Abstract
TLR ligands are among the key stimuli driving the optimal dendritic cell (DC) maturation critical for strong and efficacious T cell priming. In this study, we show that part of this effect occurs via increased TCR triggering. Pretreatment of DCs with TLR ligands resulted in the triggering of many more TCRs in responding CD8+ T cells. Importantly, even when DCs expressed the same amount of cognate peptide-MHC (pMHC) molecules, TLR ligand treatment resulted in down-regulation of larger numbers of TCR molecules. This was independent of the up-regulation of costimulatory, adhesion or cytokine molecules or the amount of noncognate pMHCs. Rather, DCs pretreated with TLR ligands exhibited increased stability of cognate pMHCs, enabling extended TCR triggering. These findings are of potential importance to T cell vaccination.
Activation of CD8+ T cells is initiated by recognition of specific peptide-MHC complexes (pMHC)3 on the surface of an APC, chiefly a dendritic cell (DC), by the TCRs on T cells. CD8+ T cell responses are highly sensitive and specific and can be triggered by very few or even one antigenic pMHC expressed among a sea of irrelevant, mostly endogenous pMHC (1). Amplification of TCR signaling by a few pMHCs is achieved through serial engagement of many TCRs (>200) by a single pMHC (2). Paradoxically, the binding affinity between TCR-pMHC is low, raising questions of how exquisite specificity can be achieved in that context. It was proposed that there are optimal dissociation binding kinetics that facilitate TCR triggering, allowing an individual TCR to be engaged long enough to initiate a downstream signaling cascade, yet short enough to allow its neighboring TCR molecule to bind to the same pMHC complex and initiate a similar productive signaling cascade. Summation of these signals from adjacent TCR molecules would lead to T cell activation (3–5). This model of T cell activation by kinetic proofreading of pMHCs by sequential TCR interactions has received considerable experimental support, and there is evidence that activation thresholds for different CTL responses are met only when a defined amount of TCR signaling has occurred in a given time window (6).
Other signals also help activate T cells, and integration of these signals by the T cell depends on the context in which the T cell is activated. DCs are the most efficient APCs for the activation of CD8+ T cell responses. To optimally activate CD8+ T cells, DCs need to undergo maturation, including up-regulation of the MHC, costimulatory molecules, and cytokines involved in priming. DC maturation is initiated upon infection by the interaction of conserved motifs on pathogens and their products with sensor molecules that include TLRs (7). There are 11 described members of the TLR family that recognize ligands such as LPS (TLR4), dsRNA (TLR3), and unmethylated CpG motifs (TLR9). Because CpG is being considered as a vaccine component (8), we investigated whether and how stimulating a DC with CpG alters the magnitude and sensitivity of antigenic stimulation in CD8+ T cells. In this study, we show that pulsing of DCs with TLR ligands results in an increased TCR triggering. CpG-mediated increase in serial triggering was not due to up-regulation of MHC class I or costimulatory molecules but rather to a longer half-life of pMHCs expressed on the surface. These results uncover a novel mechanism that contributes to the effectiveness of T cell stimulation by TLR ligands.
Materials and Methods
Mice, reagents, and flow cytofluorometric analysis (FCM)
C57BL/6-NCR (B6) and B6.TAP1−/−mice (9) were purchased from the National Cancer Institute colony (Frederick, MD) and The Jackson Laboratory, respectively. Mice transgenic for rearranged TCR specific for the HSV-1 glycoprotein B498–505 and OVA 257–264 presented by H-2Kb on the B6 genetic background (lines gBT-I and OT-I, respectively) were provided by Dr. F. R. Carbone (University of Melbourne, Melbourne, Australia). All experiments were approved by the Institutional Animal Care and Use Committee (Oregon Health and Science University, Beaverton, OR). The gB-8 (SSIEFARL) and OVA-8 (SIINFEKL) peptides were obtained from Invitrogen. Anti-Kb-OVA (25.D16; Ref. 10) was provided by Dr. R. N. Germain (National Institute of Allergy and Infectious Diseases, Bethesda, MD). All other Abs used for FCM were purchased from either BD Pharmingen or eBioscience and analyzed using the FACS LSR II instrument (BD Immunocytometry Systems) and FloJo software (Tree Star), exactly as described (11). Neutralizing Ab against CD54, CD80, and CD86 (eBioscience), poly(I:C) (GE Healthcare), LPS (L6529; Sigma-Aldrich), and CpG (HC4033; Cell Sciences) were purchased from the respective sources.
