants tested immediately performed better (M = .74, SE = .04) on the conjunction memory test than those tested a day later (M = .57, SE = .05), F(1,46) = 7.09, p<.05, ηp2=.13. There were no other significant effects. Thus, this experiment revealed an emotional harbinger effect, in which memory for contextual information associated with neutral cues that previously predicted negative events is impaired, even when no negative event occurs after the cue. Increasing the test delay did not significantly affect the magnitude of the emotional harbinger effect.
Figure 2.
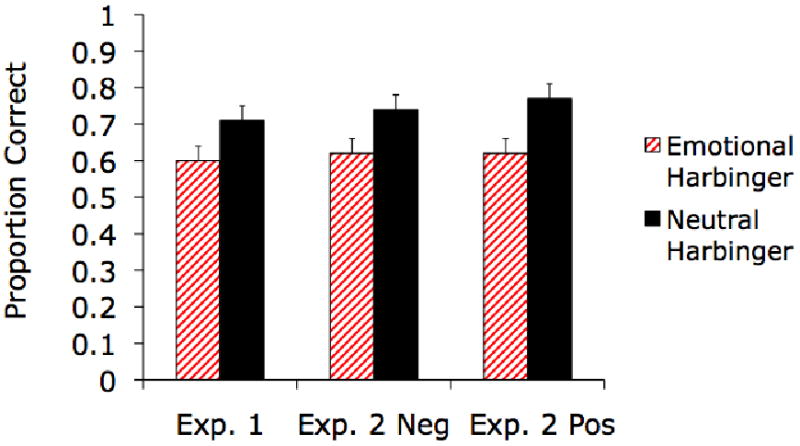
Memory for which digits had been associated with tones was lower for tones that had previously predicted arousing negative pictures than for tones that had previously predicted neutral pictures in Experiments 1 and 2. In Experiment 2, this emotional harbinger effect was extended to positive arousing pictures.
Experiment 2
Experiment 1 revealed that associative memory was worse for cues that previously predicted negative pictures than for cues that previously predicted neutral pictures. One important question is whether this effect is specific to negative harbinger cues or whether associative memory is also impaired for positive harbinger cues. Thus, in Experiment 2, we repeated Experiment 1 in one condition and compared it with another condition in which the tones predicted either positive or neutral pictures. In Experiment 2, we gave the memory test either immediately or 45 minutes later.
Method
Participants
One hundred and ten undergraduates participated for course credit (M age = 21.10, SD = 4.78, 46 males and 64 females). There were 55 participants in both the immediate condition and 24-hour test condition, with 27 or 28 participants in each condition of the 2 (negative/neutral vs. positive/neutral) × 2 (immediate vs. 24-hour test) design (with 36-50% male participants in each cell).
Materials
In the negative-neutral condition, we used the same materials as in Experiment 1. In the positive-neutral condition, we substituted 64 high arousal positive images (M arousal = 5.68 SD = .68; M valence = 7.30, SD = .38) for the high arousal negative images. The average arousal rating for the positive and negative images did not differ significantly from each other, t(126) = .15, ns.
Procedure
The procedure was the same as in Experiment 1 except that the delay group completed their memory test after a 45-min interval rather than a day later.
Results and Discussion
We analyzed the proportion of correct responses on the conjunction memory test using a 2 (Neutral/Emotional Harbinger Tone) × 2 (Negative/Positive Condition) × 2 (Delay) ANOVA. Memory for sound-digit pairings was worse for sounds that previously predicted arousing pictures (M = .62, SE = .03) than for sounds that previously predicted neutral pictures (M = .76, SE = .03), F(1,106) = 23.25, p<.001, ηp2=.18. There were no other significant effects (all F<1) and, as shown in Figure 2, associative memory for emotional harbinger sounds was impaired in both the negative and positive arousal conditions. Thus, anticipating arousing events appears to be the key factor leading to the memory impairment, not just anticipating negative events. In addition, as in the previous experiment, test delay did not significantly modulate this harbinger effect.
Why would anticipating arousal lead to memory impairment? One possibility is that participants focus on the emotional harbinger at the expense of peripheral information, in the same way that memory narrowing occurs for stimuli that are inherently arousing (Reisberg & Heuer, 2004). In a recent review, Mather (2007) pointed out that arousal-enhanced memory binding for inherently arousing stimuli (such as emotional pictures or words) is usually only seen for the features of the arousing object itself, such as its location or color (see also Kensinger, 2007). In contrast, people show little benefit (and sometimes even impairment) in memory for the associations between something arousing and external details, such as what other items were shown at the same time or what the background looked like. This arousal-enhanced binding for within-object features but not between-object features appears to result from the focused attention that arousing objects attract, increasing the likelihood that perceptual binding processes will be successful.
Thus, if emotional harbingers also elicit more focused attention, memory binding may be enhanced for the features of the harbinger cue itself (such as its color or location) but not for associations between the harbinger cue and extrinsic information that is spatially separated from the harbinger cue. In Experiments 3 and 4, we examined whether the emotional harbinger effect was due to focused attention on the harbinger cue, impairing memory for nearby information but not memory for information overlapping with the cue.