Generation of bone marrow-derived DCs (BM-DCs)
Bone marrow was harvested from C57BL/6 or TAP1−/− mice, depleted of RBC, and incubated at 106 cells/ml in RP10 medium (12) with 20 ng/ml GM-CSF (R&D Systems). After 5–6 days, loosely adherent cells were highly enriched for DCs using positive immunomagnetic selection for CD11c+ cells (Miltenyi Biotec).
In vitro stimulations
BM-DCs were plated in 96-well plates and pulsed with peptide and/or CpG (1 µM). Twelve to 15 h later, BM-DCs were washed extensively and incubated with CD8+ T cells (“untouched” CD8+ purification kit from Miltenyi Biotec) at a DC-CD8+ T cell ratio of 1:10 and analyzed 8 h later. To normalize amounts of Kb-OVA on treated BM-DCs, concentration/mean fluorescence intensity (MFI) curves were constructed by measuring the expression Kb-OVA (25.D16) at various peptide concentrations. Where indicated, BM-DCs were pretreated with 5–10 µg/ml anti-CD54 or anti-CD80 and anti-CD86 for 30 min before the addition of CD8+T cells.
MHC class I (Kb-OVA) stability assays
BM-DCs were pulsed with OVA8 peptide with or without CpG for ~15 h and stained with saturating amounts of anti-Kb-OVA (25.D16) at 4°C for 30 min. After washing off the excess Ab, BM-DCs were incubated at 37°C for the indicated amounts of time in medium alone and the amount of Kb-OVA remaining on the surface at various time points quantified by FCM.
Results and Discussion
CpG-stimulated BM-DCs trigger more CD8+ TCRs
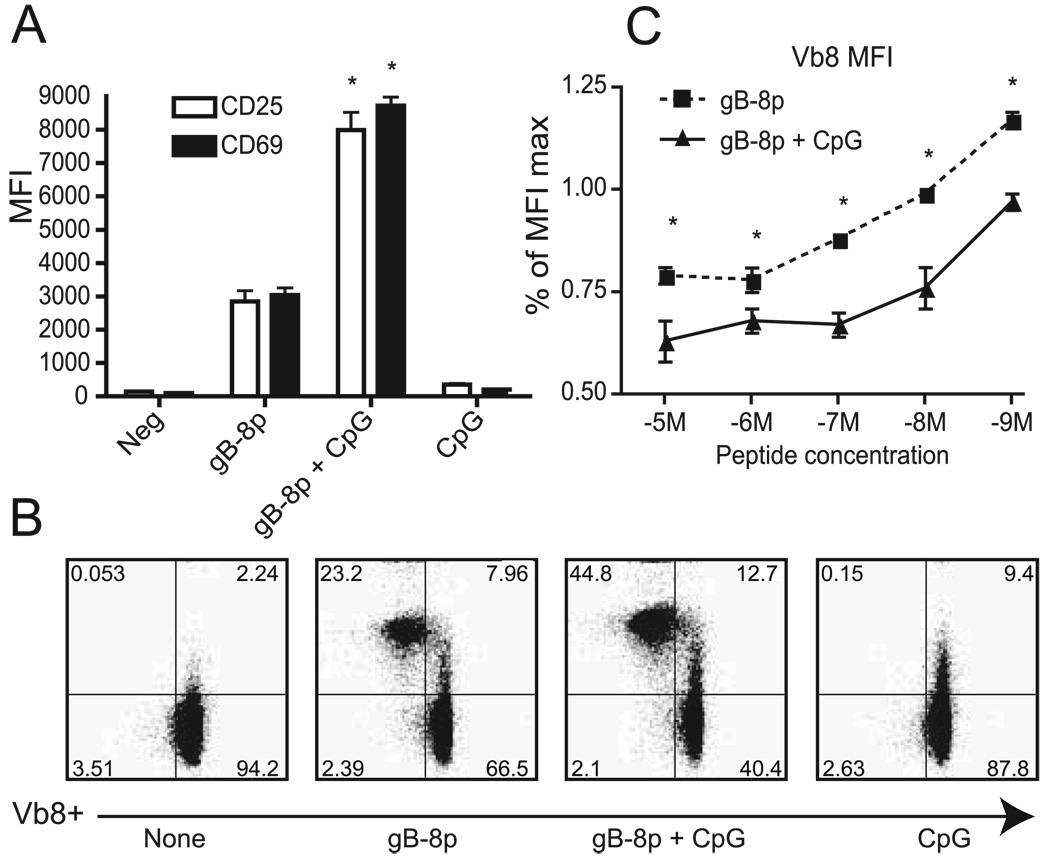
To study how TLR ligands enhance CD8+ T cell activation, we briefly (8 h) stimulated the HSV-1-specific gBT-I transgenic CD8+ T cells (13) with syngeneic CD11c+ BM-DCs pulsed with the cognate gB peptide (gB-8p) in the presence or absence of CpG. Analysis of expression levels of the early activation markers CD69 and CD25 showed that BM-DCs treated with CpG and gB-8p up-regulated these early activation markers by at least 2-fold compared with peptide alone (Fig. 1, A and B).
FIGURE 1.
Pretreatment of BM-DCs with CpG enhances CD8+ T cell activation and TCR triggering compared with peptide alone. BM-DCs were treated with gB-8P peptide and/or CpG and incubated with gBT-I CD8+ T cells for 8 h. T cell activation was analyzed by FCM. A, MFI values of CD25 and CD69 in CD8+ T cells with and without CpG treatment. Neg, Negative. B, Representative Vβ8 down-regulation on activated (CD25+) cells in the presence or the absence of peptide and CpG. C, Dose response of Vβ8 down-regulation by gBT-I CD8+ T cells depicted as the percentage relative to unpulsed DCs for each treatment. Results representative of at least four experiments. *, p < 0.05.
To determine whether the enhanced activation of CD8+ T cells by CpG was mediated by quantitative or qualitative mechanisms, we next investigated the relative number of CD8+ TCRs triggered by CpG-treated BM-DCs. Given that TCR down-modulation provides a reliable means to estimate the number of TCRs triggered, we assessed the extent of Vβ8 down-regulation in gBT-I CD8+ T cells following coculture with BM-DCs. As shown in Fig. 1, B and C, treating BM-DCs with peptide and CpG resulted in the down-regulation of significantly more TCRs compared with BM-DCs treated with peptide alone, and that was the case over a range of peptide concentrations that varied from 10−5 to 10−9 M gB-8p (Fig 1C). By contrast, no TCR down-regulation was observed in the absence of peptide or with CpG alone (Fig. 1B, leftmost and rightmost panels). Moreover, significantly more CD8 T cells up-regulated CD25 and down-regulated TCR in the presence of CpG (see Fig. 1–Fig 3). We conclude that CpG led to both quantitative and qualitative increases in CD8 activation.
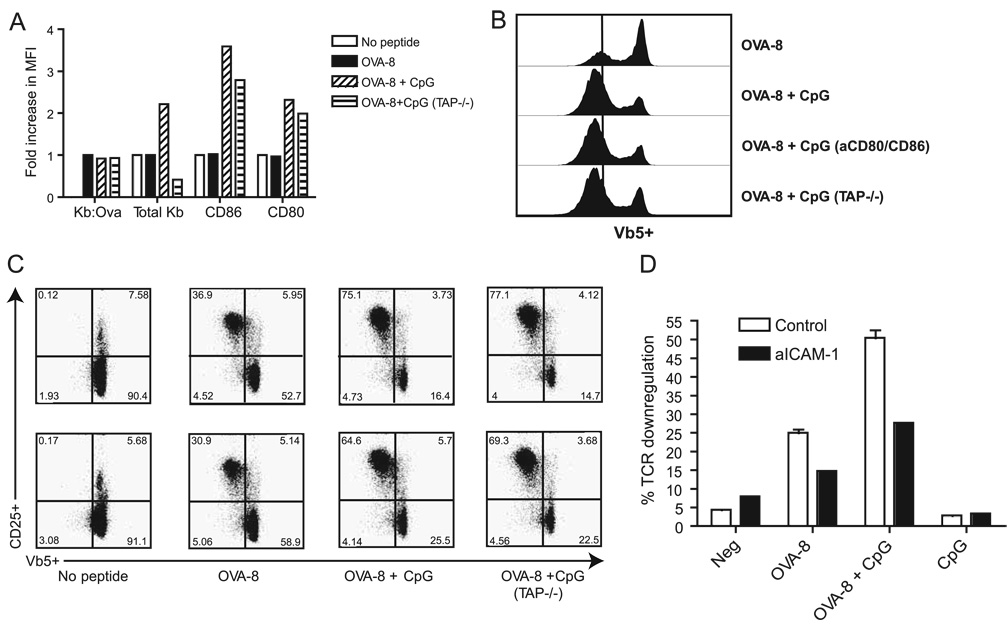
FIGURE 3.