Experiment 3
Experiments 1 and 2 revealed an emotional harbinger effect—impaired memory for information associated with a cue that previously predicted that an upcoming target would be arousing. In Experiment 3, we examined whether the harbinger effect also occurs with cues and targets from the same modality (both visual, rather than auditory and visual) and whether the degree of separation between the cue and its to-be-remembered context matters. Instead of sounds, in this experiment we used faces as the cues that predicted arousing or neutral stimuli. The associated items introduced during the subsequent study phase were hats either shown being worn or in a separate photograph next to the face (see Figures 3 and 4). At test, participants were asked to indicate whether hat-face pairs had been shown before. Because the delay interval did not influence the emotional harbinger effect in Experiments 1 and 2, we used an immediate test for all participants.
Figure 3.
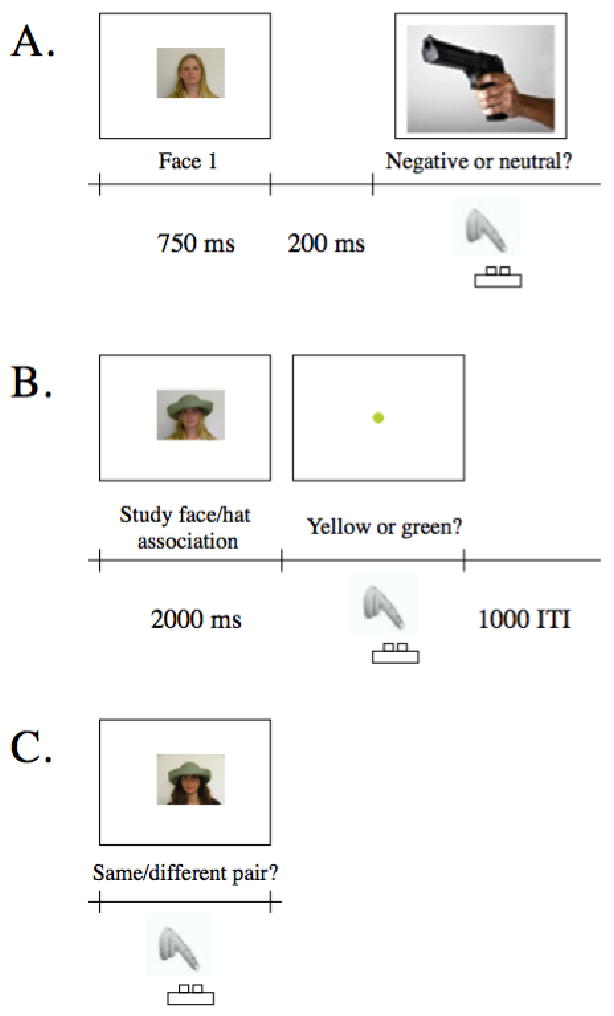
In Experiment 3, participants first completed a cue-learning phase in which certain neutral faces always preceded negative pictures (A) and other neutral faces always preceded neutral pictures. In the next phase, they studied face-hat pairings (B). In the memory test, they saw old faces and hats and indicated whether they were in the same pair or a different pair than before (C).
Figure 4.
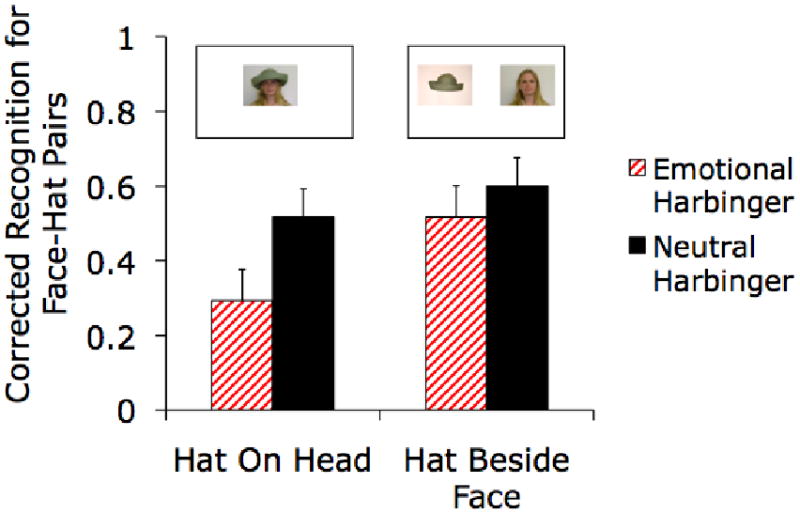
In Experiment 3, recognition of face-hat pairings from the study phase was more accurate for faces that had previously predicted neutral pictures than for those that had previously predicted arousing pictures, both when the hats were seen on the heads and when they were seen beside the faces in the study and test phases. Examples the two types of hat trials are presented (pictures are not shown to scale).
Method
Participants
Fifty-eight undergraduates participated for course credit (M age = 19.0, SD = 1.15, 23 males and 35 females). Twenty-eight were in the on-head condition (10 males) and 30 in the adjacent condition (18 males).
Materials and procedure
The procedure was based on Experiment 1, with eight neutral female face photographs substituted for the tones. As in the previous experiments, participants were not informed in advance about the predictive nature of the faces. During each trial of the initial cue-learning phase, participants viewed one of the eight face photographs for 750 ms, then after a 200 ms interval, they saw a negative or neutral picture with the “NEGATIVE” and “NEUTRAL” prompts below it (Figure 3A). As soon as they responded, the next trial began. Four of the faces always preceded negative and arousing pictures and four always preceded neutral pictures (the faces were counterbalanced across participants). We used the same number of trials and the same negative and neutral pictures as in Experiment 1.