Endogenous pMHCs and costimulatory and adhesion molecules are not responsible for increased TCR triggering by CpG-treated BM-DCs. BM-DCs isolated from wild-type and TAP−/− mice were treated with CpG and/or OVA-8. A, Expression levels of MHC and costimulatory molecules were quantitated and expressed as in Fig. 2A. B, TCR down-regulation on OT-I CD8+ T cells cocultured with BM-DCs from A. C, Representation of Vβ5−CD25+ on CD8 T cells from B. In some experiments, BM-DCs were pretreated with anti-CD80 and anti-CD86 (aCD80/CD86) (B) or anti-CD54 (aICAM-1) (D) before the addition of CD8+T cells to block the B7 or ICAM-1 costimulatory axis. Results in D are shown as percentage of CD8 cells undergoing TCR down-regulation. Similar results were obtained in at least two experiments. Neg, Negative.
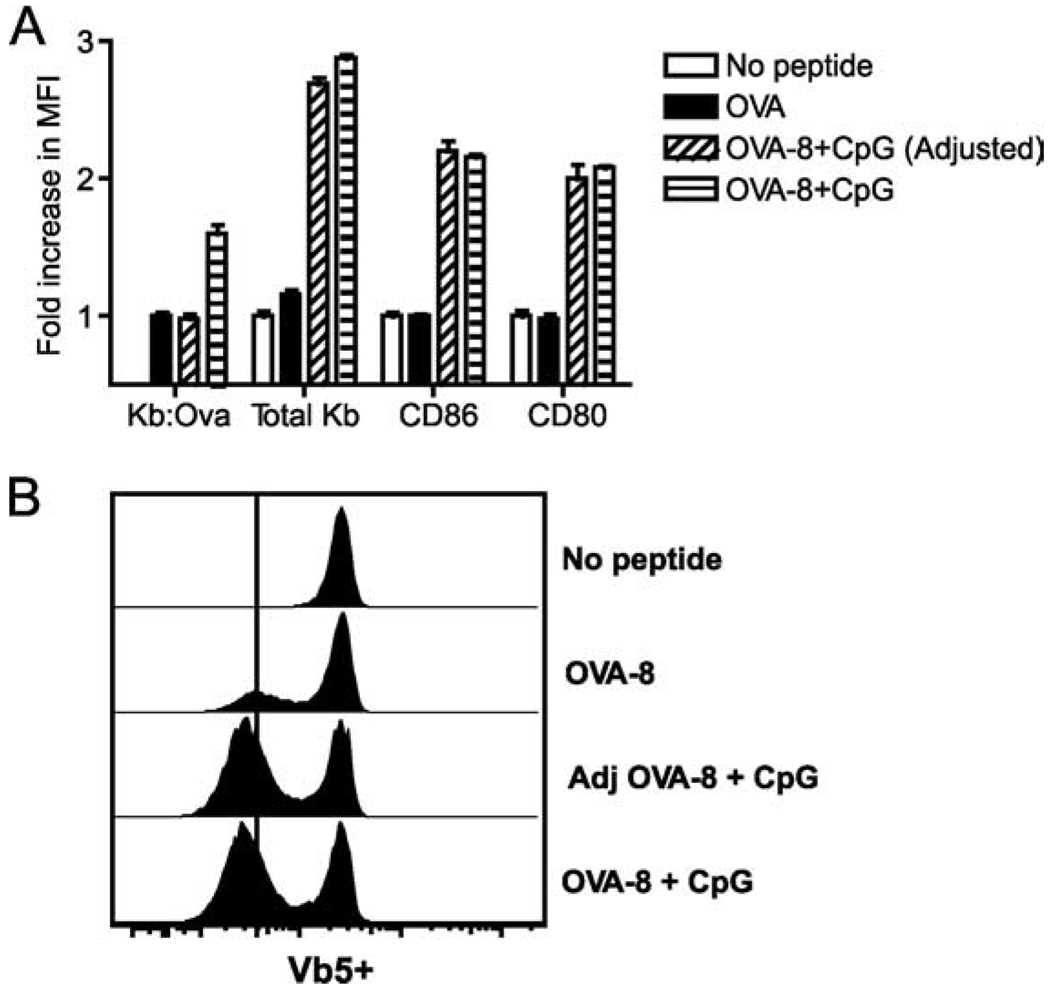
It is plausible that CpG could improve serial triggering by up-regulating MHC class I molecules on DC, allowing loading of a larger number of these molecules with the cognate peptide. This would produce an increased “determinant density”, i.e., an increase in the number of cognate pMHC complexes and the consequent increase in TCR occupancy/down-regulation. If so, equalization of the amount of cognate pMHC on the cell surface between CpG-treated and control BM-DCs should abrogate the differences in TCR triggering and down-regulation. To test this possibility and to ensure that our results are not an artifact specific for a single TCR, we used OT-I cells and the 25-D16 mAb that specifically recognizes Kb-OVA complexes (10). We adjusted the amount of the OVA-8 peptide added so that equivalent amounts (as judged by MFI) of Kb-OVA complexes were displayed on the surface of all BM-DC populations (Fig. 2A). The adjustment was in the range of 2- to 3-fold less peptide in the presence of CpG and concentrations in the range of 10−7 M. We then assessed the ability of these “cognate pMHC-normalized” BM-DCs to down-modulate TCRs in CD8+ T cells purified from OT-1 mice and observed that CpG-treated BM-DCs not only still recruited more CD8 T cells into the response, but still triggered more CD8+ TCRs (Fig 2B, lower two panels, compare the intensity of the down-regulation of OVA-8, the MFI on the vertical line, to the MFI of cells in the presence of CpG, which falls to the left of the line). These results demonstrate that the increase in TCR down-regulation in CpG-treated BM-DCs is not due to the increased expression of cognate pMHCs on the surface.
FIGURE 2.
The amount of cognate pMHC complexes does not determine the increased triggering by CpG-treated DC. A, Expression levels of MHC and costimulatory molecules are shown in BM-DCs treated with no peptide, OVA-8, OVA-8 plus CpG (OVA-8+CpG), or OVA-8 plus CpG with adjustment for specific Kb-OVA-8 expression (OVA-8+CpG (Adjusted)) as described in the text. Values are shown as mean fold increases in MFI ± SEM compared with values observed in BM-DCs treated with peptide alone. B, TCR down-regulation on OT-I CD8+ T cells cocultured with BM-DCs from A. Similar results were obtained in at least three separate experiments.
Up-regulation of endogenous pMHCs and costimulatory molecules by CpG are not responsible for the increase in TCR serial triggering
Even when cognate pMHC content was equalized, CpG-treated BM-DCs still expressed higher levels of total Kb (non-cognate pMHCs) and of the costimulatory molecules CD80 and CD86. We therefore next investigated the role of total (endogenous) pMHC in enhancing CD8+ T cell responses by CpG. Precedent for this comes from studies where weak engagements of TCR by self-pMHC were found to lower TCR signaling thresholds and/or promote TCR signal spreading in both CD4+ and CD8+ T cells activated by a few cognate pMHC complexes in the periphery (14–16). To examine this possibility, we generated BM-DCs from TAP1−/− mice that express very low levels of endogenous pMHCs. The amount of OVA-8 peptide was again adjusted so that all BM-DCs examined expressed equivalent amounts of Kb-OVA (cognate peptide) (Fig. 3A). Despite expressing only very low levels of endogenous pMHCs (20× lower than wild-type BM-DC; data not shown), TAP1−/− BM-DCs treated with CpG showed no impairments in down-regulating TCRs on CD8+ T cells purified from OT-1 mice (Fig. 3, B and C).