During the association-study phase, participants saw each face paired with a hat. Participants in the side-by-side condition saw the faces and hats on opposite sides of the screen (the center of each picture was 3” away from the center of the screen), whereas those in the on-head condition saw the people wearing the hats. In each trial, participants saw the face/hat pair for 2000 ms, followed by a dot. Like the procedure in Experiments 1 and 2, their main task was to try to remember the face-hat pairing for a later memory test. In addition, they were instructed to indicate whether the dot was green or yellow (Figure 3B). After their response, there was a 1000 ms intertrial interval before the next trial began. Each of the eight face-hat pairs was only shown once. The recognition test immediately followed the study session. Participants were presented with a face-hat pair in the same configuration as during their study session (side by side or hat on head). Half of the face-hat pairings matched the pairings seen during the study session and half were incorrectly paired but previously seen faces and hats. Participants were asked to indicate whether the faces and hats were correctly paired (“same”) or incorrectly paired (“different”) by pressing a key.
Results and Discussion
A 2 (Neutral/Emotional Harbinger Face) × 2 (Face-Hat Display Configuration) ANOVA revealed that corrected recognition (hits - false alarms) for hat-face pairs was worse for emotional harbinger faces (M = .42, SE = .06) than for neutral harbinger faces (M = .57, SE = .05), F(1,56) = 4.29, p<.05, ηp2=.07. There was not a significant interaction of face type and display configuration, (F<1; see Figure 4). Thus, this experiment replicated the emotional harbinger effect found in Experiments 1 and 2 with visual cues instead of auditory cues. Furthermore, if anything, memory impairments for the on-head hats (overlapping-location contextual details) were stronger than memory impairments for adjacent hats, suggesting that the memory impairment for contextual details is not due to a narrowing of attention onto the cue that predicts arousal.
Experiment 4
Experiment 3 revealed impaired context memory for features in the same location as the emotional harbinger—indicating that attentional narrowing that impairs memory for peripheral information cannot account for the emotional harbinger effect. However, Experiment 3 does not address the within- versus between-object distinction raised by Mather (2007). Each face was seen many times during the cue-learning face without a hat. Thus, when shown during the study phase wearing a hat, participants were likely to process the face and hat as separate objects. So, even though the hat spatially overlapped the face, it might not garner the same benefits that a within-object feature such as color or location might (e.g., Mather & Nesmith, 2008; D'Argembeau & Van der Linden, 2004; MacKay & Ahmetzanov, 2005).
Therefore, in Experiment 4, we tested whether the impaired context memory for emotional harbinger cues would extend to location memory. The associated information included the location of the harbinger face during the study phase as well as a digit also shown on the screen. At test, participants were asked to choose between two face-location pairings and also to choose between two face-digit pairings, to indicate the pairings shown before.
Method
Participants
Forty-four undergraduates participated for course credit (M age = 19.0, SD = 1.0, six males and 38 females).
Materials and procedure
The materials and procedure were the same as Experiment 3 except for the following changes. There were eight neutral faces (four male and four female) that were different than those used in Experiment 3. In the cue-learning phase, the faces appeared for 1000 ms then after a 250 ms interval, a negative or neutral picture appeared with the “NEGATIVE” and “NEUTRAL” prompts below it. As soon as participants responded, the next trial began. Two of the female and two of the male faces always preceded negative and arousing pictures and the other four faces always preceded neutral pictures (which faces were in each condition was counterbalanced across participants).
In the association-study trials, a digit appeared in the center location on the screen. Simultaneously for two seconds, one of the faces from the cue-learning phase was displayed in one of the eight other locations (locations were created by dividing the screen into a three by three grid). Participants were asked to remember each face-digit pairing and the location of each face for a later memory test. To prevent carry-over effects from the arousal of one trial to the next, they next saw a series of three yellow or green squares and had to indicate the color of each one. There were two forced-choice test sessions (order was counterbalanced across participants), one in which each face was shown in two locations and one in which each face was shown with two digits. During the face-location test, each face was shown in two of the eight possible screen locations used in the association-study trials. On each trial, one of the face location pairings was correct and the other was incorrect. During the face-digit test, each face was shown in the center of the screen with two digits below (one in the left bottom corner of the screen and one in the right bottom corner). Participants indicated with a key press whether the digit on the left or the right was previously paired with the displayed face.
Results and Discussion
A 2 (Emotional/Neutral Harbinger Face) × 2 (Location or Digit Association) ANOVA revealed that memory for the locations and associated digits was worse for emotional harbingers (M = .63, SE = .03) than for neutral harbingers (M = .74, SE = .03), F(1,44) = 13.00, p=.001, ηp2=.23. There was no significant interaction of harbinger type and location vs. digit accuracy, (F<1; see Figure 5). Thus, the emotional harbinger effect extended to impaired memory for location memory as well as between-object contextual details.
Figure 5.
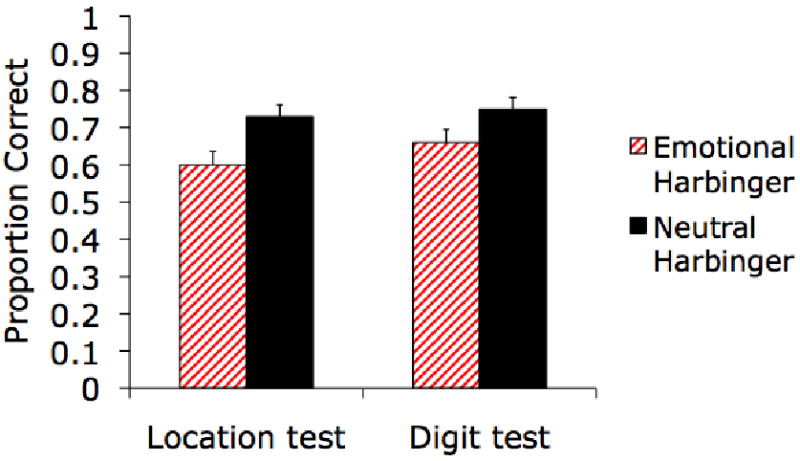
In Experiment 4, participants were better at identifying the face-location pairings and face-digit pairings from the study phase for faces that had previously predicted neutral pictures than for those that had previously predicted arousing pictures.
Experiment 5
Experiment 4 further strengthened the case that emotional harbingers yield the opposite effect on memory for contextual details as emotionally arousing pictures. In Experiment 4, location memory was worse for neutral faces that previously predicted negative pictures than for neutral faces that previously predicted neutral pictures. In contrast, a previous study that also showed pictures one at a time in different locations on the screen found that location memory was better for emotionally arousing pictures than for neutral pictures (Mather & Nesmith, 2008). However, Experiment 4 and the Mather and Nesmith (2008) study differed in several ways, including whether the associative memory encoding task was intentional (as in Experiment 4) or incidental (as in Mather & Nesmith, 2008). It is possible that arousal leads to impaired picture-location binding under intentional encoding but enhanced picture-location binding under incidental encoding. Indeed, in another paradigm in which participants maintained multiple picture-location conjunctions simultaneously in working memory while waiting for a memory test, memory was worse for the locations of arousing pictures than neutral pictures (Mather et al., 2006; Mitchell et al., 2006).
Thus, in the association-study phase of Experiment 5, we included 1) harbinger faces, half of which were neutral faces that previously predicted negative pictures and half were neutral faces that previously predicted neutral pictures, as in Experiment 3 and 4; and 2) new faces, half of which were inherently arousing and half of which were inherently neutral. Each inherently arousing new face was matched for visual appearance with one of the neutral new faces (each participant only saw either the arousing or neutral version of each new face). For instance, one matched pair consisted of a woman with two large bruises around her eyes or the same woman without the bruises. By intermixing the harbinger and the new faces during the association-study phase, we were able to see if location memory would be impaired for faces that previously predicted arousing pictures but enhanced for faces that were inherently arousing.
Faces were shown in different locations, with a shape also on the screen for four seconds. Participants were asked to learn both the locations of the faces and the face-shape associations. Given how our location memory findings from Experiment 4 contrasted with arousal-enhanced location memory from previous studies (e.g., Mather & Nesmith, 2008), we expected a significant interaction effect of face set (harbinger or new) and whether the face was emotional or not for location memory accuracy.
In addition, we tested recognition memory for the shapes themselves, to see how the emotional nature of harbinger and new faces might affect non-associative memory for bystander items. Finally, we included a test of face-shape association memory. Because previous research suggests that inherently arousing stimuli either have no impact on or impair memory for associations with other items (Mather, 2007) and Experiments 1-4 indicate that emotional harbingers impair memory for associations with other items, we predicted that there either would not be a difference in the effects of emotion for harbinger versus new faces, or that the difference would be less pronounced than for location memory.
Method
Participants
Fifty-six undergraduates participated for course credit (M age = 21.21, SD = 2.87; 18 males and 38 females).
Materials and procedure
The materials and procedure were the same as Experiment 4 except for the following changes. There were 12 neutral harbinger faces (six male and six female). In the cue-learning phase, the faces appeared for 1000 ms, then after a 250 ms interval, a negative or neutral picture appeared with the “NEGATIVE” and “NEUTRAL” prompts below it. As soon as participants responded, the next trial began. As in Experiment 4, half of the faces of each sex predicted negative and arousing pictures and the others predicted neutral pictures and which faces were in each condition was counterbalanced across participants. Each face cue appeared 12 times with a different picture of the same valence, thus in this experiment we used 72 neutral (M arousal = 2.90, SD = .44; M valence = 5.08, SD = .38) and 72 negative pictures (M arousal = 6.12, SD = .62; M valence = 2.64, SD = .60). Like the previous experiments, the pictures were from the International Affective Picture System, except for six of the neutral pictures (of houses or people) that we obtained from other sources and so are not included in the arousal and valence ratings given above.
The key change in experiment procedure was during the association-study trials; the 12 study trials for the harbinger faces were randomly intermixed with 12 study trials for new faces. Six of these new faces were arousing and the other six were neutral. These new faces were drawn from a set of 12 matched face pairs, in which each arousing face was paired with a neutral face of someone of the same approximate age, gender and race. Which face from each pair was shown was counterbalanced across participants. On each trial of the association-study phase, one of the faces appeared in one of the eight outer locations of the grid, as in Experiment 4.
In addition, an abstract geometrical object (a “geon,” Tarr, 2006) always appeared in the center of the screen in a port measuring 120 × 120 pixels. These shapes also were drawn from matched pairs; each pair had two similar shapes and participants were shown just one of these with the other one reserved for the forced-choice memory test (which one was seen during study was counterbalanced). They were asked to learn the locations of the faces as well as which shape was shown with each face.