Because all BM-DCs treated with CpG expressed elevated levels of costimulatory and adhesion molecules, we next asked whether increased signaling through these molecules may enable more TCRs to be triggered. BM-DCs were stimulated overnight as before and blocking anti-CD80 and anti-CD86 Abs were added 30 min before the addition of CD8+ T cells. Despite a complete block in the CD28/B7 costimulatory axis (as judged by complete abrogation of fluorescent staining of “blocked” cells; data not shown), CpG-treated DCs were still able to trigger many more TCRs than BM-DCs treated with peptide alone (Fig. 3C). These results are consistent with previous reports demonstrating that CD28/B7 costimulation does not operate by increasing the number of TCRs triggered but rather by other mechanisms (17). ICAM-1 (CD54) is the major adhesion molecule up-regulated by CpG in BM-DCs and is known to be critical in stabilizing CD8+T cell/DC interactions (18). To determine whether the expression levels of ICAM-1 in CpG-treated BM-DCs contributes to the increase in TCR down-regulation, ICAM-1 was also blocked with Ab before the addition of CD8+ T cells as before. This blocking resulted in a general impairment in TCR down-regulation compared with controls (Fig. 3D), but the CpG-stimulated BM-DCs still maintained their ability to down-regulate nearly 2-fold more TCRs than control BM-DCs (Fig. 3D). Overall, these results suggest that the greater number of TCRs triggered by CpG-treated BM-DCs cannot be explained by either the increased levels of the noncognate pMHCs or the up-regulation of the major costimulatory and adhesion molecules. In other experiments (not shown) we found that the cytokines IL-12p40 and IFN-I also are not involved in increased TCR triggering, based on experiments using DCs deficient in the above molecules.
Slower decay rates of pMHCs correlate with increased serial triggering and enhanced effector functions of CD8+ T cells
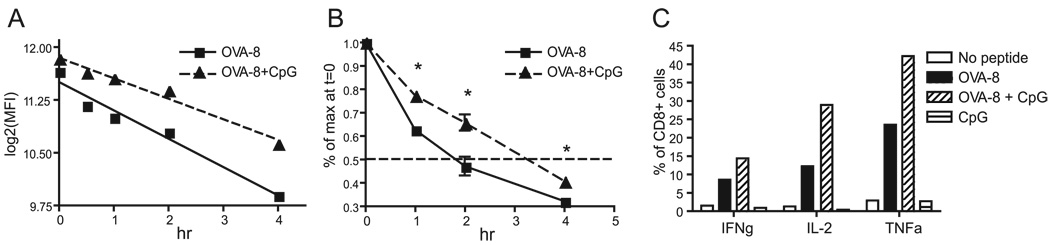
Previous reports indicate that inflammatory stimuli can modulate expression pMHC complexes by extending the half-lives of MHC molecules expressed on the surface of mature DC (19–21). We therefore hypothesized that CpG/OVA-8 pulsed BM-DCs may stabilize more pMHCs on the surface throughout the incubation period, thus allowing more TCRs on CD8+ T cells to be triggered. To examine the stability of Kb-OVA expressed on the surface of DCs, BM-DCs were stimulated and washed as before, and total amounts of Kb-OVA (MFI) were quantitated and expressed as log2 (MFI) or as percentage of initial maximal binding at various time points. Treatment of BM-DCs with CpG resulted in a greater persistence of Kb-OVA expression on the surface of these cells (Fig. 4, A and B). This enhanced stabilization of pMHCs by CpG would enable many more pMHCs to trigger TCR over time.
FIGURE 4.
BM-DCs stimulated with CpG exhibit increased MHC class I half-lives. A, BM-DCs were treated with OVA-8 with or without CpG overnight and washed, and relative expression levels (log2(MFI)) of Kb-OVA (25.D16) were quantitated and transformed at various times as indicated. B, Data from separate experiments were combined and expressed as percentage of maximum binding at time 0 for normalization purposes. C, Cytokine production (intracellular cytokine staining assay) 48 h after an 8-h stimulation of OT-I CD8+ T cells with BM-DCs from A. All results are representative of at least two experiments. IFNg, IFN-γ; TNFa, TNF-α.
Next, we sought to test whether the observed increase in TCR triggering by CpG-stimulated BM-DCs directly translated into enhanced CD8 effector functions. To that effect, BM-DCs were treated with equal amounts of OVA-8 and/or CpG as before and cocultured with CD8+ T cells in the presence of anti-CD80 and anti-CD86 blocking Abs. Following the 8 h incubation, the BM-DCs were removed from the coculture by immunomagnetic sorting and purified CD8+ T cells were cultured for an additional 2 days. Acquisition of effector functions was assessed by measuring the ability of these cells to secrete IFN-γ, TNF-α, and IL-2 when restimulated with OVA-8 peptide. As shown in Fig. 4C, CD8+ T cells stimulated for only 8 h with CpG-treated BM-DCs differentiated into nearly twice as many cytokine-producing cells, demonstrating a direct link among MHC decay rates, number of TCRs triggered, and enhanced effector functions.