Participants next completed a series of three memory tests. In the first memory test, each trial consisted of one previously seen shape shown on one side of the screen with a similar shape shown on the other side. Participants indicated which one they had seen before. In the next memory test, each trial consisted of one correctly paired face-location conjunction and the same face in a new location. Participants indicated which face-location conjunction matched the one previously seen during the study phase. Finally, participants completed a forced-choice memory test in which they indicated which of two faces had been associated with each shape they had seen before. Faces shown together were always from the same category (e.g., two negative harbinger faces).
Results and Discussion
Location-face associations
A 2 (Arousing/Non-Arousing Faces) × 2 (Harbinger/New Faces) ANOVA revealed that location memory was significantly worse for the harbinger faces (M = .61, SE = .02) than for the new faces (M = .70, SE = .02), F(1,55) = 14.45, p<.001, ηp2=.21. This main effect was qualified by an interaction of the two factors, F(1,55) = 4.59, p<.05, ηp2=.08. (see Figure 6). Location memory for the predict-arousing faces was poorer (M = .58, SE = .03) than location memory for the inherently arousing faces (M = .72, SE = .02), t(55) = 4.10, p<.001. In contrast, location memory for predict-neutral faces (M = .64, SE = .03) did not significantly differ from location memory for inherently neutral faces (M = .68, SE = .03), t(55) = 1.31, p=.19.
Figure 6.
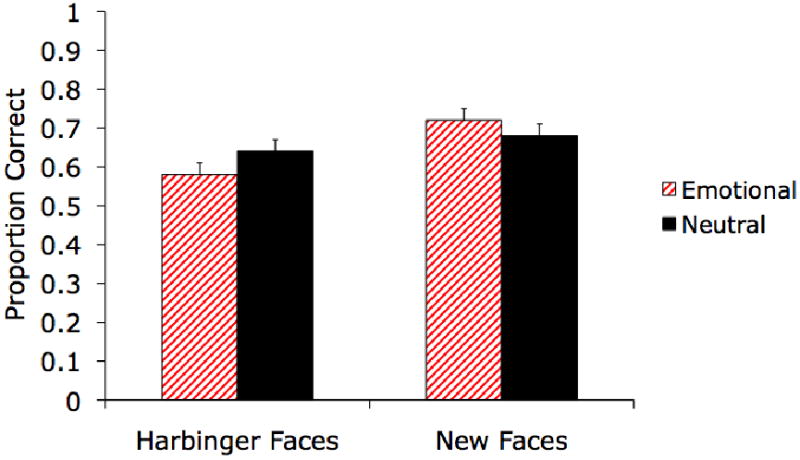
In Experiment 5, there was a significant interaction of type of face (harbinger or new) and emotion (emotional or neutral) on location memory.
Shape recognition
A 2 (Arousing/Non-Arousing Faces) × 2 (Harbinger/New Faces) ANOVA revealed no significant effects (all F<1, p>.8), with the mean forced-choice recognition .81 or .82 (SE = .02) in all conditions. Thus, whether faces previously predicted arousing pictures or were inherently arousing had no impact on recognition memory for bystander information.
Shape-face associations
A 2 (Arousing/Non-Arousing Faces) × 2 (Harbinger/New Faces) ANOVA revealed that memory for which face was shown with a shape was better for new faces (M = .71, SE = .02) than for harbinger faces (M = .64, SE = .03), F(1,55) = 5.30, p<.05, ηp2=.09. Unlike for the location-face associations, there was not a significant interaction, although planned comparisons revealed that memory for face-shape associations was worse for predict-arousing faces (M = .64, SE = .04) than for inherently arousing faces (M = .72, SE = .03), t(55) = 2.05, p<.05, but did not significantly differ for predict-neutral faces (M = .65, SE = .03) and inherently neutral faces (M = .69, SE = .03), t(55) = 1.41, p=.17. Thus, the shape-face association findings are somewhat ambiguous. We did not predict a large difference between conditions because between-item associations do not typically show much enhancement for inherently arousing items. However, in this experiment, the lack of an interaction was in part due to emotional harbinger cues showing little impairment for between-item associations compared with neutral harbinger cues. This may have been due to the fact that we tripled the number of faces shown during the association-study phase, which might have increased overall interference and reduced our ability to detect the emotional harbinger effect.
Overview
This experiment revealed that location memory was impaired for emotional harbinger faces compared with faces that were themselves emotionally arousing. Thus, it indicates that seeing a cue that previously predicted something emotionally arousing has a different effect than seeing a stimulus that is itself arousing. Also, the finding that, when averaged across emotional and neutral conditions, associative memory is worse for harbinger faces than for new faces suggests a potential mechanism for the emotional harbinger effect, as discussed further below.
General Discussion
Our findings reveal an emotional harbinger effect for context memory: When people perceive a cue they are less likely to later associate novel contextual details with it when the cue previously predicted something emotionally arousing rather than something neutral. Our experiments revealed this emotional harbinger effect for both auditory and visual harbinger cues and for harbinger cues that previously predicted either positive or negative pictures. In addition, the emotional harbinger effect generalized across a variety of different types of contextual detail.
We originally hypothesized that anticipatory arousal elicited by emotional harbinger cues would lead to better memory for the contextual details of those cues, along the same lines that the arousal elicited by an emotional word or picture can lead to better memory for its associated features such as color, location or visual detail (e.g., D'Argembeau & Van der Linden, 2004; Doerksen & Shimamura, 2001; Kensinger & Corkin, 2003; Kensinger et al., 2007b; MacKay & Ahmetzanov, 2005; MacKay et al., 2004; Mather & Nesmith, 2008). Instead we found memory impairment for which digit was associated with an emotional harbinger tone in Experiments 1 and 2. As we were testing an association between the harbinger and a separate item that was not inherent to the harbinger cue, the memory impairment for the digit potentially could be explained by attentional narrowing onto the emotional harbinger.
Therefore, in Experiment 3, we tested memory for the association between hats and emotional harbinger faces or neutral harbinger faces. Participants were less likely to remember the face-hat association for emotional harbinger faces than for neutral harbinger faces regardless of whether the hats had been shown on the head or next to it. This ruled out the possibility that the emotional harbinger effect was due to attentional narrowing, but it was still possible that the impaired memory binding would only be evident for associations between the harbinger cue and distinct items. Research with arousing words and pictures typically reveals arousal-enhanced memory binding for features—like location and color—that are part of the arousing item and not for between-item binding (Mather, 2007). Thus, in Experiment 4, we tested participants' memory for the harbinger face locations as well as for which digit they were shown with. Both types of associative memory were impaired for emotional harbinger faces, indicating that the emotional harbinger effect generalized across within- and between-object memory binding. To confirm the contrast between these findings and the arousal-enhanced within-object memory binding seen in previous studies, in Experiment 5 we directly compared location memory for inherently arousing faces to location memory for emotional harbinger faces and found that the two types of stimuli had opposite effects on memory for location.
Possible mechanism of the emotional harbinger effect
The emotional harbinger effect is particularly puzzling when considered in the context of previous findings that attention is enhanced for stimuli previously paired with aversive stimuli, compared with stimuli previously experienced but not paired with anything aversive (Armony & Dolan, 2002; Beaver et al., 2005; Van Damme et al., 2004; Van Damme et al., 2006). For instance, neutral pictures conditioned to be aversive by pairing them with aversive noise are more likely than other neutral pictures shown in the same conditioning phase to later interfere with processing of other images in a rapid visual sequence—leading to a larger “attentional blink” for pictures conditioned with an aversive noise (Smith et al., 2006). Neutral words conditioned using emotional pictures also interfere more with performance on Stroop or reasoning tasks than neutral words conditioned using neutral pictures, indicating that neutral stimuli presented with emotional pictures attract more attention later (Blanchette & Richards, 2004; Richards & Blanchette, 2004).
If, as would be expected given these previous findings, our emotional harbinger cues attracted more attention than our neutral harbinger cues, how can we explain the emotional harbinger context memory impairments? The results of Experiment 5 suggest a potential key factor in the harbinger effect. In this experiment, there was an overall impairment in associative memory for harbinger faces (see left side of Figure 6) compared with new faces (see right side of Figure 6). It is possible that the poorer associative memory performance for the harbinger face set resulted because those faces were more similar to each other than the faces in the new face set. However, a more interesting possibility is that the harbinger faces carried more baggage from the previous cue-learning phase when they appeared in the study phase. By the end of the learning phase, participants should have had a variety of associations between harbinger faces and the pictures they each preceded. These previous associations may have created proactive interference during the study phase when participants tried to make associations between the faces and the new contextual details.
Different levels of proactive interference might also account for associative memory impairments for emotional harbingers. If the initial face-picture associations were stronger for arousing pictures than for neutral pictures, proactive interference from the associations built up during the cue-learning phase would be more of an issue for emotional harbinger faces than for neutral harbinger faces and might override any effects of increased attention to those emotional harbinger faces. This potential proactive interference mechanism is particularly intriguing because it would involve interference from one type of association (e.g., face-picture) to different types of associations (e.g., face-digit or face-hat).
Relevant conditioning effects
Proactive and retroactive interference play roles in a number of previously demonstrated conditioning effects, such as extinction, counterconditioning and latent inhibition (for reviews see Bouton, 1993; Escobar et al., 2004). Although not the same as these previous conditioning effects, the effects in our studies may be caused by similar mechanisms as some of these effects.
For instance, experiencing a cue multiple times with no apparent outcome before experiencing that same cue predicting an outcome impairs learning about the cue-outcome association, an effect known as latent inhibition. Some have argued that latent inhibition is due to proactive interference from the initial association between the conditioned stimulus and its context (for a review see Wasserman & Miller, 1997).
Another potentially relevant conditioning effect is blocking; blocking occurs when a new cue is presented together with an old cue, followed by an outcome that previously was predicted by the old cue alone. The result is that people fail to associate the new cue with the outcome (Kamin, 1968). This might relate to our findings, in that the emotional harbinger cue could be blocking or overshadowing learning of new stimuli presented concurrently. A prominent theoretical account of blocking is that the old cue dominates attention, reducing attention to other cues (e.g., Kruschke, 2003; Sutherland & Mackintosh, 1971). Our findings that associative memory was impaired even when the contextual information was overlapping with (e.g., hats in Experiment 3) or intrinsic to (e.g., location in Experiments 4 and 5) the emotional harbinger argue against this type of blocking explaining our effects. More generally, our finding in Experiment 5 that recognition memory for a bystander shape was not impaired by emotional harbingers suggests that there is no global overshadowing or blocking of learning other items by emotional harbingers—instead, the impairments appear to be limited to associations between the harbinger and its context. These impairments in learning new associations would be better explained by proactive interference from previous associations to that cue than by an account that posits impairment for all new information presented with an emotional harbinger.
Future directions
These experiments raise interesting questions for future research. One question is whether increased proactive interference for emotional associations compared with neutral associations extends to other types of learning. For instance, is implicit memory for the locations of arousing harbingers impaired to the same extent as explicit memory for the locations, as suggested by previous research showing that implicit memory is also susceptible to proactive interference (Lustig & Hasher, 2001)?