Lastly, to extend and validate our findings, we examined serial triggering induced by BM-DCs pretreated with other TLR ligands. BM-DCs were pulsed with either peptide alone or in combination with LPS (0.01–10 µg/ml), poly(I:C) (0.5–50 µg/ml), or CpG (0.01–10 µM/ml) before being cocultured with CD8+ T cells. All TLR ligands promoted a greater number of TCRs triggered than BM-DCs pretreated with peptide alone across a wide range of concentrations (not shown), showing that increased serial triggering is likely an important mechanism for enhanced CD8 T cell activation by most TLR ligands. Although it is believed that TLR ligands act as adjuvants by the up-regulation of cognate pMHCs and costimulatory molecules, our data suggest that TCR sensitivity can also be significantly enhanced by slower MHC decay rates on TLR-stimulated DCs. It was recently shown that the amount of peptide (dictated by pMHC half-life) that is retained during DC migration correlates with the duration of early phases of T cell/DC interactions and T cell activation (22). Indeed, pMHC stability in DCs is likely an important factor in triggering the necessary number of TCRs and activation thresholds involved in stimulating CD8+ T cells. Therefore, in addition to other known or possible mechanisms by which TLR ligands improve priming, our results indicate that increased TCR triggering due to the increased cognate pMHC half-lives additionally contributes to efficacious CD8 priming, which could be relevant to T cell vaccination.
Acknowledgments
We thank the members of the Nikolich Laboratory for advice and support and the National Institute of Allergy and Infectious Diseases Tetramer Facility for outstanding tetramer production.
Footnotes
This work was supported by U.S. Public Health Service Grants AI-066096 (to J.N.-Ž.) and RR-0163 (to the Oregon National Primate Research Center) from the National Institute of Allergy and Infectious Diseases and National Center for Research Resources, National Institutes of Health.
Abbreviations used in this paper: pMHC, peptide-MHC complex; BM-DC, bone marrow-derived DC; DC, dendritic cell; FCM, flow cytofluorometric analysis; gB-8p, gB peptide; MFI, mean fluorescence intensity.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Eisen HN, Sykulev Y, Tsomides TJ. Antigen-specific T-cell receptors and their reactions with complexes formed by peptides with major histocompatibility complex proteins. Adv. Protein Chem. 1996;49:1–56. doi: 10.1016/s0065-3233(08)60487-8. [DOI] [PubMed] [Google Scholar]
- 2.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 3.Valitutti S, Lanzavecchia A. Serial triggering of TCRs: a basis for the sensitivity and specificity of antigen recognition. Immunol. Today. 1997;18:299–304. [PubMed] [Google Scholar]
- 4.Coombs D, Kalergis AM, Nathenson SG, Wofsy C, Goldstein B. Activated TCRs remain marked for internalization after dissociation from pMHC. Nat. Immunol. 2002;3:926–931. doi: 10.1038/ni838. [DOI] [PubMed] [Google Scholar]
- 5.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl. Acad. Sci. USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 8.Krieg AM. Development of TLR9 agonists for cancer therapy. J. Clin. Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Kaer LP, Ploegh HL, Ashton-Rickardt G, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+ T cells. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 10.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal Ab. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 11.Brien JD, Uhrlaub JL, Nikolich-Žugich J. Protective capacity and epitope specificity of CD8+ T cells responding to lethal West Nile virus infection. Eur. J. Immunol. 2007;37:1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 12.Dyall R, Vasovic LV, Molano A, Nikolić-Žugić J. CD4-independent in vivo priming of murine CTL by optimal MHC class I-restricted peptides derived from intracellular pathogens. Int. Immunol. 1995;7:1205–1212. doi: 10.1093/intimm/7.8.1205. [DOI] [PubMed] [Google Scholar]
- 13.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 14.Anikeeva N, Lebedeva T, Clapp AR, Goldman ER, Dustin ML, Mattoussi H, Sykulev Y. Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response. Proc. Natl. Acad. Sci. USA. 2006;103:16846–16851. doi: 10.1073/pnas.0607771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat. Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 17.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 18.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and DC determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno M, Citterio S, Thery C, Rittig M, Medaglini D, Pozzi G, Amigorena S, Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse DC. Proc. Natl. Acad. Sci. USA. 1998;95:5229–5234. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on DC. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 21.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J. Exp. Med. 2003;198:111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, et al. T cell sensing of antigen dose governs interactive behavior with DC and sets a threshold for T cell activation. Nat. Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]