Another question is to what extent the emotional harbinger effect is dependent on conscious awareness of the contingencies. Does the presentation of novel contextual information in the study phase have an impact on memory by violating contingency beliefs for emotional harbingers or does the memory impairment occur independently of contingency awareness? The answer to this question has implications for treatments that target anxiety disorders. Previous research on human conditioning suggests that awareness of contingency beliefs plays an important role in both learning contingencies and relearning them if they change (see Lovibond, 2004 for a review).
A related question is how easily knowledge about a cue that was once an emotional harbinger can be modified with experience. Our findings suggest that learning that involves emotional harbingers may be more resistant to modification than learning that involves neutral harbingers, because people have a harder time learning about the new contexts of emotional harbingers even with multiple learning opportunities and explicit instructions to do so.
Related to the issue of relearning, previous research indicates that the robustness of memories for the association between a conditioned stimulus such as a light or tone and a shock determines whether later presentations of the conditioned stimulus alone enhance memory for the previous association or help extinguish it (e.g., Runyan & Dash, 2005; Eisenberg et al., 2003). When given only one training session, re-presentation of the conditioned stimulus without the shock reduces later fear responses to the conditioned stimulus, whereas when given two training sessions, re-presentation of the conditioned stimulus without the shock can increase later fear responses. In our study, memory for cue-outcome associations should have been stronger for emotional harbingers than for neutral harbingers. Thus, in future studies, it would be interesting to examine whether representation of neutral harbinger cues hastens forgetting of the previously learned cue-picture associations whereas re-presentation of emotional harbinger cues strengthens cue-picture associations. If so, re-presentation of neutral harbinger cues might reduce proactive interference more than re-presentation of emotional harbinger cues, further contributing to differences in how easily people can learn new associations to the two types of cues. The nature of a predictive cue may play a crucial role in the likelihood or rate of new learning and extinction of previous learning.
Conclusion
To return to the example in the Introduction, imagine Joan had a home phone and a cell phone and received a series of emotionally arousing phone calls on her home phone and a series of neutral phone calls on her cell phone. These experiments suggest that her memory for the next time each phone rings would be influenced by this past history, with impaired memory for what else was happening when the home phone rang compared with when the cell phone rang. Furthermore, these experiments indicate that stimuli previously associated with emotionally arousing stimuli have quite different effects on memory than inherently arousing stimuli. While information that is inherently arousing can enhance memory for some types of contextual detail (for a review see Mather, 2007), our findings indicate that information that previously predicted arousal impairs memory for contextual detail. This emotional harbinger effect provides a new instance of how emotional arousal can impair memory for context, expanding on previous findings of arousal-based impairments of context memory in different paradigms (Anderson & Shimamura, 2005; Mather et al., 2006; Touryan et al., 2007).
The emotional harbinger memory impairment may carry some benefits. Emotional harbinger cues carry informational value, but the surrounding context may provide irrelevant details that could lead to prediction errors in the future. In contrast, when something emotionally arousing has just happened, and no prior history about what predicts that particular event has been established, contextual details may help establish new predictive cues that aid in future encounters.
Acknowledgments
This research was supported by NIA grant AG025340. We thank David Douglass for giving us access to lab space and the participant pool at Cabrillo College and Marcia K. Johnson and Karen J. Mitchell for comments on a previous version of the paper.
Contributor Information
Mara Mather, University of Southern California.
Marisa Knight, University of San Francisco.
References
- Anderson L, Shimamura AP. Influences of emotion on context memory while viewing film clips. American Journal of Psychology. 2005;118(3):323–337. [PubMed] [Google Scholar]
- Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: An event-related fmri study. Neuropsychologia. 2002;40(7):817–826. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, DeWall CN, Zhang LQ. How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Personality And Social Psychology Review. 2007;11(2):167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Mogg K, Bradley BP. Emotional conditioning to masked stimuli and modulation of visuospatial attention. Emotion. 2005;5(1):67–79. doi: 10.1037/1528-3542.5.1.67. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, et al. Attentional modulation of emotional stimulus processing: An fmrl study using emotional expectancy. Human Brain Mapping. 2006a;27(8):662–677. doi: 10.1002/hbm.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, et al. Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage. 2006b;30(2):588–600. doi: 10.1016/j.neuroimage.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Blanchette I, Richards A. Reasoning about emotional and neutral materials: Is logic affected by emotion? Psychological Science. 2004;15(11):745–752. doi: 10.1111/j.0956-7976.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of pavlovian learning. Psychological Bulletin. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M. Influence of affective meaning on memory for contextual information. Emotion. 2004;4(2):173–188. doi: 10.1037/1528-3542.4.2.173. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Beers JR, Schell AM, Kelly A. Allocation of cognitive processing capacity during human autonomic classical conditioning. Journal of Experimental Psychology: General. 1982;111(3):273–295. doi: 10.1037//0096-3445.111.3.273. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Mackintosh NJ. Classical conditioning in animals. Annual Review of Psychology. 1978;29:587–612. doi: 10.1146/annurev.ps.29.020178.003103. [DOI] [PubMed] [Google Scholar]
- Doerksen S, Shimamura AP. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science. 2003;301(5636):1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Escobar M, Arcediano F, Platt TL, Miller RR. Interference and time: A brief review and an integration. Reviews in the Neurosciences. 2004;15(6):415–438. doi: 10.1515/revneuro.2004.15.6.415. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An inquiry into the functions of the septo-hippocampal system. Behavioral and Brain Sciences. 1982;5(3):469–484. [Google Scholar]
- Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34(5):511–517. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Hamann SB. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Attention-like processes in classical conditioning. In: Jones MR, editor. Aversive stimulation. Coral Gables, Fla.: University of Miami Press; 1968. pp. 9–32. [Google Scholar]
- Kelly MM, Forsyth JP. Observational fear conditioning in the acquisition and extinction of attentional bias for threat: An experimental evaluation. Emotion. 2007;7(2):324–335. doi: 10.1037/1528-3542.7.2.324. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Negative emotion enhances memory accuracy: Behavioral and neuroimaging evidence. Current Directions in Psychological Science. 2007;16(4):213–218. [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory and Cognition. 2003;31(8):1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal Of Memory And Language. 2007a;56(4):575–591. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience. 2007b;19(11):1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: Neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45(13):2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, et al. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: An event-related fmri study. Neuroimage. 2003;20(2):1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, De Houwer J. Does imminent threat capture and hold attention? Emotion. 2004;4(3):312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- Kruschke JK. Attention in learning. Current Directions in Psychological Science. 2003;12:171–175. [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9(6):490–493. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. The international affective picture system (iaps): Technical manual and affective ratings. Gainesville, FL: University of Florida, The Center for Research in Psychophysiology; 1999. [Google Scholar]
- Levine LJ, Edelstein RS. Emotion and memory narrowing: A review. Cognition & Emotion in press. [Google Scholar]
- Lovibond PF. Cognitive processes in extinction. Learning and Memory. 2004;11:495–500. doi: 10.1101/lm.79604. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28(1):3–26. [PubMed] [Google Scholar]
- Lustig C, Hasher L. Implicit memory is not immune to interference. Psychological Bulletin. 2001;127(5):618–628. doi: 10.1037/0033-2909.127.5.618. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Ahmetzanov MV. Emotion, memory, and attention in the taboo stroop paradigm: An experimental analogue of flashbulb memories. Psychological Science. 2005;16:25–32. doi: 10.1111/j.0956-7976.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Shafto M, Taylor JK, Marian DE, Abrams L, Dyer JR. Relations between emotion, memory, and attention: Evidence from taboo stroop, lexical decision, and immediate memory tasks. Memory and Cognition. 2004;32(3):474–488. doi: 10.3758/bf03195840. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Gorlick M, Nesmith K. The limits of arousal's memory impairing effects on nearby information. under review. [PMC free article] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults' emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Mitchell KJ, Raye CL, Novak DL, Greene EJ, Johnson MK. Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience. 2006;18:614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal Of Memory And Language. 2008;58:449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learning and Memory. 2006;13(3):245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Mather M, Johnson MK, Raye CL, Greene EJ. A functional magnetic resonance imaging investigation of short-term source and item memory for negative pictures. Neuroreport. 2006;17(14):1543–1547. doi: 10.1097/01.wnr.0000234743.50442.e5. [DOI] [PubMed] [Google Scholar]
- Nashiro K, Mather M. How arousal affects younger and older adults' memory binding. doi: 10.1080/0361073X.2011.536746. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Ryan L, Hoscheidt S, Jacobs WJ, Nadel L. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 2006;14(1):1–16. doi: 10.1080/09658210500139176. [DOI] [PubMed] [Google Scholar]
- Phelps EA. The human amygdala and awareness: Interactions between emotion and cognition. In: Gazzaniga MS, editor. The cognitive neurosciences. 3rd. Cambridge: MIT Press; 2004. pp. 1005–1015. [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. Memory for emotional events. In: Reisberg D, Hertel P, editors. Memory and emotion. N.Y.: Oxford University Press; 2004. pp. 3–41. [Google Scholar]
- Rescorla RA, Holland PC. Behavioral studies of associative learning in animals. Annual Review of Psychology. 1982;33:265–308. [Google Scholar]
- Richards A, Blanchette I. Independent manipulation of emotion in an emotional stroop task using classical conditioning. Emotion. 2004;4(3):275–281. doi: 10.1037/1528-3542.4.3.275. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus. 2005;15(3):333–339. doi: 10.1002/hipo.20055. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106:538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, Zald DH. An emotion-induced attentional blink elicited by aversively conditioned stimuli. Emotion. 2006;6(3):523–527. doi: 10.1037/1528-3542.6.3.523. [DOI] [PubMed] [Google Scholar]
- Sutherland NS, Mackintosh NJ. Mechanisms of animal discrimination learning. New York: Academic Press; 1971. [Google Scholar]
- Tarr MJ. Geon stimuli. 2006 Retrieved August, 2006, from http://www.tarrlab.org/
- Touryan SR, Marian DE, Shimamura AP. Effect of negative emotional pictures on associative memory for peripheral information. Memory. 2007;15(2):154–166. doi: 10.1080/09658210601151310. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C, Koster EHW. Hypervigilance to learned pain signals: A componential analysis. Journal Of Pain. 2006;7(5):346–357. doi: 10.1016/j.jpain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Lorenz J, Eccleston C, Koster EHW, De Clercq A, Crombez G. Fear-conditioned cues of impending pain facilitate attentional engagement. Neurophysiologie Clinique-Clinical Neurophysiology. 2004;34(1):33–39. doi: 10.1016/j.neucli.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Miller RR. What's elementary about associative learning? Annual Review of Psychology. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]


